Revisiting the Injury Mechanism of Goat Sperm Caused by the Cryopreservation Process from a Perspective of Sperm Metabolite Profiles
Abstract
:1. Introduction
2. Results
2.1. Metabolic Findings of Sperm Samples Using UHPLC-QTOF-MS
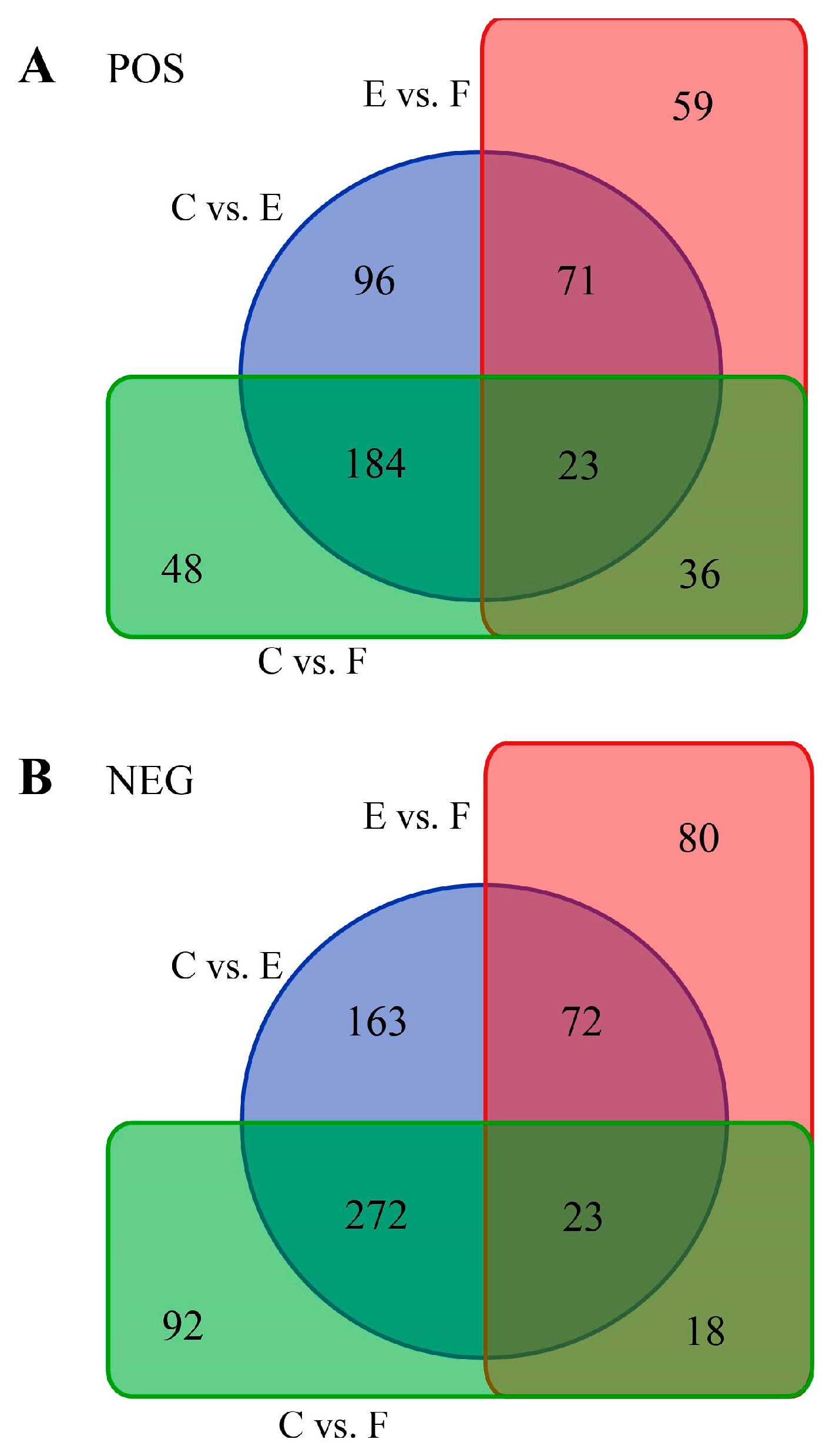
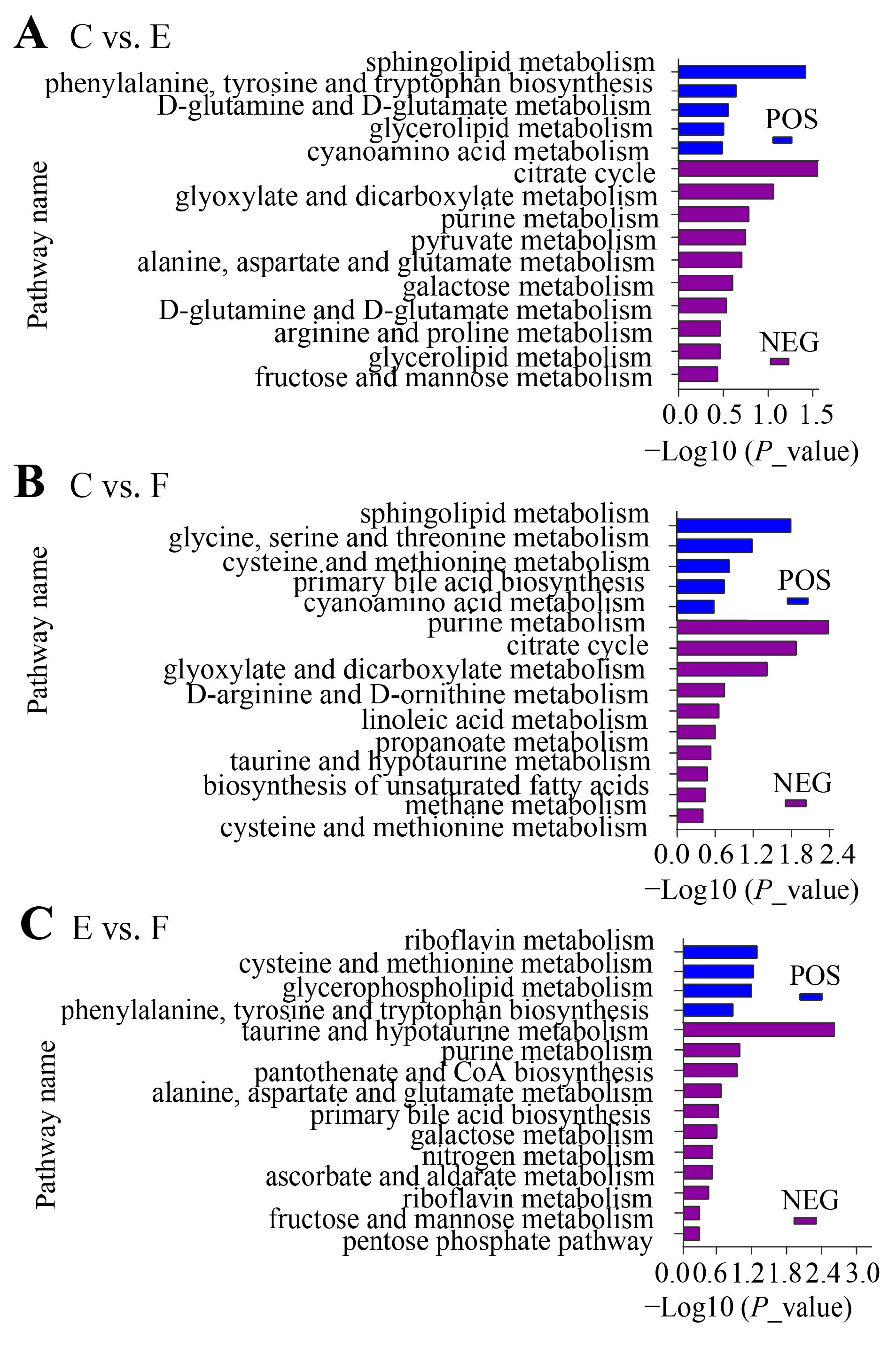
2.2. Identification and Functional Annotation of Differential Metabolites
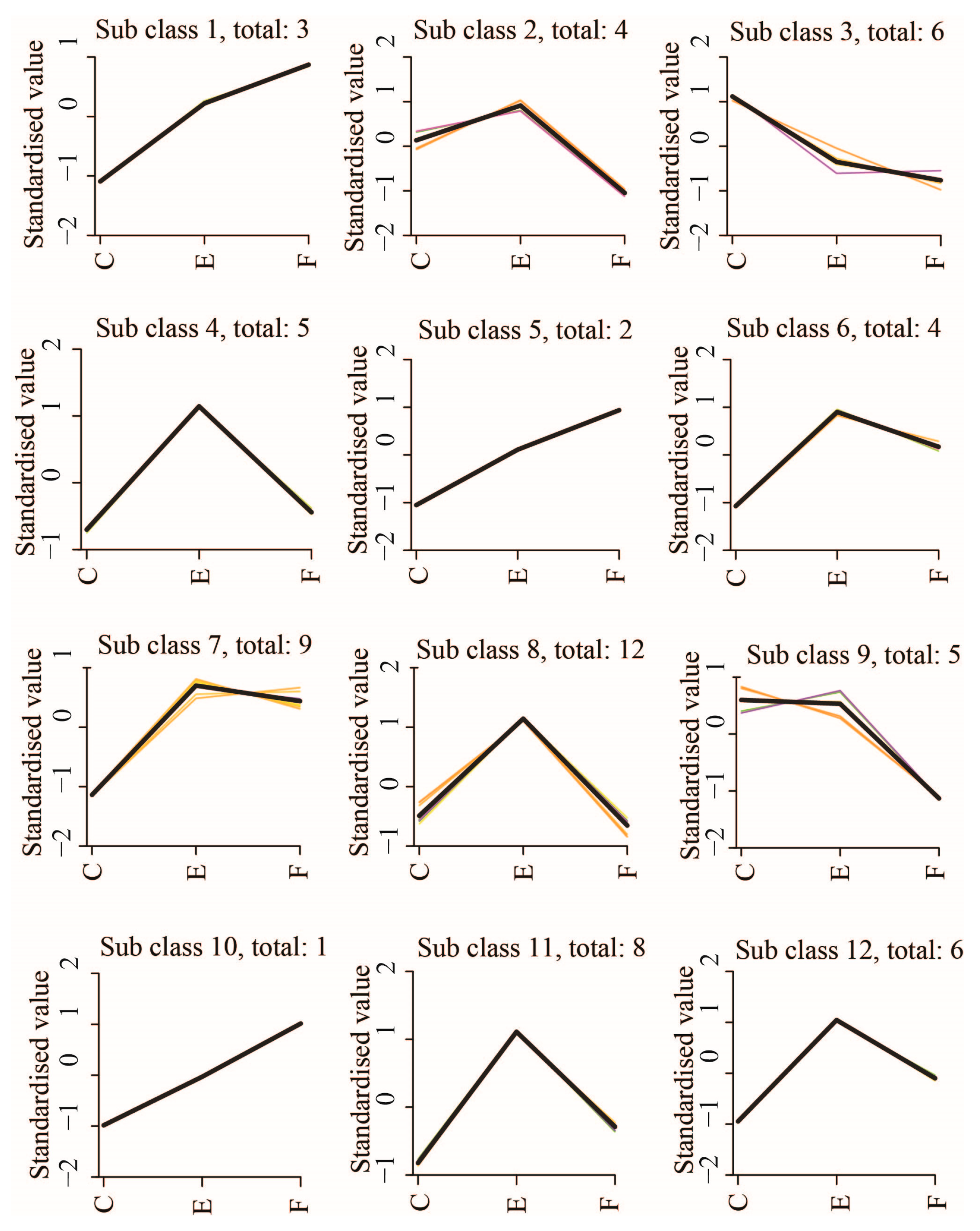
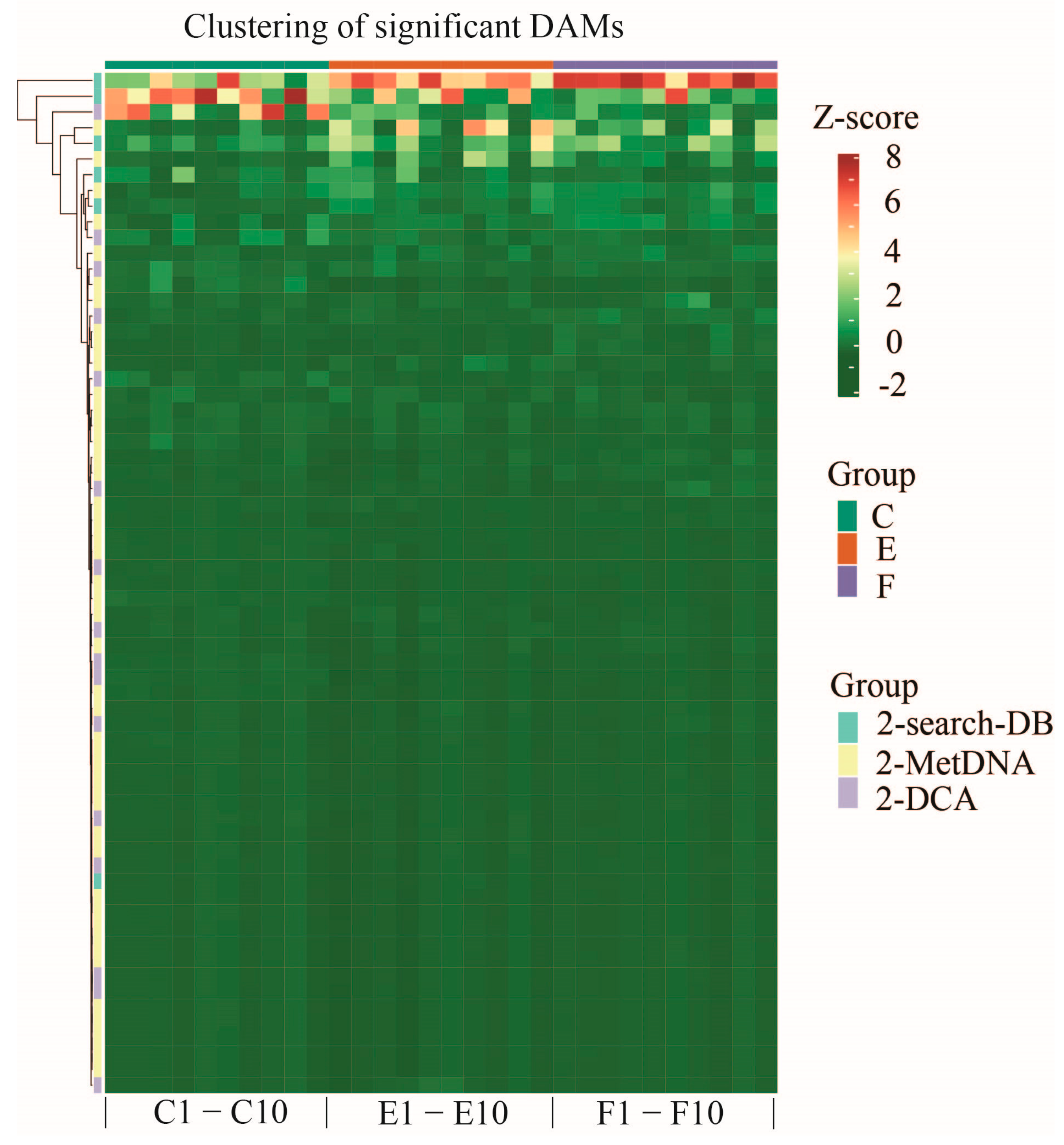
2.3. Classification and Hierarchical Clustering of Significantly DAMs
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample Collection
4.2. Cooling and Freeze-Thaw Processing
4.3. Extraction of Metabolite
4.4. UHPLC-QTOF-MS
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAMs | Differentially Abundant Metabolites |
| TCA | tricarboxylic acid |
| ATP | adenosine triphosphate |
| cAMP-PKA | cyclic adenosine monophosphate-dependent protein kinase |
| QC | quality control |
| UHPLC-QTOF-MS | ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry |
| POS | positive mode |
| PCA | principal component analysis |
| OPLS-DA | orthogonal Partial least square discriminant |
| ESI | electrospray ionization |
| ROS | reactive oxygen species |
| acetyl-CoA | acetyl coenzyme A |
| NADH | nicotinamide adenine dinucleotide |
| MUFAs | monounsaturated fatty acids |
| PUFAs | polyunsaturated fatty acids |
| LA | linoleic acid |
| AA | arachidonic acid |
| DHA | docosahexaenoic acid |
| PLA2 | phospholipase A2 |
| L-Cth | L-cystathionine |
| GSH-Px | glutathione peroxidase |
| SOD | superoxide dismutase |
| CAT | catalase |
| MDA | malondialdehyde |
References
- Arunkumar, R.; Kumaresan, A.; Sinha, M.K.; Elango, K.; Ebenezer, S.K.J.; Nag, P.; Karuthadurai, T.; Baithalu, R.K.; Mohanty, T.K.; Kumar, R.; et al. The cryopreservation process induces alterations in proteins associated with bull sperm quality: The equilibration process could be a probable critical control point. Front. Endocrinol. 2022, 13, 1064956. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Meng, L.; Liang, L.; Zhang, C. Advantages of vitrification preservation in assisted reproduction and potential influences on imprinted genes. Clin. Epigenet. 2022, 14, 141. [Google Scholar] [CrossRef]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Sathe, S.R. Laparoscopic artificial insemination technique in small ruminants-a procedure review. Front. Vet. Sci. 2018, 5, 266. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Li, C.; Ren, C.; Chen, Y.; Wang, M.; Tang, J.; Zhang, Y.; Wang, Q.; Zhang, Z. Changes on proteomic and metabolomic profiling of cryopreserved sperm effected by melatonin. J. Proteom. 2023, 273, 104791. [Google Scholar] [CrossRef]
- Upadhyay, V.R.; Ramesh, V.; Dewry, R.K.; Kumar, G.; Raval, K.; Patoliya, P. Implications of cryopreservation on structural and functional attributes of bovine spermatozoa: An overview. Andrologia 2021, 53, e14154. [Google Scholar] [CrossRef]
- Xu, B.; Wang, R.; Wang, Z.; Liu, H.; Wang, Z.; Zhang, W.; Zhang, Y.; Su, R.; Liu, Z.; Liu, Y.; et al. Evaluation of lipidomic change in goat sperm after cryopreservation. Front. Vet. Sci. 2022, 9, 1004683. [Google Scholar] [CrossRef]
- Salmon, V.M.; Leclerc, P.; Bailey, J.L. Cholesterol-loaded cyclodextrin increases the cholesterol content of goat sperm to improve cold and osmotic resistance and maintain sperm function after cryopreservation. Biol. Reprod. 2016, 94, 85. [Google Scholar] [CrossRef]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: Changes in spermatid organelles associated with development of spermatozoa. Microsc. Res. Tech. 2010, 73, 279–319. [Google Scholar] [CrossRef]
- Chauvin, T.; Xie, F.; Liu, T.; Nicora, C.D.; Yang, F.; Camp, D.N.; Smith, R.D.; Roberts, K.P. A systematic analysis of a deep mouse epididymal sperm proteome. Biol. Reprod. 2012, 87, 141. [Google Scholar] [CrossRef] [PubMed]
- Pena, F.J.; Ortiz-Rodriguez, J.M.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega-Ferrusola, C.; Martin-Cano, F.E. An integrated overview on the regulation of sperm metabolism (glycolysis-Krebs cycle-oxidative phosphorylation). Anim. Reprod. Sci. 2022, 246, 106805. [Google Scholar] [CrossRef]
- Du-Plessis, S.S.; Agarwal, A.; Mohanty, G.; Van-Der-Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Martin-Cano, F.E.; Gaitskell-Phillips, G.; Ortiz-Rodriguez, J.M.; Silva-Rodriguez, A.; Roman, A.; Rojo-Dominguez, P.; Alonso-Rodriguez, E.; Tapia, J.A.; Gil, M.C.; Ortega-Ferrusola, C.; et al. Proteomic profiling of stallion spermatozoa suggests changes in sperm metabolism and compromised redox regulation after cryopreservation. J. Proteom. 2020, 221, 103765. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chu, M.; Ma, X.; Pei, J.; Xiong, L.; Bao, P.; La, Y.; Liang, C.; Guo, X.; Wu, X.; et al. Comparative iTRAQ proteomics identified proteins in fresh and frozen thawed yak spermatozoa. Int. J. Biol. Macromol. 2023, 246, 125728. [Google Scholar] [CrossRef]
- He, Q.; Gao, F.; Wu, S.; Wang, S.; Xu, Z.; Xu, X.; Lan, T.; Zhang, K.; Quan, F. Alkaline dilution alters sperm motility in dairy goat by affecting sAC/cAMP/PKA Pathway activity. Int. J. Mol. Sci. 2023, 24, 1771. [Google Scholar] [CrossRef]
- Ould, A.Y.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys. Acta Bioenerg. 2018, 1859, 868–877. [Google Scholar] [CrossRef]
- Harrison, R.A.; Miller, N.G. cAMP-dependent protein kinase control of plasma membrane lipid architecture in boar sperm. Mol. Reprod. Dev. 2000, 55, 220–228. [Google Scholar] [CrossRef]
- Correnti, S.; Preiano, M.; Fregola, A.; Gamboni, F.; Stephenson, D.; Savino, R.; D’Alessandro, A.; Terracciano, R. Seminal plasma untargeted metabolomic and lipidomic profiling for the identification of a novel panel of biomarkers and therapeutic targets related to male infertility. Front. Pharmacol. 2023, 14, 1275832. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Kooshesh, L.; Nateghian, Z.; Aliabadi, E. Evaluation of L-carnitine potential in improvement of male fertility. J. Reprod. Infertil. 2023, 24, 69–84. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Li, Y.; Qin, S.; Cui, W.; Zhao, F.; He, M.; Jiang, Z. Progress on the roles of zinc in sperm cryopreservation. Theriogenology 2023, 211, 134–141. [Google Scholar] [CrossRef]
- Tamburrino, L.; Traini, G.; Marcellini, A.; Vignozzi, L.; Baldi, E.; Marchiani, S. Cryopreservation of human spermatozoa: Functional, molecular and clinical aspects. Int. J. Mol. Sci. 2023, 24, 4656. [Google Scholar] [CrossRef]
- Santiani, A.; Evangelista, S.; Sepulveda, N.; Risopatron, J.; Villegas, J.; Sanchez, R. Addition of superoxide dismutase mimics during cooling process prevents oxidative stress and improves semen quality parameters in frozen/thawed ram spermatozoa. Theriogenology 2014, 82, 884–889. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martin-Maestro, A.; Sanchez-Ajofrin, I.; Medina-Chavez, D.A.; Fernandez-Santos, M.R.; Garcia-Alvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm cryodamage in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Jia, B.; Larbi, A.; Lv, C.; Liang, J.; Xiang, D.; Zhang, B.; Fang, Y.; Shen, W.; Wu, G.; Quan, G. Identification and validation of ram sperm proteins associated with cryoinjuries caused by the cryopreservation process. Theriogenology 2022, 184, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, W.; Liu, C.; Wang, C.; Wang, D.; Zhu, S.; Kang, X.; Jiang, R.; Deng, L.; Li, D.; et al. Transcriptome analysis of the testes of male chickens with high and low sperm motility. Poult. Sci. 2022, 101, 102183. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, T.; Li, H.; Yan, N. Sphingomyelin synthase 2 participate in the regulation of sperm motility and apoptosis. Molecules 2020, 25, 4231. [Google Scholar] [CrossRef]
- Nissen, H.P.; Kreysel, H.W. Polyunsaturated fatty acids in relation to sperm motility. Andrologia 1983, 15, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, B.J.; Wang, S.Q.; Xu, Y.; Han, P.; Li, P.C.; Wang, Z.J.; Song, N.H.; Zhang, W.; Yin, C.J. The role of mitochondrial aconitate (ACO2) in human sperm motility. Syst. Biol. Reprod. Med. 2014, 60, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Becci, A.; Beolchini, F. Citric acid bioproduction: The technological innovation change. Crit. Rev. Biotechnol. 2020, 40, 199–212. [Google Scholar] [CrossRef]
- Hu, P.; Yuan, M.; Guo, B.; Lin, J.; Yan, S.; Huang, H.; Chen, J.L.; Wang, S.; Ma, Y. Citric Acid Promotes Immune Function by Modulating the Intestinal Barrier. Int. J. Mol. Sci. 2024, 25, 1239. [Google Scholar] [CrossRef]
- Krauze, M.; Cendrowska-Pinkosz, M.; Matusevicius, P.; Stepniowska, A.; Jurczak, P.; Ognik, K. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef]
- Aurich, A.; Specht, R.; Muller, R.A.; Stottmeister, U.; Yovkova, V.; Otto, C.; Holz, M.; Barth, G.; Heretsch, P.; Thomas, F.A.; et al. Microbiologically produced carboxylic acids used as building blocks in organic synthesis. Subcell. Biochem. 2012, 64, 391–423. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Karpukhina, O.V.; Kamzolova, S.V.; Samoilenko, V.A.; Inozemtsev, A.N. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep. Biochem. Biotechnol. 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Wassall, S.R.; Stillwell, W. Polyunsaturated fatty acid-cholesterol interactions: Domain formation in membranes. Biochim. Biophys. Acta 2009, 1788, 24–32. [Google Scholar] [CrossRef]
- Roh, K.B.; Jung, E.; Park, D.; Lee, J. Fumaric acid attenuates the eotaxin-1 expression in TNF-alpha-stimulated fibroblasts by suppressing p38 MAPK-dependent NF-ĸB signaling. Food Chem. Toxicol. 2013, 58, 423–431. [Google Scholar] [CrossRef]
- Litjens, N.H.; van Strijen, E.; van Gulpen, C.; Mattie, H.; van Dissel, J.T.; Thio, H.B.; Nibbering, P.H. In vitro pharmacokinetics of anti-psoriatic fumaric acid esters. BMC Pharmacol. 2004, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Blank, R.; Mosenthin, R.; Sauer, W.C.; Huang, S. Effect of fumaric acid and dietary buffering capacity on ileal and fecal amino acid digestibilities in early-weaned pigs. J. Anim. Sci. 1999, 77, 2974–2984. [Google Scholar] [CrossRef]
- Saratsi, A.; Samartzi, F.; Panagiotidis, I.; Basioura, A.; Tsiokos, D.; Ligda, C.; Rekkas, C.A. Post-thaw parameters of buck semen quality after soy lecithin extender supplementation with fumaric acid. Vet. Sci. 2023, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yuan, J.; Li, J.; Li, H.; Yin, K.; Wang, F.; Li, D. Overweight and underweight status are linked to specific gut microbiota and intestinal tricarboxylic acid cycle intermediates. Clin. Nutr. 2020, 39, 3189–3198. [Google Scholar] [CrossRef]
- Yan, E.; Wang, Y.; He, L.; Guo, J.; Zhang, X.; Yin, J. Effects of dietary l-malic acid supplementation on meat quality, antioxidant capacity and muscle fiber characteristics of finishing pigs. Foods 2022, 11, 3335. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Dong, X.; Hu, G.; Liu, L. Engineering rTCA pathway and C4-dicarboxylate transporter for L-malic acid production. Appl. Microbiol. Biotechnol. 2017, 101, 4041–4052. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Ye, B.; Yan, H.; Zhao, Y.; Liu, L. Effect of unsaturated fatty acids on glycation product formation pathways. Food Res. Int. 2021, 143, 110288. [Google Scholar] [CrossRef]
- Santos, J.E.; Bilby, T.R.; Thatcher, W.W.; Staples, C.R.; Silvestre, F.T. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Su, X.; Han, X.; Wen, H.; Cheng, C.; Zhang, S.; Li, W.; Cai, J.; Zheng, L.; Ma, J.; et al. Unsaturated fatty acids in mental disorders: An umbrella review of meta-analyses. Adv. Nutr. 2022, 13, 2217–2236. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef]
- Jakop, U.; Svetlichnyy, V.; Schiller, J.; Schulze, M.; Schroeter, F.; Mueller, K. In vitro supplementation with unsaturated fatty acids improves boar sperm viability after storage at 6 degrees C. Anim. Reprod. Sci. 2019, 206, 60–68. [Google Scholar] [CrossRef]
- Aurich, C.; Ortega, F.C.; Pena, V.F.; Schrammel, N.; Morcuende, D.; Aurich, J. Seasonal changes in the sperm fatty acid composition of Shetland pony stallions. Theriogenology 2018, 107, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Van Tran, L.; Malla, B.A.; Kumar, S.; Tyagi, A.K. Polyunsaturated fatty acids in male ruminant reproduction—A review. Asian-Australas. J. Anim. Sci 2017, 30, 622–637. [Google Scholar] [CrossRef]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated fatty acids drive lipid peroxidation during ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Hoxha, M.; Barbonetti, A.; Zappacosta, B. Arachidonic acid pathways and male fertility: A systematic review. Int. J. Mol. Sci. 2023, 24, 8207. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, X.; Ma, B.; Ying, H.; Shang, X.; He, B.; Zhang, Q. Abnormal arachidonic acid metabolic network may reduce sperm motility via P38 MAPK. Open Biol. 2019, 9, 180091. [Google Scholar] [CrossRef]
- Ofosu, J.; Nartey, M.A.; Mo, X.; Ye, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Fang, Y.; Zhou, G. Ram sperm cryopreservation disrupts metabolism of unsaturated fatty acids. Theriogenology 2023, 204, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.S.; Machado, W.M.; Da, F.C.; Mugabe, L.C.; Pinheiro, E.; Carneiro, I.; Rocha, L.F.; Barbosa, L.P. Docosahexaenoic acid in diluent for goat semen cryopreservation. Anim. Reprod. 2021, 18, e20210027. [Google Scholar] [CrossRef] [PubMed]
- Bwanga, C.O.; Einarsson, S.; Rodriguez-Martinez, H. Cryopreservation of boar semen. II: Effect of cooling rate and duration of freezing point plateau on boar semen frozen in mini- and maxi-straws and plastic bags. Acta Vet. Scand. 1991, 32, 455–461. [Google Scholar] [CrossRef]
- Nasiri, A.H.; Towhidi, A.; Zeinoaldini, S. Combined effect of DHA and alpha-tocopherol supplementation during bull semen cryopreservation on sperm characteristics and fatty acid composition. Andrologia 2012, 44 (Suppl. S1), 550–555. [Google Scholar] [CrossRef]
- Bunay, J.; Gallardo, L.M.; Torres-Fuentes, J.L.; Aguirre-Arias, M.V.; Orellana, R.; Sepulveda, N.; Moreno, R.D. A decrease of docosahexaenoic acid in testes of mice fed a high-fat diet is associated with impaired sperm acrosome reaction and fertility. Asian J. Androl. 2021, 23, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Mallela, S.K.; Merscher, S.; Fornoni, A. Implications of sphingolipid metabolites in kidney diseases. Int. J. Mol. Sci. 2022, 23, 4244. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.M.; Grataitong, K.; Liu, Y.B.; Wang, L.; Dai, X.; Chian, R.C. Dysregulated sphingolipid metabolism and autophagy in granulosa cells of women with endometriosis. Front. Endocrinol. 2022, 13, 906570. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, W.; Zhang, H.; Wen, X.; Yin, S.; Jia, Y. Integrated analysis of proteomics and metabolomics reveals the potential sex determination mechanism in odontobutis potamophila. J. Proteom. 2019, 208, 103482. [Google Scholar] [CrossRef]
- Sun, R.; Gu, X.; Lei, C.; Chen, L.; Chu, S.; Xu, G.; Doll, M.A.; Tan, Y.; Feng, W.; Siskind, L.; et al. Neutral ceramidase-dependent regulation of macrophage metabolism directs intestinal immune homeostasis and controls enteric infection. Cell Rep. 2022, 38, 110560. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Furuya, S. Roles of l-serine and sphingolipid synthesis in brain development and neuronal survival. Prog. Lipid Res. 2008, 47, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Murtas, G.; Marcone, G.L.; Sacchi, S.; Pollegioni, L. L-serine synthesis via the phosphorylated pathway in humans. Cell. Mol. Life Sci. 2020, 77, 5131–5148. [Google Scholar] [CrossRef]
- Matsumoto, K.; Banno, Y.; Murate, T.; Akao, Y.; Nozawa, Y. Localization of sphingosine kinase-1 in mouse sperm acrosomes. J. Histochem. Cytochem. 2005, 53, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Farley, S.; Stein, F.; Haberkant, P.; Tafesse, F.G.; Schultz, C. Trifunctional sphinganine: A new tool to dissect sphingolipid function. ACS Chem. Biol. 2024, 19, 336–347. [Google Scholar] [CrossRef]
- Ommati, M.M.; Ahmadi, H.N.; Sabouri, S.; Retana-Marquez, S.; Abdoli, N.; Rashno, S.; Niknahad, H.; Jamshidzadeh, A.; Mousavi, K.; Rezaei, M.; et al. Glycine protects the male reproductive system against lead toxicity via alleviating oxidative stress, preventing sperm mitochondrial impairment, improving kinematics of sperm, and blunting the downregulation of enzymes involved in the steroidogenesis. Environ. Toxicol. 2022, 37, 2990–3006. [Google Scholar] [CrossRef]
- Jha, K.N.; Salicioni, A.M.; Arcelay, E.; Chertihin, O.; Kumari, S.; Herr, J.C.; Visconti, P.E. Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol. Hum. Reprod. 2006, 12, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Peng, L.; Xu, J.; Guo, D.; Cao, W.; Xu, Y.; Li, S. Betaine attenuate chronic restraint stress-induced changes in testicular damage and oxidative stress in male mice. Reprod. Biol. Endocrinol. 2022, 20, 80. [Google Scholar] [CrossRef]
- Mori, N.; Ishihara, M.; Tasaki, H.; Sankai, T.; Otsuki, J. The effect of betaine for mouse sperm cryopreservation. Cryobiology 2022, 106, 157–159. [Google Scholar] [CrossRef]
- Elsheikh, N.; Omer, N.A.; Yi-Ru, W.; Mei-Qian, K.; Ilyas, A.; Abdurahim, Y.; Wang, G.L. Protective effect of betaine against lead-induced testicular toxicity in male mice. Andrologia 2020, 52, e13600. [Google Scholar] [CrossRef]
- Billah, M.M.; Khatiwada, S.; Lecomte, V.; Morris, M.J.; Maloney, C.A. Ameliorating high-fat diet-induced sperm and testicular oxidative damage by micronutrient-based antioxidant intervention in rats. Eur. J. Nutr. 2022, 61, 3741–3753. [Google Scholar] [CrossRef]
- Johnson, A.R.; Craciunescu, C.N.; Guo, Z.; Teng, Y.W.; Thresher, R.J.; Blusztajn, J.K.; Zeisel, S.H. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 2010, 24, 2752–2761. [Google Scholar] [CrossRef]
- Hanley, P.J. Elusive physiological role of prostatic acid phosphatase (PAP): Generation of choline for sperm motility via auto-and paracrine cholinergic signaling. Front. Physiol. 2023, 14, 1327769. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhu, M.; Kong, W.; Tang, C.; Du, J.; Huang, Y.; Jin, H. L-cystathionine protects against oxidative stress and DNA damage induced by oxidized low density lipoprotein in THP-1-derived macrophages. Front. Pharmacol. 2023, 14, 1161542. [Google Scholar] [CrossRef]
- Zhu, M.; Du, J.; Chen, S.; Liu, A.; Holmberg, L.; Chen, Y.; Zhang, C.; Tang, C.; Jin, H. l-cystathionine inhibits the mitochondria-mediated macrophage apoptosis induced by oxidized low density lipoprotein. Int. J. Mol. Sci. 2014, 15, 23059–23073. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Y.P.; Wu, H.; Li, Y.; Zhang, S.; Ke, J.; Yao, J.Y. Characterization of tea (Camellia sinensis L.) flower extract and insights into its antifungal susceptibilities of aspergillus flavus. BMC Complement. Med. Ther. 2023, 23, 286. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. alpha-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, M.S.; Askari, V.R.; Ghorani, V.; Jelodar, G.A.; Boskabady, M.H. The effect of Portulaca oleracea and alpha-linolenic acid on oxidant/antioxidant biomarkers of human peripheral blood mononuclear cells. Indian J. Pharmacol. 2018, 50, 177–184. [Google Scholar] [CrossRef]
- Steinritz, D.; Schmidt, A.; Simons, T.; Ibrahim, M.; Morguet, C.; Balszuweit, F.; Thiermann, H.; Kehe, K.; Bloch, W.; Bölck, B. Chlorambucil (nitrogen mustard) induced impairment of early vascular endothelial cell migration—Effects of α-linolenic acid and N-acetylcysteine. Chem.-Biol. Interact. 2014, 219, 143–150. [Google Scholar] [CrossRef]
- Pal, M.; Ghosh, M. Studies on comparative efficacy of alpha-linolenic acid and alpha-eleostearic acid on prevention of organic mercury-induced oxidative stress in kidney and liver of rat. Food Chem. Toxicol. 2012, 50, 1066–1072. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Siegel, I.; Dudkiewicz, A.B.; Friberg, J.; Suarez, M.; Gleicher, N. Inhibition of sperm motility and agglutination of sperm cells by free fatty acids in whole semen. Fertil. Steril. 1986, 45, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kaka, A.; Wahid, H.; Rosnina, Y.; Yimer, N.; Khumran, A.M.; Sarsaifi, K.; Behan, A.A.; Kaka, U.; Ebrahimi, M. alpha-Linolenic acid supplementation in BioXcell(R) extender can improve the quality of post-cooling and frozen-thawed bovine sperm. Anim. Reprod. Sci. 2015, 153, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Kim, W.H.; Cheong, H.T.; Yang, B.K.; Park, C.K. Effect of alpha-linolenic acid with bovine serum albumin or methyl-beta-cyclodextrin on membrane integrity and oxidative stress of frozen-thawed boar sperm. Dev. Reprod. 2019, 23, 11–19. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase metabolites of alpha-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016, 6, 31649. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Hale, B.J.; Bhowmick, D.; Miller, W.J.; Neufer, P.D.; Geyer, C.B. Pyruvate modulation of redox potential controls mouse sperm motility. Dev. Cell 2024, 59, 79–90. [Google Scholar] [CrossRef]
- Pohanka, M. D-lactic acid as a metabolite: Toxicology, diagnosis, and detection. Biomed. Res. Int. 2020, 17, 3419034. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Mizushima, S.; Hiyama, G.; Hirohashi, N.; Shiba, K.; Inaba, K.; Suzuki, T.; Dohra, H.; Ohnishi, T.; Sato, Y.; et al. Lactic acid is a sperm motility inactivation factor in the sperm storage tubules. Sci. Rep. 2015, 5, 17643. [Google Scholar] [CrossRef]
- Jia, B.; Memon, S.; Liang, J.; Lv, C.; Hong, Q.; Wu, G.; Quan, G. Trehalose modifies the protein profile of ram spermatozoa during cryopreservation. Theriogenology 2021, 171, 21–29. [Google Scholar] [CrossRef]
- Liu, T.; Han, Y.; Zhou, T.; Zhang, R.; Chen, H.; Chen, S.; Zhao, H. Mechanisms of ROS-induced mitochondria-dependent apoptosis underlying liquid storage of goat spermatozoa. Aging 2019, 11, 7880–7898. [Google Scholar] [CrossRef] [PubMed]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Diaz, S.; Grande-Perez, S.; Arce-Lopez, S.; Tamargo, C.; Olegario, H.C.; Perez-Cerezales, S. Changes in the cellular distribution of tyrosine phosphorylation and its relationship with the acrosomal exocytosis and plasma membrane integrity during in vitro capacitation of frozen/thawed bull spermatozoa. Int. J. Mol. Sci. 2020, 21, 2725. [Google Scholar] [CrossRef] [PubMed]
- Vozaf, J.; Svoradova, A.; Balazi, A.; Vasicek, J.; Olexikova, L.; Dujickova, L.; Makarevich, A.V.; Jurcik, R.; Duranova, H.; Chrenek, P. The Cryopreserved sperm traits of various ram breeds: Towards biodiversity conservation. Animals 2022, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Wu, Q.; Battino, M.; Bai, W.; Tian, L. Using untargeted metabolomics to profile the changes in roselle (Hibiscus sabdariffa L.) anthocyanins during wine fermentation. Food Chem. 2021, 364, 130425. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Geng, A.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, X.; Zhang, Y.; Liu, H. UHPLC-QTOF/MS-based comparative metabolomics in pectoralis major of fast-and slow-growing chickens at market ages. Food Sci. Nutr. 2022, 10, 487–498. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, L.; Zhang, T.; Ye, X.; Huang, F.; Huang, Q.; Huang, X.; Wu, J.; Zeng, J. UHPLC/MS-Based serum metabolomics reveals the mechanism of radiation-induced thrombocytopenia in mice. Int. J. Mol. Sci. 2022, 23, 7978. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin inhibits formation of mitochondrial permeability transition pores and improves oxidative phosphorylation of frozen-thawed ram sperm. Front. Endocrinol. 2019, 10, 896. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Lv, C.; Larbi, A.; Liang, J.; Yang, Q.; Wu, G.; Quan, G. Revisiting the Injury Mechanism of Goat Sperm Caused by the Cryopreservation Process from a Perspective of Sperm Metabolite Profiles. Int. J. Mol. Sci. 2024, 25, 9112. https://doi.org/10.3390/ijms25169112
Li C, Lv C, Larbi A, Liang J, Yang Q, Wu G, Quan G. Revisiting the Injury Mechanism of Goat Sperm Caused by the Cryopreservation Process from a Perspective of Sperm Metabolite Profiles. International Journal of Molecular Sciences. 2024; 25(16):9112. https://doi.org/10.3390/ijms25169112
Chicago/Turabian StyleLi, Chunyan, Chunrong Lv, Allai Larbi, Jiachong Liang, Qige Yang, Guoquan Wu, and Guobo Quan. 2024. "Revisiting the Injury Mechanism of Goat Sperm Caused by the Cryopreservation Process from a Perspective of Sperm Metabolite Profiles" International Journal of Molecular Sciences 25, no. 16: 9112. https://doi.org/10.3390/ijms25169112





