Dietary Exposure to Pesticide and Veterinary Drug Residues and Their Effects on Human Fertility and Embryo Development: A Global Overview
Abstract
1. Introduction
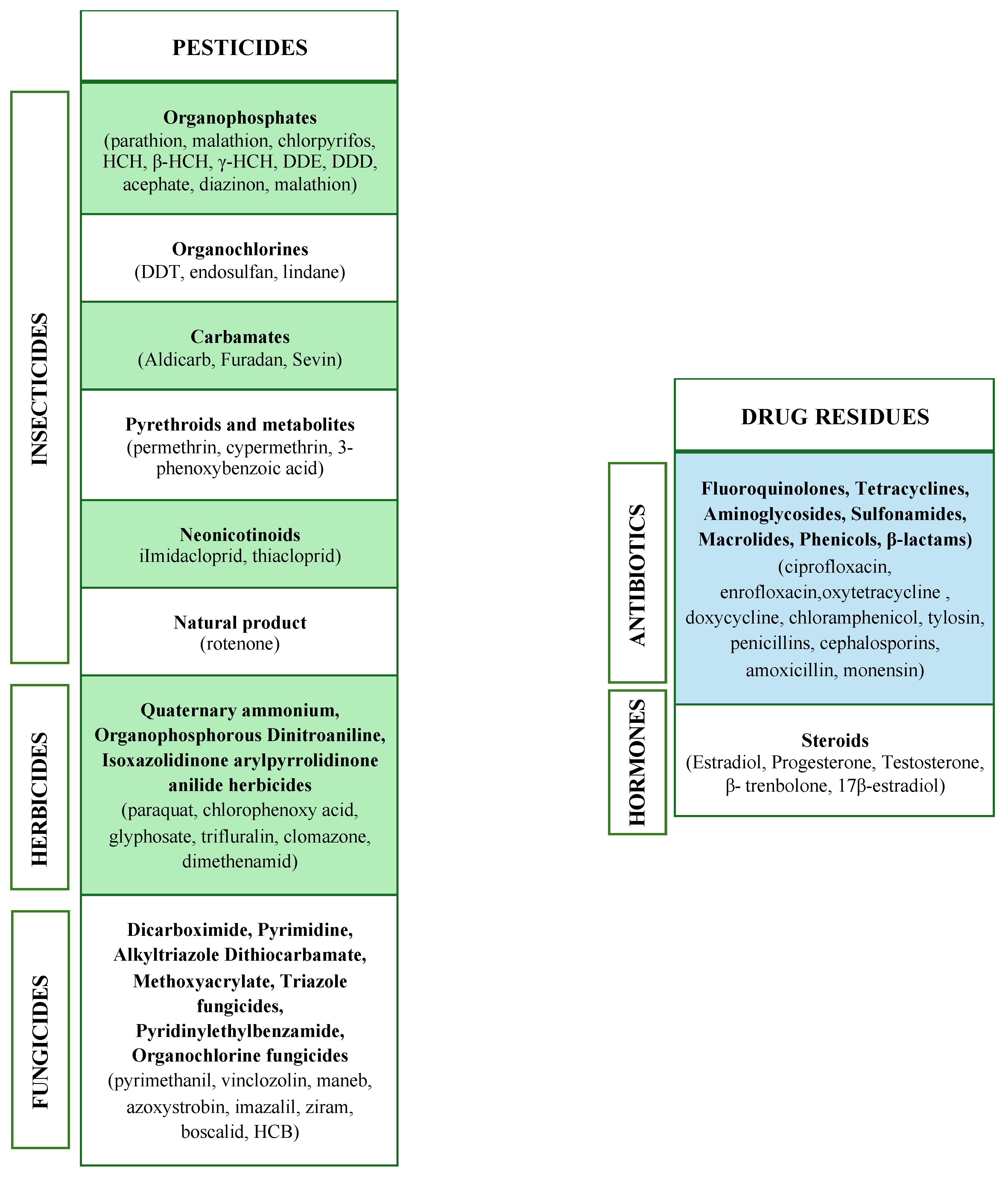
2. Food Contaminants of Agricultural and Veterinary Origin
2.1. Pesticides
2.2. Drug Residues
3. Worldwide Distribution of Pesticides and Drugs Contaminants
3.1. Pesticides
3.2. Drug Residues
4. Routes of Exposure
4.1. Oral Exposure
4.2. Other Routes of Exposure
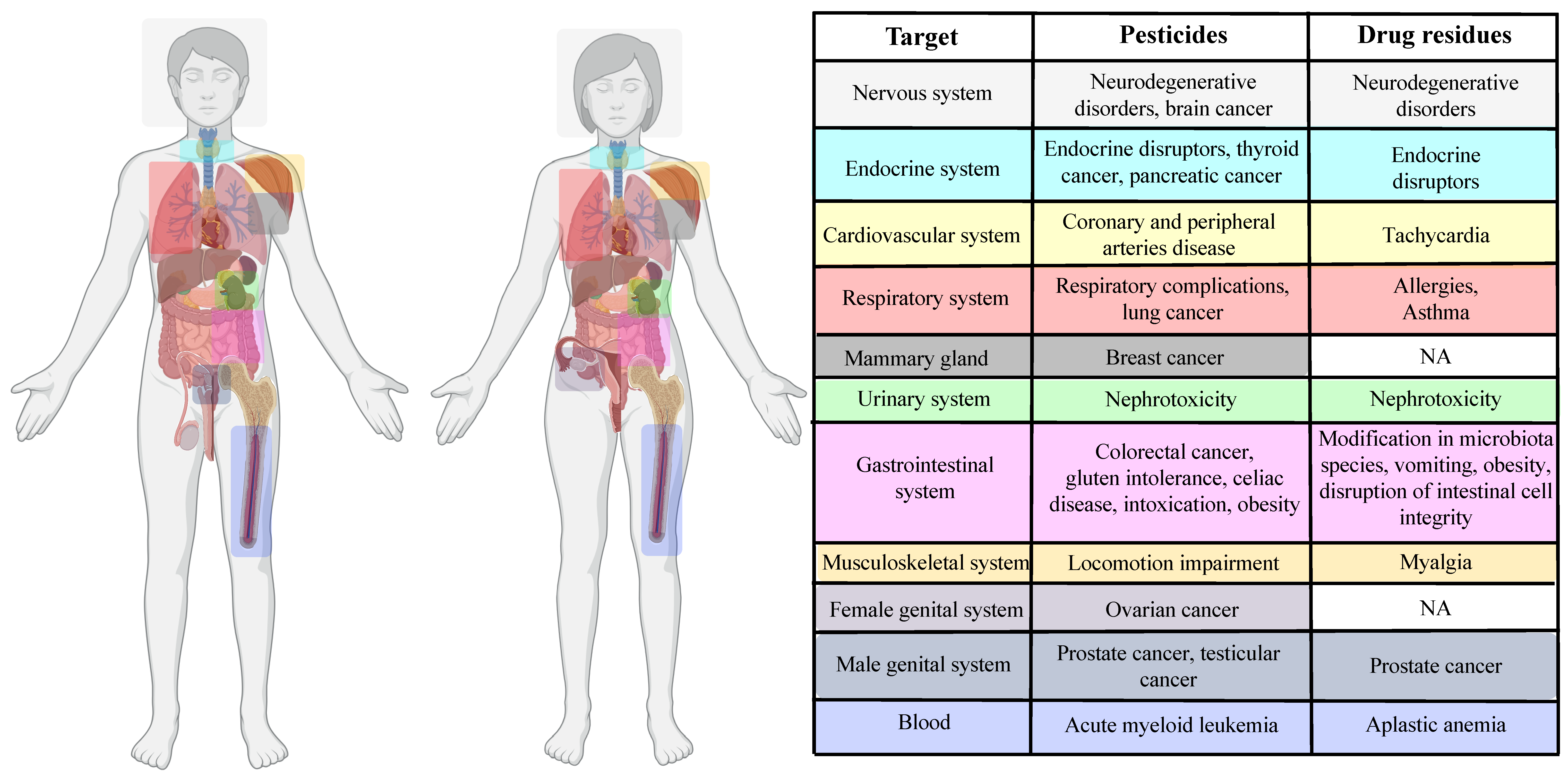
5. Impact of Pesticides and Drug Residues on Human Health at the Cellular Level
5.1. Pesticides
5.2. Drug Residues
6. Effects of Pesticide and Drug Residues on Human Fertility, Embryo Development and Transgenerational Inheritance
6.1. Adverse Effects on Female Fertility and Pregnancy
6.1.1. Pesticides

6.1.2. Antibiotics
6.2. Adverse Effects on Male Fertility
6.2.1. Pesticides
6.2.2. Antibiotics
7. Intergenerational and Transgenerational Effects
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Takele Beyene, B.T. Rational veterinary drug use: Its significance in public health. J. Vet. Med. Anim. Health 2014, 6, 302–308. [Google Scholar]
- Okeke, E.S.; Enochoghene, A.; Ezeudoka, B.C.; Kaka, S.D.; Chen, Y.; Mao, G.; ThankGod Eze, C.; Feng, W.; Wu, X. A review of heavy metal risks around e-waste sites and comparable municipal dumpsites in major African cities: Recommendations and future perspectives. Toxicology 2024, 501, 153711. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okagu, I.U.; Okoye, C.O.; Ezeorba, T.P.C. The use of calcium carbide in food and fruit ripening: Potential mechanisms of toxicity to humans and future prospects. Toxicology 2022, 468, 153112. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Shang, C.; Okeke, E.S.; Ejeromedoghene, O.; Oderinde, O.; Etafo, N.O.; Mgbechidinma, C.L.; Bakare, O.C.; Meugang, E.F. Antibiotics soil-solution chemistry: A review of environmental behavior and uptake and transformation by plants. J. Environ. Manag. 2024, 354, 120312. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Oderinde, O.; Etafo, N.O.; Kifle, G.A.; Okeke, E.S.; Ejeromedoghene, O.; Mgbechidinma, C.L.; Oke, E.A.; Raheem, S.A.; Bakare, O.C.; et al. Recent perspective of an-tibiotics remediation: A review of the principles, mechanisms, and chemistry controlling remediation from aqueous media. Sci. Total Environ. 2023, 881, 163469. [Google Scholar] [CrossRef]
- Eze, C.G.; Okeke, E.S.; Nwankwo, C.E.; Nyaruaba, R.; Anand, U.; Okoro, O.J.; Bontempi, E. Emerging contaminants in food matrices: An overview of the occurrence, pathways, impacts and detection techniques of per- and polyfluoroalkyl substances. Toxicol. Rep. 2024, 12, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Shikoray, L.; Mohamed, M.I.; Habib, I.; Matsumoto, T. Veterinary Drug Residues in the Food Chain as an Emerging Public Health Threat: Sources, Analytical Methods, Health Impacts, and Preventive Measures. Foods 2024, 13, 1629. [Google Scholar] [CrossRef]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic Chemicals and Persistent Organic Pollutants Associated with Micro-and Nanoplastics Pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food chain microplastics contamination and impact on human health: A review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- United Nation. Available online: https://www.unep.org (accessed on 28 May 2024).
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Fini, J.B.; Mughal, B.B.; Le Mevel, S.; Leemans, M.; Lettmann, M.; Spirhanzlova, P.; Affaticati, P.; Jenett, A.; Demeneix, B.A. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Sci. Rep. 2017, 7, 43786. [Google Scholar] [CrossRef]
- Rani, P.; Dhok, A. Effects of Pollution on Pregnancy and Infants. Cureus 2023, 15, e33906. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int (accessed on 12 May 2024).
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef] [PubMed]
- Danjou, A.M.N.; Perol, O.; Coste, A.; Faure, E.; Beranger, R.; Boyle, H.; Belladame, E.; Grassot, L.; Dubuis, M.; Spinosi, J.; et al. Domestic use of pesticides during early periods of development and risk of testicular germ cell tumors in adulthood: A French nationwide case-control study. Environ. Health 2021, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; Pesaresi, P.; Mizzotti, C.; Bulone, V.; Mezzetti, B.; Baraldi, E.; Masiero, S. Game-changing alternatives to conventional fungicides: Small RNAs and short peptides. Trends Biotechnol. 2022, 40, 320–337. [Google Scholar] [CrossRef]
- Goetz, A.K.; Dix, D.J. Mode of action for reproductive and hepatic toxicity inferred from a genomic study of triazole antifungals. Toxicol. Sci. 2009, 110, 449–462. [Google Scholar] [CrossRef]
- Chaabane, M.; Koubaa, M.; Soudani, N.; Elwej, A.; Grati, M.; Jamoussi, K.; Boudawara, T.; Ellouze Chaabouni, S.; Zeghal, N. Nitraria retusa fruit prevents penconazole-induced kidney injury in adult rats through modulation of oxidative stress and histopathological changes. Pharm. Biol. 2017, 55, 1061–1073. [Google Scholar] [CrossRef]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Detection of glyphosate and its metabolites in food of animal origin based on ion-chromatography-high resolution mass spectrometry (IC-HRMS). Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 592–600. [Google Scholar] [CrossRef]
- Bacanli, M.; Basaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; on behalf of the EFFORT consortium. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2019, 74, 807–816. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.X.; Li, Z.Y.; Zheng, Z.Y.; Xiang, Y.; Liu, Y.; Zhao, R.F.; Fang, J. Detection of fluoroquinolone and sulfonamide residues in poultry eggs in Kunming city, southwest China. Poult. Sci. 2022, 101, 101892. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Duan, J.; Li, X.; Xu, X.; Shi, H.; Kang, W. Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex. Int. J. Mol. Sci. 2017, 18, 1286. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, L4/43, 43–167.
- Moussa, F.; Doumiati, S.; Bernabo, N.; Barboni, B.; Jaber, F.; Mokh, S. Hormones residues in bovine animals: Sampling, analysis and health risk assessment. Steroids 2022, 181, 108994. [Google Scholar] [CrossRef]
- Kamaly, H.F.; Sharkawy, A.A. Hormonal residues in chicken and cattle meat: A risk threat the present and future consumer health. Food Chem. Toxicol. 2023, 182, 114172. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kang, D.; Lim, M.W.; Kang, C.S.; Sung, H.J. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol. Res. 2010, 26, 301–313. [Google Scholar] [CrossRef]
- Collier, R.J.; Bauman, D.E. Update on human health concerns of recombinant bovine somatotropin use in dairy cows. J. Anim. Sci. 2014, 92, 1800–1807. [Google Scholar] [CrossRef]
- Farshad, A.A.; Enferadi, M.; Bakand, S.; Jamshidi Orak, R.; Mirkazemi, R. Penicillin dust exposure and penicillin resistance among pharmaceutical workers in Tehran, Iran. Int. J. Occup. Environ. Health 2016, 22, 218–223. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Lewis, D.F. Steroid hormone receptors and dietary ligands: A selected review. Proc. Nutr. Soc. 2002, 61, 105–122. [Google Scholar] [CrossRef]
- Moussa, F.; Mokh, S.; Doumiati, S.; Barboni, B.; Bernabo, N.; Al Iskandarani, M. LC-MS/MS method for the determination of hormones: Validation, application and health risk assessment in various bovine matrices. Food Chem. Toxicol. 2020, 138, 111204. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Pestana, D.; Faria, G.; Vasconcelos, F.; Delerue-Matos, C.; Calhau, C.; Domingues, V.F. Method development for the determination of Synthetic Musks and Organophosphorus Pesticides in Human Adipose Tissue. J. Pharm. Biomed. Anal. 2020, 191, 113598. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.C.; Singh, N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef] [PubMed]
- FDA. Pesticide Residue Monitoring Program Fiscal Year 2021 Pesticide Report; FDA: Silver Spring, MD, USA, 2021. [Google Scholar]
- Lalonde, B.; Garron, C. Temporal and Spatial Analysis of Surface Water Pesticide Occurrences in the Maritime Region of Canada. Arch. Environ. Contam. Toxicol. 2020, 79, 12–22. [Google Scholar] [CrossRef]
- Hjorth, K.; Holen, K.J.B.; Andersson, A.; Christensen, H.B.; Siivinen, K.; Toome, M. Pesticide residues in fruits and vegetables from South America—A Nordic project. Food Control 2011, 22, 1701–1706. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F.; Ou, J. Global pesticide consumption and pollution: With China as a focus. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125–144. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, X.; Jones, K.C.; Jiao, C.; Sun, C.; Liu, Y.; Zhu, Y.; Zhang, Q.; Zhai, L.; Shen, Z.; et al. Pesticide risk constraints to achieving Sustainable Development Goals in China based on national modeling. NPJ Clean Water 2022, 5, 59. [Google Scholar] [CrossRef]
- Ma, C.; Wei, D.; Liu, P.; Fan, K.; Nie, L.; Song, Y.; Wang, M.; Wang, L.; Xu, Q.; Wang, J.; et al. Pesticide residues in commonly consumed vegetables in Henan Province of China in 2020. Front. Public Health 2022, 10, 901485. [Google Scholar] [CrossRef] [PubMed]
- Soman, S.; Christiansen, A.; Florinski, R.; Bharat, G.; Steindal, E.H.; Nizzetto, L.; Chakraborty, P. An up-dated status of currently used pesticides in India: Human dietary exposure from an Indian food basket. Environ. Res. 2024, 242, 117543. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Cabrera, L.C.; Di Piazza, G.; Dujardin, B.; Pastor, P.M. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar]
- De Liguoro, M.; Bona, M.D.; Gallina, G.; Capolongo, F.; Gallocchio, F.; Binato, G.; Di Leva, V. A monitoring of chemical contaminants in waters used for field irrigation and livestock watering in the Veneto region (Italy), using bioassays as a screening tool. Environ. Sci. Pollut. Res. Int. 2014, 21, 3546–3557. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M. Evaluation of toxic metal (Hg, Cd, Pb), polychlorinated biphenyl (PCBs), and pesticide (DDTs) levels in aromatic herbs collected in selected areas of Southern Italy. Environ. Sci. Pollut. Res. Int. 2014, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Occurrence of organochlorine pesticide residues in biological and environmental matrices in Africa: A two-decade review. Heliyon 2020, 6, e03518. [Google Scholar] [CrossRef]
- Ssemugabo, C.; Bradman, A.; Ssempebwa, J.C.; Sille, F.; Guwatudde, D. Pesticide Residues in Fresh Fruit and Vegetables from Farm to Fork in the Kampala Metropolitan Area, Uganda. Environ. Health Insights 2022, 16, 11786302221111866. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/publications/i/item/9789240057586 (accessed on 20 April 2024).
- Soares, V.M.; Pereira, J.G.; Barreto, F.; Jank, L.; Rau, R.B.; Dias Ribeiro, C.B.; Dos Santos Castilhos, T.; Tomaszewski, C.A.; Hillesheim, D.R.; Mondadori, R.G.; et al. Residues of Veterinary Drugs in Animal Products Commercialized in the Border Region of Brazil, Argentina, and Uruguay. J. Food Prot. 2022, 85, 980–986. [Google Scholar] [CrossRef]
- Houtman, C.J.; ten Broek, R.; de Jong, K.; Pieterse, B.; Kroesbergen, J. A multicomponent snapshot of pharmaceuticals and pesticides in the river Meuse basin. Environ. Toxicol. Chem. 2013, 32, 2449–2459. [Google Scholar] [CrossRef]
- Lopez-Serna, R.; Kasprzyk-Hordern, B.; Petrovic, M.; Barcelo, D. Multi-residue enantiomeric analysis of pharmaceuticals and their active metabolites in the Guadalquivir River basin (South Spain) by chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 5859–5873. [Google Scholar] [CrossRef]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Munoz, G.; Duy, S.V.; Do, D.T.; Bayen, S.; Sauvé, S. Analysis of sulfonamides, fluoroquinolones, tetracyclines, triphenylmethane dyes and other veterinary drug residues in cultured and wild seafood sold in Montreal, Canada. J. Food Compos. Anal. 2020, 94, 103630. [Google Scholar] [CrossRef]
- Chau, H.T.C.; Kadokami, K.; Duong, H.T.; Kong, L.; Nguyen, T.T.; Nguyen, T.Q.; Ito, Y. Occurrence of 1153 organic micropollutants in the aquatic environment of Vietnam. Environ. Sci. Pollut. Res. Int. 2018, 25, 7147–7156. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.H.; Chen, L.; Meng, X.Z.; Duan, Y.P.; Zhang, Z.S.; Zeng, E.Y. Occurrence and human health risk of wastewater-derived pharmaceuticals in a drinking water source for Shanghai, East China. Sci. Total Environ. 2014, 490, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Rai, P.; Singh, A.K.; Verma, P.; Gupta, S. Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environ. Monit. Assess. 2014, 186, 6663–6682. [Google Scholar] [CrossRef]
- Ergen, A.M.; Yalcin, S.S. Unexpected drug residuals in human milk in Ankara, capital of Turkey. BMC Pregnancy Childbirth 2019, 19, 348. [Google Scholar] [CrossRef]
- Olatoye, O.; Kayode, S.T. Oxytetracycline residues in retail chicken eggs in Ibadan, Nigeria. Food Addit. Contam. Part B Surveill. 2012, 5, 255–259. [Google Scholar] [CrossRef]
- Darwish, W.S.; Eldaly, E.A.; El-Abbasy, M.T.; Ikenaka, Y.; Nakayama, S.; Ishizuka, M. Antibiotic residues in food: The African scenario. Jpn. J. Vet. Res. 2013, 61, S13–S22. [Google Scholar]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Heymann, A.K.; Schnabel, K.; Billenkamp, F.; Buhler, S.; Frahm, J.; Kersten, S.; Huther, L.; Meyer, U.; von Soosten, D.; Trakooljul, N.; et al. Effects of glyphosate residues and different concentrate feed proportions in dairy cow rations on hepatic gene expression, liver histology and biochemical blood parameters. PLoS ONE 2021, 16, e0246679. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Schledorn, P.; Schrödl, W.; Hoppe, H.W.; Lutz, W.; Shehata, A.A. Detection of Glyphosate Residues in Animals and Humans. J. Environ. Anal. Toxicol. 2014, 4, 1000210. [Google Scholar]
- von Soosten, D.; Meyer, U.; Huther, L.; Danicke, S.; Lahrssen-Wiederholt, M.; Schafft, H.; Spolders, M.; Breves, G. Excretion pathways and ruminal disappearance of glyphosate and its degradation product aminomethylphosphonic acid in dairy cows. J. Dairy Sci. 2016, 99, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Foldager, L.; Winters, J.F.M.; Norskov, N.P.; Sorensen, M.T. Impact of feed glyphosate residues on broiler breeder egg production and egg hatchability. Sci. Rep. 2021, 11, 19290. [Google Scholar] [CrossRef]
- Connolly, A.; Basinas, I.; Jones, K.; Galea, K.S.; Kenny, L.; McGowan, P.; Coggins, M.A. Characterising glyphosate exposures among amenity horticulturists using multiple spot urine samples. Int. J. Hyg. Environ. Health 2018, 221, 1012–1022. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Banerjee, K.; Utture, S.; Kamble, N.; Rao, B.M.; Panda, S.K.; Mathew, S. Assessment of polyaromatic hydrocarbons and pesticide residues in domestic and imported pangasius (Pangasianodon hypophthalmus) fish in India. J. Sci. Food Agric. 2016, 96, 2373–2377. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.M.; Henriksson, P.J.; Murray, F.J.; Little, D.C.; Dalsgaard, A.; Van den Brink, P.J. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412–413, 231–243. [Google Scholar] [CrossRef]
- Song, S.; Zhu, K.; Han, L.; Sapozhnikova, Y.; Zhang, Z.; Yao, W. Residue Analysis of 60 Pesticides in Red Swamp Crayfish Using QuEChERS with High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 5031–5038. [Google Scholar] [CrossRef]
- Oerlemans, A.; Figueiredo, D.M.; Mol, J.G.J.; Nijssen, R.; Anzion, R.B.M.; van Dael, M.F.P.; Duyzer, J.; Roeleveld, N.; Russel, F.G.M.; Vermeulen, R.C.H.; et al. Personal exposure assessment of pesticides in residents: The association between hand wipes and urinary biomarkers. Environ. Res. 2021, 199, 111282. [Google Scholar] [CrossRef]
- Bouwman, H.; Sereda, B.; Meinhardt, H.M. Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ. Pollut. 2006, 144, 902–917. [Google Scholar] [CrossRef]
- Ben, Y.; Hu, M.; Zhang, X.; Wu, S.; Wong, M.H.; Wang, M.; Andrews, C.B.; Zheng, C. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water. Water Res. 2020, 175, 115699. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Ismail, N.A.H.; Haron, D.E.M.; Yusoff, F.M.; Praveena, S.M.; Aris, A.Z. Pharmaceuticals, hormones, plasticizers, and pesticides in drinking water. J. Hazard. Mater. 2022, 424 Pt A, 127327. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Magu, M.M.; Muendo, B.M. Occurrence of antibiotics residues in hospital wastewater, wastewater treatment plant, and in surface water in Nairobi County, Kenya. Environ. Monit. Assess. 2019, 192, 18. [Google Scholar] [CrossRef] [PubMed]
- Valdes, M.E.; Santos, L.; Rodriguez Castro, M.C.; Giorgi, A.; Barcelo, D.; Rodriguez-Mozaz, S.; Ame, M.V. Distribution of antibiotics in water, sediments and biofilm in an urban river (Cordoba, Argentina, LA). Environ. Pollut. 2021, 269, 116133. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Mas, L.I.; Aparicio, V.C.; De Gerónimo, E.; Costa, J.L. Pesticides in water sources used for human consumption in the semiarid region of Argentina. SN Appl. Sci. 2020, 2, 691. [Google Scholar] [CrossRef]
- Lin, Z.; Vahl, C.I.; Riviere, J.E. Human Food Safety Implications of Variation in Food Animal Drug Metabolism. Sci. Rep. 2016, 6, 27907. [Google Scholar] [CrossRef] [PubMed]
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Van Cuong, N.; Nhung, N.T.; Nghia, N.H.; Mai Hoa, N.T.; Trung, N.V.; Thwaites, G.; Carrique-Mas, J. Antimicrobial Consumption in Medicated Feeds in Vietnamese Pig and Poultry Production. Ecohealth 2016, 13, 490–498. [Google Scholar] [CrossRef]
- Peeters, L.E.; Daeseleire, E.; Devreese, M.; Rasschaert, G.; Smet, A.; Dewulf, J.; Heyndrickx, M.; Imberechts, H.; Haesebrouck, F.; Butaye, P.; et al. Residues of chlortetracycline, doxycycline and sulfadiazine-trimethoprim in intestinal content and feces of pigs due to cross-contamination of feed. BMC Vet. Res. 2016, 12, 209. [Google Scholar] [CrossRef]
- Baazize-Ammi, D.; Dechicha, A.S.; Tassist, A.; Gharbi, I.; Hezil, N.; Kebbal, S.; Morsli, W.; Beldjoudi, S.; Saadaoui, M.R.; Guetarni, D. Screening and quantification of antibiotic residues in broiler chicken meat and milk in the central region of Algeria. Rev. Sci. Tech. 2019, 38, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the antibiotic residues in poultry meat in some of the EU countries and selection of the best compositions of lactic acid bacteria and essential oils against Salmonella enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef]
- Turnipseed, S.B.; Storey, J.M.; Lohne, J.J.; Andersen, W.C.; Burger, R.; Johnson, A.S.; Madson, M.R. Wide-Scope Screening Method for Multiclass Veterinary Drug Residues in Fish, Shrimp, and Eel Using Liquid Chromatography-Quadrupole High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 7252–7267. [Google Scholar] [CrossRef]
- Available online: www.efsa.europa.eu/en/news/efsa-reassesses-safety-feed-additive-ethoxyquin (accessed on 2 June 2024).
- Msibi, S.S.; Chen, C.Y.; Chang, C.P.; Chen, C.J.; Chiang, S.Y.; Wu, K.Y. High pesticide inhalation exposure from multiple spraying sources amongst applicators in Eswatini, Southern Africa. Pest Manag. Sci. 2021, 77, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Merrill, A.K.; Eckard, M.L.; Marvin, E.; Conrad, K.; Welle, K.; Oberdorster, G.; Sobolewski, M.; Cory-Slechta, D.A. Paraquat Inhalation, a Translationally Relevant Route of Exposure: Disposition to the Brain and Male-Specific Olfactory Impairment in Mice. Toxicol. Sci. 2021, 180, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; Ahmed, I.A.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.; Torres-Jardon, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [PubMed]
- Hamsan, H.; Ho, Y.B.; Zaidon, S.Z.; Hashim, Z.; Saari, N.; Karami, A. Occurrence of commonly used pesticides in personal air samples and their associated health risk among paddy farmers. Sci. Total Environ. 2017, 603–604, 381–389. [Google Scholar] [CrossRef]
- Hamscher, G.; Pawelzick, H.T.; Sczesny, S.; Nau, H.; Hartung, J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ. Health Perspect. 2003, 111, 1590–1594. [Google Scholar] [CrossRef]
- Macfarlane, E.; Carey, R.; Keegel, T.; El-Zaemay, S.; Fritschi, L. Dermal exposure associated with occupational end use of pesticides and the role of protective measures. Saf. Health Work 2013, 4, 136–141. [Google Scholar] [CrossRef]
- Slocum, A.C.; Shern, L.C. Spray deposition patterns during simulated work activities by lawn care specialists. J. Environ. Sci. Health B 1991, 26, 259–278. [Google Scholar] [CrossRef]
- Wang, X.; Murison, J.; Wang, J.; Leong, G.; Wu, Z.; Li, Q. Dermal exposure assessment to trinexapac-ethyl: A case study of workers in golf course in Hawaii, USA. Environ. Sci. Pollut. Res. Int. 2021, 28, 1072–1076. [Google Scholar] [CrossRef]
- Lebailly, P.; Bouchart, V.; Baldi, I.; Lecluse, Y.; Heutte, N.; Gislard, A.; Malas, J.P. Exposure to pesticides in open-field farming in France. Ann. Occup. Hyg. 2009, 53, 69–81. [Google Scholar] [PubMed]
- Bluemlein, K.; Nowak, N.; Ellinghusen, B.; Gerling, S.; Badorrek, P.; Hansen, T.; Hohlfeld, J.M.; Paul, R.; Schuchardt, S. Occupational exposure to veterinary antibiotics: Pharmacokinetics of enrofloxacin in humans after dermal, inhalation and oral uptake—A clinical study. Environ. Toxicol. Pharmacol. 2023, 100, 104139. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Faas, J.; Krska, R.; Khiaosa-Ard, R.; Zebeli, Q. Residues of pesticides and veterinary drugs in diets of dairy cattle from conventional and organic farms in Austria. Environ. Pollut. 2023, 316 Pt 2, 120626. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Papetti, A. Advances in the Analysis of Veterinary Drug Residues in Food Matrices by Capillary Electrophoresis Techniques. Molecules 2019, 24, 4617. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef]
- de Oliveira, F.G.R.; Candian, M.; Lucchette, F.F.; Salgon, J.L.; Sales, A. A technical note on the relationship between ultrasonic velocity and moisture content of Brazilian hardwood (Goupia glabra). Build. Environ. 2005, 40, 297–300. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, A.S.; Singh, S.M.; Bharti, P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res. Int. 2010, 43, 2027–2030. [Google Scholar] [CrossRef]
- Available online: https://www.oecd.org/publications/oecd-fao-agricultural-outlook-19991142.htm (accessed on 2 June 2024).
- Lari, S.; Jonnalagadda, P.R.; Yamagani, P.; Medithi, S.; Vanka, J.; Pandiyan, A.; Naidu, M.; Jee, B. Assessment of dermal exposure to pesticides among farmers using dosimeter and hand washing methods. Front. Public Health 2022, 10, 957774. [Google Scholar] [CrossRef]
- Silva, V.; Gai, L.; Harkes, P.; Tan, G.; Ritsema, C.J.; Alcon, F.; Contreras, J.; Abrantes, N.; Campos, I.; Baldi, I.; et al. Pesticide residues with hazard classifications relevant to non-target species including humans are omnipresent in the environment and farmer residences. Environ. Int. 2023, 181, 108280. [Google Scholar] [CrossRef]
- Mueller, W.; Jones, K.; Fuhrimann, S.; Ahmad, Z.; Sams, C.; Harding, A.H.; Povey, A.; Atuhaire, A.; Basinas, I.; van Tongeren, M.; et al. Factors influencing occupational exposure to pyrethroids and glyphosate: An analysis of urinary biomarkers in Malaysia, Uganda and the United Kingdom. Environ. Res. 2024, 242, 117651. [Google Scholar] [CrossRef]
- Bus, J.S.; Cagen, S.Z.; Olgaard, M.; Gibson, J.E. A mechanism of paraquat toxicity in mice and rats. Toxicol. Appl. Pharmacol. 1976, 35, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Ghorab, M.S.K. Toxicological Effects of Organophosphates Pesticides. Int. J. Environ. Monit. Anal. 2015, 3, 218–220. [Google Scholar]
- Garcia, J.; Ventura, M.I.; Requena, M.; Hernandez, A.F.; Parron, T.; Alarcon, R. Association of reproductive disorders and male congenital anomalies with environmental exposure to endocrine active pesticides. Reprod. Toxicol. 2017, 71, 95–100. [Google Scholar] [CrossRef]
- Larsen, A.E.; Gaines, S.D.; Deschenes, O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 2017, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Addissie, Y.A.; Kruszka, P.; Troia, A.; Wong, Z.C.; Everson, J.L.; Kozel, B.A.; Lipinski, R.J.; Malecki, K.M.C.; Muenke, M. Prenatal exposure to pesticides and risk for holoprosencephaly: A case-control study. Environ. Health 2020, 19, 65. [Google Scholar] [CrossRef]
- Bast, A.; Semen, K.O.; Drent, M. Pulmonary toxicity associated with occupational and environmental exposure to pesticides and herbicides. Curr. Opin. Pulm. Med. 2021, 27, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Bhadauriya, P.; Parihar, R.; Ganesh, S. Pesticides DEET, fipronil and maneb induce stress granule assembly and translation arrest in neuronal cells. Biochem. Biophys. Rep. 2021, 28, 101110. [Google Scholar] [CrossRef]
- Witczak, A.; Pohorylo, A.; Abdel-Gawad, H. Endocrine-Disrupting Organochlorine Pesticides in Human Breast Milk: Changes during Lactation. Nutrients 2021, 13, 229. [Google Scholar] [CrossRef]
- Gea, M.; Zhang, C.; Tota, R.; Gilardi, G.; Di Nardo, G.; Schiliro, T. Assessment of Five Pesticides as Endocrine-Disrupting Chemicals: Effects on Estrogen Receptors and Aromatase. Int. J. Environ. Res. Public Health 2022, 19, 1959. [Google Scholar] [CrossRef]
- Iteire, K.A.; Sowole, A.T.; Ogunlade, B. Exposure to pyrethroids induces behavioral impairments, neurofibrillary tangles and tau pathology in Alzheimer’s type neurodegeneration in adult Wistar rats. Drug Chem. Toxicol. 2022, 45, 839–849. [Google Scholar] [CrossRef]
- Cavalier, H.; Trasande, L.; Porta, M. Exposures to pesticides and risk of cancer: Evaluation of recent epidemiological evidence in humans and paths forward. Int. J. Cancer 2023, 152, 879–912. [Google Scholar] [CrossRef]
- Richardson, J.R.; Quan, Y.; Sherer, T.B.; Greenamyre, J.T.; Miller, G.W. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005, 88, 193–201. [Google Scholar] [CrossRef]
- Hoffman, L.; Trombetta, L.; Hardej, D. Ethylene bisdithiocarbamate pesticides Maneb and Mancozeb cause metal overload in human colon cells. Environ. Toxicol. Pharmacol. 2016, 41, 78–88. [Google Scholar] [CrossRef]
- Stykel, M.G.; Humphries, K.; Kirby, M.P.; Czaniecki, C.; Wang, T.; Ryan, T.; Bamm, V.; Ryan, S.D. Nitration of microtubules blocks axonal mitochondrial transport in a human pluripotent stem cell model of Parkinson’s disease. FASEB J. 2018, 32, 5350–5364. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Fang, Y.; Du, Z.; Yan, Z.; Yuan, X.; Dai, L.; Yu, T.; Xiong, M.; Tian, Y.; et al. Exposure to the environmentally toxic pesticide maneb induces Parkinson’s disease-like neurotoxicity in mice: A combined proteomic and metabolomic analysis. Chemosphere 2022, 308 Pt 2, 136344. [Google Scholar] [CrossRef]
- Calaf, G.M. Role of organophosphorous pesticides and acetylcholine in breast carcinogenesis. Semin. Cancer Biol. 2021, 76, 206–217. [Google Scholar] [CrossRef]
- Zeng, F.; Lerro, C.; Lavoue, J.; Huang, H.; Siemiatycki, J.; Zhao, N.; Ma, S.; Deziel, N.C.; Friesen, M.C.; Udelsman, R.; et al. Occupational exposure to pesticides and other biocides and risk of thyroid cancer. Occup. Environ. Med. 2017, 74, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.T.; Wathieu, H.; Glasgow, E.; Peran, I.; Parasido, E.; Li, T.; Simbulan-Rosenthal, C.M.; Rosenthal, D.; Medvedev, A.V.; Makarov, S.S.; et al. A novel chemo-phenotypic method identifies mixtures of salpn, vitamin D3, and pesticides involved in the development of colorectal and pancreatic cancer. Ecotoxicol. Environ. Saf. 2022, 233, 113330. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, E.Y.; Kim, J.; Park, E.; Oh, J.K.; Lim, M.K. Occupational Exposure to Pesticides and Lung Cancer Risk: A Propensity Score Analyses. Cancer Res. Treat. 2022, 54, 130–139. [Google Scholar] [CrossRef]
- Pardo, L.A.; Beane Freeman, L.E.; Lerro, C.C.; Andreotti, G.; Hofmann, J.N.; Parks, C.G.; Sandler, D.P.; Lubin, J.H.; Blair, A.; Koutros, S. Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ. Health 2020, 19, 30. [Google Scholar] [CrossRef]
- Renier, M.; Hippert, J.; Louis-Bastien, W.; Tual, S.; Meryet-Figuiere, M.; Vigneron, N.; Marcotullio, E.; Baldi, I.; Lebailly, P.; AGRICAN Group. Agricultural exposure and risk of ovarian cancer in the AGRIculture and CANcer (AGRICAN) cohort. Occup. Environ. Med. 2024, 81, 75–83. [Google Scholar] [CrossRef]
- Sharma, T.; Sirpu Natesh, N.; Pothuraju, R.; Batra, S.K.; Rachagani, S. Gut microbiota: A non-target victim of pesticide-induced toxicity. Gut Microbes 2023, 15, 2187578. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Gordon, J.I. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 2006, 103, 10011–10016. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.C.; Lee, I.K.; Moon, H.B.; Chang, Y.S.; Jacobs, D.R., Jr.; Lee, D.H. Associations among organochlorine pesticides, Methanobacteriales, and obesity in Korean women. PLoS ONE 2011, 6, e27773. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and uses of chlorpyrifos in the United States. Rev. Environ. Contam. Toxicol. 2014, 231, 13–34. [Google Scholar]
- Wang, B.; Tsakiridis, E.E.; Zhang, S.; Llanos, A.; Desjardins, E.M.; Yabut, J.M.; Green, A.E.; Day, E.A.; Smith, B.K.; Lally, J.S.V.; et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue. Nat. Commun. 2021, 12, 5163. [Google Scholar] [CrossRef]
- Hallal, N.; El Khayat El Sabbouri, H.; Salami, A.; Ramadan, W.; Khachfe, H.; Moustafa, M.E.; Khalil, M.; Joumaa, W.H. Impacts of prolonged chlorpyrifos exposure on locomotion and slow-and fast- twitch skeletal muscles contractility in rats. Toxicol. Rep. 2019, 6, 598–606. [Google Scholar] [CrossRef]
- Wahab, A.; Hod, R.; Ismail, N.H.; Omar, N. The effect of pesticide exposure on cardiovascular system: A systematic review. Int. J. Community Med. Public Health 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Zuin, M.; Trentini, A.; Marsillach, J.; D’Amuri, A.; Bosi, C.; Roncon, L.; Passaro, A.; Zuliani, G.; Mackness, M.; Cervellati, C. Paraoxonase-1 (PON-1) Arylesterase Activity Levels in Patients with Coronary Artery Disease: A Meta-Analysis. Dis. Markers 2022, 2022, 4264314. [Google Scholar] [CrossRef]
- Nie, J.; Zhou, J.; Shen, Y.; Lin, R.; Hu, H.; Zeng, K.; Bi, H.; Huang, M.; Yu, L.; Zeng, S.; et al. Studies on the interaction of five triazole fungicides with human renal transporters in cells. Toxicol. Vitr. 2023, 88, 105555. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg. Neurol. Int. 2015, 6, 45. [Google Scholar]
- Beyene, T. Veterinary Drug Residues in Food-animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol. 2016, 7, 1000285. [Google Scholar] [CrossRef]
- Sadighara, P.; Rostami, S.; Shafaroodi, H.; Sarshogi, A.; Mazaheri, Y.; Sadighara, M. The effect of residual antibiotics in food on intestinal microbiota: A systematic review. Front. Sustain. Food Syst. 2023, 7, 1163885. [Google Scholar] [CrossRef]
- Chen, R.A.; Wu, W.K.; Panyod, S.; Liu, P.Y.; Chuang, H.L.; Chen, Y.H.; Lyu, Q.; Hsu, H.C.; Lin, T.L.; Shen, T.D.; et al. Dietary Exposure to Antibiotic Residues Facilitates Metabolic Disorder by Altering the Gut Microbiota and Bile Acid Composition. mSystems 2022, 7, e0017222. [Google Scholar] [CrossRef]
- Petersen, L.; Rogers, C. Aminoglycoside-induced hearing deficits—A review of cochlear ototoxicity. S. Afr. Fam. Pract. 2015, 57, 1–6. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, D.W.; Han, S.; Kang, H.S.; Suh, J.H.; Oh, H.S.; Hwang, M.S.; Moon, G.; Park, Y.; Hong, J.H.; et al. Veterinary drug, 17beta-trenbolone promotes the proliferation of human prostate cancer cell line through the Akt/AR signaling pathway. Chemosphere 2018, 198, 364–369. [Google Scholar] [CrossRef]
- Tuck, S.; Furey, A.; Danaher, M. Analysis of Anthelmintic and Anticoccidial Drug Residues in Animal-Derived Foods. In Chemical Analysis of Non-antimicrobial Veterinary Drug Residues in Food; Wiley: Hoboken, NJ, USA, 2016; pp. 245–309. [Google Scholar]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari, N.S.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; Gao, H.; Wang, B.; Huang, K.; Wu, X.; Liang, C.; Yan, S.; Han, Y.; Ding, P.; Wang, W.; et al. Urinary tetracycline antibiotics exposure during pregnancy and maternal thyroid hormone parameters: A repeated measures study. Sci. Total Environ. 2022, 838 Pt 2, 156146. [Google Scholar] [CrossRef]
- Sebastian, R.; Raghavan, S.C. Exposure to Endosulfan can result in male infertility due to testicular atrophy and reduced sperm count. Cell Death Discov. 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, C.; Liu, L.; Xia, G.; Qiao, H. Disrupting effects of polychlorinated biphenyls on gonadal development and reproductive functions in chickens. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2002, 37, 509–519. [Google Scholar] [CrossRef]
- Faja, F.; Esteves, S.; Pallotti, F.; Cicolani, G.; Di Chiano, S.; Delli Paoli, E.; Lenzi, A.; Lombardo, F.; Paoli, D. Environmental disruptors and testicular cancer. Endocrine 2022, 78, 429–435. [Google Scholar] [CrossRef]
- Tijani, A.S.; Daba, T.M.; Ubong, I.A.; Olufunke, O.; Ani, E.J.; Farombi, E.O. Rutin attenuated hexachlorobenzene-induced testicular injury via regulation of oxidative stress, steroidogenic enzymes and apoptotic process in male rats. Eur. J. Med. Chem. Rep. 2024, 10, 100121. [Google Scholar] [CrossRef]
- Hou, L.; Fu, Y.; Zhao, C.; Fan, L.; Hu, H.; Yin, S. Ciprofloxacin and enrofloxacin can cause reproductive toxicity via endocrine signaling pathways. Ecotoxicol. Environ. Saf. 2022, 244, 114049. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2018, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hassan, M.M.; Chowdhury, S. Determination of antibiotic residues in milk and assessment of human health risk in Bangladesh. Heliyon 2021, 7, e07739. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, Z.; Fang, Z.; Cravotto, G. Sonolytic degradation kinetics and mechanisms of antibiotics in water and cow milk. Ultrason. Sonochem. 2023, 99, 106518. [Google Scholar] [CrossRef]
- Abhishek Sharma, A.K. Multi-residue detection of antibiotics in migratory goat milk and human health risk assessment in Western Himalayan region, India. J. Food Compos. Anal. 2024, 125, 105815. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.M.; Jorgensen, N.; Main, K.M.; Lidegaard, O.; Priskorn, L.; Holmboe, S.A.; Brauner, E.V.; et al. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum. Exp. Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef]
- Thorson, J.L.M.; Beck, D.; Ben Maamar, M.; Nilsson, E.E.; Skinner, M.K. Epigenome-wide association study for pesticide (Permethrin and DEET) induced DNA methylation epimutation biomarkers for specific transgenerational disease. Environ. Health 2020, 19, 109. [Google Scholar] [CrossRef]
- Sanchez, F.; Smitz, J. Molecular control of oogenesis. Biochim. Biophys. Acta 2012, 1822, 1896–1912. [Google Scholar] [CrossRef] [PubMed]
- Rimon-Dahari, N.; Yerushalmi-Heinemann, L.; Alyagor, L.; Dekel, N. Ovarian Folliculogenesis. Results Probl. Cell Differ. 2016, 58, 167–190. [Google Scholar]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar]
- Bretveld, R.W.; Thomas, C.M.; Scheepers, P.T.; Zielhuis, G.A.; Roeleveld, N. Pesticide exposure: The hormonal function of the female reproductive system disrupted? Reprod. Biol. Endocrinol. 2006, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.E.; Burr, S.A. Pyrethrins/Pyrethroids. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1152–1158. [Google Scholar]
- Available online: https://www.atsdr.cdc.gov (accessed on 20 April 2024).
- Kotil, T.; Yon, N.D. The effects of permethrin on rat ovarian tissue morphology. Exp. Toxicol. Pathol. 2015, 67, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Radwan, P.; Wielgomas, B.; Radwan, M.; Karwacka, A.; Kaluzny, P.; Piskunowicz, M.; Dziewirska, E.; Hanke, W. Exposure to pyrethroid pesticides and ovarian reserve. Environ. Int. 2020, 144, 106028. [Google Scholar] [CrossRef] [PubMed]
- Radwan, P.; Wielgomas, B.; Radwan, M.; Krasinski, R.; Kilanowicz-Sapota, A.; Banaszczyk, R.; Jurewicz, J. Synthetic Pyrethroids Exposure and Embryological Outcomes: A Cohort Study in Women from Fertility Clinic. Int. J. Environ. Res. Public Health 2022, 19, 5117. [Google Scholar] [CrossRef]
- Medithi, S.; Kasa, Y.D.; Jee, B.; Venkaiah, K.; Jonnalagadda, P.R. Alterations in reproductive hormone levels among farm women and their children occupationally exposed to organophosphate pesticides. Women Health 2022, 62, 454–464. [Google Scholar] [CrossRef]
- Wu, Y.; Weng, X.; Liu, S.; Tan, Y.; Liang, H.; Li, Y.; Wen, L.; Chen, Q.; Jing, C. Associations of single and multiple organophosphate pesticide exposure with female infertility in the USA: Data from the 2015–2018 National Health and Nutrition Examination Survey. Environ. Sci. Pollut. Res. Int. 2023, 30, 23411–23421. [Google Scholar] [CrossRef]
- Bjorvang, R.D.; Hassan, J.; Stefopoulou, M.; Gemzell-Danielsson, K.; Pedrelli, M.; Kiviranta, H.; Rantakokko, P.; Ruokojarvi, P.; Lindh, C.H.; Acharya, G.; et al. Persistent organic pollutants and the size of ovarian reserve in reproductive-aged women. Environ. Int. 2021, 155, 106589. [Google Scholar] [CrossRef]
- Campagna, C.; Sirard, M.A.; Ayotte, P.; Bailey, J.L. Impaired maturation, fertilization, and embryonic development of porcine oocytes following exposure to an environmentally relevant organochlorine mixture. Biol. Reprod. 2001, 65, 554–560. [Google Scholar] [CrossRef]
- Pollock, T.; Weaver, R.E.; Ghasemi, R.; deCatanzaro, D. A mixture of five endocrine-disrupting chemicals modulates concentrations of bisphenol A and estradiol in mice. Chemosphere 2018, 193, 321–328. [Google Scholar] [CrossRef]
- Petit, C.; Chevrier, C.; Durand, G.; Monfort, C.; Rouget, F.; Garlantezec, R.; Cordier, S. Impact on fetal growth of prenatal exposure to pesticides due to agricultural activities: A prospective cohort study in Brittany, France. Environ. Health 2010, 9, 71. [Google Scholar] [CrossRef]
- Dopavogui, L.; Cadoret, F.; Loison, G.; El Fouikar, S.; Frenois, F.X.; Giton, F.; Ellero-Simatos, S.; Lasserre, F.; Polizzi, A.; Rives, C.; et al. Pre- and Postnatal Dietary Exposure to a Pesticide Cocktail Disrupts Ovarian Functions in 8-Week-Old Female Mice. Int. J. Mol. Sci. 2022, 23, 7525. [Google Scholar] [CrossRef] [PubMed]
- La Sala, G.; Farini, D.; De Felici, M. Proapoptotic effects of lindane on mouse primordial germ cells. Toxicol. Sci. 2009, 108, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Pan, W.; Wang, B.; Liu, Y.; Gan, H.; Li, M.; Liao, T.; Yang, X.; Yang, Q.; Huang, C.; et al. Association between antibiotic exposure and the risk of infertility in women of childbearing age: A case-control study. Ecotoxicol. Environ. Saf. 2023, 249, 114414. [Google Scholar] [CrossRef]
- Minguez-Alarcon, L.; Christou, G.; Messerlian, C.; Williams, P.L.; Carignan, C.C.; Souter, I.; Ford, J.B.; Calafat, A.M.; Hauser, R.; Team, E.S. Urinary triclosan concentrations and diminished ovarian reserve among women undergoing treatment in a fertility clinic. Fertil. Steril. 2017, 108, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Ge, L.; Han, Z.; Hao, X.; Zhang, M.L.; Zhang, X.J.; Zhou, C.J.; Zhang, D.J.; Liang, C.G. Oral administration of olaquindox negatively affects oocytes quality and reproductive ability in female mice. Ecotoxicol. Environ. Saf. 2020, 201, 110826. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, Y.; Hu, W.; Liu, Y.; Wang, H. Prenatal amoxicillin exposure induces developmental toxicity in fetal mice and its characteristics. J. Environ. Sci. 2024, 137, 287–301. [Google Scholar] [CrossRef]
- Ji, G.; Xia, Y.; Gu, A.; Shi, X.; Long, Y.; Song, L.; Wang, S.; Wang, X. Effects of non-occupational environmental exposure to pyrethroids on semen quality and sperm DNA integrity in Chinese men. Reprod. Toxicol. 2011, 31, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Sobala, W.; Piskunowicz, M.; Radwan, P.; Bochenek, M.; Hanke, W. The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Syst. Biol. Reprod. Med. 2015, 61, 37–43. [Google Scholar] [CrossRef]
- Toshima, H.; Suzuki, Y.; Imai, K.; Yoshinaga, J.; Shiraishi, H.; Mizumoto, Y.; Hatakeyama, S.; Onohara, C.; Tokuoka, S. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: A pilot study on exposure and semen quality. Int. J. Hyg. Environ. Health 2012, 215, 502–506. [Google Scholar] [CrossRef]
- Imai, K.; Yoshinaga, J.; Yoshikane, M.; Shiraishi, H.; Mieno, M.N.; Yoshiike, M.; Nozawa, S.; Iwamoto, T. Pyrethroid insecticide exposure and semen quality of young Japanese men. Reprod. Toxicol. 2014, 43, 38–44. [Google Scholar] [CrossRef]
- Hamed, M.A.; Akhigbe, T.M.; Adeogun, A.E.; Adesoye, O.B.; Akhigbe, R.E. Impact of organophosphate pesticides exposure on human semen parameters and testosterone: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1227836. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Y.; Wu, S.; Zhu, Q.; Li, X.; Liu, S.; Huang, T.; Li, H.; Ge, R.S. Acephate interferes with androgen synthesis in rat immature Leydig cells. Chemosphere 2020, 245, 125597. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Y.; Miao, Y.; Liu, C.; Deng, Y.L.; Chen, P.P.; Zhang, M.; Cui, F.P.; Shi, T.; Lu, T.T.; Liu, C.J.; et al. Serum multiple organochlorine pesticides in relation to testosterone concentrations among Chinese men from an infertility clinic. Chemosphere 2022, 299, 134469. [Google Scholar] [CrossRef] [PubMed]
- Yucra, S.; Gasco, M.; Rubio, J.; Gonzales, G.F. Semen quality in Peruvian pesticide applicators: Association between urinary organophosphate metabolites and semen parameters. Environ. Health 2008, 7, 59. [Google Scholar] [CrossRef]
- Pant, N.; Pant, A.B.; Chaturvedi, P.K.; Shukla, M.; Mathur, N.; Gupta, Y.K.; Saxena, D.K. Semen quality of environmentally exposed human population: The toxicological consequence. Environ. Sci. Pollut. Res. Int. 2013, 20, 8274–8281. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Dai, Y.; Zhang, J.; Wu, Z.; Li, S.; Zhou, Z. Associations between exposure to pesticides mixture and semen quality among the non-occupationally exposed males: Four statistical models. Environ. Res. 2024, 257, 119400. [Google Scholar] [CrossRef]
- Farombi, E.O.; Ugwuezunmba, M.C.; Ezenwadu, T.T.; Oyeyemi, M.O.; Ekor, M. Tetracycline-induced reproductive toxicity in male rats: Effects of vitamin C and N-acetylcysteine. Exp. Toxicol. Pathol. 2008, 60, 77–85. [Google Scholar] [CrossRef]
- Karaman, M.; Budak, H.; Ciftci, M. Amoxicillin and gentamicin antibiotics treatment adversely influence the fertility and morphology through decreasing the Dazl gene expression level and increasing the oxidative stress. Arch. Physiol. Biochem. 2019, 125, 447–455. [Google Scholar] [CrossRef]
- Kubsad, D.; Nilsson, E.E.; King, S.E.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Assessment of Glyphosate Induced Epigenetic Transgenerational Inheritance of Pathologies and Sperm Epimutations: Generational Toxicology. Sci. Rep. 2019, 9, 6372. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Pesticide and insect repellent mixture (permethrin and DEET) induces epigenetic transgenerational inheritance of disease and sperm epimutations. Reprod. Toxicol. 2012, 34, 708–719. [Google Scholar] [CrossRef]
- Zeh, J.A.; Bonilla, M.M.; Adrian, A.J.; Mesfin, S.; Zeh, D.W. From father to son: Transgenerational effect of tetracycline on sperm viability. Sci. Rep. 2012, 2, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, L.; Hu, Y.; Tse, L.A.; Wang, Y.; Qin, K.; Ding, G.; Zhou, Y.; Yu, X.; Ouyang, F.; et al. Exposure to Organophosphate Pesticides and Menstrual Cycle Characteristics in Chinese Preconceptional Women. Am. J. Epidemiol. 2020, 189, 375–383. [Google Scholar] [CrossRef]
- Sampaio, C.F.; Prates, K.V.; Siervo, G.; Mathias, P.C.F.; Fernandes, G.S.A. Impairment of testicular development in rats exposed to acephate during maternal gestation and lactation. Environ. Sci. Pollut. Res. Int. 2020, 27, 5482–5488. [Google Scholar] [CrossRef]
- Lin, K.J.; Mitchell, A.A.; Yau, W.P.; Louik, C.; Hernandez-Diaz, S. Maternal exposure to amoxicillin and the risk of oral clefts. Epidemiology 2012, 23, 699–705. [Google Scholar] [CrossRef]
- Versporten, A.; Bruyndonckx, R.; Adriaenssens, N.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; on behalf of the ESAC-Net study group. Consumption of tetracyclines, sulphonamides and trimethoprim, and other antibacterials in the community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76 (Suppl. 2), ii45–ii59. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef] [PubMed]
- Rosario, R.; Adams, I.R.; Anderson, R.A. Is there a role for DAZL in human female fertility? Mol. Hum. Reprod. 2016, 22, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.Y.; Rosen, M.P.; Nelson, L.M.; Turek, P.J.; Witte, J.S.; Cramer, D.W.; Cedars, M.I.; Reijo-Pera, R.A. Novel missense mutations of the Deleted-in-AZoospermia-Like (DAZL) gene in infertile women and men. Reprod. Biol. Endocrinol. 2006, 4, 40. [Google Scholar] [CrossRef]
- Miska, E.A.; Ferguson-Smith, A.C. Transgenerational inheritance: Models and mechanisms of non-DNA sequence-based inheritance. Science 2016, 354, 59–63. [Google Scholar] [CrossRef]
- Mango, S.E. Generations of longevity. Nature 2011, 479, 302–303. [Google Scholar] [CrossRef]
- Whitelaw, L.D.E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar]
- Kelce, W.R.; Monosson, E.; Gamcsik, M.P.; Laws, S.C.; Gray, L.E., Jr. Environmental hormone disruptors: Evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol. Appl. Pharmacol. 1994, 126, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Ben Maamar, M.; Skinner, M.K. Role of epigenetic transgenerational inheritance in generational toxicology. Environ. Epigenet. 2022, 8, dvac001. [Google Scholar] [CrossRef]
- Duhig, K.E.; Myers, J.; Seed, P.T.; Sparkes, J.; Lowe, J.; Hunter, R.M.; Shennan, A.H.; Chappell, L.C.; on behalf of the PARROT trial group. Placental growth factor testing to assess women with suspected pre-eclampsia: A multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 2019, 393, 1807–1818. [Google Scholar] [CrossRef]
- Argaw-Denboba, A.; Schmidt, T.S.B.; Di Giacomo, M.; Ranjan, B.; Devendran, S.; Mastrorilli, E.; Lloyd, C.T.; Pugliese, D.; Paribeni, V.; Dabin, J.; et al. Paternal microbiome perturbations impact offspring fitness. Nature 2024, 629, 652–659. [Google Scholar] [CrossRef]
- Singh, D.V.; Ali, R.; Anita Kulsum, M.; Bhat, R.A. Ecofriendly approaches for remediation of pesticides in contaminated environs. Bioremediation Biotechnol. 2020, 3, 173–194. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colopi, A.; Guida, E.; Cacciotti, S.; Fuda, S.; Lampitto, M.; Onorato, A.; Zucchi, A.; Balistreri, C.R.; Grimaldi, P.; Barchi, M. Dietary Exposure to Pesticide and Veterinary Drug Residues and Their Effects on Human Fertility and Embryo Development: A Global Overview. Int. J. Mol. Sci. 2024, 25, 9116. https://doi.org/10.3390/ijms25169116
Colopi A, Guida E, Cacciotti S, Fuda S, Lampitto M, Onorato A, Zucchi A, Balistreri CR, Grimaldi P, Barchi M. Dietary Exposure to Pesticide and Veterinary Drug Residues and Their Effects on Human Fertility and Embryo Development: A Global Overview. International Journal of Molecular Sciences. 2024; 25(16):9116. https://doi.org/10.3390/ijms25169116
Chicago/Turabian StyleColopi, Ambra, Eugenia Guida, Silvia Cacciotti, Serena Fuda, Matteo Lampitto, Angelo Onorato, Alice Zucchi, Carmela Rita Balistreri, Paola Grimaldi, and Marco Barchi. 2024. "Dietary Exposure to Pesticide and Veterinary Drug Residues and Their Effects on Human Fertility and Embryo Development: A Global Overview" International Journal of Molecular Sciences 25, no. 16: 9116. https://doi.org/10.3390/ijms25169116
APA StyleColopi, A., Guida, E., Cacciotti, S., Fuda, S., Lampitto, M., Onorato, A., Zucchi, A., Balistreri, C. R., Grimaldi, P., & Barchi, M. (2024). Dietary Exposure to Pesticide and Veterinary Drug Residues and Their Effects on Human Fertility and Embryo Development: A Global Overview. International Journal of Molecular Sciences, 25(16), 9116. https://doi.org/10.3390/ijms25169116








