Sodium Tungstate Promotes Neurite Outgrowth and Confers Neuroprotection in Neuro2a and SH-SY5Y Cells
Abstract
1. Introduction
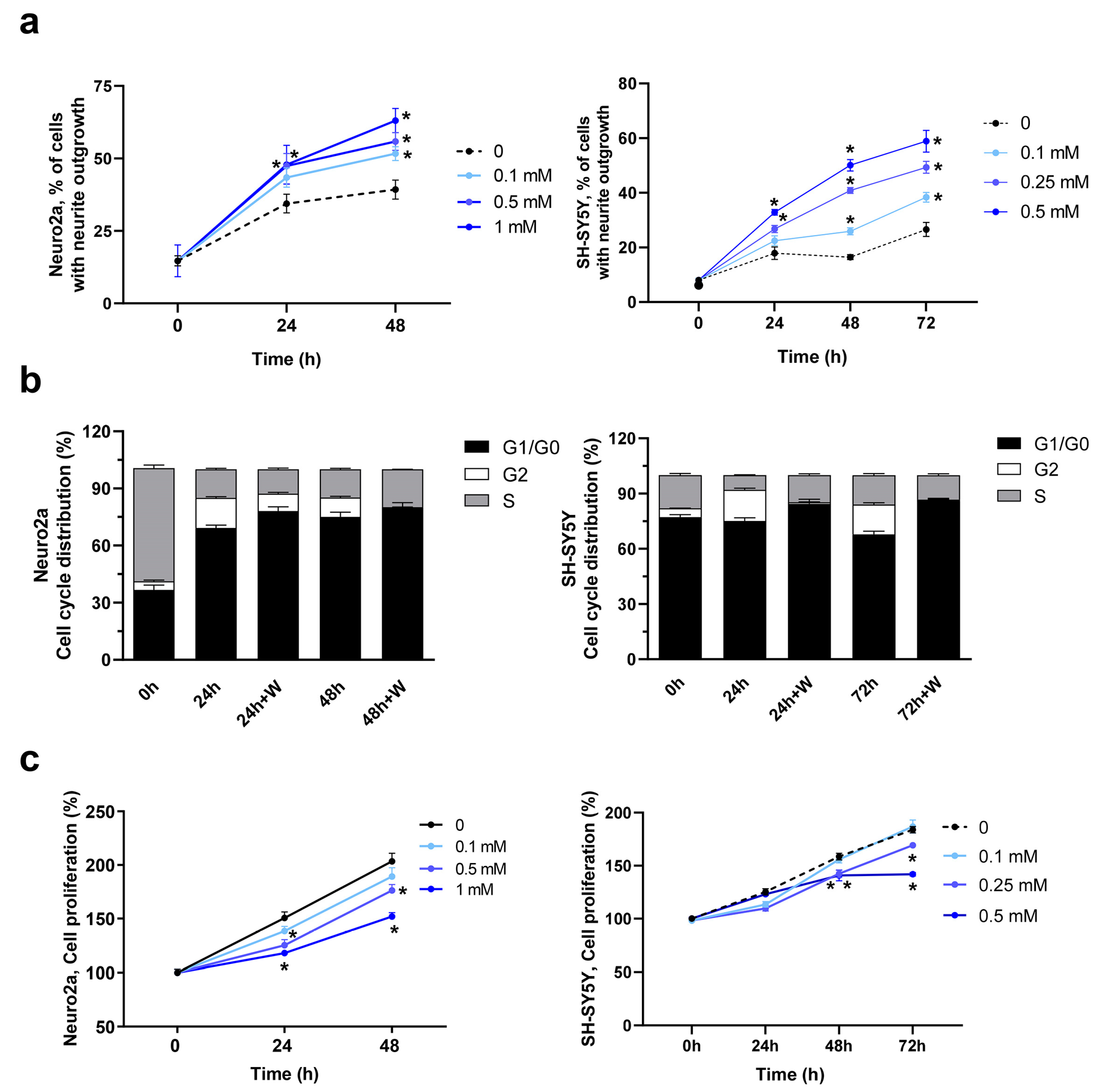
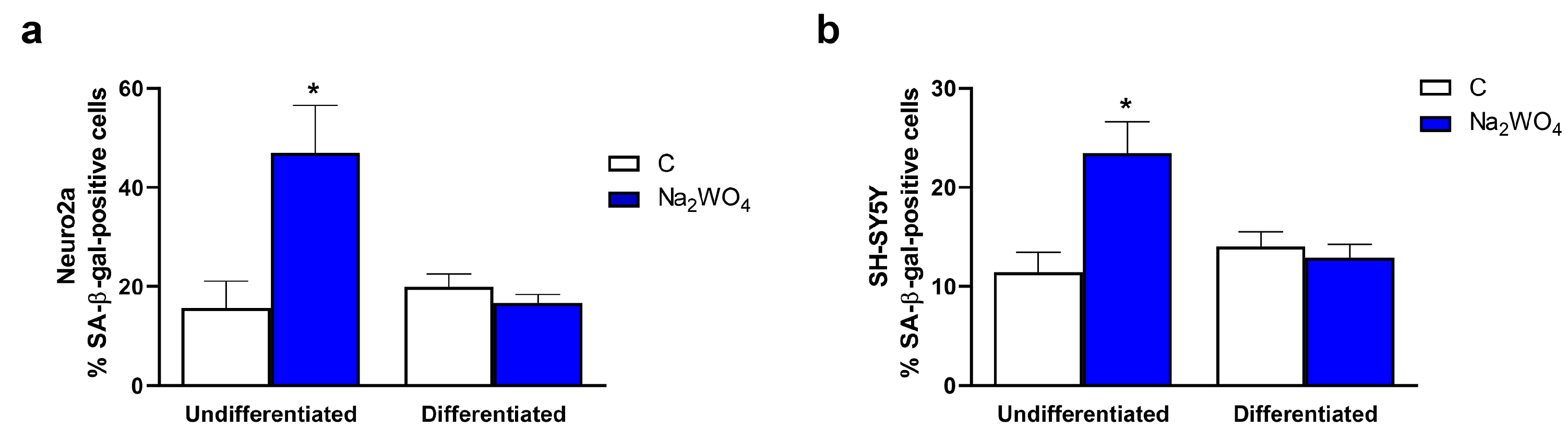
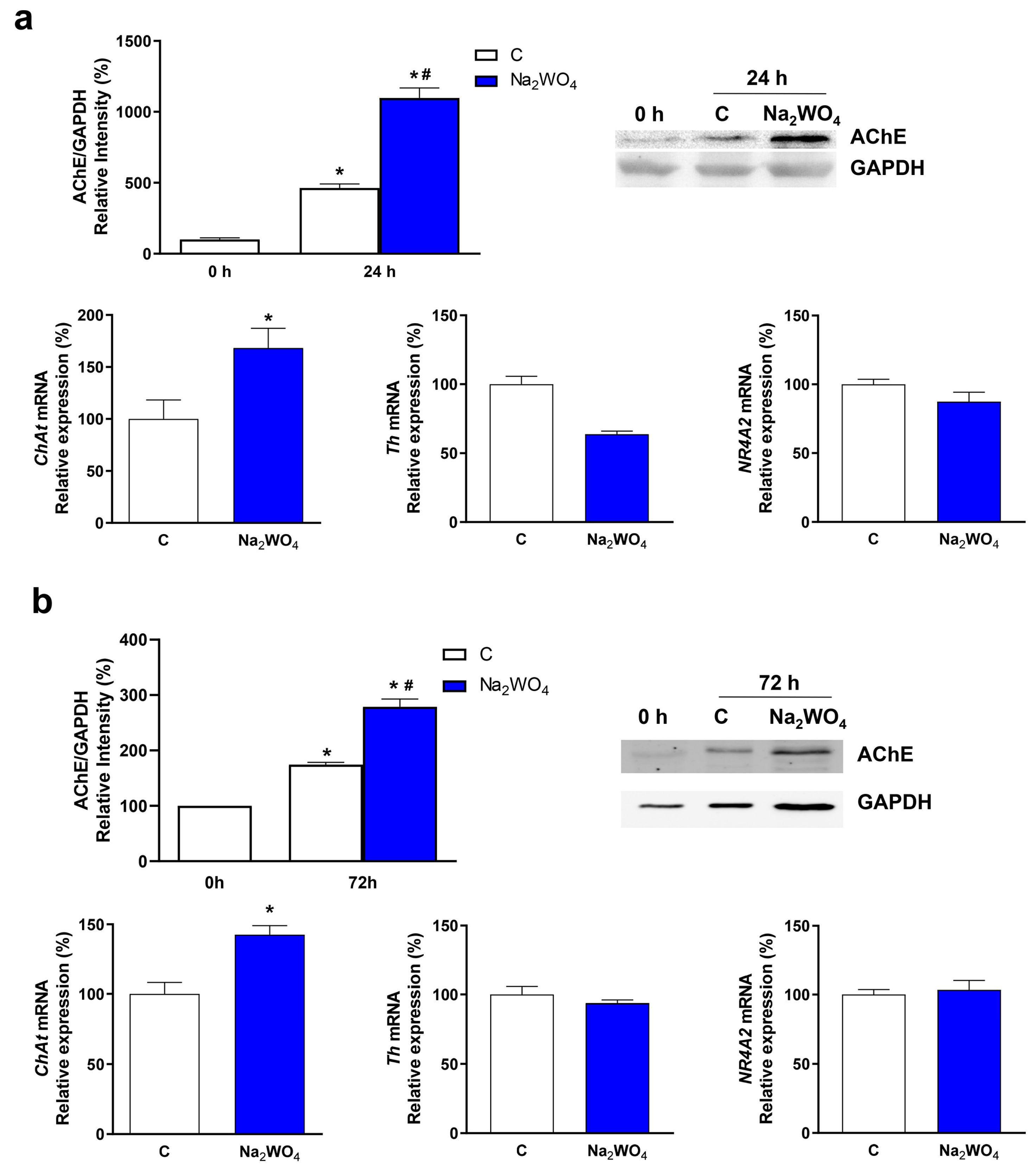
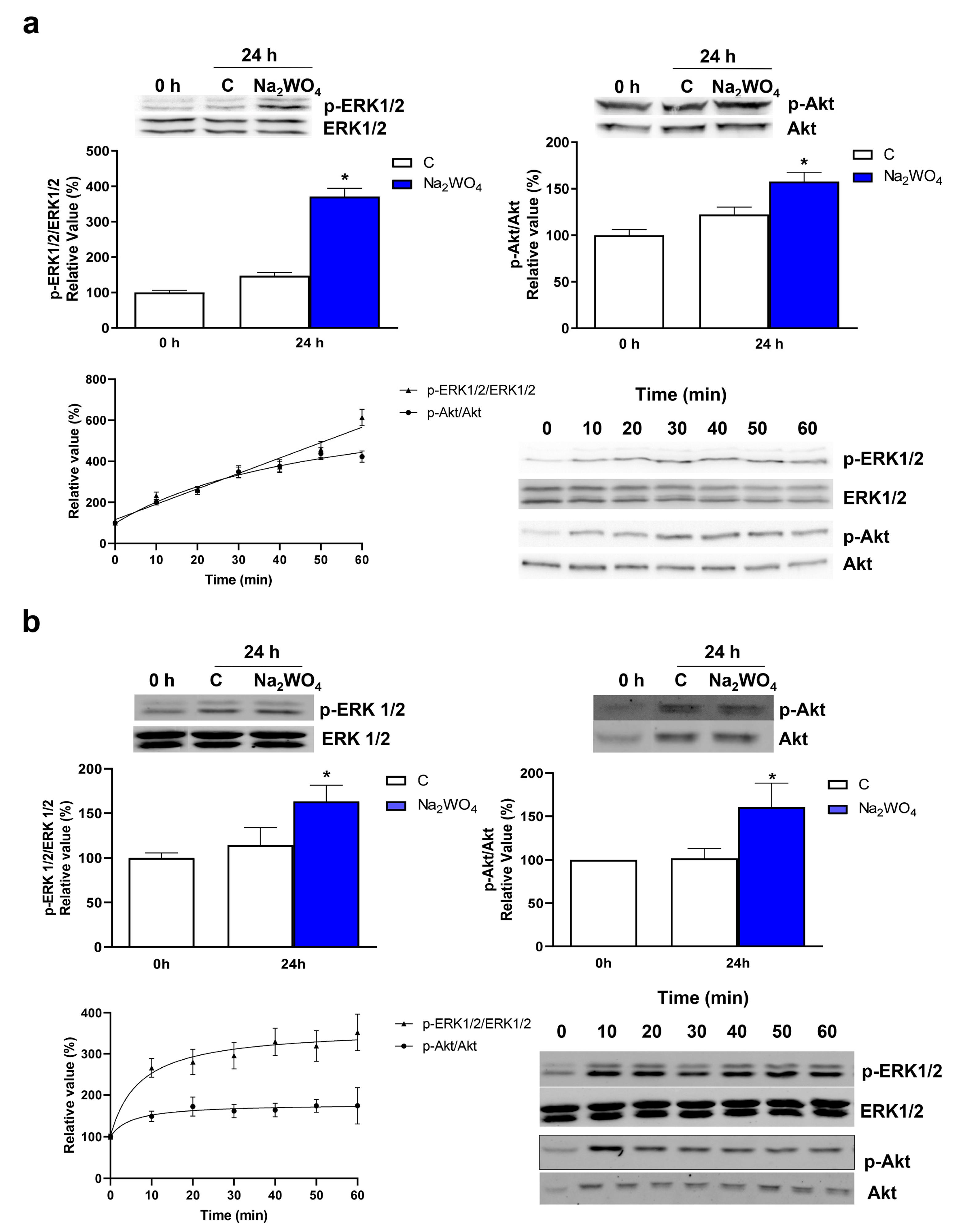
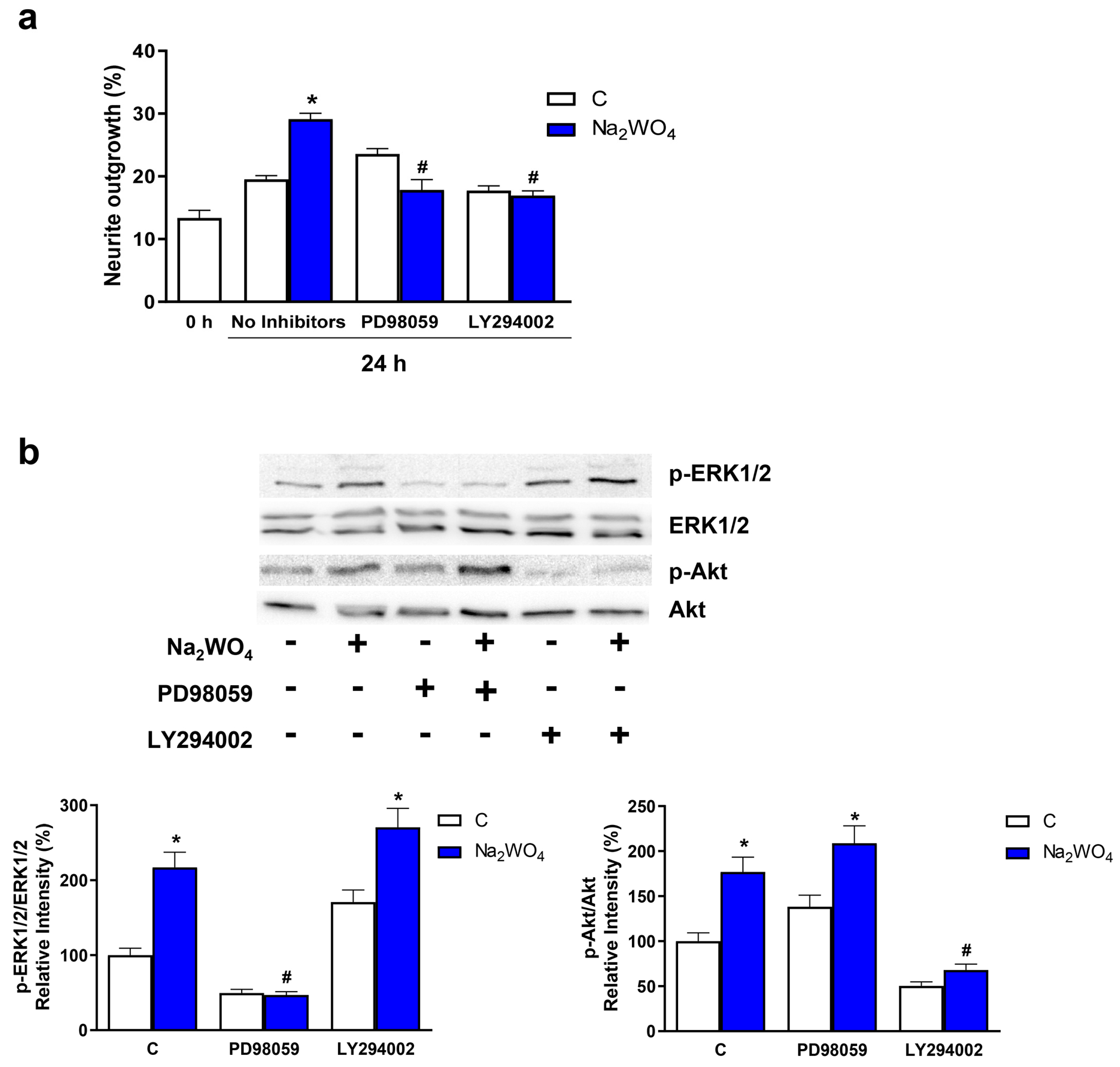
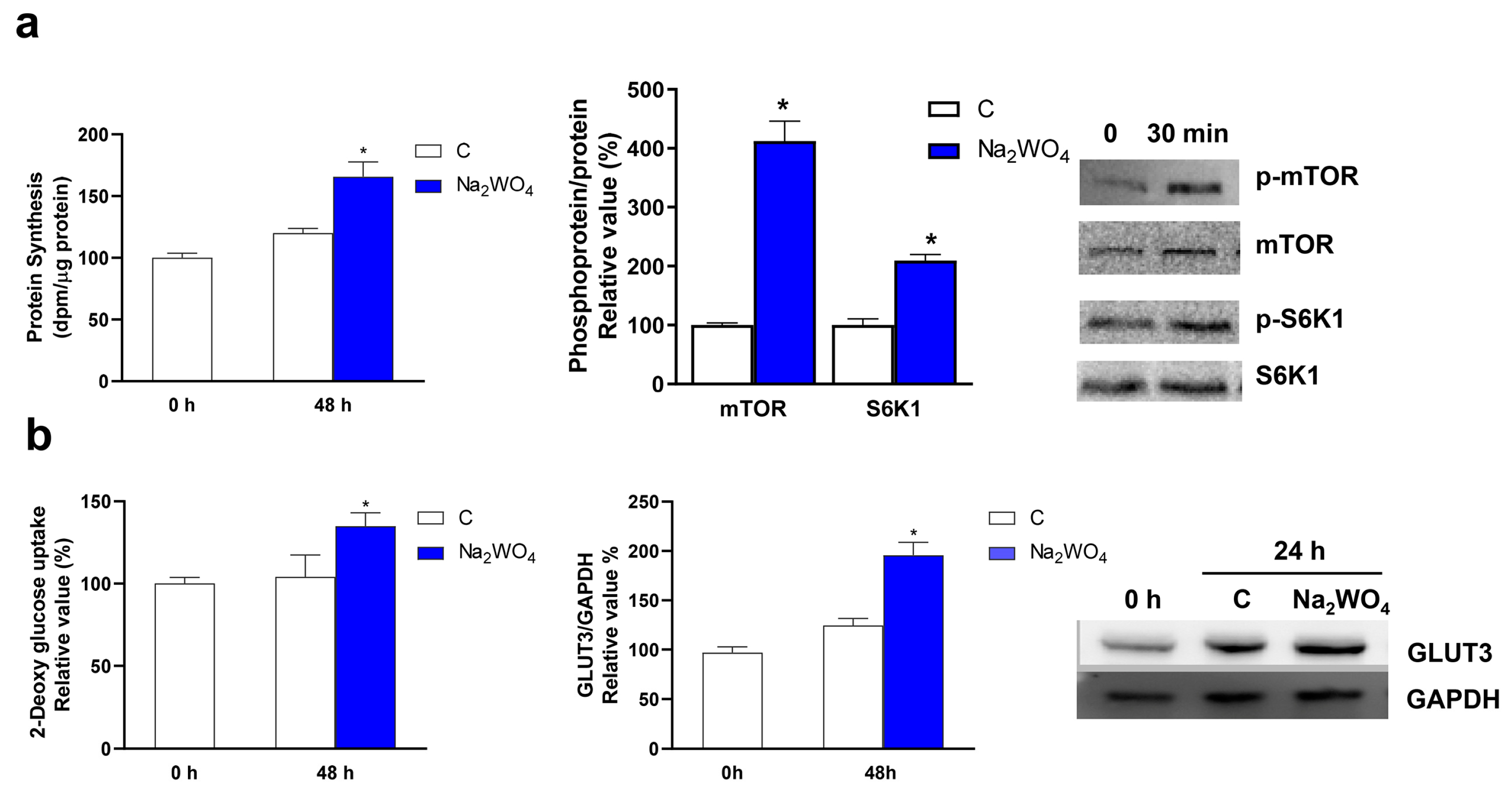
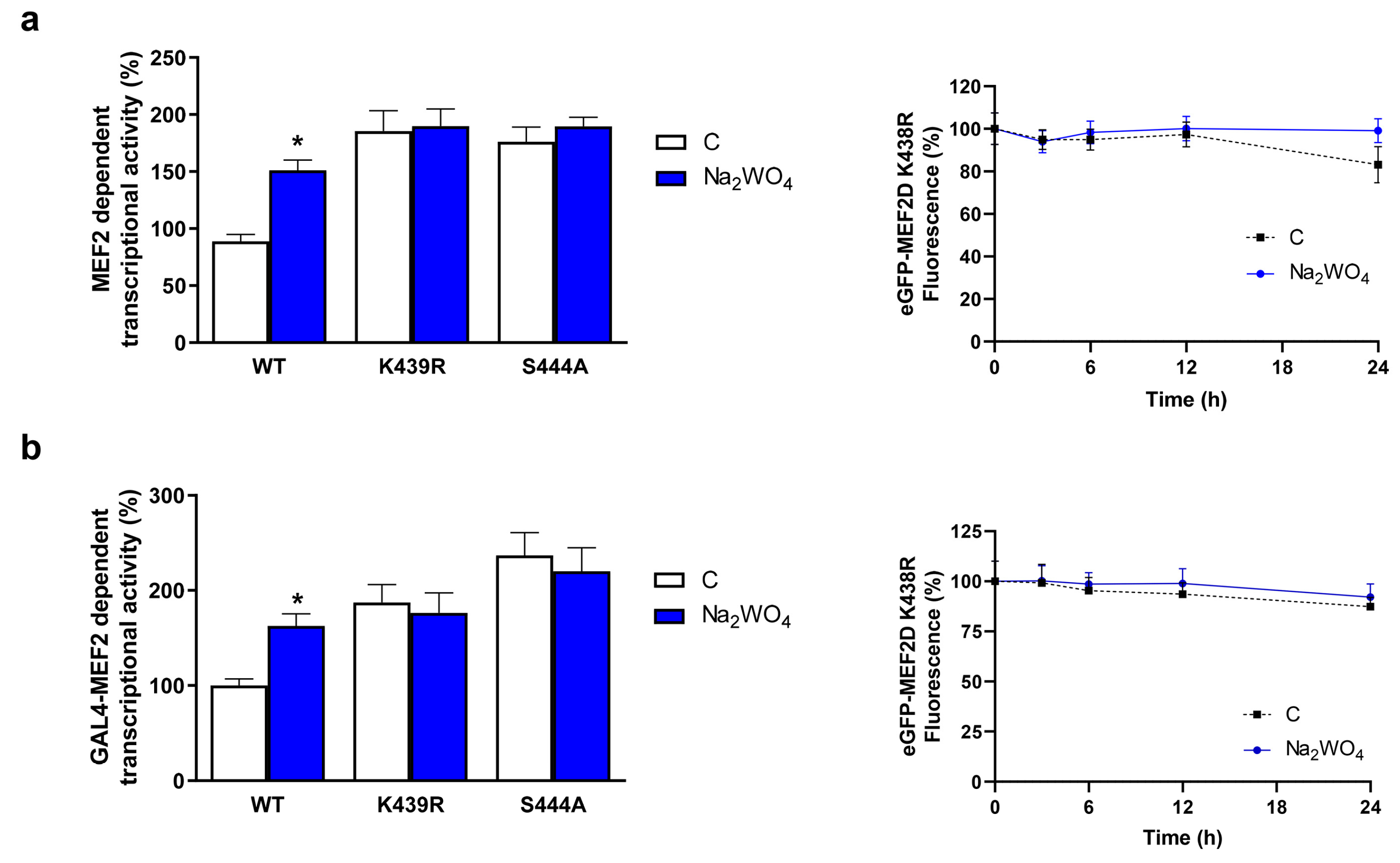
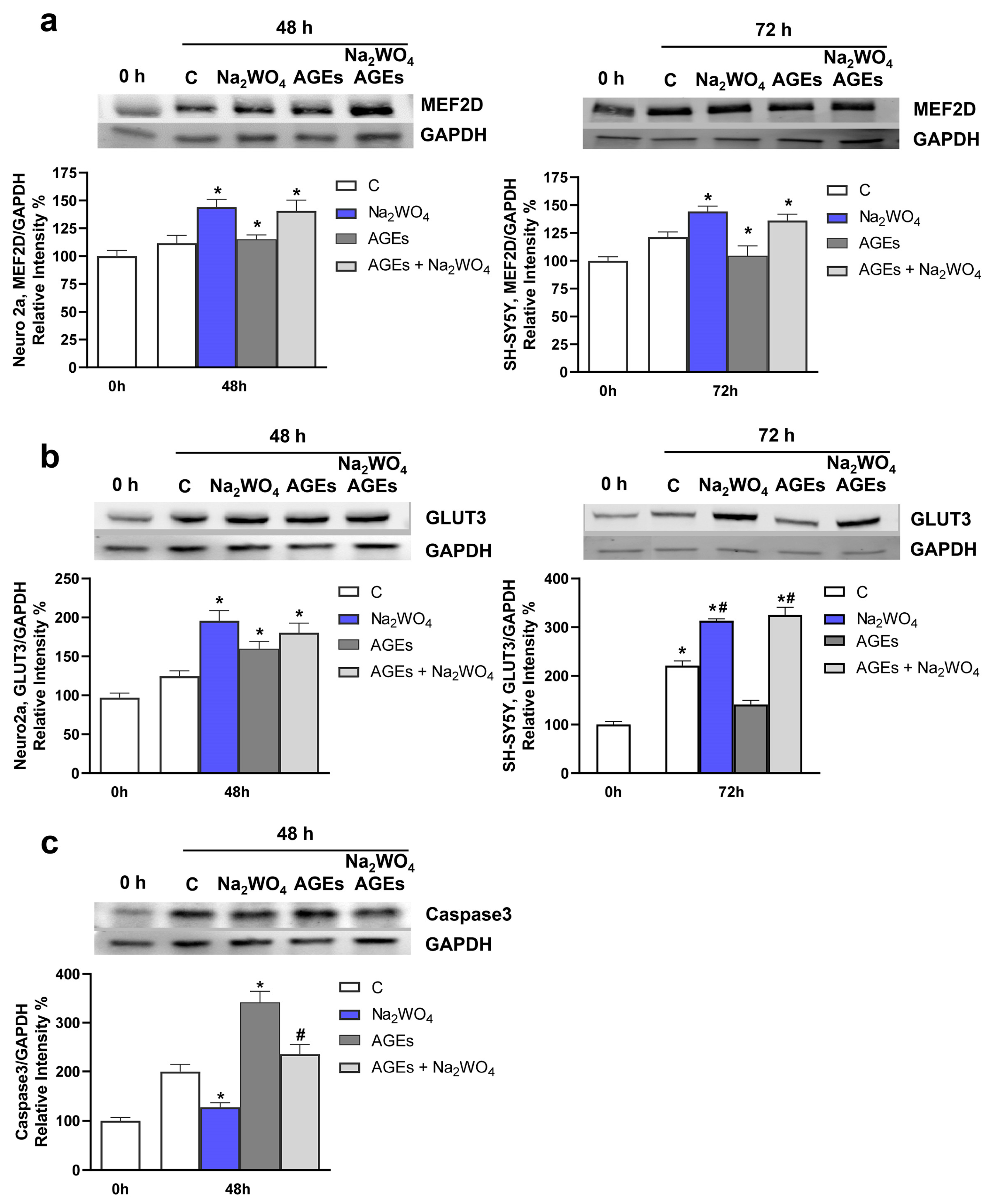
2. Results and Discussion
3. Materials and Methods
3.1. Cell Culture
3.2. Cell Neurite Outgrowth Assays and Proliferation
3.3. Cell Cycle Analysis
3.4. Protein Analysis
3.5. RNA Extraction and RT-qPCR
3.6. MEF2D Expression Vectors, Reporter Gene Constructs, and Transcriptional Assays
3.7. Senescence
3.8. Determination of Protein Synthesis and 2-Deoxy-D-[1−3H]glucose Uptake
3.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hrvoj-Mihic, B.; Bienvenu, T.; Stefanacci, L.; Muotri, A.R.; Semendeferi, K. Evolution, development, and plasticity of the human brain: From molecules to bones. Front. Hum. Neurosci. 2013, 7, 707. [Google Scholar] [CrossRef]
- Liu, H.H.; Jan, Y.N. Mechanisms of neurite repair. Curr. Opin. Neurobiol. 2020, 63, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Read, D.E.; Gorman, A.M. Involvement of Akt in neurite outgrowth. Cell Mol. Life Sci. 2009, 66, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. A Critical Kinase Cascade in Neurological Disorders: PI 3-K., Akt, and mTOR. Future Neurol. 2012, 7, 733–748. [Google Scholar] [CrossRef]
- El Ouaamari, Y.; Van den Bos, J.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jin, M.L.; An, H.K.; Kim, K.S.; Ko, M.J.; Kim, C.M.; Choi, Y.W.; Lee, Y.C. Emodin induces neurite outgrowth through PI3K/Akt/GSK−3beta-mediated signaling pathways in Neuro2a cells. Neurosci. Lett. 2015, 588, 101–107. [Google Scholar] [CrossRef]
- Wu, P.Y.; Lin, Y.C.; Chang, C.L.; Lu, H.T.; Chin, C.H.; Hsu, T.T.; Chu, D.; Sun, S.H. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro−2a neuroblastoma cells. Cell Signal. 2009, 21, 881–891. [Google Scholar] [CrossRef]
- Yanaka, N.; Nogusa, Y.; Fujioka, Y.; Yamashita, Y.; Kato, N. Involvement of membrane protein GDE2 in retinoic acid-induced neurite formation in Neuro2A cells. FEBS Lett. 2007, 581, 712–718. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 108, 53193. [Google Scholar]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Maiuolo, J.; Costanzo, P.; Masullo, M.; D’Errico, A.; Nasso, R.; Bonacci, S.; Mollace, V.; Oliverio, M.; Arcone, R. Hydroxytyrosol-Donepezil Hybrids Play a Protective Role in an In Vitro Induced Alzheimer’s Disease Model and in Neuronal Differentiated Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 13461. [Google Scholar] [CrossRef] [PubMed]
- Bertinat, R.; Nualart, F.; Li, X.; Yanez, A.J.; Gomis, R. Preclinical and Clinical Studies for Sodium Tungstate: Application in Humans. J. Clin. Cell. Immunol. 2015, 6, 285. [Google Scholar]
- Zafra, D.; Nocito, L.; Dominguez, J.; Guinovart, J.J. Sodium tungstate activates glycogen synthesis through a non-canonical mechanism involving G-proteins. FEBS Lett. 2013, 587, 291–296. [Google Scholar] [CrossRef]
- Giron, M.D.; Sevillano, N.; Vargas, A.M.; Dominguez, J.; Guinovart, J.J.; Salto, R. The glucose-lowering agent sodium tungstate increases the levels and translocation of GLUT4 in L6 myotubes through a mechanism associated with ERK1/2 and MEF2D. Diabetologia 2008, 51, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alvarez, J.; Barbera, A.; Nadal, B.; Barcelo-Batllori, S.; Piquer, S.; Claret, M.; Guinovart, J.J.; Gomis, R. Stable and functional regeneration of pancreatic beta-cell population in nSTZ-rats treated with tungstate. Diabetologia 2004, 47, 470–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dominguez, J.E.; Munoz, M.C.; Zafra, D.; Sanchez-Perez, I.; Baque, S.; Caron, M.; Mercurio, C.; Barbera, A.; Perona, R.; Gomis, R.; et al. The antidiabetic agent sodium tungstate activates glycogen synthesis through an insulin receptor-independent pathway. J. Biol. Chem. 2003, 278, 42785–42794. [Google Scholar] [CrossRef] [PubMed]
- Amigo-Correig, M.; Barcelo-Batllori, S.; Piquer, S.; Soty, M.; Pujadas, G.; Gasa, R.; Bortolozzi, A.; Carmona, M.C.; Gomis, R. Sodium tungstate regulates food intake and body weight through activation of the hypothalamic leptin pathway. Diabetes Obes. Metab. 2011, 13, 235–242. [Google Scholar] [CrossRef]
- Salto, R.; Vilchez, J.D.; Cabrera, E.; Guinovart, J.J.; Giron, M.D. Activation of ERK by sodium tungstate induces protein synthesis and prevents protein degradation in rat L6 myotubes. FEBS Lett. 2014, 588, 2246–2254. [Google Scholar] [CrossRef]
- Salto, R.; Vilchez, J.D.; Giron, M.D.; Cabrera, E.; Campos, N.; Manzano, M.; Rueda, R.; Lopez-Pedrosa, J.M. beta-Hydroxy-beta-Methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS ONE 2015, 10, e0135614. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, J.J.; Cai, Z.N.; Xu, C.J. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62. Int. J. Mol. Med. 2018, 42, 2481–2488. [Google Scholar] [CrossRef]
- Galderisi, U.; Jori, F.P.; Giordano, A. Cell cycle regulation and neural differentiation. Oncogene 2003, 22, 5208–5219. [Google Scholar] [CrossRef]
- Hindley, C.; Philpott, A. Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem. J. 2012, 444, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Sun, L.; Wang, X. Evidence and perspectives of cell senescence in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111327. [Google Scholar] [CrossRef] [PubMed]
- Dickey, C.A.; De Mesquita, D.D.; Morgan, D.; Pennypacker, K.R. Induction of memory-associated immediate early genes by nerve growth factor in rat primary cortical neurons and differentiated mouse Neuro2A cells. Neurosci. Lett. 2004, 366, 10–14. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Yao, Y.; Li, J.; Zhang, X.; Li, C.; Cheng, Y.; Ding, G.; Liu, L.; Ding, Z. Essential role of ERK activation in neurite outgrowth induced by alpha-lipoic acid. Biochim. Biophys. Acta 2011, 1813, 827–838. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Li, J.; Wang, X.; Yao, Y.; Zhang, X.; Li, C.; Cheng, Y.; Ding, G.; Liu, L.; et al. MEK/ERKs signaling is essential for lithium-induced neurite outgrowth in N2a cells. Int. J. Dev. Neurosci. 2011, 29, 415–422. [Google Scholar] [CrossRef]
- Zoungrana, L.I.; Krause-Hauch, M.; Wang, H.; Fatmi, M.K.; Bates, L.; Li, Z.; Kulkarni, P.; Ren, D.; Li, J. The Interaction of mTOR and Nrf2 in Neurogenesis and Its Implication in Neurodegenerative Diseases. Cells 2022, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Fao, L.; Mota, S.; Rego, A.C. Mitochondrial function and dynamics in neural stem cells and neurogenesis: Implications for neurodegenerative diseases. Ageing Res. Rev. 2022, 80, 101667. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Tan, C.; Mo, L.; Jiang, J.; Zhou, W.; Du, J.; Zhou, X.; Liu, X.; Chen, L. Glucose transporter 3 in neuronal glucose metabolism: Health and diseases. Metabolism 2021, 123, 154869. [Google Scholar] [CrossRef]
- Agostini, M.; Romeo, F.; Inoue, S.; Niklison-Chirou, M.V.; Elia, A.J.; Dinsdale, D.; Morone, N.; Knight, R.A.; Mak, T.W.; Melino, G. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 2016, 23, 1502–1514. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Kim, M.S.; Adachi, M.; Morris, M.J.; Qi, X.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N.; Kavalali, E.T.; Monteggia, L.M. In vivo analysis of MEF2 transcription factors in synapse regulation and neuronal survival. PLoS ONE 2012, 7, e34863. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, B.; Chen, G.; Huang, Y.; Tian, Z.; Zhang, L.; Tian, L.; Fu, Q. MEF2D Participates in Microglia-Mediated Neuroprotection in Cerebral Ischemia-Reperfusion Rats. Shock 2022, 57, 118–130. [Google Scholar] [CrossRef]
- Wang, N.; Yang, W.; Li, L.; Tian, M. MEF2D upregulation protects neurons from oxygen-glucose deprivation/re-oxygenation-induced injury by enhancing Nrf2 activation. Brain Res. 2020, 1741, 146878. [Google Scholar] [CrossRef]
- Chen, X.; Gao, B.; Ponnusamy, M.; Lin, Z.; Liu, J. MEF2 signaling and human diseases. Oncotarget 2017, 8, 112152–112165. [Google Scholar] [CrossRef]
- Lam, B.Y.; Chawla, S. MEF2D expression increases during neuronal differentiation of neural progenitor cells and correlates with neurite length. Neurosci. Lett. 2007, 427, 153–158. [Google Scholar] [CrossRef]
- Lisek, M.; Przybyszewski, O.; Zylinska, L.; Guo, F.; Boczek, T. The Role of MEF2 Transcription Factor Family in Neuronal Survival and Degeneration. Int. J. Mol. Sci. 2023, 24, 3120. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Tremblay, A.M.; Xiao, L.; Yang, Q.; Ma, K.; Nie, J.; Mao, Z.; Wu, Z.; Giguere, V.; Yang, X.J. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J. Biol. Chem. 2006, 281, 4423–4433. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Nagasaki, S.C.; Suzuki, Y.; Hirano, Y.; Imayoshi, I. Optimization of Light-Inducible Gal4/UAS Gene Expression System in Mammalian Cells. iScience 2020, 23, 101506. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Ludwig, S.C.; Kuhla, B.; Munch, G.; Vollmar, B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer’s disease brain. Neurobiol. Aging 2015, 36, 753–761. [Google Scholar] [CrossRef]
- Schmidt, A.; Kuhla, B.; Bigl, K.; Munch, G.; Arendt, T. Cell cycle related signaling in Neuro2a cells proceeds via the receptor for advanced glycation end products. J. Neural. Transm. 2007, 114, 1413–1424. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Qiu, Y.; Liu, B.; Qi, H.; Li, Z.; Kong, H.; Kong, L. The downregulation of thioredoxin accelerated Neuro2a cell apoptosis induced by advanced glycation end product via activating several pathways. Neurochem. Int. 2015, 87, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Reddy Addi, U.; Jakhotia, S.; Reddy, S.S.; Reddy, G.B. Age-related neuronal damage by advanced glycation end products through altered proteostasis. Chem. Biol. Interact. 2022, 355, 109840. [Google Scholar] [CrossRef]
- Souza, C.G.; Riboldi, B.P.; Hansen, F.; Moreira, J.D.; Souza, D.G.; de Assis, A.M.; Brum, L.M.; Perry, M.L.; Souza, D.O. Chronic sulforaphane oral treatment accentuates blood glucose impairment and may affect GLUT3 expression in the cerebral cortex and hypothalamus of rats fed with a highly palatable diet. Food Funct. 2013, 4, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Giron-Gonzalez, M.D.; Morales-Portillo, A.; Salinas-Castillo, A.; Lopez-Jaramillo, F.J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F.; Salto-Gonzalez, R. Engineered glycated amino dendritic polymers as specific nonviral gene delivery vectors targeting the receptor for advanced glycation end products. Bioconjug. Chem. 2014, 25, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Khiem, D.; Cyster, J.G.; Schwarz, J.J.; Black, B.L. A p38 MAPK-MEF2C pathway regulates B-cell proliferation. Proc. Natl. Acad. Sci. USA 2008, 105, 17067–17072. [Google Scholar] [CrossRef] [PubMed]
- Kucinska, M.; Giron, M.D.; Piotrowska, H.; Lisiak, N.; Granig, W.H.; Lopez-Jaramillo, F.J.; Salto, R.; Murias, M.; Erker, T. Novel Promising Estrogenic Receptor Modulators: Cytotoxic and Estrogenic Activity of Benzanilides and Dithiobenzanilides. PLoS ONE 2016, 11, e0145615. [Google Scholar] [CrossRef]
- Kao, S.H.; Wang, W.L.; Chen, C.Y.; Chang, Y.L.; Wu, Y.Y.; Wang, Y.T.; Wang, S.P.; Nesvizhskii, A.I.; Chen, Y.J.; Hong, T.M.; et al. Analysis of Protein Stability by the Cycloheximide Chase Assay. Bio Protoc. 2015, 5, e1374. [Google Scholar] [CrossRef]
- Itahana, K.; Itahana, Y.; Dimri, G.P. Colorimetric detection of senescence-associated beta galactosidase. Methods Mol. Biol. 2013, 965, 143–156. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Martin, N.; Girón, M.D.; Vílchez, J.D.; Salto, R. Sodium Tungstate Promotes Neurite Outgrowth and Confers Neuroprotection in Neuro2a and SH-SY5Y Cells. Int. J. Mol. Sci. 2024, 25, 9150. https://doi.org/10.3390/ijms25179150
Montero-Martin N, Girón MD, Vílchez JD, Salto R. Sodium Tungstate Promotes Neurite Outgrowth and Confers Neuroprotection in Neuro2a and SH-SY5Y Cells. International Journal of Molecular Sciences. 2024; 25(17):9150. https://doi.org/10.3390/ijms25179150
Chicago/Turabian StyleMontero-Martin, Nora, María D. Girón, José D. Vílchez, and Rafael Salto. 2024. "Sodium Tungstate Promotes Neurite Outgrowth and Confers Neuroprotection in Neuro2a and SH-SY5Y Cells" International Journal of Molecular Sciences 25, no. 17: 9150. https://doi.org/10.3390/ijms25179150
APA StyleMontero-Martin, N., Girón, M. D., Vílchez, J. D., & Salto, R. (2024). Sodium Tungstate Promotes Neurite Outgrowth and Confers Neuroprotection in Neuro2a and SH-SY5Y Cells. International Journal of Molecular Sciences, 25(17), 9150. https://doi.org/10.3390/ijms25179150







