Differences in Bone Metabolism between Children with Prader–Willi Syndrome during Growth Hormone Treatment and Healthy Subjects: A Pilot Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biochemical Methods
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heksch, R.; Kamboj, M.; Anglin, K.; Obrynba, K. Review of Prader-Willi syndrome: The endocrine approach. Transl. Pediatr. 2017, 6, 274–285. [Google Scholar] [CrossRef]
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi syndrome—Clinical genetics, diagnosis and teatment approaches: An update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef]
- Bakker, N.E.; Kuppens, R.J.; Siemensma, E.P.; Tummers-de Lind van Wijngaarden, R.F.; Festen, D.A.; Bindels-de Heus, G.C.; Bocca, G.; Haring, D.A.; Hoorweg-Nijman, J.J.; Houdijk, E.C.; et al. Bone mineral density in children and adolescents with Prader-Willi syndrome: A longitudinal study during puberty and 9 years of growth hormone treatment. J. Clin. Endocrinol. Metab. 2015, 100, 1609–1618. [Google Scholar] [CrossRef]
- Oto, Y.; Murakami, N.; Inoue, T.; Matsubara, K.; Saima, S.; Ogata, H.; Ihara, H.; Nagai, T.; Matsubara, T. Growth hormone treatment and bone mineral density in pediatric patients with Prader-Willi syndrome. J. Pediatr. Endocrinol. Metab. 2021, 34, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Grugni, G.; Piacente, L.; Delvecchio, M.; Ventura, A.; Giordano, P.; Grano, M.; D’Amato, G.; Laforgia, D.; Crinò, A.; et al. Analysis of Circulating Mediators of Bone Remodeling in Prader-Willi Syndrome. Calcif. Tissue Int. 2018, 102, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Q.; Du, P.; Chen, X.; Zhang, Y. Roles of vitamin K-dependent protein in biomineralization (Review). Int. J. Mol. Med. 2024, 53, 6. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of emerging vitamin K-dependent proteins: Growth arrest-specific protein 6, Gla-rich protein and periostin (Review). Int. J. Mol. Med. 2021, 47, 2. [Google Scholar] [CrossRef]
- Rousseau, J.C.; Sornay-Rendu, E.; Bertholon, C.; Chapurlat, R.; Garnero, P. Serum periostin is associated with fracture risk in postmenopausal women: A 7-year prospective analysis of the OFELY study. J. Clin. Endocrinol. Metab. 2014, 99, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Voisin, S.; Al Saedi, A.; Phu, S.; Brennan-Speranza, T.; Parker, L.; Eynon, N.; Hiam, D.; Yan, X.; Scott, D.; et al. Osteocalcin and its forms across the lifespan in adult men. Bone 2020, 130, 115085. [Google Scholar] [CrossRef]
- Martiniakova, M.; Biro, R.; Kovacova, V.; Babikova, M.; Zemanova, N.; Mondockova, V.; Omelka, R. Current knowledge of bone-derived factor osteocalcin: Its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases. J. Mol. Med. 2024, 102, 435–452. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Karampatsou, S.I.; Paltoglou, G.; Genitsaridi, S.M.; Kassari, P.; Charmandari, E. The effect of a comprehensive life-style intervention program of diet and exercise on four bone-derived proteins, FGF-23, osteopontin, NGAL and sclerostin, in overweight or obese children and adolescents. Nutrients 2022, 14, 3772. [Google Scholar] [CrossRef] [PubMed]
- Weghuber, D.; Mangge, H.; Hochbrugger, E.; Stulnig, T.M. Impact of age and metabolic syndrome on the adipokine profile in childhood and adult obesity. Exp. Clin. Endocrinol. Diabetes 2014, 122, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.F.S.; Dechichi, P.; Limirio, P.H.J.O. Impact of childhood obesity on bone metabolism. Pediatr. Endocrinol. Rev. 2020, 17, 308–316. [Google Scholar] [PubMed]
- Marini, F.; Giusti, F.; Palmini, G.; Brandi, M.L. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos. Int. 2023, 34, 213–238. [Google Scholar] [CrossRef]
- Vestergaard, P.; Kristensen, K.; Bruun, J.M.; Østergaard, J.R.; Heickendorff, L.; Mosekilde, L.; Richelsen, B. Reduced bone mineral density and increased bone turnover in Prader-Willi syndrome compared with controls matched for sex and body mass index--a cross-sectional study. J. Pediatr. 2004, 144, 614–619. [Google Scholar] [CrossRef]
- Butler, M.G.; Haber, L.; Mernaugh, R.; Carlson, M.G.; Price, R.; Feurer, I.D. Decreased bone mineral density in Prader-Willi syndrome: Comparison with obese subjects. Am. J. Med. Genet. 2001, 103, 216–222. [Google Scholar] [CrossRef][Green Version]
- van Nieuwpoort, I.C.; Twisk, J.W.R.; Curfs, L.M.G.; Lips, P.; Drent, M.L. Body composition, adipokines, bone mineral density and bone remodeling markers in relation to IGF-1 levels in adults with Prader-Willi syndrome. Int. J. Pediatr. Endocrinol. 2018, 2018, 1. [Google Scholar] [CrossRef]
- Rubin, D.A.; Wilson, K.S.; Orsso, C.E.; Gertz, E.R.; Haqq, A.M.; Castner, D.M.; Dumont-Driscoll, M. A 24-Week physical activity intervention increases bone mineral content without changes in bone markers in youth with PWS. Genes 2020, 11, 984. [Google Scholar] [CrossRef]
- Brunetti, G.; D’Amato, G.; Chiarito, M.; Tullo, A.; Colaianni, G.; Colucci, S.; Grano, M.; Faienza, M.F. An update on the role of RANKL-RANK/osteoprotegerin and WNT-ß-catenin signaling pathways in pediatric diseases. World J. Pediatr. 2019, 15, 4–11. [Google Scholar] [CrossRef]
- Jarosz, M. Normy Żywienia dla Populacji Polskiej i Ich Zastosowanie; National Food and Nutrition Institute: Warsaw, Poland, 2020; pp. 26–148. [Google Scholar]
- Gajewska, J.; Szamotulska, K.; Klemarczyk, W.; Chełchowska, M.; Strucińska, M.; Ambroszkiewicz, J. Circulating levels of nesfatin-1 and spexin in children with Prader-Willi Syndrome during growth hormone treatment and dietary intervention. Nutrients 2023, 15, 1240. [Google Scholar] [CrossRef]
- Erhardt, É.; Molnár, D. Prader-Willi Syndrome: Possibilities of weight gain prevention and treatment. Nutrients 2022, 14, 1950. [Google Scholar] [CrossRef]
- Maser, R.E.; Lenhard, M.J.; Pohlig, R.T.; Balagopal, P.B.; Abdel-Misih, R. Effect of parathyroidectomy on osteopontin and undercarboxylated osteocalcin in patients with primary hyperparathyroidism. Endocr. Res. 2018, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Saki, F.; Sheikhi, A.; Omrani, G.H.R.; Karimi, H.; Dabbaghmanesh, M.H.; Mousavinasab, S.N. Evaluation of bone mineral density in children with type I diabetes mellitus and relationship to serum levels of osteopontin. Prog. Drug Res. 2017, 67, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Shi, T.; Han, X.; Chen, D.; Xu, Z.; Shi, D.; Teng, H.; Jiang, Q. Serum levels of leptin, osteopontin, and sclerostin in patients with and without knee osteoarthritis. Clin. Rheumatol. 2020, 40, 287–294. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Osmark, P.; Kuulasmaa, T.; Pilgaard, K.; Omar, B.; Brøns, C.; Kotova, O.; Zetterqvist, A.V.; Stancáková, A.; Jonsson, A.; et al. Link between GIP and osteopontin in adipose tissue and insulin resistance. Diabetes 2013, 62, 2088–2094. [Google Scholar] [CrossRef]
- Gajewska, J.; Ambroszkiewicz, J.; Szamotulska, K.; Rowicka, G.; Strucińska, M.; Klemarczyk, W.; Chełchowska, M. Associations between oxidant/antioxidant status and circulating adipokines in non-obese children with Prader-Willi Syndrome. Antioxidants 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Mazière, C.; Gomila, C.; Mazière, J.C. Oxidized low-density lipoprotein increases osteopontin expression by generation of oxidative stress. Free Radic. Biol. Med. 2010, 48, 1382–1387. [Google Scholar] [CrossRef]
- Stock, M.; Schett, G. Vitamin K-dependent proteins in skeletal development and disease. Int. J. Mol. Sci. 2021, 22, 9328. [Google Scholar] [CrossRef]
- Horiuchi, T.; Kazama, H.; Araki, A.; Inoue, J.; Hosoi, T.; Onouchi, T.; Mizuno, S.; Ito, H.; Orimo, H. Impaired gamma carboxylation of osteocalcin in elderly women with type II diabetes mellitus: Relationship between increase in undercarboxylated osteocalcin levels and low bone mineral density. J. Bone Miner. Metab. 2004, 22, 236–240. [Google Scholar] [CrossRef]
- Ziemińska, M.; Pawlak, D.; Sieklucka, B.; Chilkiewicz, K.; Pawlak, K. Vitamin K-dependent carboxylation of osteocalcin in bone-ally or adversary of bone mineral status in rats with experimental chronic kidney disease? Nutrients 2022, 14, 4082. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Grüner, D.; Worch, H.; Pompe, W.; Lichte, H.; El Khassawna, T.; Heiss, C.; Wenisch, S.; Kniep, R. First evidence of octacalcium phosphate@osteocalcin nanocomplex as skeletal bone component directing collagen triple-helix nanofibril mineralization. Sci. Rep. 2018, 8, 13696. [Google Scholar] [CrossRef]
- Uchida, Y.; Irie, K.; Fukuhara, D.; Kataoka, K.; Hattori, T.; Ono, M.; Ekuni, D.; Kubota, S.; Morita, M. Commensal microbiota enhance both osteoclast and osteoblast activities. Molecules 2018, 23, 1517. [Google Scholar] [CrossRef]
- Liu, C.; Wo, J.; Zhao, Q.; Wang, Y.; Wang, B.; Zhao, W. Association between serum total osteocalcin level and type 2 diabetes mellitus: A systematic review and meta-analysis. Horm. Metab. Res. 2015, 47, 813–819. [Google Scholar] [CrossRef]
- Lin, X.; Parker, L.; McLennan, E.; Hayes, A.; McConell, G.; Brennan-Speranza, T.C.; Levinger, I. Undercarboxylated osteocalcin improves insulin-stimulated glucose uptake in muscles of corticosterone-treated mice. J. Bone. Miner. Res. 2019, 34, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Tanabe, Y.; Kuroyanagi, Y.; Kaneko, K. Decreased undercarboxylated osteocalcin in children with type 2 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2016, 29, 879–884. [Google Scholar] [CrossRef]
- Madeo, S.F.; Zagaroli, L.; Vandelli, S.; Calcaterra, V.; Crinò, A.; De Sanctis, L.; Faienza, M.F.; Fintini, D.; Guazzarotti, L.; Licenziati, M.R.; et al. Endocrine features of Prader-Willi syndrome: A narrative review focusing on genotype-phenotype correlation. Front Endocrinol. 2024, 15, 1382583. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.; Garnero, P. The multiple facets of periostin in bone metabolism. Osteoporos. Int. 2012, 23, 1199–1212. [Google Scholar] [CrossRef]
- Kim, B.J.; Rhee, Y.; Kim, C.H.; Baek, K.H.; Min, Y.K.; Kim, D.Y.; Ahn, S.H.; Kim, H.; Lee, S.H.; Lee, S.Y.; et al. Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: Clinical evidence for the different effects of periostin depending on the skeletal site. Bone 2015, 81, 435–441. [Google Scholar] [CrossRef]
- Yoshihara, T.; Morimoto, T.; Hirata, H.; Murayama, M.; Nonaka, T.; Tsukamoto, M.; Toda, Y.; Kobayashi, T.; Izuhara, K.; Mawatari, M. Mechanisms of tissue degeneration mediated by periostin in spinal degenerative diseases and their implications for pathology and diagnosis: A review. Front Med. 2023, 10, 1276900. [Google Scholar] [CrossRef]
- Duchamp de Lageneste, O.; Colnot, C. Periostin in bone regeneration. Adv. Exp. Med. Biol. 2019, 1132, 49–61. [Google Scholar] [PubMed]
- Chapurlat, R.D.; Confavreux, C.B. Novel biological markers of bone: From bone metabolism to bone physiology. Rheumatology 2016, 55, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef] [PubMed]
- Pires, G.O.; Vieira, I.O.; Hernandes, F.R.; Teixeira, A.L.; Oliveira, I.B.; Dominguez, W.V.; Dos Reis, L.M.; Montenegro, F.M.; Moysés, R.M.; Carvalho, A.B.; et al. Effects of parathyroidectomy on the biology of bone tissue in patients with chronic kidney disease and secondary hyperparathyroidism. Bone 2019, 121, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Cai, J. Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice. Open Life Sci. 2023, 18, 20220663. [Google Scholar] [CrossRef]
- Galli, C.; Piergianni, M.; Piemontese, M.; Lumetti, S.; Ravanetti, F.; Cacchioli, A.; Macaluso, G.M.; Passeri, G. Periostin improves cell adhesion to implantable biomaterials and osteoblastic differentiation on implant titanium surfaces in a topography-dependent fashion. J. Biomed. Mater Res. A. 2014, 102, 3855–3861. [Google Scholar] [CrossRef]
- Bakker, N.E.; van Doorn, J.; Renes, J.S.; Donker, G.H.; Hokken-Koelega, A.C. IGF-1 levels, complex formation, and IGF bioactivity in growth hormone-treated children with Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 3041–3049. [Google Scholar] [CrossRef]
- Gaddas, M.; Périn, L.; Le Bouc, Y. Evaluation of IGF1/IGFBP3 molar ratio as an effective tool for assessing the safety of growth hormone therapy in small-for-gestational-age, growth hormone-deficient and Prader-Willi children. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 253–261. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, J.M.; Ning, G.; Zhao, Y.J.; Chen, Y.; Sun, L.H.; Zhang, L.Z.; Xu, M.Y.; Chen, J.L. Relationships between insulin-like growth factor-I (IGF-I) and OPG, RANKL, bone mineral density in healthy Chinese women. Osteoporos. Int. 2008, 19, 221–226. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Chen, X.; Wang, Z.; Zheng, S.; Cheng, Y.; Liu, S.; Hao, L. The role of insulin-like growth factor-1 in bone remodeling: A review. Int. J. Biol. Macromol. 2023, 238, 124125. [Google Scholar] [CrossRef]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M. Dieta 5.0 Software for Individual and Group Nutrition Assessment and Diet Planning; National Food and Nutrition Institute: Warsaw, Poland, 2015. [Google Scholar]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Światek-Leśniak, A.; Litwin, M. Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 year of age. Stand. Med. 2015, 12, 119–135. [Google Scholar]
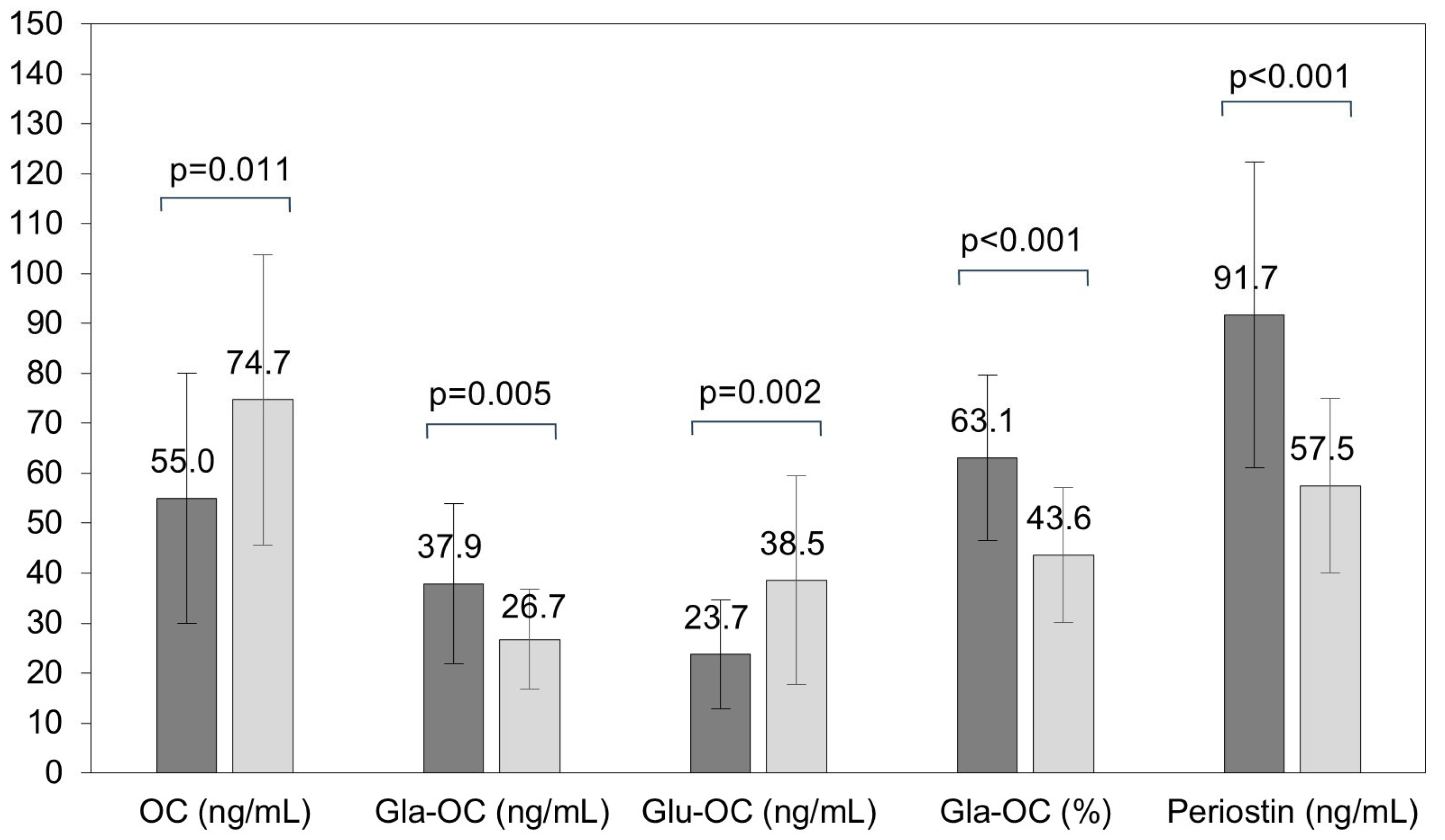

| Children with PWS n = 24 | Healthy Children n = 30 | p-Value | |
|---|---|---|---|
| Age (year) | 6.1 ± 3.1 | 6.5 ± 3.0 | 0.586 |
| Girls/Boys | 12/12 | 15/15 | − |
| Height (cm) | 113.1 ± 22.9 | 117.2 ± 18.7 | 0.470 |
| Weight (kg) | 20.3 ± 9.2 | 22.2 ± 8.3 | 0.425 |
| BMI (kg/m2) | 15.2 ± 1.6 | 15.7 ± 1.6 | 0.234 |
| BMI Z-score | −0.54 ± 0.71 | −0.26 ± 0.63 | 0.128 |
| n = 15 | n = 15 | ||
| Fat mass (%) | 20.6 ± 4.2 | 19.1 ± 4.8 | 0.992 |
| Fat mass (kg) | 5.12 ± 2.0 | 3.86 ± 1.3 | 0.061 |
| TBLH-BMC (kg) | 0.57 ± 0.17 | 0.47 ± 0.15 | 0.089 |
| TBLH-BMD (g/cm2) | 0.57 ± 0.07 | 0.64 ± 0.06 | 0.018 |
| TBLH-BMD Z-score | −0.95 ± 0.54 | −0.48 ± 0.69 | 0.044 |
| Children with PWS n = 24 | Healthy Children n = 30 | p-Value | |
|---|---|---|---|
| BALP (U/L) | 126.3 ± 34.9 | 126.3 ± 39.4 | 0.996 |
| Osteopontin (ng/mL) | 59.3 (48.9–85.8) | 50.6 (35.3–67.5) | 0.032 |
| OC/CTX-I | 0.35 ± 0.15 | 0.46 ± 0.15 | 0.006 |
| CTX-I (ng/mL) | 1.68 ± 0.52 | 1.64 ± 0.45 | 0.813 |
| OPG (pmol/L) | 4.28 ± 0.84 | 4.56 ± 1.28 | 0.361 |
| Sclerostin (ng/mL) | 0.45 ± 0.26 | 0.42 ± 0.18 | 0.646 |
| IGF-I (ng/mL) | 299.6 (136.8–333.0) | 134.4 (108.2–194.5) | 0.002 |
| IGF-I/t-IGFBP-3 molar ratio | 0.24 ± 0.08 | 0.16 ± 0.06 | <0.001 |
| PTH (pg/mL) | 18.4 ± 9.2 | 20.5 ± 9.0 | 0.414 |
| 25-hydroxyvitamin D (ng/mL) | 35.9 ± 9.0 | 23.9 ± 8.6 | <0.001 |
| Calcium (mmol/L) | 2.59 ± 0.09 | 2.59 ± 0.07 | 0.970 |
| Phosphorus (mmol/L) | 1.67 ± 0.15 | 1.72 ± 0.10 | 0.155 |
| Magnesium (mmol/L) | 0.89 ± 0.05 | 0.90 ± 0.02 | 0.112 |
| Children with PWS n = 24 | Healthy Children n = 30 | p-Value | |
|---|---|---|---|
| Energy (kcal/day) | 1033 ± 322 | 1453 ± 396 | <0.001 |
| Energy (% of EER) | 70.5 ± 14.5 | 93.2 ± 19.9 | <0.001 |
| Proteins (% of energy) | 17.5 ± 4.4 | 13.4 ±2.3 | <0.001 |
| Carbohydrates (% of energy) | 51.3 ± 7.1 | 53.7 ± 5.5 | 0.164 |
| Fat (% of energy) | 29.7 ± 5.5 | 32.8 ± 5.2 | 0.041 |
| Protein (g/day) | 45.5 ± 18.7 | 46.7 ± 17.8 | 0.817 |
| Animal protein (g/day) | 31.4 ± 15.3 | 30.4 ± 11.3 | 0.787 |
| Plant protein (g/day) | 13.4 ± 5.9 | 16.2 ± 6.9 | 0.122 |
| Carbohydrates (g/day) | 144.7 ± 45.9 | 197.7 ± 65.4 | 0.001 |
| Fat (g/day) | 35.0 ± 14.5 | 51.3 ± 19.6 | 0.001 |
| Vitamin D (µg/day) | 8.08 (3.09–15.31) | 1.90 (1.32–2.47) | <0.001 |
| Vitamin D (% of AI) | 54 (19–102) | 13 (9–17) | <0.001 |
| Calcium (mg/day) | 795.5 ± 261.3 | 508.7 ± 272.2 | <0.001 |
| Calcium (% of EAR) | 113 ± 41 | 63 ± 32 | <0.001 |
| Phosphorus (mg/day) | 1004.3 ± 313.2 | 805.8 ± 310.5 | 0.028 |
| Phosphorus (% of EAR) | 209 ± 62 | 168 ± 75 | 0.039 |
| Magnesium (mg/day) | 204.9 ± 76.3 | 182.5 ± 76.3 | 0.299 |
| Magnesium (% of EAR) | 202 ± 62 | 160 ± 67 | 0.027 |
| Children with PWS | |||||
| Glu-OC | Gla-OC | Gla-OC% | Periostin | ||
| OC | r (p) | 0.580 (0.003) | 0.512 (0.010) | −0.195 (0.360) | 0.446 (0.029) |
| partial r (p) | 0.613 (0.002) | 0.485 (0.019) | −0.225 (0.303) | 0.473 (0.023) | |
| Gla-OC | r (p) | −0.006 (0.976) | X | 0.640 (0.001) | 0.419 (0.042) |
| partial r (p) | −0.003 (0.988) | X | 0.642 (0.001) | 0.434 (0.040) | |
| OC/CTX-I | r (p) | 0.433 (0.035) | 0.412 (0.045) | −0.021 (0.921) | 0.553 (0.005) |
| partial r (p) | 0.441 (0.035) | 0.394 (0.063) | −0.031 (0.888) | 0.562 (0.005) | |
| BALP | r (p) | −0.105 (0.627) | 0.217 (0.309) | 0.130 (0.545) | −0.118 (0.584) |
| partial r (p) | −0.104 (0.637) | 0.214 (0.327) | 0.128 (0.560) | −0.117 (0.594) | |
| OPG | r (p) | 0.172 (0.421) | −0.181 (0.398) | −0.121 (0.524) | 0.480 (0.018) |
| partial r (p) | 0.174 (0.427) | −0.136 (0.536) | −0.109 (0.622) | 0.494 (0.017) | |
| Sclerostin | r (p) | 0.366 (0.079) | 0.325 (0.121) | −0.061 (0.775) | 0.083 (0.700) |
| partial r (p) | 0.274 (0.206) | 0.274 (0.206) | −0.094 (0.670) | 0.098 (0.657) | |
| Healthy Children | |||||
| Glu-OC | Gla-OC | Gla-OC% | Periostin | ||
| OC | r (p) | 0.846 (<0.001) | 0.216 (0.253) | −0.590 (0.001) | 0.060 (0.752) |
| partial r (p) | 0.752 (<0.001) | 0.551 (0.002) | −0.333 (0.077) | 0.154 (0.443) | |
| Gla-OC | r (p) | −0.0003 (0.999) | X | 0.451 (0.012) | 0.260 (0.165) |
| partial r (p) | 0.287 (0.131) | X | 0.331 (0.080) | 0.242 (0.206) | |
| OC/CTX-I | r (p) | 0.677 (<0.001) | −0.012 (0.948) | −0.592 (0.001) | −0.064 (0.736) |
| partial r (p) | 0.579 (0.001) | 0.152 (0.433) | −0.458 (0.012) | −0.024 (0.902) | |
| BALP | r (p) | 0.311 (0.094) | −0.013 (0.945) | −0.318 (0.087) | 0.131 (0.492) |
| partial r (p) | 0.368 (0.049) | 0.001 (0.995) | −0.377 (0.044) | 0.136 (0.483) | |
| OPG | r (p) | −0.218 (0.247) | 0.095 (0.618) | 0.253 (0.178) | −0.061 (0.749) |
| partial r (p) | −0.129 (0.504) | 0.035 (0.858) | 0.174 (0.366) | −0.082 (0.674) | |
| Sclerostin | r (p) | −0.116 (0.542) | −0.008 (0.996) | 0.145 (0.445) | −0.062 (0.743) |
| partial r (p) | −0.308 (0.104) | 0.057 (0.767) | 0.349 (0.064) | −0.045 (0.817) | |
| Children with PWS | ||||||
|---|---|---|---|---|---|---|
| Crude | Age-Adjusted | |||||
| Beta | 95%CI | p-Value | Beta | 95% CI | p-Value | |
| OC | 0.08 | −0.20–0.18 | 0.111 | 0.16 | 0.04–0.28 | 0.013 |
| Gla-OC | 0.08 | −0.003–0.16 | 0.059 | 0.12 | 0.004–0.23 | 0.042 |
| Glu-OC | −0.02 | −0.07–0.03 | 0.469 | 0.04 | −0.03–0.10 | 0.252 |
| Gla% | −0.002 | −0.08–0.08 | 0.964 | 0.03 | −0.10–0.16 | 0.690 |
| OC/CTX-I | 0.001 | −0.00002–0.001 | 0.058 | 0.0009 | 0.0002–0.002 | 0.017 |
| Periostin | 0.10 | −0.03–0.23 | 0.127 | 0.15 | −0.04–0.34 | 0.122 |
| Healthy Children | ||||||
| OC | 0.19 | 0.06–0.32 | 0.006 | 0.11 | −0.07–0.29 | 0.215 |
| Gla−OC | 0.01 | −0.04–0.06 | 0.669 | 0.08 | 0.02–0.14 | 0.008 |
| Glu-OC | 0.12 | 0.01–0.22 | 0.032 | 0.10 | −0.03–0.23 | 0.134 |
| Gla% | −0.05 | −0.11–0.02 | 0.145 | 0.02 | −0.05–0.09 | 0.544 |
| OC/CTX-I | 0.0003 | −0.0001–0.0009 | 0.124 | 0.0002 | −0.0009–0.001 | 0.723 |
| Periostin | 0.02 | −0.07–0.11 | 0.686 | 0.02 | −0.12–0.16 | 0.733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, J.; Chełchowska, M.; Szamotulska, K.; Klemarczyk, W.; Strucińska, M.; Ambroszkiewicz, J. Differences in Bone Metabolism between Children with Prader–Willi Syndrome during Growth Hormone Treatment and Healthy Subjects: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 9159. https://doi.org/10.3390/ijms25179159
Gajewska J, Chełchowska M, Szamotulska K, Klemarczyk W, Strucińska M, Ambroszkiewicz J. Differences in Bone Metabolism between Children with Prader–Willi Syndrome during Growth Hormone Treatment and Healthy Subjects: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(17):9159. https://doi.org/10.3390/ijms25179159
Chicago/Turabian StyleGajewska, Joanna, Magdalena Chełchowska, Katarzyna Szamotulska, Witold Klemarczyk, Małgorzata Strucińska, and Jadwiga Ambroszkiewicz. 2024. "Differences in Bone Metabolism between Children with Prader–Willi Syndrome during Growth Hormone Treatment and Healthy Subjects: A Pilot Study" International Journal of Molecular Sciences 25, no. 17: 9159. https://doi.org/10.3390/ijms25179159
APA StyleGajewska, J., Chełchowska, M., Szamotulska, K., Klemarczyk, W., Strucińska, M., & Ambroszkiewicz, J. (2024). Differences in Bone Metabolism between Children with Prader–Willi Syndrome during Growth Hormone Treatment and Healthy Subjects: A Pilot Study. International Journal of Molecular Sciences, 25(17), 9159. https://doi.org/10.3390/ijms25179159









