Anticancer Activity and Mechanism of Action of Couroupita guianensis Bark Decoction in Gastric Adenocarcinoma Cancer Cell Line
Abstract
:1. Introduction
2. Results
2.1. Production of Total Decoction (LDCG) of C. guianensis and Fractions and Evaluation of the Activity on Gastric Adenocarcinoma Cells’ Viability and Proliferation
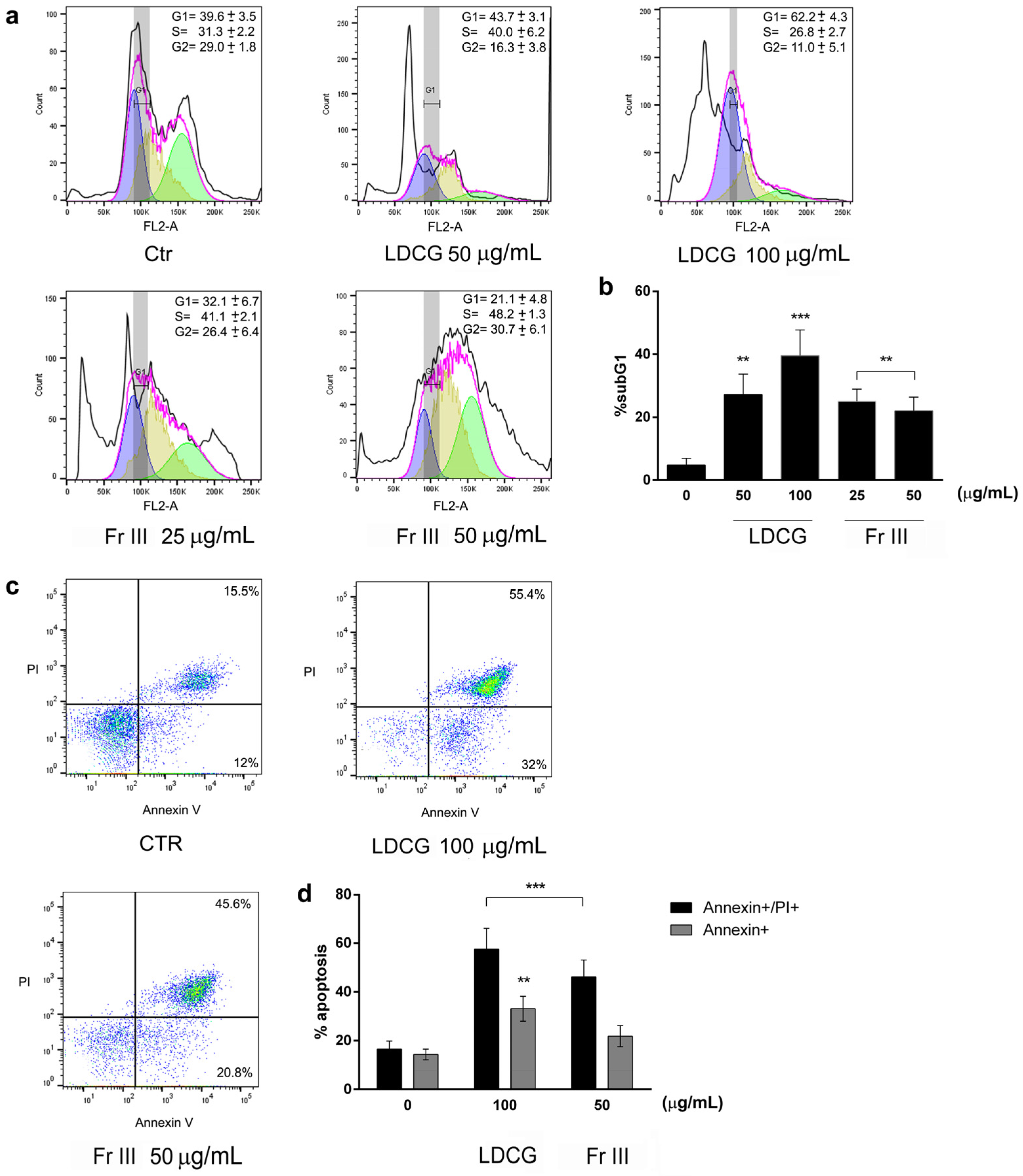
2.2. LDCG and Fraction III Directly Affect Cell Proliferation through Cell Cycle Blockade and Apoptosis Induction
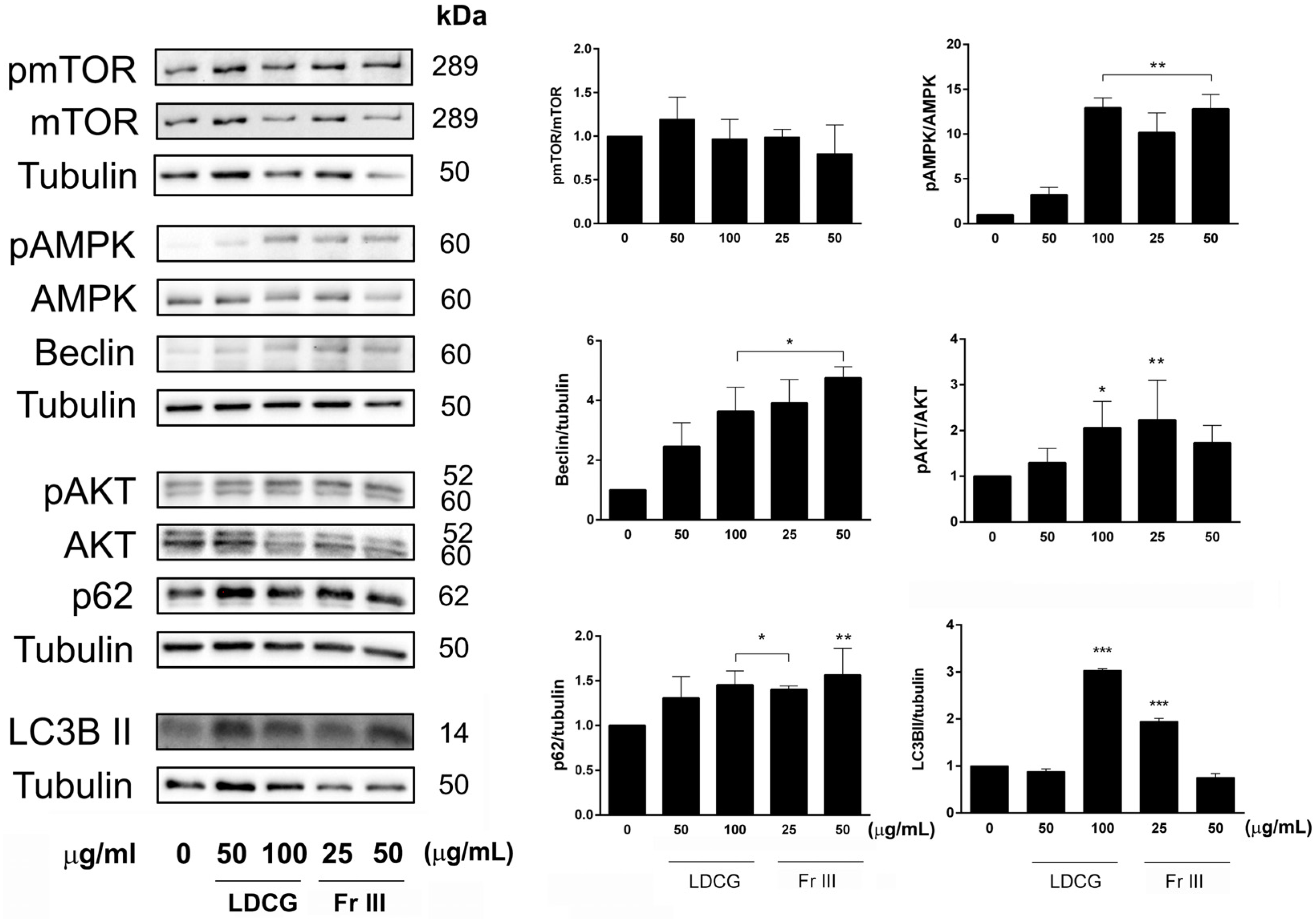
2.3. LDCG and Fr III Affect Key Cell Cycle Control Proteins and Promote the Expression of Apoptosis Machinery
2.4. LDCG and Fr III Promote the Autophagic Process
3. Discussion
4. Materials and Methods
4.1. Plant Material, Decoction Preparation and HPLC-UV-HRMSn Analysis
4.2. Cell Culture and Treatments
4.3. Cell Viability Assay
4.4. BrdU Assay for Cell Proliferation Determination
4.5. Flow Cytometry Assays
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soni, P.K. Couroupita Guianensis Aubl. (Lecythidaceae): A Medicinal Plant: Ayurvedic and Modern View. Int. J. Multidiscip. Res. 2023, 5, 1–13. [Google Scholar]
- Ramalakshmi, C.; Kalirajan, A.; Ranjitsingh, A.J.A.; Kalirajan, K. Bioprospecting of medicinal plant Couroupita guianensis for its potential anti-ulcer activity. Int. J. Appl. Biol. Pharm. Technol. 2014, 53, 226–232. [Google Scholar]
- Sheba, L.A.; Anuradha, V. An updated review on Couroupita guianensis Aubl: A sacred plant of India with myriad medicinal properties. J. Herbmed Pharmacol. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Leandro de França Ferreira, É.; Pereira de Carvalho Oliveira, J.; Silva de Araújo, M.R.; Rai, M.; Chaves, M.H. Phytochemical profile and ethnopharmacological applications of Lecythidaceae: An overview. J. Ethnopharmacol. 2021, 274, 114049. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Balachandran, C.; Raj, M.K.; Duraipandiyan, V.; Muthukumar, C.; Ignacimuthu, S.; Ali Khan, I.; Rajput, V. Antimicrobial, antimycobacterial and antibiofilm properties of Couroupita guianensis Aubl. fruit extract. BMC Complement. Altern. Med. 2012, 12, 242. [Google Scholar] [CrossRef]
- Venkatraman, A.; Sheba, L. Antioxidant potential and chromatographic profiling of Couroupita guianensis fruit pulp. J. Adv. Sci. Res. 2022, 13, 286–293. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Fernandes, S.B.; Fingolo, C.E.; Boylan, F.; Fernandes, P.D. Anti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis Aublet leaves. J. Ethnopharmacol. 2013, 146, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Akther, T.; Khan, M.S.; Srinivasan, H. Novel Silver Nanoparticles Synthesized from Anthers of Couroupita guianensis Aubl. Control Growth and Biofilm Formation in Human Pathogenic Bacteria. Nano Biomed. Eng. 2018, 10, 250–257. [Google Scholar] [CrossRef]
- Singh, R.; Hano, C.; Tavanti, F.; Sharma, B. Biogenic Synthesis and Characterization of Antioxidant and Antimicrobial Silver Nanoparticles Using Flower Extract of Couroupita guianensis Aubl. Materials 2021, 14, 6854. [Google Scholar] [CrossRef]
- Babu, V.; Arokiyaraj, S.; Sakthi Sri, S.P.; George, M.; Ragavan, R.M.; Dharmalingam, D.; Oh, T.; Ramasundaram, S.; Agastian, P. Antibacterial, Antioxidant, Larvicidal and Anticancer Activities of Silver Nanoparticles Synthesized Using Extracts from Fruits of Lagerstroemia speciosa and Flowers of Couroupita guianensis. Molecules 2022, 27, 7792. [Google Scholar] [CrossRef]
- Muthulakshmi, V.; Dhilip Kumar, C.; Sundrarajan, M. Green synthesis of ionic liquid mediated neodymium oxide nanoparticles via Couroupita guianensis abul leaves extract with its biological applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Pisanti, S.; Martinelli, R.; Celano, R.; Mencherini, T.; Re, T.; Aquino, R.P. Couroupita guianensis bark decoction: From Amazonian medicine to the UHPLC-HRMS chemical profile and its role in inflammation processes and re-epithelialization. J. Ethnopharm. 2023, 313, 116579. [Google Scholar] [CrossRef]
- Sheba, L.A.; Anuradha, V.; Ali, M.S.; Yogananth, N. Wound Healing Potential of Couroupita guianensis Aubl. Fruit Pulp Investigated on Excision Wound Model. Appl. Biochem. Biotechnol. 2023, 195, 6516–6536. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, P.M.; Guntuku, G. In vitro Cytotoxic and Antibacterial Activity of Various Flower Extracts of Couroupita guianensis. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 113–117. [Google Scholar]
- Premanathan, M.; Radhakrishnan, S.; Kolanjiappar, K.; Singaravelu, G.; Thirumalaiarasu, V.; Sivakumar, T.; Kathiresan, K. Antioxidant & anticancer activities of isatin (1H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis Aubl. Indian J. Med. Res. 2012, 136, 822–826. [Google Scholar]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modeling study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Citro, M.; Caputo, M.; Pisanti, S.; Martinelli, R. Psychological Stress and Cancer: New Evidence of An Increasingly Strong Link. Transl. Med. UniSa 2020, 23, 53–57. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Ratti, M.; Orlandi, E.; Toscani, I.; Vecchia, S.; Anselmi, E.; Hahne, J.C.; Ghidini, M.; Citterio, C. Emerging Therapeutic Targets and Future Directions in Advanced Gastric Cancer: A Comprehensive Review. Cancers 2024, 16, 2692. [Google Scholar] [CrossRef]
- Esposito, T.; Pisanti, S.; Mauro, L.; Mencherini, T.; Martinelli, R.; Aquino, R.P. Activity of Colocasia esculenta (Taro) Corms against Gastric Adenocarcinoma Cells: Chemical Study and Molecular Characterization. Int. J. Mol. Sci. 2023, 25, 252. [Google Scholar] [CrossRef]
- Mrakovcic, M.; Fröhlich, L.F. p53-Mediated Molecular Control of Autophagy in Tumor Cells. Biomolecules 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, R.; Ciaglia, E.; Amodio, G.; Picardi, P.; Proto, M.C.; Gazzerro, P.; Laezza, C.; Remondelli, P.; Bifulco, M.; Pisanti, S. N6-isopentenyladenosine dual targeting of AMPK and Rab7 prenylation inhibits melanoma growth through the impairment of autophagic flux. Cell Death Differ. 2018, 25, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Kihara, M.; Omoloso, A.D. Antibiotic Activity of Couroupita guianensis. J. Herbs Spices Med. Plants 2003, 10, 95–108. [Google Scholar] [CrossRef]
- Geetha, M.; Saluja, A.K.; Shankar, M.B.; Mehta, R.S. Analgesic and anti-inflammatory activity of Couroupita guianensis Aubl. J. Nat. Remedies 2004, 4, 52–55. [Google Scholar] [CrossRef]
- Nagananda, G.S.; Shivashankar, M.; Rajeshwari, S.; Rajath, S.; Chandan, N. Comparative antioxidant and antimicrobial studies of cold and hot bark hydromethanolic extract of Couroupita guianensis Aubl. Res. Pharm. 2013, 3, 6–13. [Google Scholar]
- Pinheiro, M.M.; Bessa, S.O.; Fingolo, C.E.; Kuster, R.M.; Matheus, M.E.; Menezes, F.S.; Fernandes, P.D. Antinociceptive activity of fractions from Couroupita guianensis Aubl. leaves. J. Ethnopharmacol. 2010, 127, 407–413. [Google Scholar] [CrossRef]
- Prabhu, V.; Ravi, S. Quantification of quercetin and stigmasterol of Couroupita guianensis aubl by HPTLC method and in-vitro cytototoxic activity by MTT assay of the methanol extract against HeLa, NIH 3T3 and HepG2 cancer cell lines. Int. J. Pharm. Pharm. Sci. 2012, 4, 126–130. [Google Scholar]
- Cheshomi, H.; Bahrami, A.R.; Rafatpanah, H.; Matin, M.M. The effects of ellagic acid and other pomegranate (Punica granatum L.) derivatives on human gastric cancer AGS cells. Hum. Exp. Toxicol. 2022, 41, 9603271211064534. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An Update on the Potential Mechanism of Gallic Acid as an Antibacterial and Anticancer Agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chiu, Y.M.; Ho, T.Y.; Hsieh, C.T.; Shieh, D.C.; Lee, Y.J.; Tsay, G.J.; Wu, Y.Y. Gallic Acid Induces Apoptosis in Human Gastric Adenocarcinoma Cells. Anticancer Res. 2018, 38, 2057–2067. [Google Scholar]
- Owczarek, A.; Różalski, M.; Krajewska, U.; Olszewska, M.A. Rare Ellagic Acid Sulphate Derivatives from the Rhizome of Geum rivale L.—Structure, Cytotoxicity, and Validated HPLC-PDA Assay. Appl. Sci. 2017, 7, 400. [Google Scholar] [CrossRef]
- Manurung, J.; Kappen, J.; Schnitzler, J.; Frolov, A.; Wessjohann, L.A.; Agusta, A.; Muellner-Riehl, A.N.; Franke, K. Analysis of Unusual Sulfated Constituents and Anti-infective Properties of Two Indonesian Mangroves, Lumnitzera littorea and Lumnitzera racemose (Combretaceae). Separations 2021, 8, 82. [Google Scholar] [CrossRef]
- Yu, H.; Jeong, H.; Yang, K.Y.; Cho, J.Y.; Hong, I.K.; Nam, S.H. Synthesis of ellagic acid glucoside using glucansucrase from Leuconostoc and characterization of this glucoside as a functional neuroprotective agent. AMB Express 2021, 11, 108. [Google Scholar] [CrossRef]
- Onodera, K.; Tsuha, K.; Yasumoto-Hirose, M.; Tsuha, K.; Hanashiro, K.; Naoki, H.; Yasumoto, T. Okicamelliaside, an extraordinarily potent anti-degranulation glucoside isolated from leaves of Camellia japonica. Biosci. Biotechnol. Biochem. 2010, 74, 2532–2534. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Liu, K.X.; Zhang, M.; Shen, F.K.; Ye, L.L.; Wu, W.B.; Hou, X.T.; Hao, E.W.; Hou, Y.Y.; Bai, G. Okicamelliaside targets the N-terminal chaperone pocket of HSP90 disrupts the chaperone protein interaction of HSP90-CDC37 and exerts antitumor activity. Acta Pharmacol. Sin. 2022, 43, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, I.; Tapia, O.; Espinoza, J.A.; Leal, P.; Buchegger, K.; Sandoval, A.; Bizama, C.; Araya, J.C.; Peek, R.M.; Roa, J.C. The Gene Expression Status of the PI3K/AKT/mTOR Pathway in Gastric Cancer Tissues and Cell Lines. Pathol. Oncol. Res. 2016, 22, 797–805. [Google Scholar] [CrossRef]
- Hassan, A.M.I.A.; Zhao, Y.; Chen, X.; He, C. Blockage of Autophagy for Cancer Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 7459. [Google Scholar] [CrossRef]
- Kim, S.M.; Vetrivel, P.; Ha, S.E.; Kim, H.H.; Kim, J.A.; Kim, G.S. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J. Nutr. Biochem. 2020, 83, 427. [Google Scholar] [CrossRef]
- Zhong, J.; Fang, L.; Chen, R.; Xu, J.; Guo, D.; Guo, C.; Guo, C.; Chen, J.; Chen, C.; Wang, X. Polysaccharides from sporoderm-removed spores of Ganoderma lucidum induce apoptosis in human gastric cancer cells via disruption of autophagic flux. Oncol. Lett. 2021, 21, 425. [Google Scholar] [CrossRef]
- Paes, A.S.; Koga, R.C.R.; Sales, P.F.; Santos Almeida, H.K.; Teixeira, T.A.C.C.; Carvalho, J.C.T. Phytocompounds from Amazonian Plant Species against Acute Kidney Injury: Potential Nephroprotective Effects. Molecules 2023, 28, 6411. [Google Scholar] [CrossRef]
- Simomura, V.L.; Miorando, D.; de Oliveira, B.M.M.; Mânica, A.; Bohnen, L.C.; Buzatto, M.V.; Kunst, F.M.; Ansolin, L.D.; Somensi, L.B.; Vidal Gutiérrez, M.; et al. Aqueous extract of the bark of Uncaria tomentosa, an amazonian medicinal plant, promotes gastroprotection and accelerates gastric healing in rats. J. Ethnopharmacol. 2024, 321, 117542. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Martinelli, R.; Massaro, M.; Calabriso, N.; Scoditti, E.; Maffia, M.; Verri, T.; Gatta, V.; De Caterina, R. Nutrigenomic Effect of Hydroxytyrosol in Vascular Endothelial Cells: A Transcriptomic Profile Analysis. Nutrients 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Citro, M.; Pisanti, S.; Caputo, M.; Martinelli, R. Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol. Int. J. Mol. Sci. 2021, 22, 2438. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.V.; Chambers, S.H.; Smith, P.J. A pragmatic approach to the analysis of DNA histograms with a definable G1 peak. Cytometry 1987, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Martinelli, R.; Gatta, V.; Scoditti, E.; Pellegrino, M.; Carluccio, M.A.; Calabriso, N.; Buonomo, T.; Stuppia, L.; Storelli, C.; et al. Transcriptome-based identification of new anti-inflammatory and vasodilating properties of the n-3 fatty acid docosahexaenoic acid in vascular endothelial cell under proinflammatory conditions [corrected]. PLoS ONE 2015, 10, e0129652. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisanti, S.; Penna, S.; Sposito, S.; Esposito, T.; Mencherini, T.; Celano, R.; Re, T.; Aquino, R.P.; Martinelli, R. Anticancer Activity and Mechanism of Action of Couroupita guianensis Bark Decoction in Gastric Adenocarcinoma Cancer Cell Line. Int. J. Mol. Sci. 2024, 25, 9183. https://doi.org/10.3390/ijms25179183
Pisanti S, Penna S, Sposito S, Esposito T, Mencherini T, Celano R, Re T, Aquino RP, Martinelli R. Anticancer Activity and Mechanism of Action of Couroupita guianensis Bark Decoction in Gastric Adenocarcinoma Cancer Cell Line. International Journal of Molecular Sciences. 2024; 25(17):9183. https://doi.org/10.3390/ijms25179183
Chicago/Turabian StylePisanti, Simona, Serena Penna, Silvia Sposito, Tiziana Esposito, Teresa Mencherini, Rita Celano, Tania Re, Rita Patrizia Aquino, and Rosanna Martinelli. 2024. "Anticancer Activity and Mechanism of Action of Couroupita guianensis Bark Decoction in Gastric Adenocarcinoma Cancer Cell Line" International Journal of Molecular Sciences 25, no. 17: 9183. https://doi.org/10.3390/ijms25179183






