TRPA1-Related Diseases and Applications of Nanotherapy
Abstract
:1. Introduction
2. TRPA1 Structure
3. Physiological Roles of TRPA1
3.1. TRPA1’s Role in Pain
3.2. TRPA1’s Role in Itching
3.3. TRPA1’s Role in Temperature
4. Diseases Associated with TRPA1
4.1. Alzheimer’s Disease (AD) and TRPA1
4.2. Migraine and TRPA1
4.3. Asthma and TRPA1
4.4. Diabetes Mellitus (DM) and TRPA1
4.5. Cancer and TRPA1
4.6. Renal Failure and TRPA1
4.7. Post-Operative Ileus (POI) and TRPA1
4.8. Coronavirus Disease (COVID-19) and TRPA1
5. Application of Nanomedicine to Ion Channels-Related Diseases
Nanoparticles Used in the Latest NDS for Disease Treatment Targeting Ion Channels
6. Application of Nanoparticles in TRPA1-Related Diseases
6.1. Treatment Methods to Inhibit TRPA1
6.1.1. Periocular Mechanical Allodynia (PMA)
6.1.2. Breast Cancer
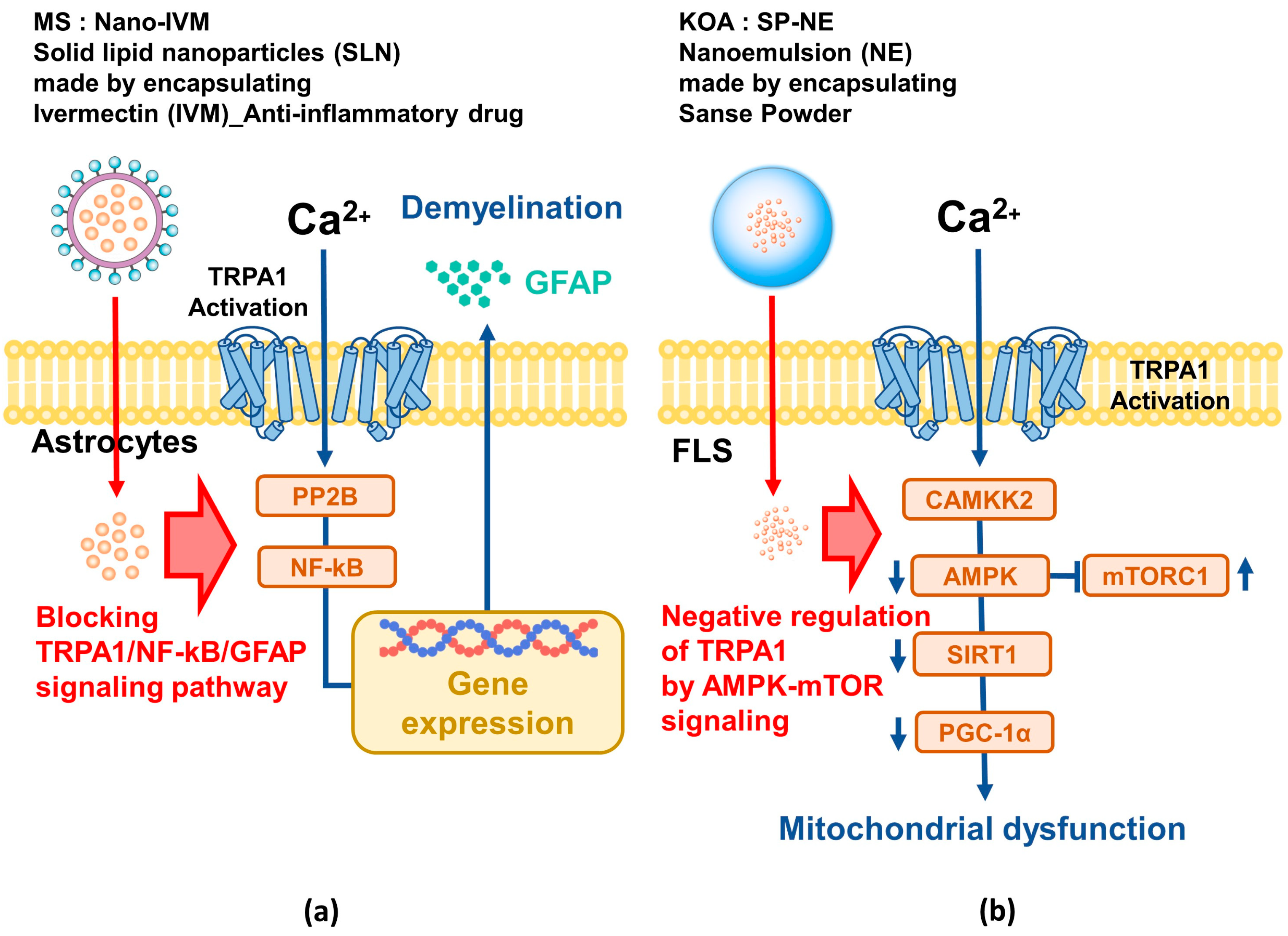
6.1.3. Multiple Sclerosis (MS)
6.1.4. Knee Osteoarthritis (KOA)
6.2. Treatment Methods to Activate TRPA1
6.2.1. Tumor
6.2.2. Diabetes
7. Conclusions
Funding
Conflicts of Interest
References
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Yelshanskaya, M.V.; Sobolevsky, A.I. Ligand-Binding Sites in Vanilloid-Subtype TRP Channels. Front. Pharmacol. 2022, 13, 900623. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 1992, 8, 643–651. [Google Scholar] [CrossRef]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef]
- Asghar, M.Y.; Tornquist, K. Transient Receptor Potential Canonical (TRPC) Channels as Modulators of Migration and Invasion. Int. J. Mol. Sci. 2020, 21, 1739. [Google Scholar] [CrossRef]
- Reyes-Garcia, J.; Carbajal-Garcia, A.; Montano, L.M. Transient receptor potential cation channel subfamily V (TRPV) and its importance in asthma. Eur. J. Pharmacol. 2022, 915, 174692. [Google Scholar] [CrossRef]
- Huang, Y.; Fliegert, R.; Guse, A.H.; Lu, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85, 102111. [Google Scholar] [CrossRef]
- Jaslan, D.; Bock, J.; Krogsaeter, E.; Grimm, C. Evolutionary Aspects of TRPMLs and TPCs. Int. J. Mol. Sci. 2020, 21, 4181. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef]
- Sidi, S.; Friedrich, R.W.; Nicolson, T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 2003, 301, 96–99. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Earley, S.; Santana, L.F.; Lederer, W.J. The physiological sensor channels TRP and piezo: Nobel Prize in Physiology or Medicine 2021. Physiol Rev 2022, 102, 1153–1158. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert. Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- Gao, S.; Kaudimba, K.K.; Guo, S.; Zhang, S.; Liu, T.; Chen, P.; Wang, R. Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Cardiovascular Diseases. Front. Physiol. 2020, 11, 836. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Du, Q.; Gu, J.; Wu, J.; Liu, Q.; Li, Z.; Zhang, T.; Xu, J.; Xie, R. Research Progress on TRPA1 in Diseases. J. Membr. Biol. 2023, 256, 301–316. [Google Scholar] [CrossRef]
- Milici, A.; Talavera, K. TRP Channels as Cellular Targets of Particulate Matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef]
- Landini, L.; Souza Monteiro de Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef]
- Viana, F. TRPA1 channels: Molecular sentinels of cellular stress and tissue damage. J. Physiol. 2016, 594, 4151–4169. [Google Scholar] [CrossRef]
- Cordero-Morales, J.F.; Gracheva, E.O.; Julius, D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc. Natl. Acad. Sci. USA 2011, 108, E1184–E1191. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 525, 552. [Google Scholar] [CrossRef]
- Habgood, M.; Seiferth, D.; Zaki, A.M.; Alibay, I.; Biggin, P.C. Atomistic mechanisms of human TRPA1 activation by electrophile irritants through molecular dynamics simulation and mutual information analysis. Sci. Rep. 2022, 12, 4929. [Google Scholar] [CrossRef]
- Startek, J.B.; Milici, A.; Naert, R.; Segal, A.; Alpizar, Y.A.; Voets, T.; Talavera, K. The Agonist Action of Alkylphenols on TRPA1 Relates to Their Effects on Membrane Lipid Order: Implications for TRPA1-Mediated Chemosensation. Int. J. Mol. Sci. 2021, 22, 3368. [Google Scholar] [CrossRef]
- Legrand, C.; Merlini, J.M.; de Senarclens-Bezencon, C.; Michlig, S. New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci. Rep. 2020, 10, 11238. [Google Scholar] [CrossRef]
- Araki, M.; Kanda, N.; Iwata, H.; Sagae, Y.; Masuda, K.; Okuno, Y. Identification of a new class of non-electrophilic TRPA1 agonists by a structure-based virtual screening approach. Bioorg Med. Chem. Lett. 2020, 30, 127142. [Google Scholar] [CrossRef]
- Rhyu, M.R.; Kim, Y.; Lyall, V. Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1. Int. J. Mol. Sci. 2021, 22, 3360. [Google Scholar] [CrossRef]
- Del Prete, D.; Caprioglio, D.; Appendino, G.; Minassi, A.; Schiano-Moriello, A.; Di Marzo, V.; De Petrocellis, L. Discovery of non-electrophilic capsaicinoid-type TRPA1 ligands. Bioorg Med. Chem. Lett. 2015, 25, 1009–1011. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Viana, F. Chemosensory properties of the trigeminal system. ACS Chem. Neurosci. 2011, 2, 38–50. [Google Scholar] [CrossRef]
- Fujita, F.; Moriyama, T.; Higashi, T.; Shima, A.; Tominaga, M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br. J. Pharmacol. 2007, 151, 153–160. [Google Scholar] [CrossRef]
- Mizushima, T.; Obata, K.; Katsura, H.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Mashimo, T.; Noguchi, K. Noxious cold stimulation induces mitogen-activated protein kinase activation in transient receptor potential (TRP) channels TRPA1- and TRPM8-containing small sensory neurons. Neuroscience 2006, 140, 1337–1348. [Google Scholar] [CrossRef]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihofner, C. TRPA1 and TRPM8 activation in humans: Effects of cinnamaldehyde and menthol. Neuroreport 2005, 16, 955–959. [Google Scholar] [CrossRef]
- Koivisto, A. Sustained TRPA1 activation in vivo. Acta Physiol. 2012, 204, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Hogestatt, E.D. Trpa1. Handb. Exp. Pharmacol. 2014, 222, 583–630. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Derbenev, A.V.; Zsombok, A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin. Immunopathol. 2016, 38, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Garrison, S.R.; Stucky, C.L. The dynamic TRPA1 channel: A suitable pharmacological pain target? Curr. Pharm. Biotechnol. 2011, 12, 1689–1697. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Rodriguez, R.; Kaushik, S.B.; Yosipovitch, G. Transient receptor potential channels and dermatological disorders. Curr. Top. Med. Chem. 2013, 13, 335–343. [Google Scholar] [CrossRef]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hu, H. TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals 2018, 11, 100. [Google Scholar] [CrossRef]
- Dong, X.; Dong, X. Peripheral and Central Mechanisms of Itch. Neuron 2018, 98, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dong, X. Trp channels and itch. Semin. Immunopathol. 2016, 38, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Miyake, T.; Nakamura, S.; Zhao, M.; So, K.; Inoue, K.; Numata, T.; Takahashi, N.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat. Commun. 2016, 7, 12840. [Google Scholar] [CrossRef]
- Chen, J.; Kang, D.; Xu, J.; Lake, M.; Hogan, J.O.; Sun, C.; Walter, K.; Yao, B.; Kim, D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 2013, 4, 2501. [Google Scholar] [CrossRef]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Sinica, V.; Vlachova, V. Transient receptor potential ankyrin 1 channel: An evolutionarily tuned thermosensor. Physiol. Res. 2021, 70, 363–381. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Pernet-Gallay, K.; Buisson, A.; Albrieux, M. Astrocyte-neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 2022, 145, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, D.; Scheller, A.; Kirchhoff, F. From Physiology to Pathology of Cortico-Thalamo-Cortical Oscillations: Astroglia as a Target for Further Research. Front. Neurol. 2021, 12, 661408. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, Y.S.; Kim, T.H.; Jin, M.U.; Ahn, D.K.; Noguchi, K.; Bae, Y.C. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J. Chem. Neuroanat. 2012, 45, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Lee, H.T.; Lin, H.C.; Tsay, H.J.; Tsai, F.C.; Shyue, S.K.; Lee, T.S. Role of transient receptor potential ankyrin 1 channels in Alzheimer’s disease. J. Neuroinflammation 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, B.; Shang, D.; Zhang, K.; Yan, X.; Zhang, X. Ion Channel Dysfunction in Astrocytes in Neurodegenerative Diseases. Front. Physiol. 2022, 13, 814285. [Google Scholar] [CrossRef]
- Ovey, I.S.; Naziroglu, M. Effects of homocysteine and memantine on oxidative stress related TRP cation channels in in-vitro model of Alzheimer’s disease. J. Recept. Signal Transduct. Res. 2021, 41, 273–283. [Google Scholar] [CrossRef]
- Ozsimsek, A.; Ovey, I.S. Potential Effects of Melatonin on TRPA1 Channels in the Prevention and Treatment of Alzheimer’s Disease. Noro Psikiyatr. Ars. 2022, 59, 188–192. [Google Scholar] [CrossRef]
- Sampaio, G.; Vieira-Neto, A.E.; Leite, G.O.; Campos, A.R. Melatonin promotes orofacial antinociception in adult zebrafish by modulating TRP channels. Physiol. Behav. 2023, 269, 114238. [Google Scholar] [CrossRef]
- Prodhan, A.; Cavestro, C.; Kamal, M.A.; Islam, M.A. Melatonin and Sleep Disturbances in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2021, 20, 736–754. [Google Scholar] [CrossRef]
- Masood, T.; Lakatos, S.; Rosta, J. Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity. Int. J. Mol. Sci. 2023, 24, 5375. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Magni, G.; Zanaboni, A.M.; Riboldi, B.; Francavilla, M.; Nativi, C.; Ceruti, S.; Tassorelli, C. Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine. Int. J. Mol. Sci. 2022, 23, 14085. [Google Scholar] [CrossRef]
- Thakore, P.; Alvarado, M.G.; Ali, S.; Mughal, A.; Pires, P.W.; Yamasaki, E.; Pritchard, H.A.; Isakson, B.E.; Tran, C.H.T.; Earley, S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife 2021, 10, e63040. [Google Scholar] [CrossRef]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Earley, S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. Elife 2018, 7, e35316. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, M.; Ottolini, M.; Sonkusare, S.K. Role of TRP ion channels in cerebral circulation and neurovascular communication. Neurosci. Lett. 2021, 765, 136258. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Pechadre, J.C.; John, G.W. Tanacetum parthenium and Salix alba (Mig-RL) combination in migraine prophylaxis: A prospective, open-label study. Clin. Drug Investig. 2006, 26, 287–296. [Google Scholar] [CrossRef]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef]
- Iannone, L.F.; Nassini, R.; Patacchini, R.; Geppetti, P.; De Logu, F. Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief. Temperature 2023, 10, 50–66. [Google Scholar] [CrossRef]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Balestrini, A.; Joseph, V.; Dourado, M.; Reese, R.M.; Shields, S.D.; Rouge, L.; Bravo, D.D.; Chernov-Rogan, T.; Austin, C.D.; Chen, H.; et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 2021, 218, e20201637. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, X.; Yue, Q.; Hu, F.; Zhang, Y.; Zhu, C. The neuro-immune interaction in airway inflammation through TRPA1 expression in CD4+ T cells of asthmatic mice. Int. Immunopharmacol. 2020, 86, 106696. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Boden, G. Effects of free fatty acids (FFA) on glucose metabolism: Significance for insulin resistance and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2003, 111, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Kong, X.; Tu, Y.; Li, B.; Zhang, L.; Feng, L.; Wang, L.; Zhang, L.; Zhou, H.; Hua, X.; Ma, X. Roux-en-Y gastric bypass enhances insulin secretion in type 2 diabetes via FXR-mediated TRPA1 expression. Mol. Metab. 2019, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, J.; Cai, T.; McCarthy, R.; Eschbach, M.D., 2nd; Wang, Y.; Zhao, Y.; Yi, Z.; Zang, K.; Yuan, Y.; et al. Estrogen metabolites increase nociceptor hyperactivity in a mouse model of uterine pain. JCI Insight 2022, 7, e149107. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Frederico, M.J.S.; Cipriani, A.; Heim, J.B.A.; Mendes, A.K.B.; Aragon, M.; Gaspar, J.M.; De Alencar, N.M.N.; Silva, F. Electrophilic Agonists Modulate the Transient Receptor Potential Ankyrin-1 Channels Mediated by Insulin and Glucagon-like Peptide-1 Secretion for Glucose Homeostasis. Pharmaceuticals 2023, 16, 1167. [Google Scholar] [CrossRef]
- Khare, P.; Mahajan, N.; Singh, D.P.; Kumar, V.; Kumar, V.; Mangal, P.; Boparai, R.K.; Gesing, A.; Bhadada, S.K.; Sharma, S.S.; et al. Allicin, a dietary trpa1 agonist, prevents high fat diet-induced dysregulation of gut hormones and associated complications. Food Funct. 2021, 12, 11526–11536. [Google Scholar] [CrossRef]
- Alhazmi, L.S.S.; Bawadood, M.A.A.; Aljohani, A.M.S.; Alzahrani, A.A.R.; Moshref, L.; Trabulsi, N.; Moshref, R. Pain Management in Breast Cancer Patients: A Multidisciplinary Approach. Cureus 2021, 13, e15994. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Pereira, G.C.; Brum, E.D.S.; Silva, C.R.; Antoniazzi, C.T.D.; Ardisson-Araujo, D.; Oliveira, S.M.; Trevisan, G. Role of TRPA1 expressed in bone tissue and the antinociceptive effect of the TRPA1 antagonist repeated administration in a breast cancer pain model. Life Sci. 2021, 276, 119469. [Google Scholar] [CrossRef] [PubMed]
- Marcotti, A.; Fernandez-Trillo, J.; Gonzalez, A.; Vizcaino-Escoto, M.; Ros-Arlanzon, P.; Romero, L.; Vela, J.M.; Gomis, A.; Viana, F.; de la Pena, E. TRPA1 modulation by Sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy. Brain 2023, 146, 475–491. [Google Scholar] [CrossRef]
- Eller, O.C.; Yang, X.; Fuentes, I.M.; Pierce, A.N.; Jones, B.M.; Brake, A.D.; Wang, R.; Dussor, G.; Christianson, J.A. Voluntary Wheel Running Partially Attenuates Early Life Stress-Induced Neuroimmune Measures in the Dura and Evoked Migraine-Like Behaviors in Female Mice. Front. Physiol. 2021, 12, 665732. [Google Scholar] [CrossRef] [PubMed]
- Antoniazzi, C.T.D.; Nassini, R.; Rigo, F.K.; Milioli, A.M.; Bellinaso, F.; Camponogara, C.; Silva, C.R.; de Almeida, A.S.; Rossato, M.F.; De Logu, F.; et al. Transient receptor potential ankyrin 1 (TRPA1) plays a critical role in a mouse model of cancer pain. Int. J. Cancer 2019, 144, 355–365. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Souza Monteiro de Araujo, D.; Ugolini, F.; Iannone, L.F.; Vannucchi, M.; Portelli, F.; Landini, L.; Titiz, M.; De Giorgi, V.; Geppetti, P.; et al. The TRPA1 Channel Amplifies the Oxidative Stress Signal in Melanoma. Cells 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Falleni, M.; Savi, F.; Tosi, D.; Agape, E.; Cerri, A.; Moneghini, L.; Bulfamante, G.P. M1 and M2 macrophages’ clinicopathological significance in cutaneous melanoma. Melanoma Res. 2017, 27, 200–210. [Google Scholar] [CrossRef]
- Lee, H.; Ferguson, A.L.; Quek, C.; Vergara, I.A.; Pires daSilva, I.; Allen, R.; Gide, T.N.; Conway, J.W.; Koufariotis, L.T.; Hayward, N.K.; et al. Intratumoral CD16+ Macrophages Are Associated with Clinical Outcomes of Patients with Metastatic Melanoma Treated with Combination Anti-PD-1 and Anti-CTLA-4 Therapy. Clin. Cancer Res. 2023, 29, 2513–2524. [Google Scholar] [CrossRef]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003 e1007. [Google Scholar] [CrossRef]
- Lameire, N.H.; Bagga, A.; Cruz, D.; De Maeseneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; Van Biesen, W.; et al. Acute kidney injury: An increasing global concern. Lancet 2013, 382, 170–179. [Google Scholar] [CrossRef]
- Wu, C.K.; Lin, J.F.; Lee, T.S.; Kou, Y.R.; Tarng, D.C. Role of TRPA1 in Tissue Damage and Kidney Disease. Int. J. Mol. Sci. 2021, 22, 3415. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Geng, Y.; Song, Y. Transient receptor potential ankyrin 1 protects against sepsis-induced kidney injury by modulating mitochondrial biogenesis and mitophagy. Am. J. Transl. Res. 2018, 10, 4163–4172. [Google Scholar] [PubMed]
- Ma, S.; Wang, D.H. Knockout of Trpa1 Exacerbates Renal Ischemia-Reperfusion Injury With Classical Activation of Macrophages. Am. J. Hypertens. 2021, 34, 110–116. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, J.W.; Bai, Y.R.; Li, R.L.; Wu, C.J.; Peng, W. Targeting TRPV1 and TRPA1: A feasible strategy for natural herbal medicines to combat postoperative ileus. Pharmacol. Res. 2023, 196, 106923. [Google Scholar] [CrossRef]
- Kono, T.; Koseki, T.; Chiba, S.; Ebisawa, Y.; Chisato, N.; Iwamoto, J.; Kasai, S. Colonic vascular conductance increased by Daikenchuto via calcitonin gene-related peptide and receptor-activity modifying protein 1. J. Surg. Res. 2008, 150, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.L.; Qi, Y.X.; Yang, T.; Lin, Y.Y.; Li, S.M.; Li, Y.J.; Xu, Q.W.; Sun, Y.B.; Li, W.M.; Chen, X.Z.; et al. Early oral nutrition improves postoperative ileus through the TRPA1/CCK1-R-mediated mast cell-nerve axis. Ann. Transl. Med. 2020, 8, 179. [Google Scholar] [CrossRef]
- Lubbers, T.; Luyer, M.D.; de Haan, J.J.; Hadfoune, M.; Buurman, W.A.; Greve, J.W. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann. Surg. 2009, 249, 481–487. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kubota, K.; Ohbuchi, K.; Kaneko, A.; Ohno, N.; Mase, A.; Matsushima, H.; Yamamoto, M.; Miyano, K.; Uezono, Y.; et al. Transient receptor potential ankyrin 1 agonists improve intestinal transit in a murine model of postoperative ileus. Neurogastroenterol. Motil. 2016, 28, 1792–1805. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Rivas, I.; Romaguera, D.; Quijal, M.; Czarlewski, W.; Vidal, A.; Fonseca, J.A.; Ballester, J.; Anto, J.M.; Basagana, X.; et al. Association between consumption of fermented vegetables and COVID-19 mortality at a country level in Europe. medRxiv 2020. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Rivas, I.; Romaguera, D.; Quijal-Zamorano, M.; Czarlewski, W.; Vidal, A.; Fonseca, J.A.; Ballester, J.; Anto, J.M.; Basagana, X.; et al. Association between consumption of vegetables and COVID-19 mortality at a country level in Europe. medRxiv 2020. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Czarlewski, W.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; Vidal, A.; Sheikh, A.; Akdis, C.A.; et al. Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy 2021, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, Y.; Khan, N.A. Immunity-Boosting Spices and the Novel Coronavirus. ACS Chem. Neurosci. 2020, 11, 1696–1698. [Google Scholar] [CrossRef]
- Mohammed, A.; Islam, M.S. Spice-Derived Bioactive Ingredients: Potential Agents or Food Adjuvant in the Management of Diabetes Mellitus. Front. Pharmacol. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef]
- Stafford, N. Covid-19: Why Germany’s case fatality rate seems so low. BMJ 2020, 369, m1395. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Iaccarino, G.; Czarlewski, W.; Haahtela, T.; Anto, A.; Akdis, C.A.; Blain, H.; Canonica, G.W.; Cardona, V.; et al. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jimenez-Villegas, J.; Escoll, M.; Fernandez-Gines, R.; Garcia Yague, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Jawad, M.J.; Ahjel, S.W.; Singh, R.B.; Singh, J.; Awad, S.M.; Hadi, N.R. The Nrf2 Activator (DMF) and Covid-19: Is there a Possible Role? Med. Arch. 2020, 74, 134–138. [Google Scholar] [CrossRef]
- McCord, J.M.; Hybertson, B.M.; Cota-Gomez, A.; Gao, B. Nrf2 Activator PB125(R) as a Potential Therapeutic Agent Against COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gu, X.; Yu, N.; Pang, X.; Zhang, W.; Zhang, J.; Zhang, Y. EXPRESS: Products of oxidative stress and TRPA1 expression in the brainstem of rats after lung ischemia-reperfusion injury. Pulm. Circ. 2019, 9, 2045894019865169. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen polysulfide (H(2)S(n)) signaling along with hydrogen sulfide (H(2)S) and nitric oxide (NO). J. Neural Transm. 2016, 123, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; MacColl Garfinkel, A.E.; Li, Y.; Benowitz, L.I.; Cepko, C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Investig. 2015, 125, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Zhang, B.; He, X.M.; Li, D.D.; Shi, J.S.; Zhang, F. Naringenin targets on astroglial Nrf2 to support dopaminergic neurons. Pharmacol. Res. 2019, 139, 452–459. [Google Scholar] [CrossRef]
- Zhu, L.; Li, D.; Chen, C.; Wang, G.; Shi, J.; Zhang, F. Activation of Nrf2 signaling by Icariin protects against 6-OHDA-induced neurotoxicity. Biotechnol. Appl. Biochem. 2019, 66, 465–471. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019, 109, 2145–2154. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Hegron, A.; Landini, L.; Jensen, D.D.; Latorre, R.; Ding, J.; Marini, M.; Souza Monteiro de Araujo, D.; Ramirez-Garcia, P.; et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 2022, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, P.D.; Retamal, J.S.; Shenoy, P.; Imlach, W.; Sykes, M.; Truong, N.; Constandil, L.; Pelissier, T.; Nowell, C.J.; Khor, S.Y.; et al. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019, 14, 1150–1159. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Li, B.; Ren, S.; Gao, D.; Li, N.; Wu, M.; Yuan, H.; Zhou, M.; Xing, C. Photothermal Conjugated Polymer Nanoparticles for Suppressing Breast Tumor Growth by Regulating TRPA1 Ion Channels. Adv. Healthc. Mater. 2022, 11, e2102506. [Google Scholar] [CrossRef]
- Khaledi, E.; Noori, T.; Mohammadi-Farani, A.; Sureda, A.; Dehpour, A.R.; Yousefi-Manesh, H.; Sobarzo-Sanchez, E.; Shirooie, S. Trifluoperazine reduces cuprizone-induced demyelination via targeting Nrf2 and IKB in mice. Eur. J. Pharmacol. 2021, 909, 174432. [Google Scholar] [CrossRef] [PubMed]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Fakhri, S.; Sobarzo-Sanchez, E.; Farzaei, M.H.; Kupeli Akkol, E.; Khodarahmi, Z.; Hosseini, S.Z.; Alavi, S.D.; et al. The role of glycogen synthase kinase 3 beta in multiple sclerosis. Biomed. Pharmacother. 2020, 132, 110874. [Google Scholar] [CrossRef] [PubMed]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Wang, P.; Xie, K.; Wang, C.; Bi, J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 2014, 72, 249–254. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Alavi, S.D.; Hosseini, S.Z.; Korani, S.; Sureda, A.; Esmaeili, J.; Shirooie, S. Synthesis and evaluation of the effects of solid lipid nanoparticles of ivermectin and ivermectin on cuprizone-induced demyelination via targeting the TRPA1/NF-kB/GFAP signaling pathway. Iran. J. Basic. Med. Sci. 2023, 26, 1272–1282. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Miller, R.J.; Malfait, A.M.; Miller, R.E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, P.; Xing, R.; Zhao, L.; Li, X.; Zhang, L.; Xiao, Y. Transient Receptor Potential Ankyrin 1 (TRPA1) Mediates Lipopolysaccharide (LPS)-Induced Inflammatory Responses in Primary Human Osteoarthritic Fibroblast-Like Synoviocytes. Inflammation 2018, 41, 700–709. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinozzi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a Highly Stable Carlina acaulis Essential Oil Nanoemulsion for Managing Lobesia botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef]
- Wu, P.; Huang, Z.; Shan, J.; Luo, Z.; Zhang, N.; Yin, S.; Shen, C.; Xing, R.; Mei, W.; Xiao, Y.; et al. Interventional effects of the direct application of “Sanse powder” on knee osteoarthritis in rats as determined from lipidomics via UPLC-Q-Exactive Orbitrap MS. Chin. Med. 2020, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Liu, Z.; Zhang, L.; Xing, R.; Yin, S.; Li, X.; Zhang, N.; Wang, P. Sanse Powder Essential Oil Nanoemulsion Negatively Regulates TRPA1 by AMPK/mTOR Signaling in Synovitis: Knee Osteoarthritis Rat Model and Fibroblast-Like Synoviocyte Isolates. Mediat. Inflamm. 2021, 2021, 4736670. [Google Scholar] [CrossRef]
- Dai, J.; Hu, J.J.; Dong, X.; Chen, B.; Dong, X.; Liu, R.; Xia, F.; Lou, X. Deep Downregulation of PD-L1 by Caged Peptide-Conjugated AIEgen/miR-140 Nanoparticles for Enhanced Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202117798. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Wang, P.; Zhu, F.; Wu, M.; Luo, Q.; Zhang, Y.; Liu, X. Tumor Microenvironment-Responsive Yolk-Shell NaCl@Virus-Inspired Tetrasulfide-Organosilica for Ion-Interference Therapy via Osmolarity Surge and Oxidative Stress Amplification. ACS Nano 2022, 16, 7380–7397. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Liu, M.; Meng, J.; Liu, S.; Wang, S.; Jia, R.; Wang, Y.; Ma, G.; Wei, W.; Tian, Z. MOFs-based nanoagent enables dual mitochondrial damage in synergistic antitumor therapy via oxidative stress and calcium overload. Nat. Commun. 2021, 12, 6399. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tian, H.; Li, L.; Li, B.; Yang, M.; Zhou, L.; Jiang, H.; Li, Q.; Wang, W.; Nice, E.C.; et al. Nanoengineering a Zeolitic Imidazolate Framework-8 Capable of Manipulating Energy Metabolism against Cancer Chemo-Phototherapy Resistance. Small 2022, 18, e2204926. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yuan, L.; Zhang, Y.; Kuang, J.; Song, W.; Lou, X.; Xia, F.; Yoon, J. Photo-Controlled Calcium Overload from Endogenous Sources for Tumor Therapy. Angew. Chem. Int. Ed. 2024, 63, e202317578. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Tang, Y.; Yang, X.; Liu, Y.; Huang, D.; Zhang, Y.; Li, C.; Wang, Q. Advanced Near-Infrared Light for Monitoring and Modulating the Spatiotemporal Dynamics of Cell Functions in Living Systems. Adv. Sci. 2020, 7, 1903783. [Google Scholar] [CrossRef]
- Fu, X.C.; Huang, Y.M.; Zhao, H.; Zhang, E.D.; Shen, Q.; Di, Y.F.; Lv, F.T.; Liu, L.B.; Wang, S. Near-Infrared-Light Remote-Controlled Activation of Cancer Immunotherapy Using Photothermal Conjugated Polymer Nanoparticles. Adv. Mater. 2021, 33, 2102570. [Google Scholar] [CrossRef]
- Wang, Z.J.; Qu, S.Y.; Gao, D.; Shao, Q.; Nie, C.Y.; Xing, C.F. A Strategy of On-Demand Immune Activation for Antifungal Treatment Using Near-Infrared Responsive Conjugated Polymer Nanoparticles. Nano Lett. 2023, 23, 326–335. [Google Scholar] [CrossRef]
- Li, N.; Gao, Y.J.; Li, B.Y.; Gao, D.; Geng, H.; Li, S.L.; Xing, C.F. Remote Manipulation of ROS-Sensitive Calcium Channel Using Near-Infrared-Responsive Conjugated Oligomer Nanoparticles for Enhanced Tumor Therapy. Nano Lett. 2022, 22, 5427–5433. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Wang, Y.Y.; Gao, D.; Ren, S.X.; Li, L.; Li, N.; An, H.L.; Zhu, T.T.; Yang, Y.K.; Zhang, H.L.; et al. Photothermal Modulation of Depression-Related Ion Channel Function through Conjugated Polymer Nanoparticles. Adv. Funct. Mater. 2021, 31, 2010757. [Google Scholar] [CrossRef]
- Wu, X.; Yang, F.; Cai, S.; Pu, K.Y.; Hong, G.S. Nanotransducer-Enabled Deep-Brain Neuromodulation with NIR-II Light. Acs Nano 2023, 17, 7941–7952. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, Z.; Li, S.L.; Huang, Z.M.; Wan, Y.P.; Cao, C.; Lin, S.E.; Wu, L.; Zhou, J.; Liao, L.S.; et al. Molecular Programming of NIR-IIb-Emissive Semiconducting Small Molecules for In Vivo High-Contrast Bioimaging Beyond 1500 nm. Adv. Mater. 2022, 34, e2201263. [Google Scholar] [CrossRef]
- Wei, J.C.; Liu, Y.; Yu, J.; Chen, L.; Luo, M.; Yang, L.L.; Li, P.; Li, S.L.; Zhang, X.H. Conjugated Polymers: Optical Toolbox for Bioimaging and Cancer Therapy. Small 2021, 17, 2103127. [Google Scholar] [CrossRef]
- Li, X.Z.; Fang, F.; Sun, B.; Yin, C.; Tan, J.H.; Wan, Y.P.; Zhang, J.F.; Sun, P.F.; Fan, Q.L.; Wang, P.F.; et al. Near-infrared small molecule coupled with rigidness and flexibility for high-performance multimodal imaging-guided photodynamic and photothermal synergistic therapy. Nanoscale Horiz. 2021, 6, 177–185. [Google Scholar] [CrossRef]
- He, S.S.; Jiang, Y.Y.; Li, J.C.; Pu, K.Y. Semiconducting Polycomplex Nanoparticles for Photothermal Ferrotherapy of Cancer. Angew. Chem. Int. Ed. 2020, 59, 10633–10638. [Google Scholar] [CrossRef]
- Li, N.; Li, C.; Li, B.Y.; Li, C.Q.; Zhao, Q.; Huang, Z.M.; Shu, Y.; Qu, X.W.; Wang, B.Q.; Li, S.L.; et al. Dual Activation of Calcium Channels Using Near-Infrared Responsive Conjugated Oligomer Nanoparticles for Precise Regulation of Blood Glucose Homeostasis. Nano Lett. 2023, 23, 10608–10616. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D. TRPA1-Related Diseases and Applications of Nanotherapy. Int. J. Mol. Sci. 2024, 25, 9234. https://doi.org/10.3390/ijms25179234
Yang D. TRPA1-Related Diseases and Applications of Nanotherapy. International Journal of Molecular Sciences. 2024; 25(17):9234. https://doi.org/10.3390/ijms25179234
Chicago/Turabian StyleYang, Dongki. 2024. "TRPA1-Related Diseases and Applications of Nanotherapy" International Journal of Molecular Sciences 25, no. 17: 9234. https://doi.org/10.3390/ijms25179234





