Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice
Abstract
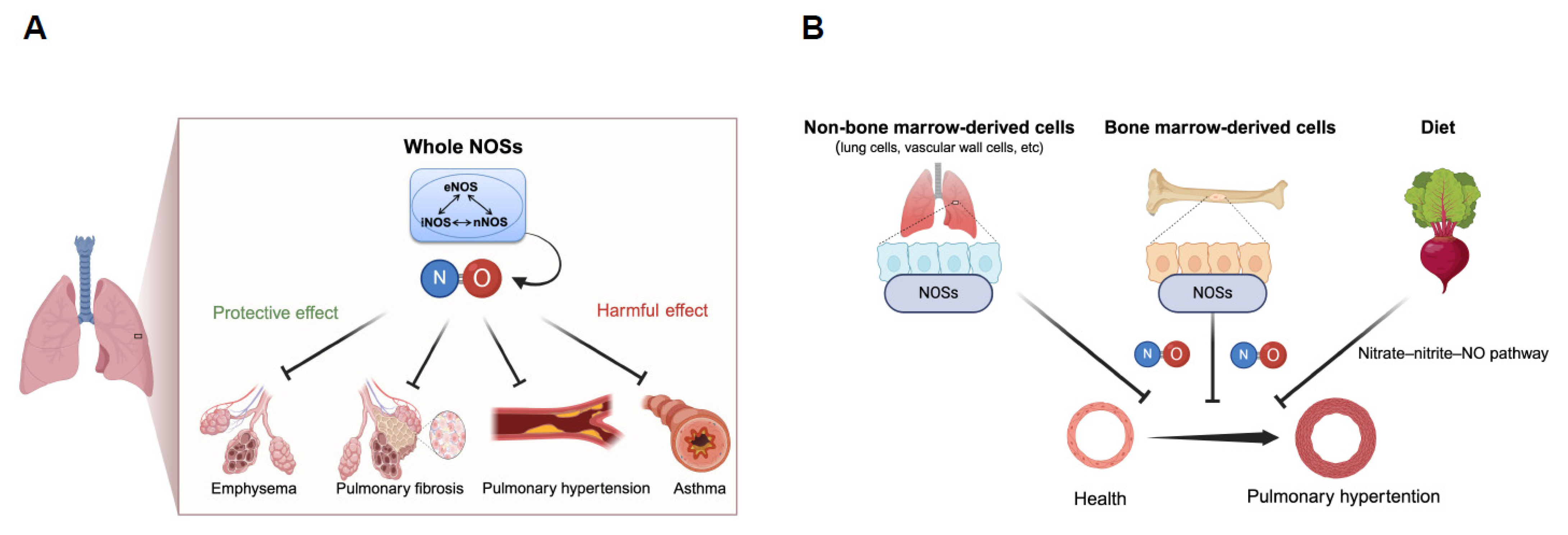
:1. Introduction
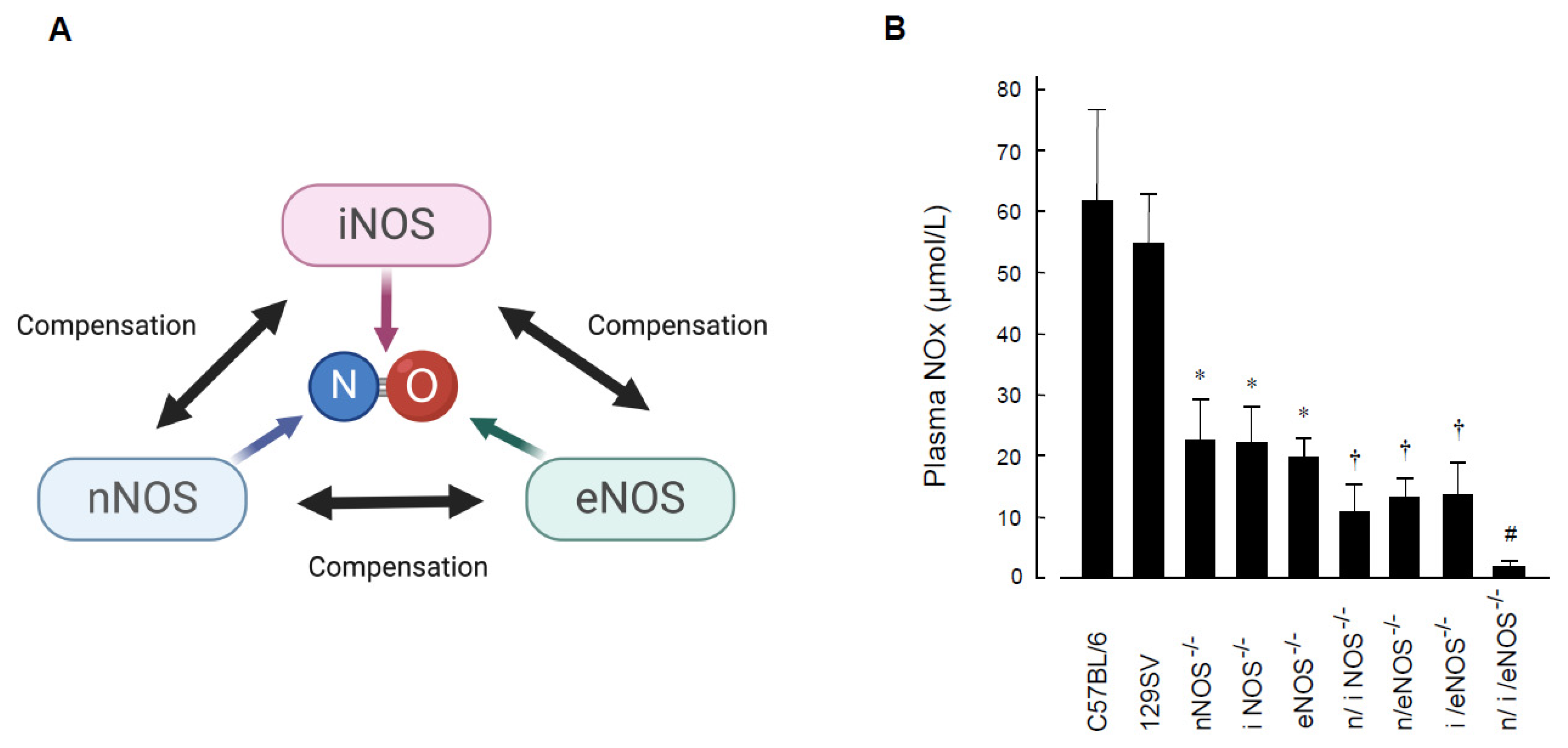
2. Diverse Roles of Nitric Oxide Synthases in Respiratory Diseases
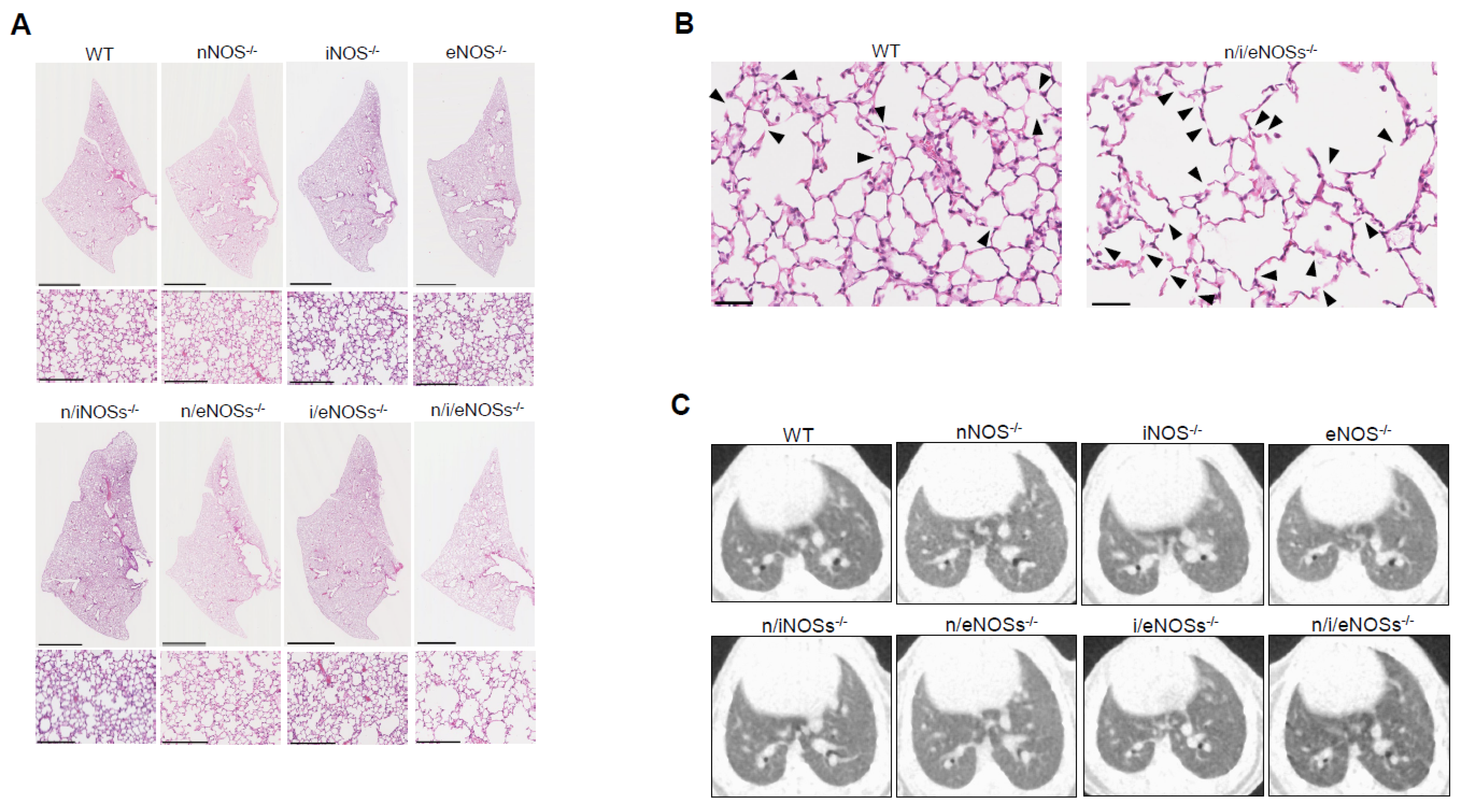
2.1. Preventive Role of Nitric Oxide Synthases in Spontaneous Pulmonary Emphysema
2.2. Protective Role of Nitric Oxide Synthases in Pulmonary Fibrosis
2.3. Protective Role of Nitric Oxide Synthases in Pulmonary Hypertension
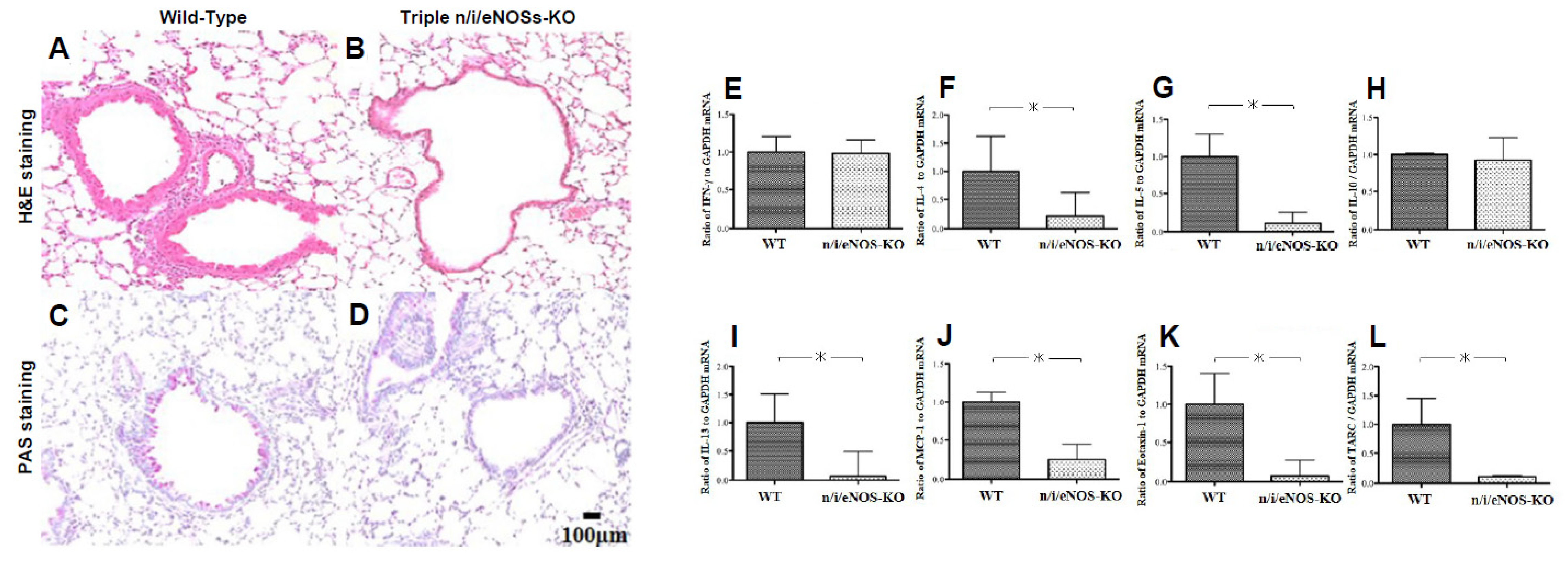
2.4. Opposing Detrimental Role of Nitric Oxide Synthases in Asthma
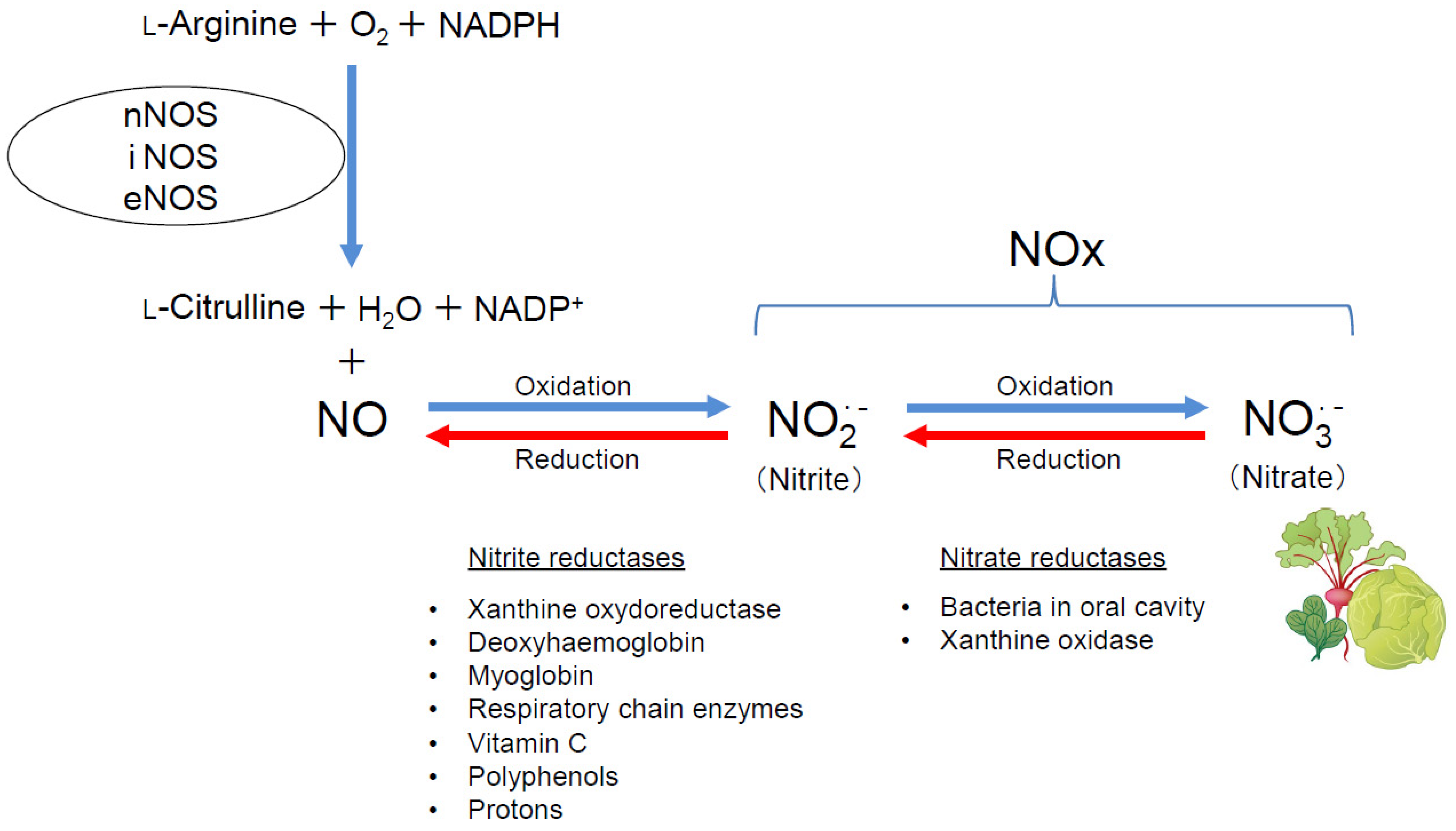
3. Current State of Development of Inorganic Nitrate as a New Drug for Respiratory Diseases
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef]
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- Ignarro, L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 535–560. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Murad, F. What are the molecular mechanisms for the antiproliferative effects of nitric oxide and cGMP in vascular smooth muscle? Circulation 1997, 95, 1101–1103. [Google Scholar] [CrossRef]
- Ogoshi, T.; Tsutsui, M.; Kido, T.; Sakanashi, M.; Naito, K.; Oda, K.; Ishimoto, H.; Yamada, S.; Wang, K.Y.; Toyohira, Y.; et al. Protective Role of Myelocytic Nitric Oxide Synthases against Hypoxic Pulmonary Hypertension in Mice. Am. J. Respir. Crit. Care Med. 2018, 198, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, M.; Shimokawa, H.; Otsuji, Y.; Yanagihara, N. Pathophysiological relevance of NO signaling in the cardiovascular system: Novel insight from mice lacking all NO synthases. Pharmacol. Ther. 2010, 128, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Brindicci, C.; Kharitonov, S.A.; Ito, M.; Elliott, M.W.; Hogg, J.C.; Barnes, P.J.; Ito, K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 21–30. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Podzuweit, T.; Bocker, W.; Samoilova, V.E.; Thomas, S.; Wellner, M.; Baba, H.A.; Robenek, H.; Schnekenburger, J.; Lerch, M.M. Vascular smooth muscle and nitric oxide synthase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 500–508. [Google Scholar] [CrossRef]
- Kobzik, L.; Bredt, D.S.; Lowenstein, C.J.; Drazen, J.; Gaston, B.; Sugarbaker, D.; Stamler, J.S. Nitric oxide synthase in human and rat lung: Immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 1993, 9, 371–377. [Google Scholar] [CrossRef]
- Antosova, M.; Mokra, D.; Pepucha, L.; Plevkova, J.; Buday, T.; Sterusky, M.; Bencova, A. Physiology of nitric oxide in the respiratory system. Physiol. Res. 2017, 66, S159–S172. [Google Scholar] [CrossRef]
- Hampl, V.; Herget, J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol. Rev. 2000, 80, 1337–1372. [Google Scholar] [CrossRef]
- Prado, C.M.; Martins, M.A.; Tibério, I.F.L.C. Nitric Oxide in Asthma Physiopathology. ISRN Allergy 2011, 2011, 832560. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef]
- Buxton, I.L.; Cheek, D.J.; Eckman, D.; Westfall, D.P.; Sanders, K.M.; Keef, K.D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ. Res. 1993, 72, 387–395. [Google Scholar] [CrossRef]
- Heim, K.F.; Thomas, G.; Ramwell, P.W. Effect of substituted arginine compounds on superoxide production in the rabbit aorta. J. Pharmacol. Exp. Ther. 1991, 257, 1130–1135. [Google Scholar]
- Peterson, D.A.; Peterson, D.C.; Archer, S.; Weir, E.K. The non specificity of specific nitric oxide synthase inhibitors. Biochem. Biophys. Res. Commun. 1992, 187, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Ramwell, P.W. NW-nitro L-arginine benzyl ester, a potent irreversible inhibitor of endothelium dependent relaxation. Biochem. Biophys. Res. Commun. 1991, 179, 1677–1682. [Google Scholar] [CrossRef]
- Suda, O.; Tsutsui, M.; Morishita, T.; Tanimoto, A.; Horiuchi, M.; Tasaki, H.; Huang, P.L.; Sasaguri, Y.; Yanagihara, N.; Nakashima, Y. Long-term treatment with N(omega)-nitro-L-arginine methyl ester causes arteriosclerotic coronary lesions in endothelial nitric oxide synthase-deficient mice. Circulation 2002, 106, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Suda, O.; Tsutsui, M.; Morishita, T.; Tasaki, H.; Ueno, S.; Nakata, S.; Tsujimoto, T.; Toyohira, Y.; Hayashida, Y.; Sasaguri, Y.; et al. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: Involvement of renin-angiotensin system and oxidative stress. Arter. Thromb. Vasc. Biol. 2004, 24, 1682–1688. [Google Scholar] [CrossRef]
- Huang, A.; Sun, D.; Shesely, E.G.; Levee, E.M.; Koller, A.; Kaley, G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H429–H436. [Google Scholar] [CrossRef] [PubMed]
- Lamping, K.G.; Nuno, D.W.; Shesely, E.G.; Maeda, N.; Faraci, F.M. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1906–H1912. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Ayata, C.; Waeber, C.; Huang, P.L.; Moskowitz, M.A. Neuronal NOS-cGMP-dependent ACh-induced relaxation in pial arterioles of endothelial NOS knockout mice. Am. J. Physiol. 1998, 274, H411–H415. [Google Scholar] [CrossRef]
- Meng, W.; Ma, J.; Ayata, C.; Hara, H.; Huang, P.L.; Fishman, M.C.; Moskowitz, M.A. ACh dilates pial arterioles in endothelial and neuronal NOS knockout mice by NO-dependent mechanisms. Am. J. Physiol. 1996, 271, H1145–H1150. [Google Scholar] [CrossRef] [PubMed]
- Benkhoff, S.; Loot, A.E.; Pierson, I.; Sturza, A.; Kohlstedt, K.; Fleming, I.; Shimokawa, H.; Grisk, O.; Brandes, R.P.; Schröder, K. Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arter. Thromb. Vasc. Biol. 2012, 32, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Tsutsui, M.; Shimokawa, H.; Suda, O.; Morishita, T.; Shibata, K.; Yatera, Y.; Sabanai, K.; Tanimoto, A.; Nagasaki, M.; et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation 2008, 117, 2211–2223. [Google Scholar] [CrossRef]
- Duplain, H.; Burcelin, R.; Sartori, C.; Cook, S.; Egli, M.; Lepori, M.; Vollenweider, P.; Pedrazzini, T.; Nicod, P.; Thorens, B.; et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 2001, 104, 342–345. [Google Scholar] [CrossRef]
- Morishita, T.; Tsutsui, M.; Shimokawa, H.; Sabanai, K.; Tasaki, H.; Suda, O.; Nakata, S.; Tanimoto, A.; Wang, K.-Y.; Ueta, Y.; et al. Nephrogenic diabetes insipidus in mice lacking all nitric oxide synthase isoforms. Proc. Natl. Acad. Sci. USA 2005, 102, 10616–10621. [Google Scholar] [CrossRef]
- Tsutsui, M.; Nakata, S.; Shimokawa, H.; Otsuji, Y.; Yanagihara, N. Spontaneous myocardial infarction and nitric oxide synthase. Trends. Cardiovasc. Med. 2008, 18, 275–279. [Google Scholar] [CrossRef]
- Tsutsui, M.; Shimokawa, H.; Morishita, T.; Nakashima, Y.; Yanagihara, N. Development of genetically engineered mice lacking all three nitric oxide synthases. J. Pharmacol. Sci. 2006, 102, 147–154. [Google Scholar] [CrossRef]
- Tsutsui, M.; Tanimoto, A.; Tamura, M.; Mukae, H.; Yanagihara, N.; Shimokawa, H.; Otsuji, Y. Significance of nitric oxide synthases: Lessons from triple nitric oxide synthases null mice. J. Pharmacol. Sci. 2015, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Tsutsui, M.; Noguchi, S.; Iha, Y.; Naito, K.; Ogoshi, T.; Nishida, C.; Tahara, M.; Yamashita, H.; Wang, K.-Y.; et al. Spontaneous pulmonary emphysema in mice lacking all three nitric oxide synthase isoforms. Sci. Rep. 2021, 11, 22088. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yatera, K.; Wang, K.-Y.; Oda, K.; Akata, K.; Yamasaki, K.; Kawanami, T.; Ishimoto, H.; Toyohira, Y.; Shimokawa, H.; et al. Nitric oxide exerts protective effects against bleomycin-induced pulmonary fibrosis in mice. Respir. Res. 2014, 15, 92. [Google Scholar] [CrossRef]
- Akata, K.; Yatera, K.; Wang, K.Y.; Naito, K.; Ogoshi, T.; Noguchi, S.; Kido, T.; Toyohira, Y.; Shimokawa, H.; Yanagihara, N.; et al. Decreased Bronchial Eosinophilic Inflammation and Mucus Hypersecretion in Asthmatic Mice Lacking All Nitric Oxide Synthase Isoforms. Lung 2016, 194, 121–124. [Google Scholar] [CrossRef]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.D.; Coxson, H.O.; Pillai, S.G.; Agustí, A.G.N.; Calverley, P.M.A.; Donner, C.F.; Make, B.J.; Müller, N.L.; Rennard, S.I.; Vestbo, J.; et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 500–505. [Google Scholar] [CrossRef]
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M.; Gillespie, S.; Burney, P.; Mannino, D.M.; Menezes, A.M.B.; Sullivan, S.D.; Lee, T.A.; Weiss, K.B.; et al. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- Lanzetti, M.; da Costa, C.A.; Nesi, R.T.; Barroso, M.V.; Martins, V.; Victoni, T.; Lagente, V.; Pires, K.M.P.; e Silva, P.M.R.; Resende, A.C.; et al. Oxidative stress and nitrosative stress are involved in different stages of proteolytic pulmonary emphysema. Free Radic. Biol. Med. 2012, 53, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Seimetz, M.; Parajuli, N.; Pichl, A.; Veit, F.; Kwapiszewska, G.; Weisel, F.C.; Milger, K.; Egemnazarov, B.; Turowska, A.; Fuchs, B.; et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011, 147, 293–305. [Google Scholar] [CrossRef]
- Boyer, L.; Plantier, L.; Dagouassat, M.; Lanone, S.; Goven, D.; Caramelle, P.; Berrehar, F.; Kerbrat, S.; Dinh-Xuan, A.T.; Crestani, B.; et al. Role of nitric oxide synthases in elastase-induced emphysema. Lab. Investig. 2011, 91, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Valença, S.S.; Rueff-Barroso, C.R.; Pimenta, W.A.; Melo, A.C.; Nesi, R.T.; Silva, M.A.S.; Porto, L.C. L-NAME and L-arginine differentially ameliorate cigarette smoke-induced emphysema in mice. Pulm. Pharmacol. Ther. 2011, 24, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Allcock, G.H.; Warner, T.D. Comparison of effects of chronic and acute administration of NG-nitro-L-arginine methyl ester to the rat on inhibition of nitric oxide-mediated responses. Br. J. Pharmacol. 1995, 114, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Kneidinger, N.; Yildirim, A.; Callegari, J.; Takenaka, S.; Stein, M.M.; Dumitrascu, R.; Bohla, A.; Bracke, K.R.; Morty, R.E.; Brusselle, G.G.; et al. Activation of the WNT/β-catenin pathway attenuates experimental emphysema. Am. J. Respir. Crit. Care Med. 2011, 183, 723–733. [Google Scholar] [CrossRef]
- Mandel, H.; Shemer, R.; Borochowitz, Z.U.; Okopnik, M.; Knopf, C.; Indelman, M.; Drugan, A.; Tiosano, D.; Gershoni-Baruch, R.; Choder, M.; et al. SERKAL syndrome: An autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am. J. Hum. Genet. 2008, 82, 39–47. [Google Scholar] [CrossRef]
- Green, F.H. Overview of pulmonary fibrosis. Chest 2002, 122, 334s–339s. [Google Scholar] [CrossRef]
- Chung, M.P.; Monick, M.M.; Hamzeh, N.Y.; Butler, N.S.; Powers, L.S.; Hunninghake, G.W. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am. J. Respir. Cell Mol. Biol. 2003, 29, 375–380. [Google Scholar] [CrossRef]
- Yoshimura, S.; Nishimura, Y.; Nishiuma, T.; Yamashita, T.; Kobayashi, K.; Yokoyama, M. Overexpression of nitric oxide synthase by the endothelium attenuates bleomycin-induced lung fibrosis and impairs MMP-9/TIMP-1 balance. Respirology 2006, 11, 546–556. [Google Scholar] [CrossRef]
- Genovese, T.; Cuzzocrea, S.; Di Paola, R.; Failla, M.; Mazzon, E.; Sortino, M.A.; Frasca, G.; Gili, E.; Crimi, N.; Caputi, A.P.; et al. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir. Res. 2005, 6, 58. [Google Scholar] [CrossRef]
- Yildirim, Z.; Turkoz, Y.; Kotuk, M.; Armutcu, F.; Gurel, A.; Iraz, M.; Ozen, S.; Aydogdu, I.; Akyol, O. Effects of aminoguanidine and antioxidant erdosteine on bleomycin-induced lung fibrosis in rats. Nitric Oxide Biol. Chem. 2004, 11, 156–165. [Google Scholar] [CrossRef]
- Davis, D.W.; Weidner, D.A.; Holian, A.; McConkey, D.J. Nitric oxide-dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J. Exp. Med. 2000, 192, 857–869. [Google Scholar] [CrossRef]
- Vyas-Read, S.; Shaul, P.W.; Yuhanna, I.S.; Willis, B.C. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L212–L221. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Aono, Y.; Azuma, M.; Kishi, J.; Takezaki, A.; Kishi, M.; Makino, H.; Okazaki, H.; Uehara, H.; Izumi, K.; et al. Antifibrotic effects of focal adhesion kinase inhibitor in bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 536–543. [Google Scholar] [CrossRef]
- Keil, A.; Blom, I.E.; Goldschmeding, R.; Rupprecht, H.D. Nitric oxide down-regulates connective tissue growth factor in rat mesangial cells. Kidney Int. 2002, 62, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Hurdman, J.; Condliffe, R.; Elliot, C.A.; Davies, C.; Hill, C.; Wild, J.M.; Capener, D.; Sephton, P.; Hamilton, N.; Armstrong, I.J.; et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur. Respir. J. 2012, 39, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Fagan, K.A.; Tyler, R.C.; Sato, K.; Fouty, B.W.; Morris, K.G.; Huang, P.L.; McMurtry, I.F.; Rodman, D.M. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am. J. Physiol. 1999, 277, 472. [Google Scholar] [CrossRef]
- Quinlan, T.R.; Li, D.; Laubach, V.E.; Shesely, E.G.; Zhou, N.; Johns, R.A. eNOS-deficient mice show reduced pulmonary vascular proliferation and remodeling to chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, 641. [Google Scholar] [CrossRef] [PubMed]
- Steudel, W.; Scherrer-Crosbie, M.; Bloch, K.D.; Weimann, J.; Huang, P.L.; Jones, R.C.; Picard, M.H.; Zapol, W.M. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J. Clin. Investig. 1998, 101, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Reviews. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Farha, S.; Asosingh, K.; Xu, W.; Sharp, J.; George, D.; Comhair, S.; Park, M.; Tang, W.H.W.; Loyd, J.E.; Theil, K.; et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: A link to the intrinsic myeloid abnormalities. Blood 2011, 117, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Yates, D.; Robbins, R.A.; Logan-Sinclair, R.; Shinebourne, E.A.; Barnes, P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994, 343, 133–135. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, G.T.; MacLean, J.A.; Hamada, K.; Mehta, S.; Scott, J.A.; Jiao, A.; Yandava, C.N.; Kobzik, L.; Wolyniec, W.W.; Fabian, A.J.; et al. Contribution of Nitric Oxide Synthases 1, 2, and 3 to Airway Hyperresponsiveness and Inflammation in a Murine Model of Asthma. J. Exp. Med. 1999, 189, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Karupiah, G.; Hogan, S.P.; Foster, P.S.; Ramsay, A.J. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase 2. J. Immunol. 1999, 162, 445–452. [Google Scholar] [CrossRef]
- Broeke, R.T.; De Crom, R.; Van Haperen, R.; Verweij, V.; Leusink-Muis, T.; Van Ark, I.; De Clerck, F.; Nijkamp, F.P.; Folkerts, G. Overexpression of endothelial nitric oxide synthase suppresses features of allergic asthma in mice. Respir. Res. 2006, 7, 58. [Google Scholar] [CrossRef]
- Feder, L.S.; Stelts, D.; Chapman, R.W.; Manfra, D.; Crawley, Y.; Jones, H.; Minnicozzi, M.; Fernandez, X.; Paster, T.; Egan, R.W.; et al. Role of nitric oxide on eosinophilic lung inflammation in allergic mice. Am. J. Respir. Cell Mol. Biol. 1997, 17, 436–442. [Google Scholar] [CrossRef]
- Blease, K.; Kunkel, S.L.; Hogaboam, C.M. Acute inhibition of nitric oxide exacerbates airway hyperresponsiveness, eosinophilia and C-C chemokine generation in a murine model of fungal asthma. Inflamm. Res. 2000, 49, 297–304. [Google Scholar] [CrossRef]
- Wang, J.; Feng, F.; Zhao, Y.; Bai, L.; Xu, Y.; Wei, Y.; He, H.; Zhou, X. Dietary nitrate supplementation to enhance exercise capacity in patients with COPD: Evidence from a meta-analysis of randomized controlled trials and a network pharmacological analysis. Respir. Med. 2024, 222, 107498. [Google Scholar] [CrossRef]
- Henrohn, D.; Björkstrand, K.; Lundberg, J.O.; Granstam, S.-O.; Baron, T.; Ingimarsdóttir, I.J.; Hedenström, H.; Malinovschi, A.; Wernroth, M.-L.; Jansson, M.; et al. Effects of Oral Supplementation With Nitrate-Rich Beetroot Juice in Patients With Pulmonary Arterial Hypertension-Results From BEET-PAH, an Exploratory Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J. Card. Fail. 2018, 24, 640–653. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogoshi, T.; Yatera, K.; Mukae, H.; Tsutsui, M. Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice. Int. J. Mol. Sci. 2024, 25, 9317. https://doi.org/10.3390/ijms25179317
Ogoshi T, Yatera K, Mukae H, Tsutsui M. Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice. International Journal of Molecular Sciences. 2024; 25(17):9317. https://doi.org/10.3390/ijms25179317
Chicago/Turabian StyleOgoshi, Takaaki, Kazuhiro Yatera, Hiroshi Mukae, and Masato Tsutsui. 2024. "Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice" International Journal of Molecular Sciences 25, no. 17: 9317. https://doi.org/10.3390/ijms25179317
APA StyleOgoshi, T., Yatera, K., Mukae, H., & Tsutsui, M. (2024). Role of Nitric Oxide Synthases in Respiratory Health and Disease: Insights from Triple Nitric Oxide Synthases Knockout Mice. International Journal of Molecular Sciences, 25(17), 9317. https://doi.org/10.3390/ijms25179317





