Diet-Induced Obesity Induces Transcriptomic Changes in Neuroimmunometabolic-Related Genes in the Striatum and Olfactory Bulb
Abstract
:1. Introduction
2. Results
2.1. DEGs in the Striatal Tissue
2.2. DEGs in the Olfactory Tissue
2.3. Weighted Gene Co-Expression Network Associated with Free Fatty Acids
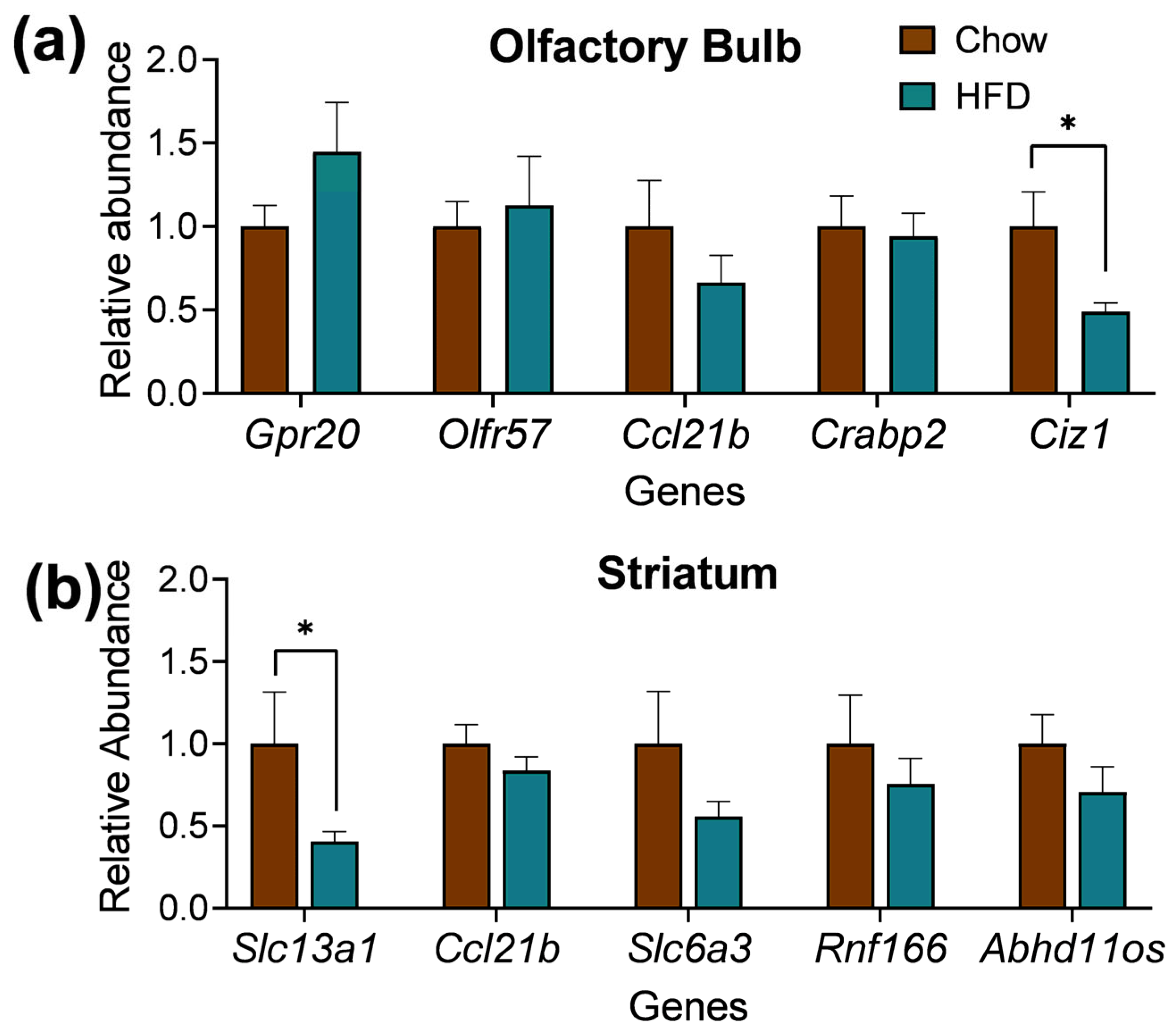
2.4. DEGs Quantitative Real-Time PCR (qPCR) of Striatum and Olfactory Bulb
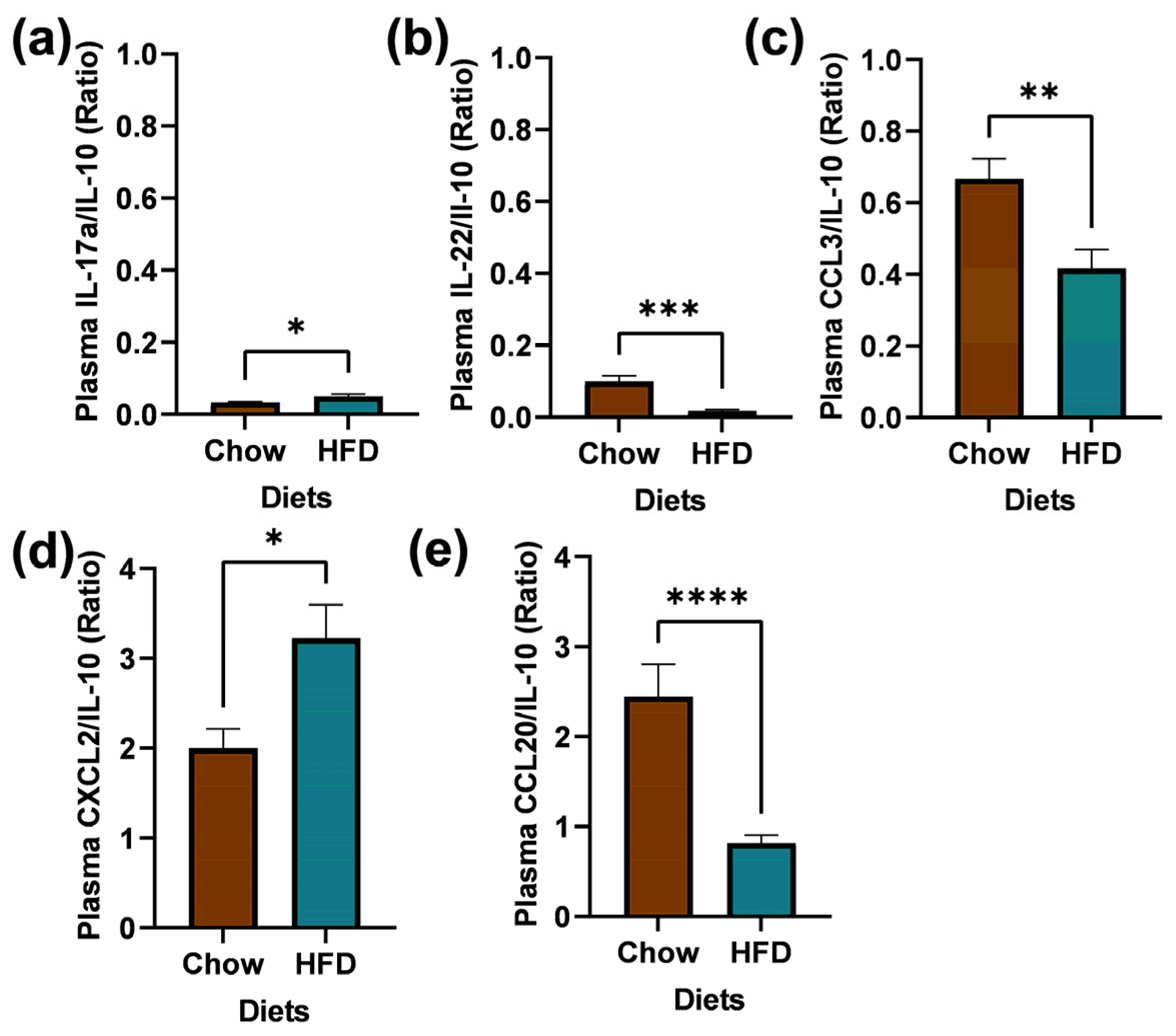
2.5. Plasma Collection and Biomarkers Measurement
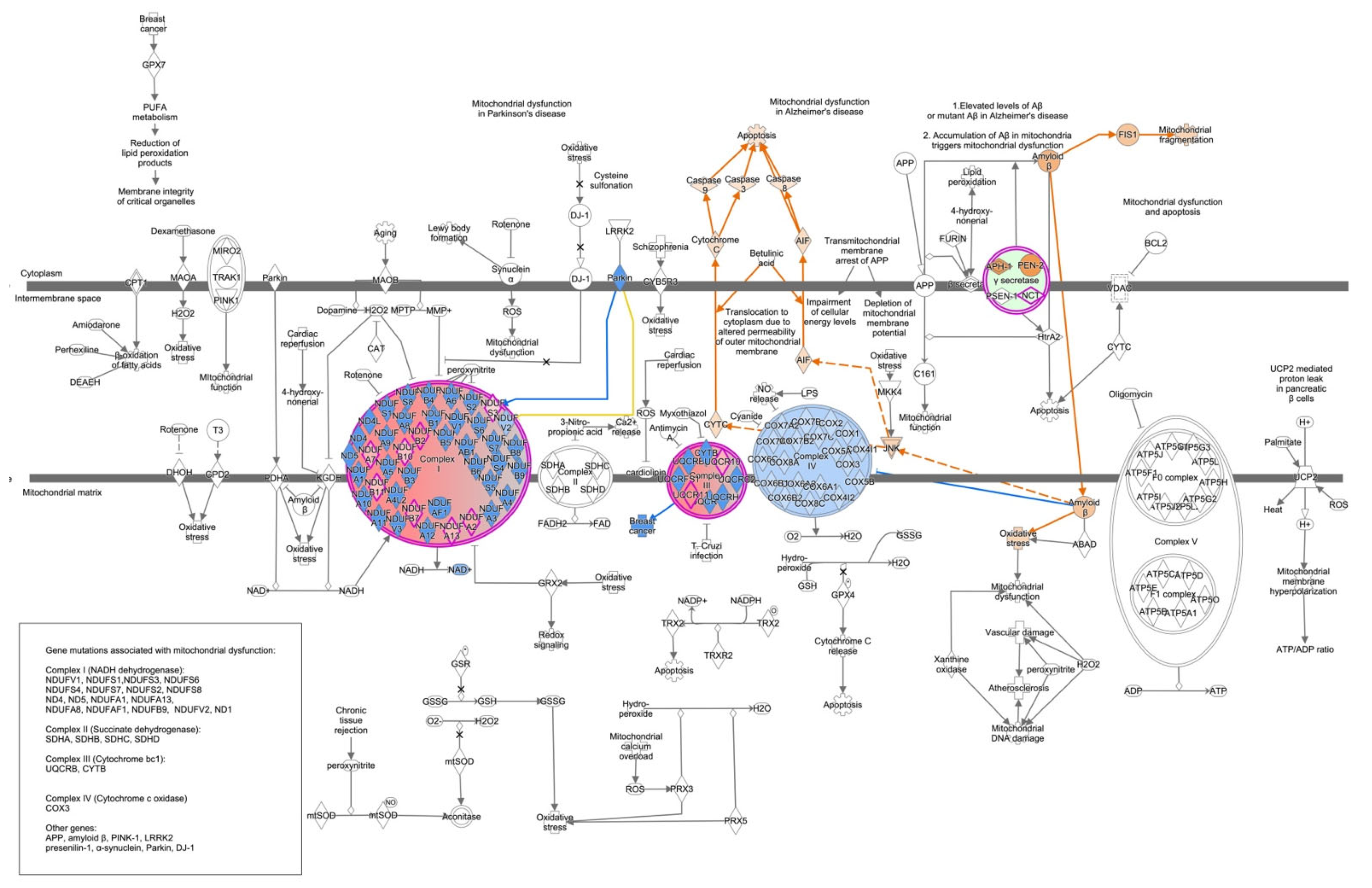
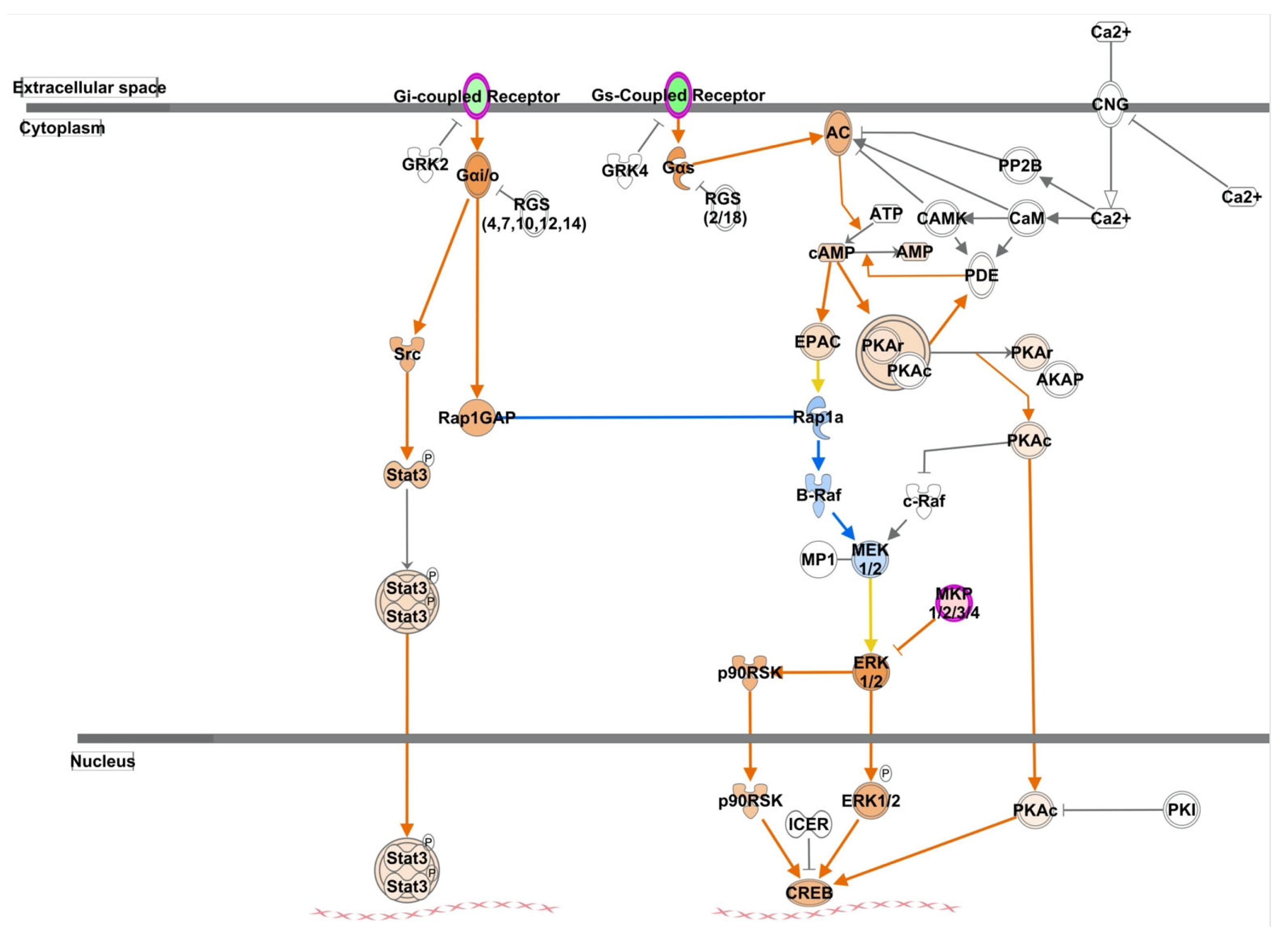
3. Discussion
4. Materials and Methods
4.1. Animal Handling and Diets
4.2. Sample Collection
4.3. Biological Measurements
4.4. Activity Measurements
4.5. RNA Sequencing
4.6. Analysis of RNA Sequencing
4.7. Enrichment Analysis
4.8. Ingenuity Pathway Analysis (IPA)
4.9. Construction of Weighted Gene Co-Expression Networks
4.10. Identification of Hub Genes
4.11. Quantitative Real-Time PCR (qPCR) of Striatum and Olfactory Bulb
4.12. Plasma Collection and Biomarker Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Caballero, B. The Global Epidemic of Obesity: An Overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef]
- Erlanson-Albertsson, C. Appetite regulation and energy balance. Acta Paediatr. Suppl. 2005, 94, 40–41. [Google Scholar] [CrossRef]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176. [Google Scholar] [CrossRef]
- Ubeda-Bañon, I.; Novejarque, A.; Mohedano-Moriano, A.; Pro-Sistiaga, P.; de la Rosa-Prieto, C.; Insausti, R.; Martinez-Garcia, F.; Lanuza, E.; Martinez-Marcos, A. Projections from the posterolateral olfactory amygdala to the ventral striatum: Neural basis for reinforcing properties of chemical stimuli. BMC Neurosci. 2007, 8, 103. [Google Scholar] [CrossRef]
- Purves, D.; Williams, S.M. Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.A.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab. 2017, 26, 198–211.e195. [Google Scholar] [CrossRef]
- Yoon, G.; Cho, K.A.; Song, J.; Kim, Y.K. Transcriptomic Analysis of High Fat Diet Fed Mouse Brain Cortex. Front. Genet. 2019, 10, 83. [Google Scholar] [CrossRef]
- Dalby, M.J.; Aviello, G.; Ross, A.W.; Walker, A.W.; Barrett, P.; Morgan, P.J. Diet induced obesity is independent of metabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamic expression of the acute phase protein, SerpinA3N. Sci. Rep. 2018, 8, 15648. [Google Scholar] [CrossRef]
- Sack, M.N. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc. Res. 2006, 72, 210–219. [Google Scholar] [CrossRef]
- Miyata, S.; Mori, Y.; Tohyama, M. PRMT1 and Btg2 regulates neurite outgrowth of Neuro2a cells. Neurosci. Lett. 2008, 445, 162–165. [Google Scholar] [CrossRef]
- Levy, D.E.; Lee, C.-k. What does Stat3 do? J. Clin. Investig. 2002, 109, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Vazquez, A.; Plaisier, C.L.; Geng, R.; Haas, B.E.; Lee, J.; Greevenbroek, M.M.; van der Kallen, C.; de Bruin, T.W.A.; Taskinen, M.-R.; Alagramam, K.N.; et al. A nonsynonymous SNP within PCDH15 is associated with lipid traits in familial combined hyperlipidemia. Hum. Genet. 2010, 127, 83–89. [Google Scholar] [CrossRef]
- Xing, P.C.; An, P.; Hu, G.Y.; Wang, D.L.; Zhou, M.J. LncRNA MIAT Promotes Inflammation and Oxidative Stress in Sepsis-Induced Cardiac Injury by Targeting miR-330-5p/TRAF6/NF-κB Axis. Biochem. Genet. 2020, 58, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liang, Q.; Song, X.; Zhang, Z.; Lindtner, S.; Li, Z.; Wen, Y.; Liu, G.; Guo, T.; Qi, D.; et al. SP8 and SP9 coordinately promote D2-type medium spiny neuron production by activating Six3 expression. Development 2018, 145, dev165456. [Google Scholar] [CrossRef]
- Camp, A.J.; Wijesinghe, R. Calretinin: Modulator of neuronal excitability. Int. J. Biochem. Cell. Biol. 2009, 41, 2118–2121. [Google Scholar] [CrossRef]
- Kanwal, M.; Smahel, M.; Olsen, M.; Smahelova, J.; Tachezy, R. Aspartate β-hydroxylase as a target for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 163. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Xu, B.; Bi, Y.; Wu, C.; Ru, B.; Sun, B.; Bai, X. Recent advances in research on aspartate β-hydroxylase (ASPH) in pancreatic cancer: A brief update. Bosn. J. Basic Med. Sci. 2018, 18, 297–304. [Google Scholar] [CrossRef]
- Tang, C.; Hou, Y.; Wang, H.; Wang, K.; Xiang, H.; Wan, X.; Xia, Y.; Li, J.; Wei, W.; Xu, S.; et al. Aspartate β-hydroxylase disrupts mitochondrial DNA stability and function in hepatocellular carcinoma. Oncogenesis 2017, 6, e362. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.T.; Tajsic, T.; Mellad, J.A.; Searles, R.; Zhang, Q.; Shanahan, C.M. Novel nuclear nesprin-2 variants tether active extracellular signal-regulated MAPK1 and MAPK2 at promyelocytic leukemia protein nuclear bodies and act to regulate smooth muscle cell proliferation. J. Biol. Chem. 2010, 285, 1311–1320. [Google Scholar] [CrossRef]
- Gonçalves, J.C.; Quintremil, S.; Yi, J.; Vallee, R.B. Nesprin-2 Recruitment of BicD2 to the Nuclear Envelope Controls Dynein/Kinesin-Mediated Neuronal Migration In Vivo. Curr. Biol. 2020, 30, 3116–3129.e3114. [Google Scholar] [CrossRef]
- Gil-Yarom, N.; Radomir, L.; Sever, L.; Kramer, M.P.; Lewinsky, H.; Bornstein, C.; Blecher-Gonen, R.; Barnett-Itzhaki, Z.; Mirkin, V.; Friedlander, G.; et al. CD74 is a novel transcription regulator. Proc. Natl. Acad. Sci. USA 2017, 114, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.Y.; Kranzusch, P.J.; Cate, J.H.D. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 2015, 522, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Kondás, K.; Szláma, G.; Trexler, M.; Patthy, L. Both WFIKKN1 and WFIKKN2 have high affinity for growth and differentiation factors 8 and 11. J. Biol. Chem. 2008, 283, 23677–23684. [Google Scholar] [CrossRef]
- Xu, H.; Kaul, S.; Kallinger, R.; Thomas, M.J.; Schill, R.L.; Sahoo, D.; Sorci-Thomas, M.G. PCPE2 and SR-BI Partner to Impact Accumulation of Fat in Mice. bioRxiv 2018. [Google Scholar] [CrossRef]
- Yuting, Y.; Lifeng, F.; Qiwei, H. Secreted modular calcium-binding protein 2 promotes high fat diet (HFD)-induced hepatic steatosis through enhancing lipid deposition, fibrosis and inflammation via targeting TGF-β1. Biochem. Biophys. Res. Commun. 2019, 509, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, C.-C.; Song, X.-P.; Wang, H.-M.; Zhou, H.; Sun, Y.; Wang, X.-K.; Hou, S.; Pei, F.-Y. Suppression of SMOC2 reduces bleomycin (BLM)-induced pulmonary fibrosis by inhibition of TGF-β1/SMADs pathway. Biomed. Pharmacother. 2018, 105, 841–847. [Google Scholar] [CrossRef]
- Takazawa, I.; Larange, A.; Cheroutre, H. Cellular retinoic acid binding protein 2 (CRABP2) serves as a critical regulator in immune regulation and inflammation. J. Immunol. 2019, 202, 124.123. [Google Scholar] [CrossRef]
- Coenye, T. Do results obtained with RNA-sequencing require independent verification? Biofilm 2021, 3, 100043. [Google Scholar] [CrossRef]
- Shen, H.Y.; Chen, J.F. Adenosine A(2A) receptors in psychopharmacology: Modulators of behavior, mood and cognition. Curr. Neuropharmacol. 2009, 7, 195–206. [Google Scholar] [CrossRef]
- Wu, C. Human Brain Transcriptome Profiles in Obesity. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, 2017. [Google Scholar]
- Wang, G.J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusil, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Thanos, P.K.; Logan, J.; Alexoff, D.; Ding, Y.S.; Wong, C.; Ma, Y.; et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage 2008, 42, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.M.; Zald, D.H.; Ansari, M.S.; Li, R.; Cowan, R.L. Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse 2014, 68, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, A.V.; O’Neal, T.J.; Friend, D.M. Do Dopaminergic Impairments Underlie Physical Inactivity in People with Obesity? Front. Hum. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
- Matikainen-Ankney, B.A.; Kravitz, A.V. Persistent effects of obesity: A neuroplasticity hypothesis. Ann. N. Y. Acad. Sci. 2018, 1428, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, A.; Margas, W.M.; Covasa, M. Altered dopamine D2 receptor function and binding in obese OLETF rat. Brain Res. Bull. 2008, 75, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Michaelides, M.; Piyis, Y.K.; Wang, G.J.; Volkow, N.D. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 2008, 62, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.M.; Devarakonda, K.; O’Neal, T.J.; Skirzewski, M.; Papazoglou, I.; Kaplan, A.R.; Liow, J.S.; Guo, J.; Rane, S.G.; Rubinstein, M.; et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab. 2017, 25, 312–321. [Google Scholar] [CrossRef]
- Venteclef, N.; Jakobsson, T.; Steffensen, K.R.; Treuter, E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol. Metab. 2011, 22, 333–343. [Google Scholar] [CrossRef]
- Picklo, M.J.; Long, E.K.; Vomhof-DeKrey, E.E. Glutathionyl systems and metabolic dysfunction in obesity. Nutr. Rev. 2015, 73, 858–868. [Google Scholar] [CrossRef]
- Cho, Y.G.; Park, K.H.; Kim, C.W.; Hur, Y.I. The Relationship between Serum Gamma-glutamyltransferase Level and Overweight in Korean Urban Children. Korean J. Fam. Med. 2011, 32, 182–188. [Google Scholar] [CrossRef]
- Coku, V.; Shkembi, X. Serum Gamma-glutamyltransferase and Obesity: Is there a Link? Med. Arch. 2018, 72, 112–115. [Google Scholar] [CrossRef]
- Andrich, D.E.; Melbouci, L.; Ou, Y.; Auclair, N.; Mercier, J.; Grenier, J.C.; Lira, F.S.; Barreiro, L.B.; Danialou, G.; Comtois, A.S.; et al. A Short-Term High-Fat Diet Alters Glutathione Levels and IL-6 Gene Expression in Oxidative Skeletal Muscles of Young Rats. Front. Physiol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Banks, A.S.; McAllister, F.E.; Camporez, J.P.; Zushin, P.J.; Jurczak, M.J.; Laznik-Bogoslavski, D.; Shulman, G.I.; Gygi, S.P.; Spiegelman, B.M. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 2015, 517, 391–395. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Kammoun, H.L.; Kraakman, M.J.; Kowalski, G.M.; Bruce, C.R.; Febbraio, M.A. PKR is not obligatory for high-fat diet-induced obesity and its associated metabolic and inflammatory complications. Nat. Commun. 2016, 7, 10626. [Google Scholar] [CrossRef]
- Shupp, A.; Casimiro, M.C.; Pestell, R.G. Biological functions of CDK5 and potential CDK5 targeted clinical treatments. Oncotarget 2017, 8, 17373–17382. [Google Scholar] [CrossRef]
- Yao, L.; Herlea-Pana, O.; Heuser-Baker, J.; Chen, Y.; Barlic-Dicen, J. Roles of the chemokine system in development of obesity, insulin resistance, and cardiovascular disease. J. Immunol. Res. 2014, 2014, 181450. [Google Scholar] [CrossRef]
- Schmitt, L.O.; Gaspar, J.M. Obesity-Induced Brain Neuroinflammatory and Mitochondrial Changes. Metabolites 2023, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- de Paula, G.C.; Brunetta, H.S.; Engel, D.F.; Gaspar, J.M.; Velloso, L.A.; Engblom, D.; de Oliveira, J.; de Bem, A.F. Hippocampal Function Is Impaired by a Short-Term High-Fat Diet in Mice: Increased Blood-Brain Barrier Permeability and Neuroinflammation as Triggering Events. Front. Neurosci. 2021, 15, 734158. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef]
- St Laurent, G.; Shtokalo, D.; Tackett, M.R.; Yang, Z.; Vyatkin, Y.; Milos, P.M.; Seilheimer, B.; McCaffrey, T.A.; Kapranov, P. On the importance of small changes in RNA expression. Methods 2013, 63, 18–24. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.R.; Zhang, B.; Fang, Z.; Mischel, P.S.; Horvath, S.; Nelson, S.F. Gene connectivity, function, and sequence conservation: Predictions from modular yeast co-expression networks. BMC Genom. 2006, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Matikainen-Ankney, B.A.; Garmendia-Cedillos, M.; Ali, M.; Krynitsky, J.; Salem, G.; Miyazaki, N.L.; Pohida, T.; Kravitz, A.V. Rodent Activity Detector (RAD), an Open Source Device for Measuring Activity in Rodent Home Cages. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Falcon, S.; Gentleman, R. Using GOstats to test gene lists for GO term association. Bioinformatics 2007, 23, 257–258. [Google Scholar] [CrossRef]
- Joseph, P.V.; Jaime-Lara, R.B.; Wang, Y.; Xiang, L.; Henderson, W.A. Comprehensive and Systematic Analysis of Gene Expression Patterns Associated with Body Mass Index. Sci. Rep. 2019, 9, 7447. [Google Scholar] [CrossRef] [PubMed]
- Ravasz, E.; Somera, A.L.; Mongru, D.A.; Oltvai, Z.N.; Barabási, A.L. Hierarchical organization of modularity in metabolic networks. Science 2002, 297, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
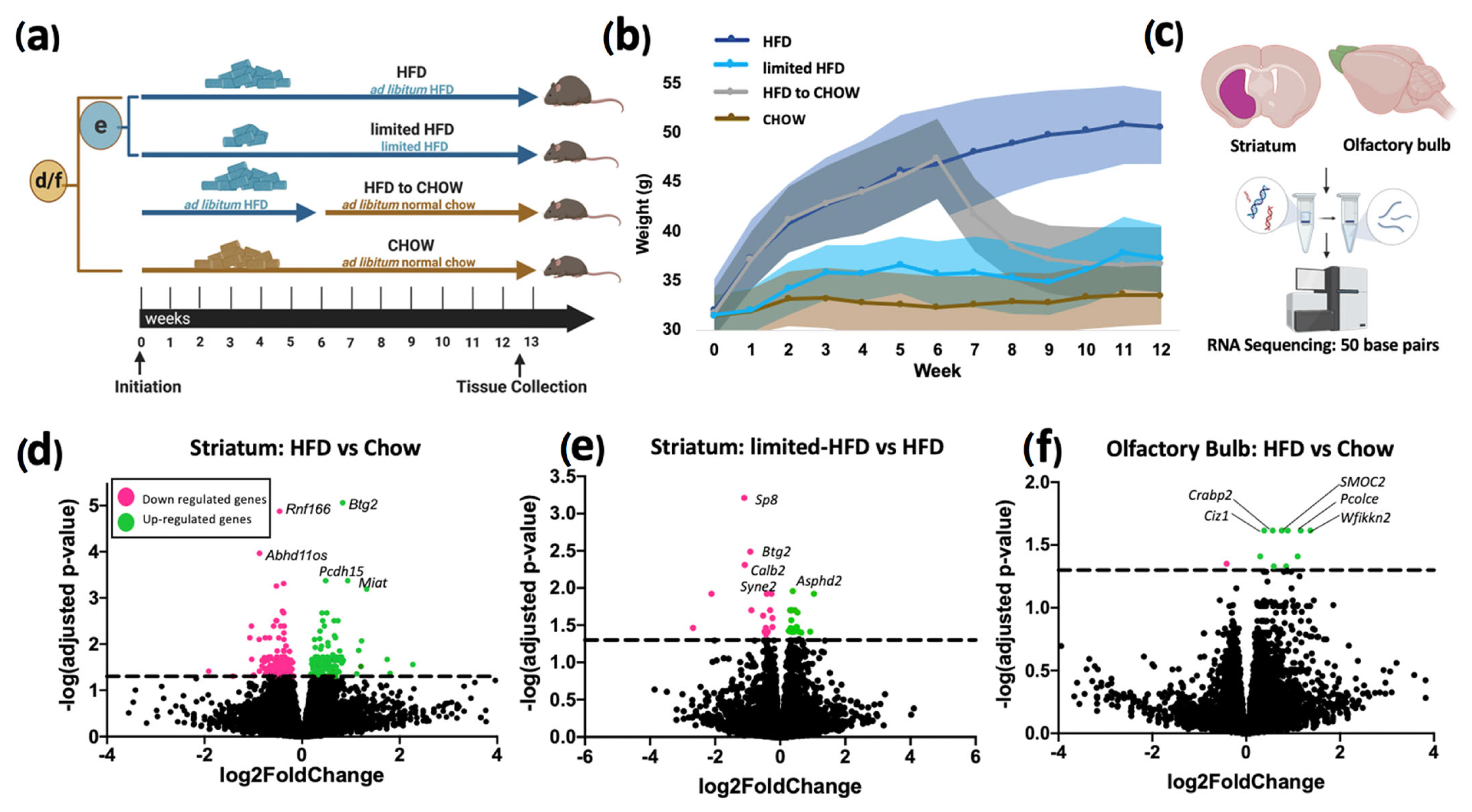


| Striatum: HFD vs. Chow | ||
|---|---|---|
| Gene | Logarithm of Fold Change | FDR-Adjusted p-Value |
| Btg2 | 0.8318072 | 8.66 × 10−6 |
| Rnf166 | −0.4600948 | 1.32 × 10−5 |
| Abhd11os | −0.8731612 | 0.00010783 |
| Pcdh15 | 0.9363582 | 0.000422444 |
| Miat | 0.4841786 | 0.000422444 |
| Gng10 | −0.3782426 | 0.000485772 |
| Tesc | −0.5258876 | 0.000551012 |
| Col12a1 | 1.3235917 | 0.000638062 |
| Hmgn2 | −0.4035872 | 0.001924701 |
| Dcx | 0.4088739 | 0.002103913 |
| Striatum: Limited HFD vs. HFD | ||
| Sp8 | −1.105220599 | 0.000614072 |
| Btg2 | −0.920027322 | 0.003250265 |
| Calb2 | −1.087379338 | 0.00490404 |
| Asphd2 | 0.387021075 | 0.010988066 |
| Flywch2 | 1.035088914 | 0.011978881 |
| Gng12 | −0.274927851 | 0.011978881 |
| Scgn | −2.114478585 | 0.011978881 |
| Syne2 | −0.414699518 | 0.011978881 |
| Adamts1 | −0.885121012 | 0.019887626 |
| Hmgn5 | −0.31248415 | 0.019887626 |
| Striatum: HFD vs. Chow | ||
|---|---|---|
| Gene | Logarithm of Fold Change | FDR-Adjusted p-Value |
| Smoc2 | 0.8872776 | 0.02427586 |
| Ciz1 | 0.3847122 | 0.02427586 |
| Pcolce | 1.1616673 | 0.02427586 |
| Wfikkn2 | 1.3674595 | 0.02427586 |
| Crabp2 | 0.7584510 | 0.02427586 |
| Fn1 | 0.5652448 | 0.02427586 |
| Ndrg1 | 0.2978863 | 0.03900604 |
| Anpep | 1.0962185 | 0.03900604 |
| 1500015A07Rik | −0.4177287 | 0.04459341 |
| Steap3 | 0.5881312 | 0.04674888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaime-Lara, R.B.; Colina-Prisco, C.; De Jesus Vega, M.; Williams, S.; Usdin, T.; Matikainen-Ankney, B.; Kinkead, A.; Brooks, B.; Wang, Y.; Franks, A.T.; et al. Diet-Induced Obesity Induces Transcriptomic Changes in Neuroimmunometabolic-Related Genes in the Striatum and Olfactory Bulb. Int. J. Mol. Sci. 2024, 25, 9330. https://doi.org/10.3390/ijms25179330
Jaime-Lara RB, Colina-Prisco C, De Jesus Vega M, Williams S, Usdin T, Matikainen-Ankney B, Kinkead A, Brooks B, Wang Y, Franks AT, et al. Diet-Induced Obesity Induces Transcriptomic Changes in Neuroimmunometabolic-Related Genes in the Striatum and Olfactory Bulb. International Journal of Molecular Sciences. 2024; 25(17):9330. https://doi.org/10.3390/ijms25179330
Chicago/Turabian StyleJaime-Lara, Rosario B., Claudia Colina-Prisco, Marcel De Jesus Vega, Sarah Williams, Ted Usdin, Bridget Matikainen-Ankney, Alayna Kinkead, Brianna Brooks, Yupeng Wang, Alexis T. Franks, and et al. 2024. "Diet-Induced Obesity Induces Transcriptomic Changes in Neuroimmunometabolic-Related Genes in the Striatum and Olfactory Bulb" International Journal of Molecular Sciences 25, no. 17: 9330. https://doi.org/10.3390/ijms25179330
APA StyleJaime-Lara, R. B., Colina-Prisco, C., De Jesus Vega, M., Williams, S., Usdin, T., Matikainen-Ankney, B., Kinkead, A., Brooks, B., Wang, Y., Franks, A. T., Kravitz, A., & Joseph, P. V. (2024). Diet-Induced Obesity Induces Transcriptomic Changes in Neuroimmunometabolic-Related Genes in the Striatum and Olfactory Bulb. International Journal of Molecular Sciences, 25(17), 9330. https://doi.org/10.3390/ijms25179330






