Apoptosis–Cell Cycle–Autophagy Molecular Mechanisms Network in Heterogeneous Aggressive Phenotype Prostate Hyperplasia Primary Cell Cultures Have a Prognostic Role
Abstract
:1. Introduction
2. Results
2.1. Caspases 3/7 Activity
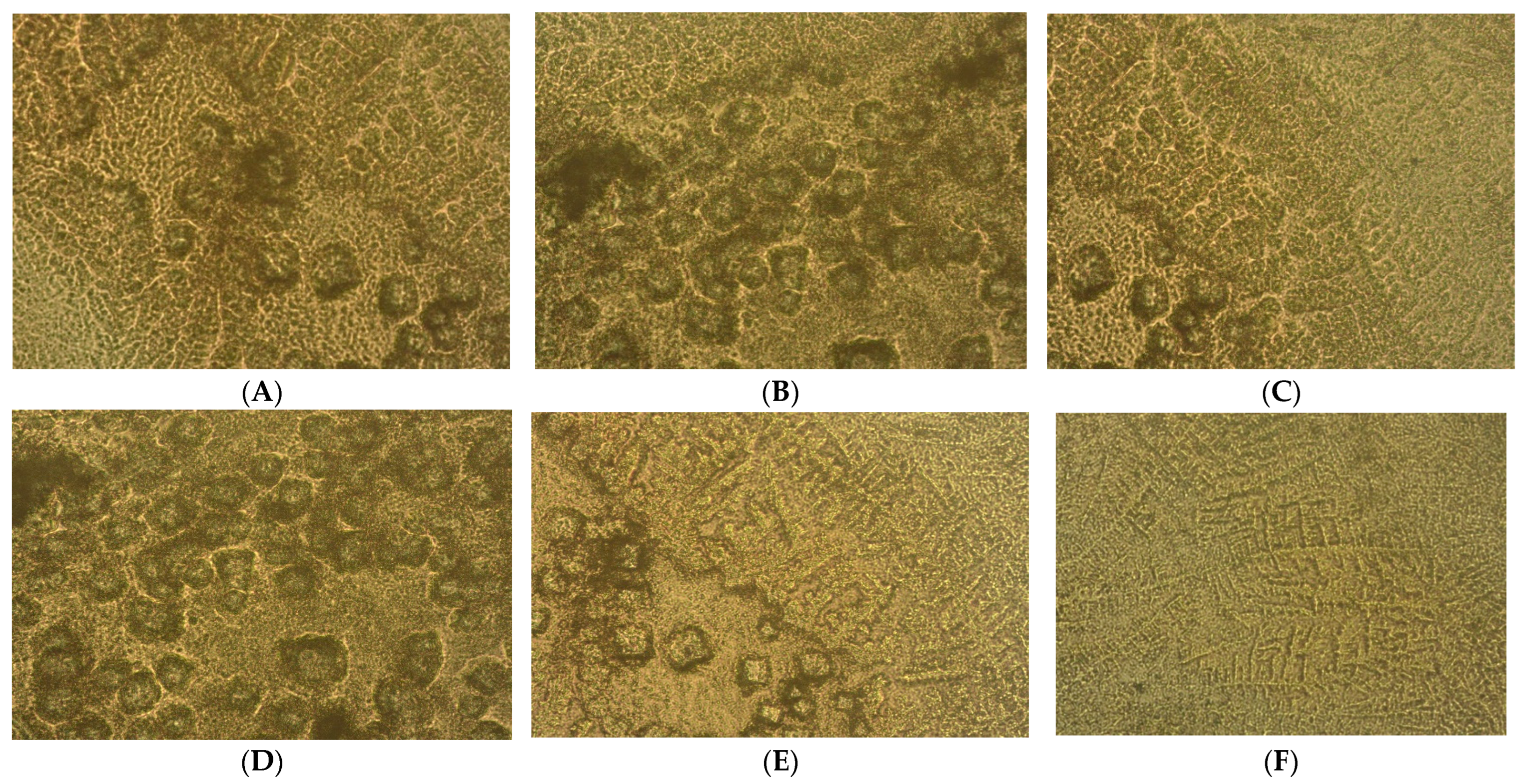
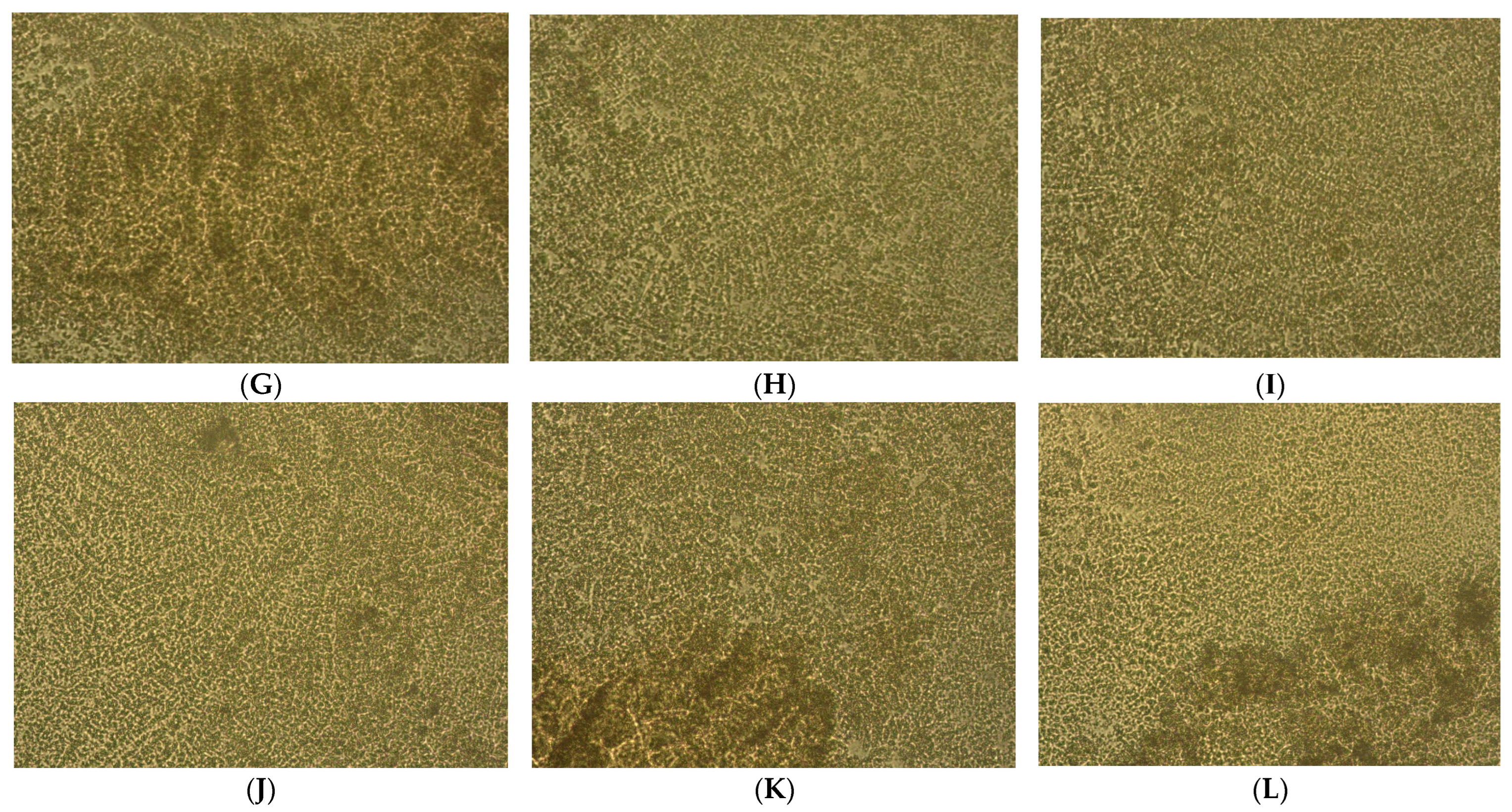
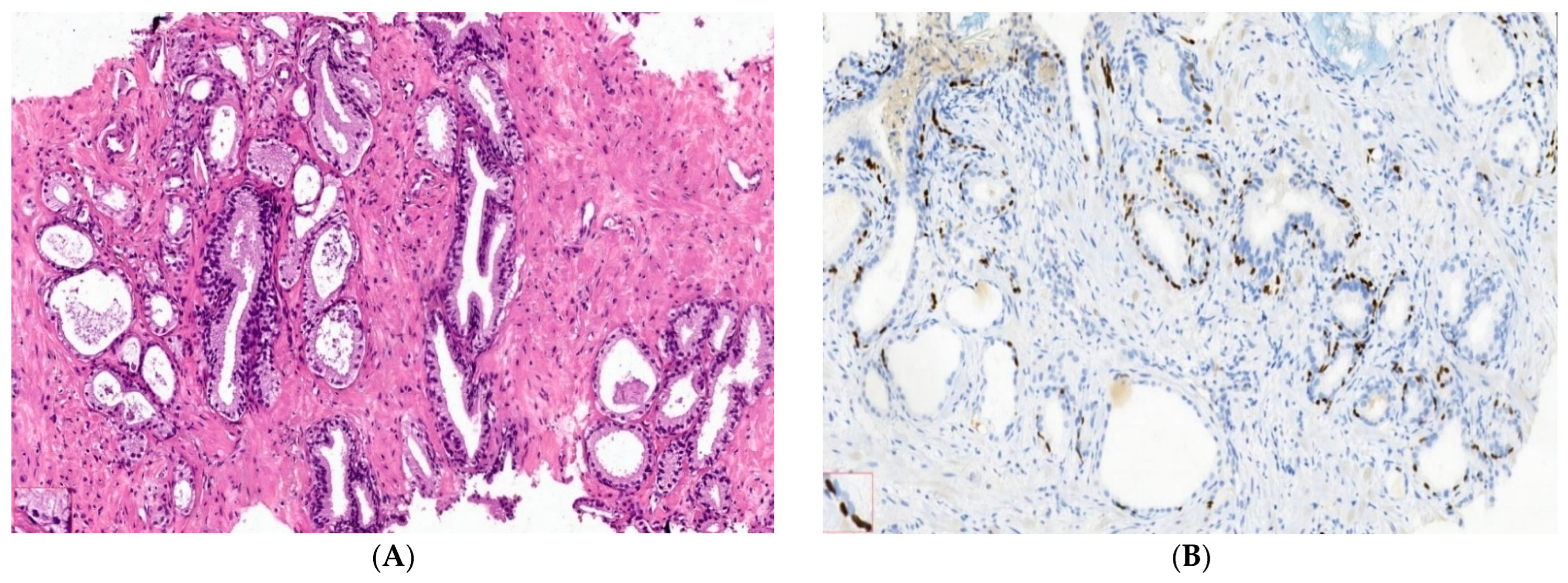
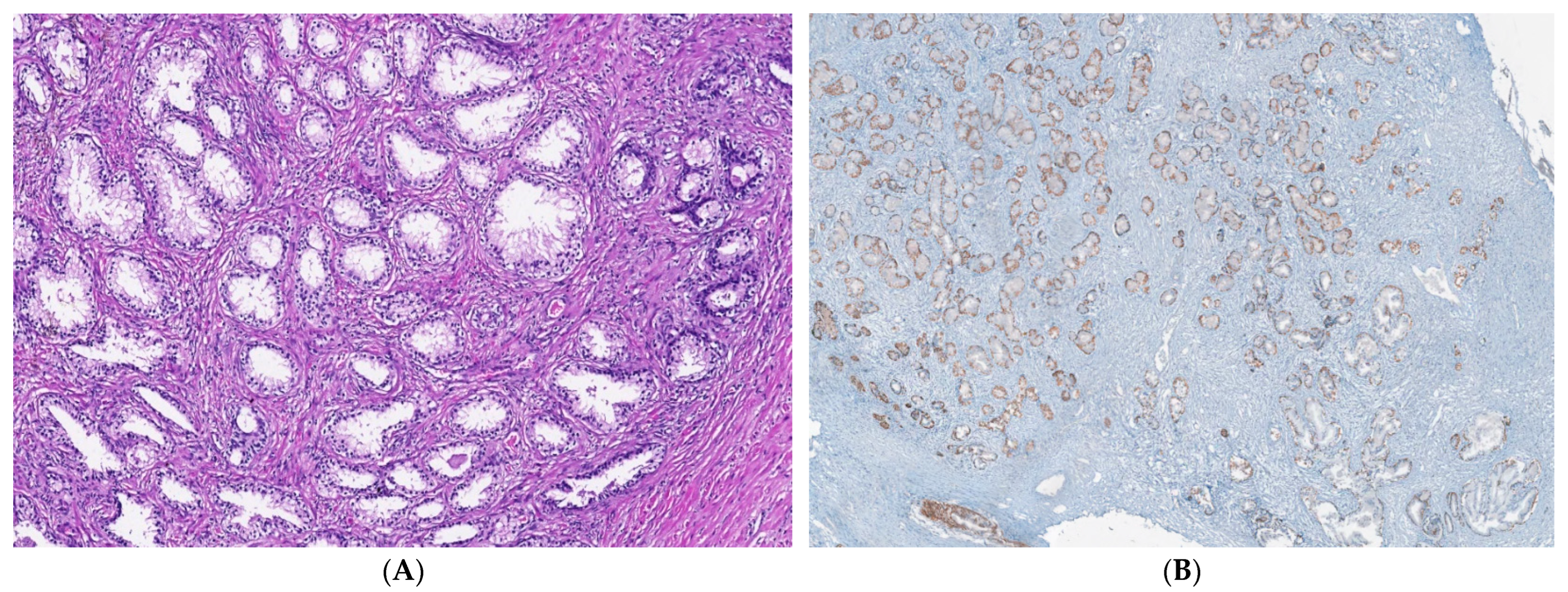
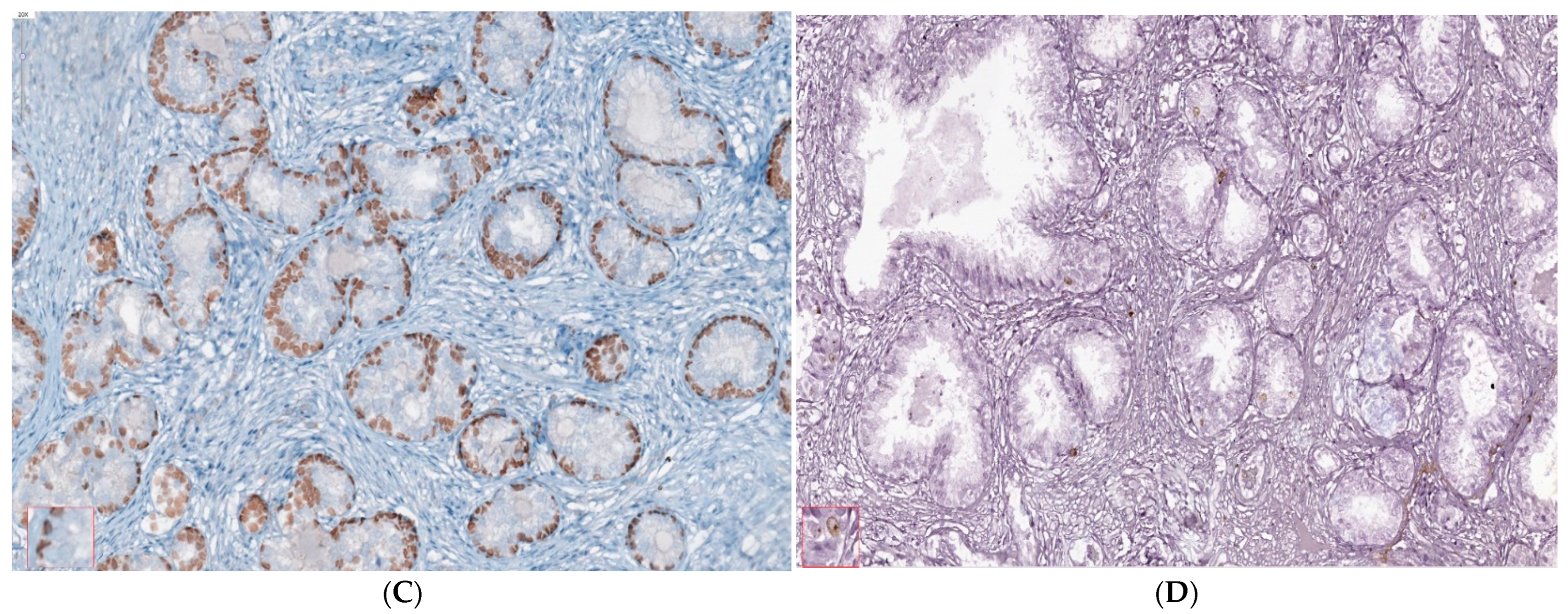
2.2. Microenvironment Characterization of Nodular Hyperplasia and Adenoma Samples
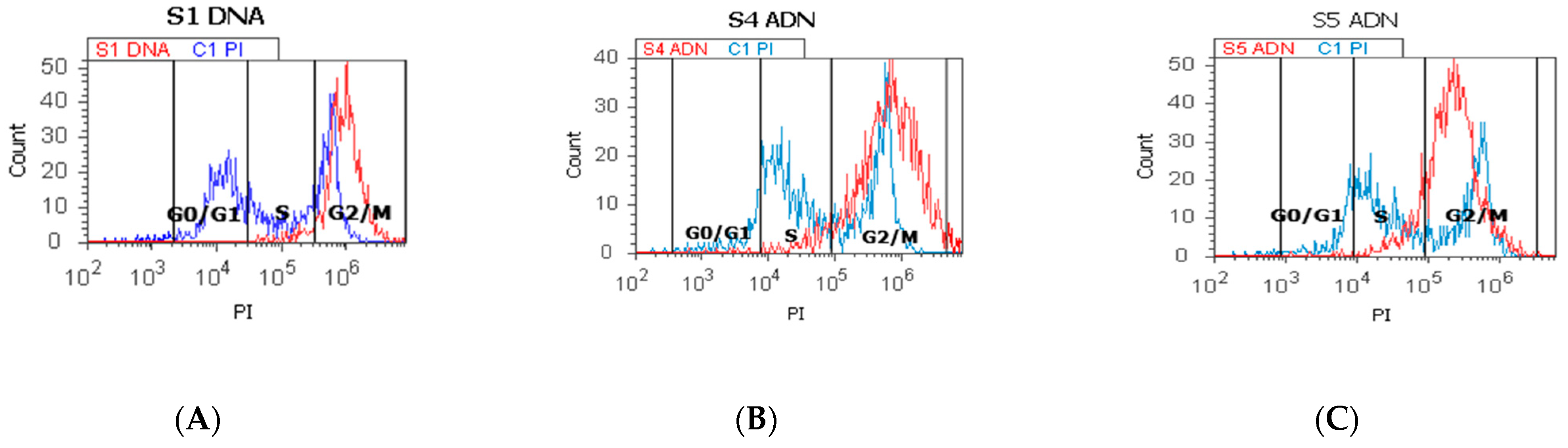
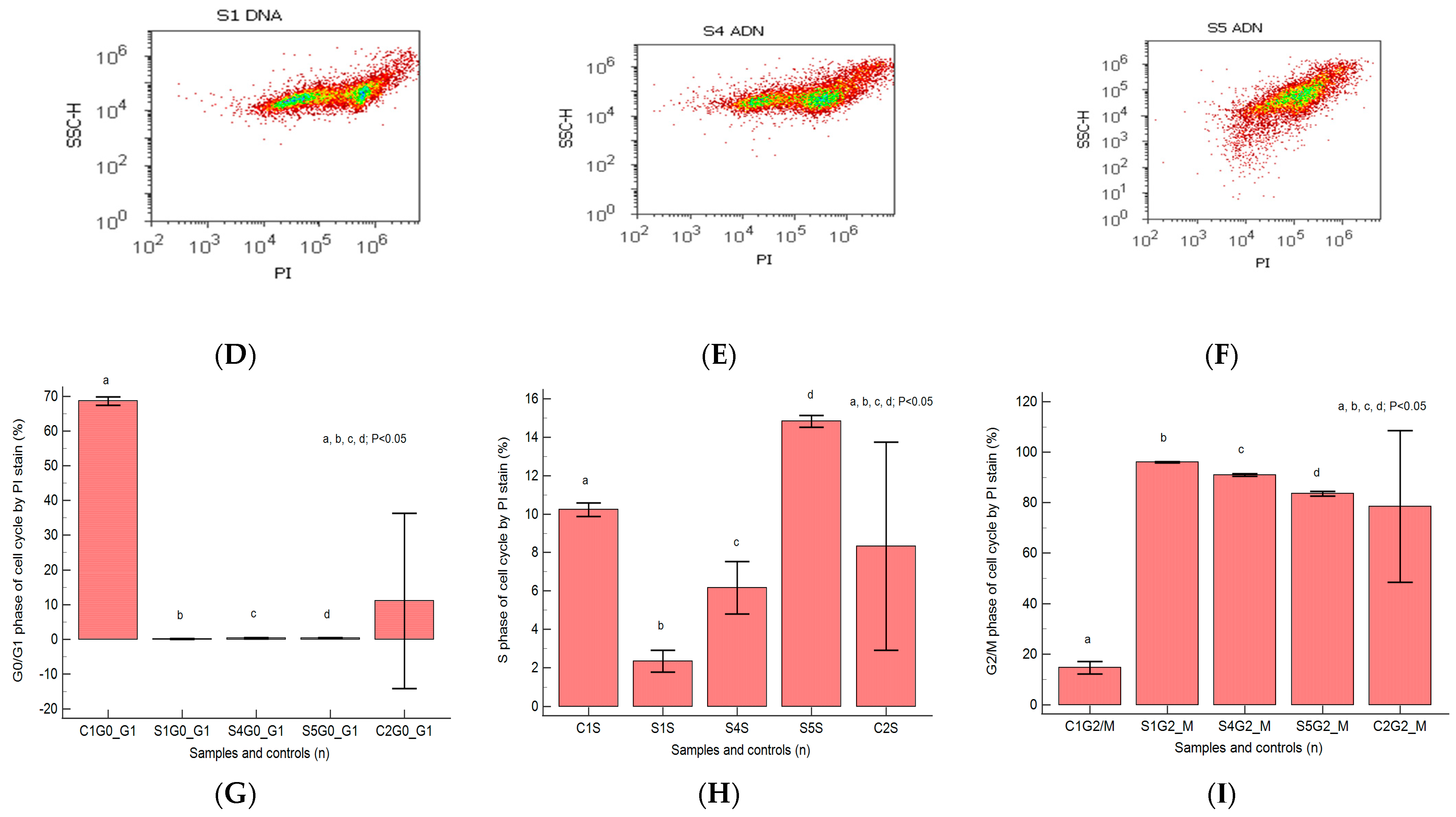
2.3. Cell Cycle
2.4. Cell Adhesion
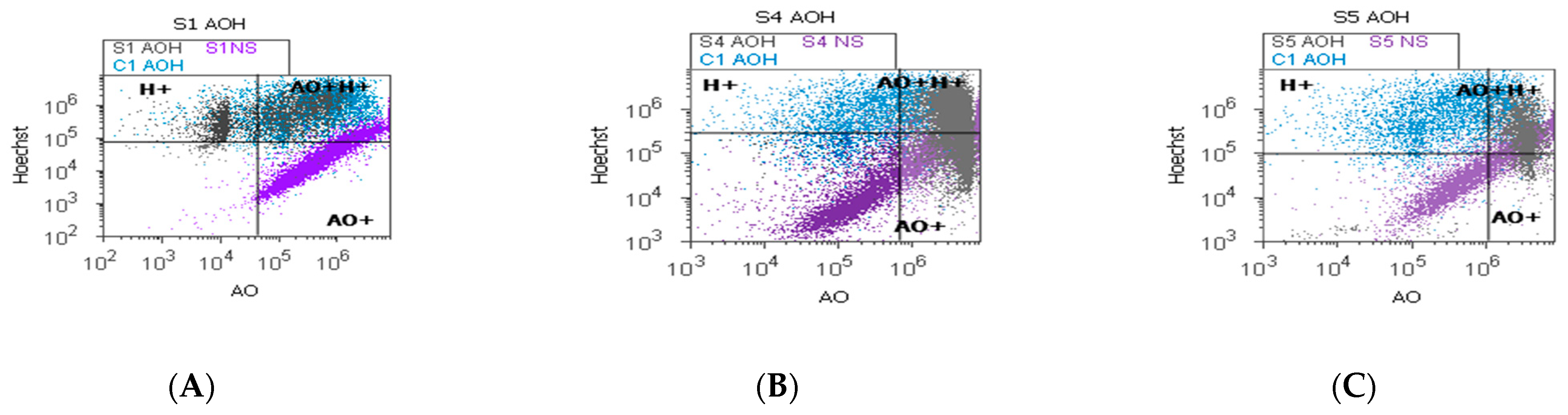
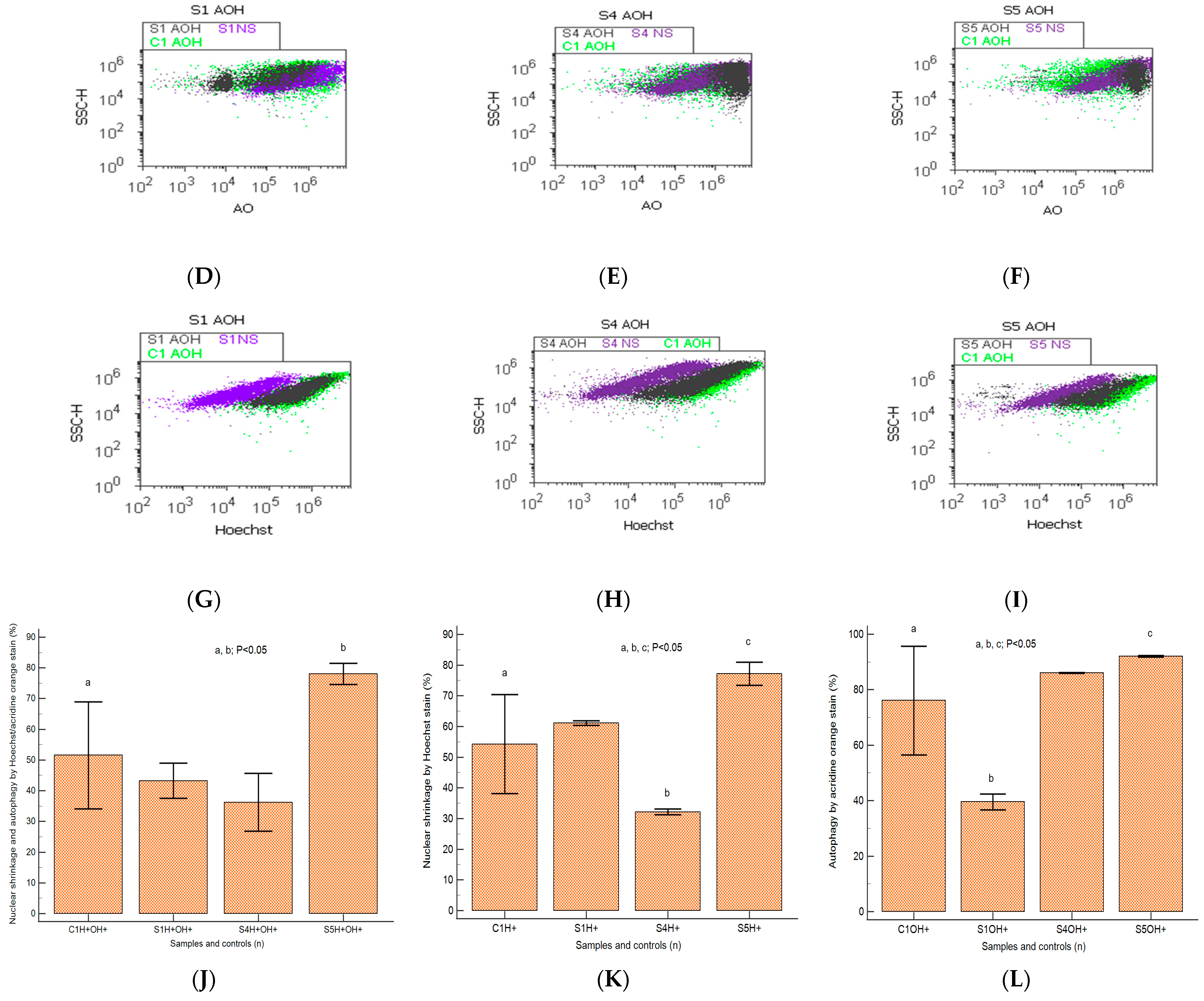
2.5. Autophagy and Nuclear Shrinkage
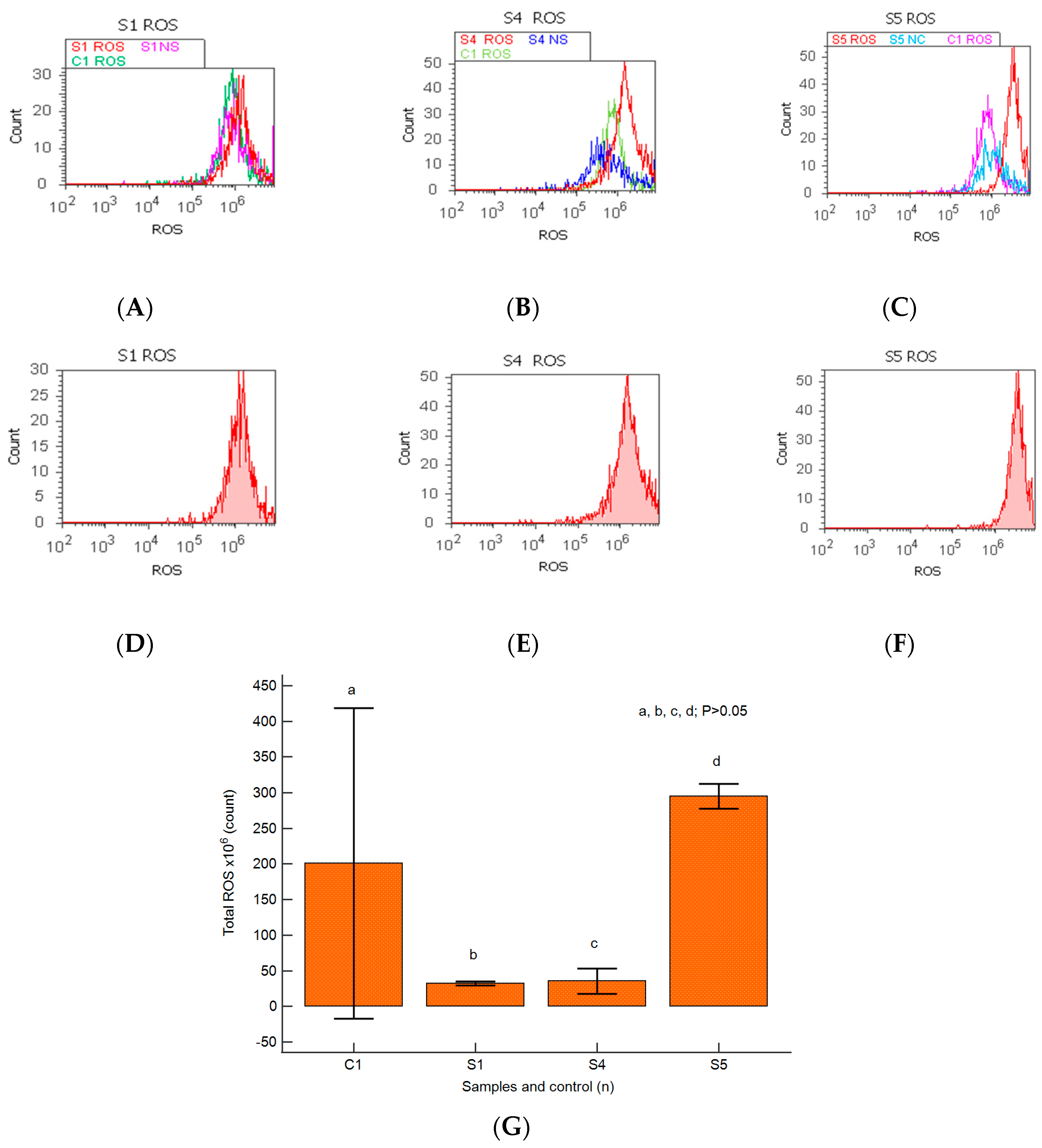
2.6. Oxidative Stress
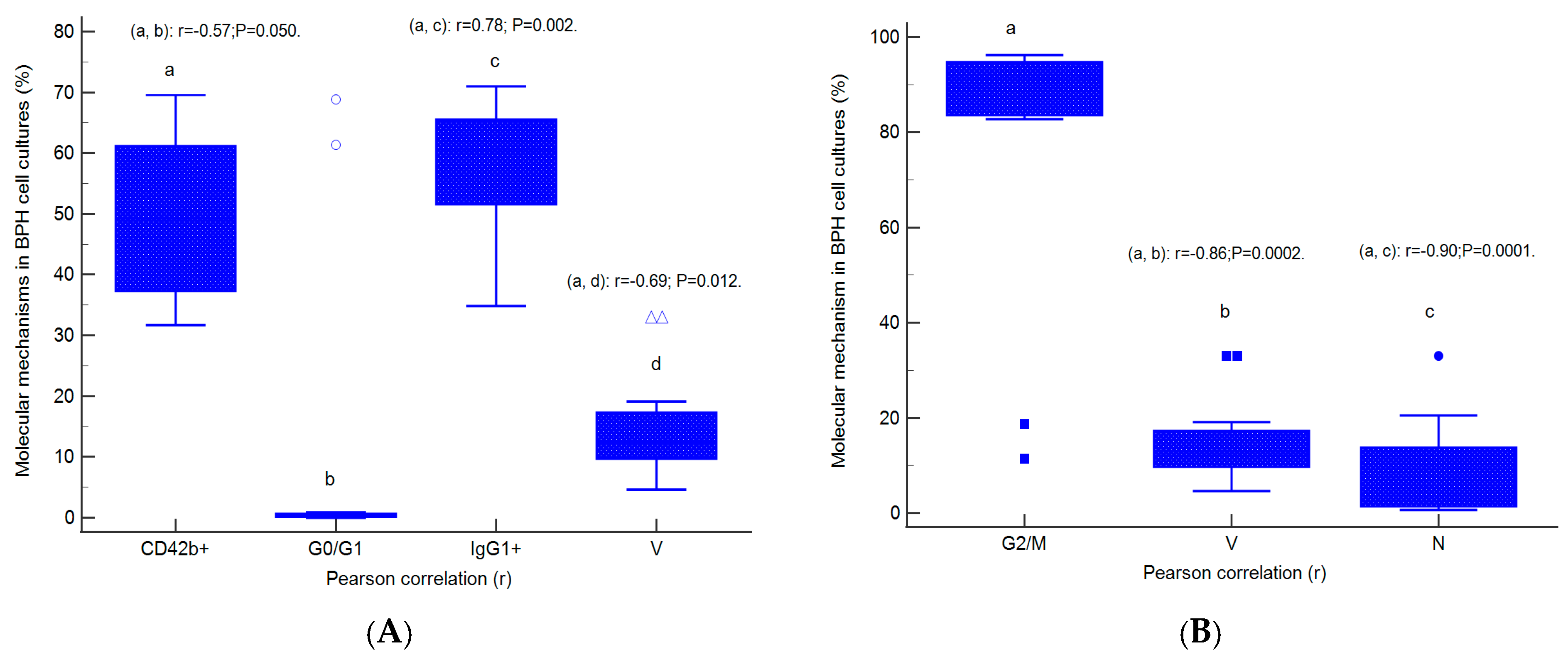
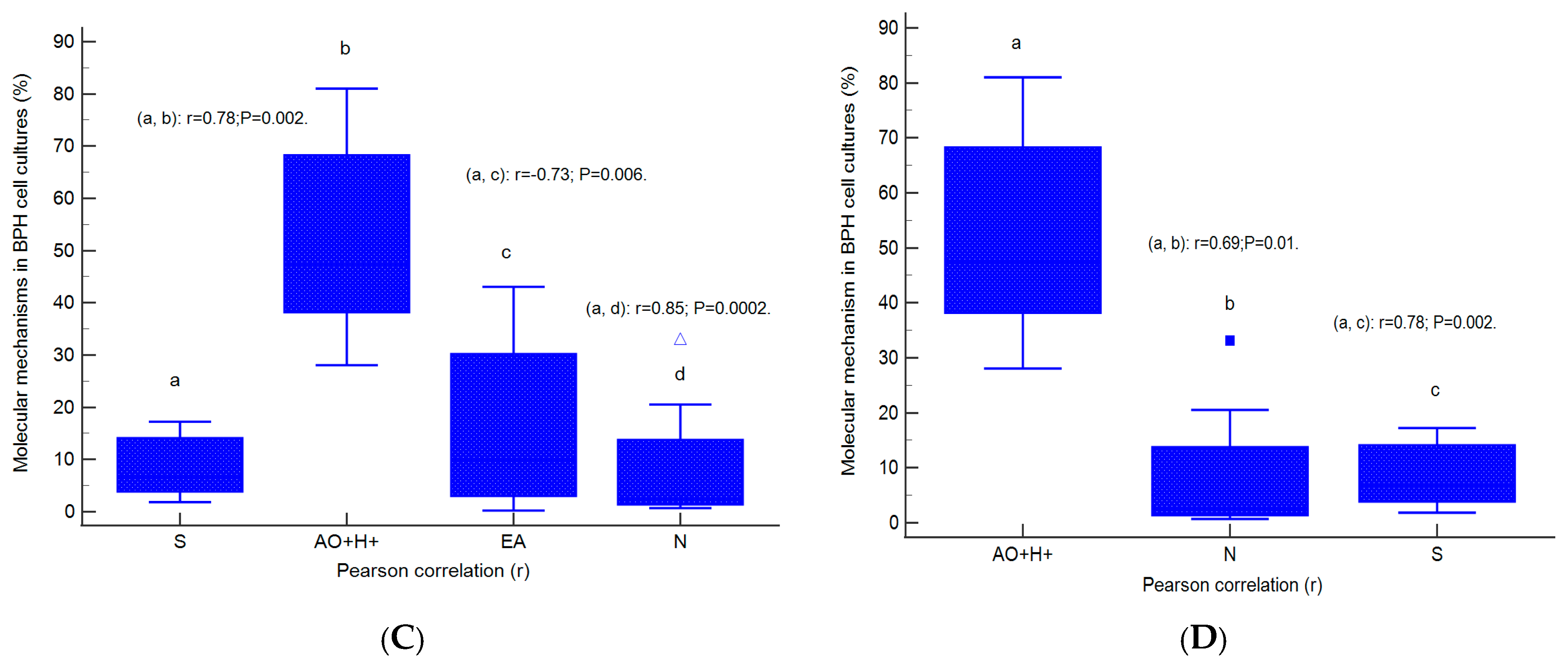
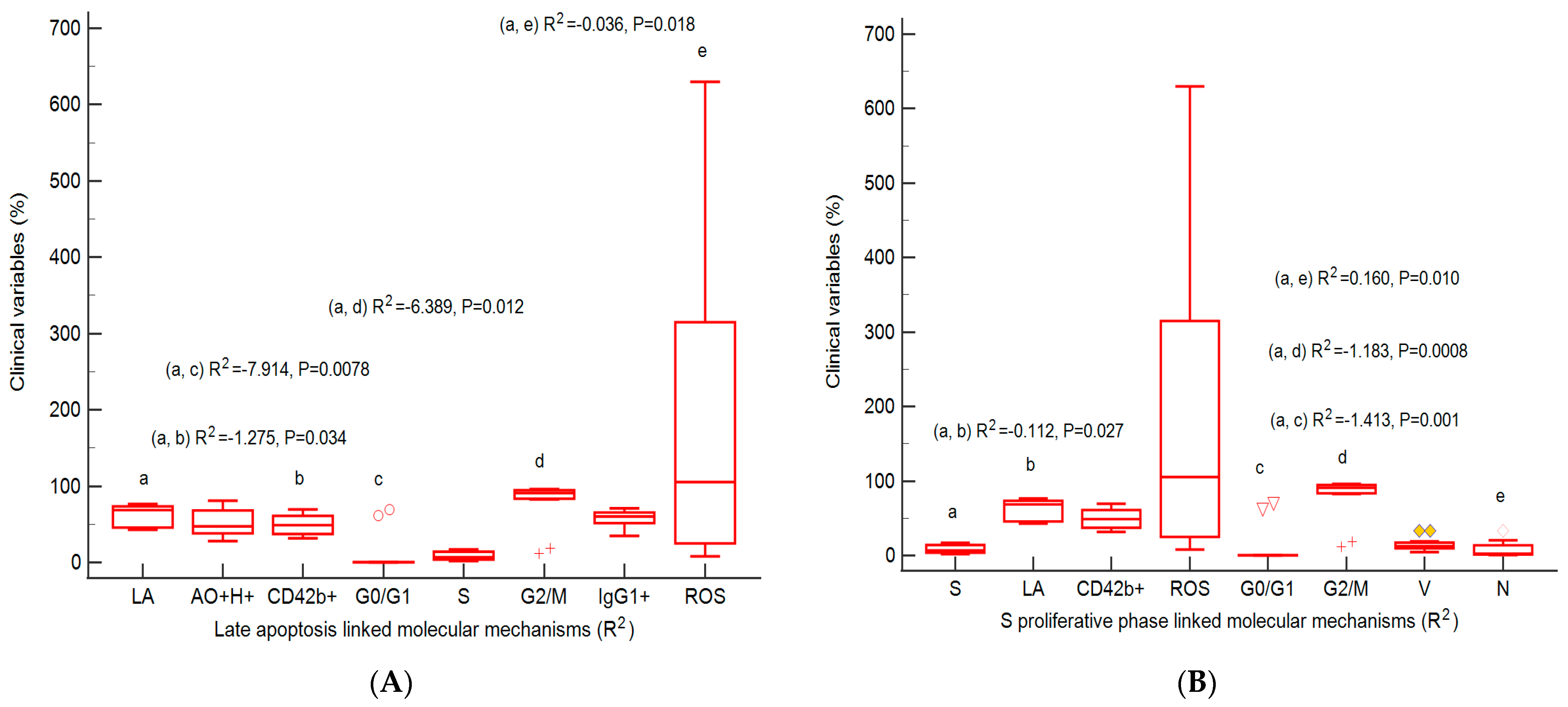
2.7. Correlations between Apoptosis, Cell Cycle, Adhesion Glycoprotein Expression, Autophagy, and Nuclear Shrinkage in Different BPH Cell Cultures with Aggressive Phenotype
2.8. Prognostic Role of Molecular Mechanisms Network in Heterogeneous BPH Cell Cultures
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Prostate Primary Cell Cultures
4.3. Samples and Controls
4.4. Nodular Hyperplasia and Adenoma Microenvironment Characterization by IHC Methods
4.5. Equipment
4.6. Methods
4.6.1. Caspase-3/7 Activity
4.6.2. Cell Cycle
4.6.3. CD42b Adhesion Glycoprotein
4.6.4. Nuclear Shrinkage and Lysosomal Activity
4.6.5. Total Reactive Oxygen Species (ROS)
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.; Cockett, A.; Cussenot, O.; Griffiths, K.; Isaacs, W.; Schalken, J. Regulation of prostate growth. In Fifth International Consultation on Benign Prostatic Hyperplasia; Chatelain, C., Denis, L., Foo, J.K.T., Eds.; Health Publications: Plymouth, UK, 2001; pp. 81–106. [Google Scholar]
- Degterev, A.; Boyce, M.; Yuan, J.A. decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef] [PubMed]
- Di Silverio, F.; Gentile, V.; De Matteis, A.; Mariotti, G.; Giuseppe, V.; Luigi, P.A.; Sciarra, A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: A retrospective analysis. Eur. Urol. 2003, 23, 164–175. [Google Scholar] [CrossRef]
- Novara, G.; Galfano, A.; Berto, R.B.; Ficarra, V.; Navarrete, R.V.; Artibani, W. Inflammation, Apoptosis, and BPH: What is the Evidence? Eur. Urol. Suppl. 2006, 5, 401–409. [Google Scholar] [CrossRef]
- De Marzo, A.M.; DeWeese, T.L.; Platz, E.A.; Meeker, A.K.; Nakayama, M.; Epstein, J.I.; Isaacs, W.B.; Nelson, W.G. Pathological and molecular mechanisms of prostate carcinogenesis: Implications for diagnosis, detection, prevention, and treatment. J. Cell Biochem. 2004, 91, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.; Korsmeyer, S. Cell death: Critical control points. Cell Prolif. 2004, 116, 205–219. [Google Scholar]
- Thangapazham, R.L.; Gaddipati, J.P.; Rajeshkumar, N.V.; Sharma, A.; Singh, A.K.; Ives, J.A.; Maheshwari, R.K.; Jonas, W.B. Homeopathic Medicines Do Not Alter Growth and Gene Expression in Prostate and Breast Cancer Cells In Vitro. Integr. Cancer Ther. 2006, 5, 356–361. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Jackson, A.L.; Loeb, L.A. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat. Res. 2001, 477, 7–21. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Bendas, G.; Borsig, L. Cancer Cell Adhesion and Metastasis: Selectins, Integrins, and the Inhibitory Potential of Heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Karp, C.; Strohecker, A.M.; Guo, Y.; Mathew, R. Role of autophagy in suppression of inflammation and cancer. Curr. Opin. Cell Biol. 2010, 22, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Kongara, S.; Beaudoin, B.; Karp, C.M.; Bray, K.; Degenhardt, K.; Chen, G.; Jin, S.; White, E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes. Dev. 2007, 21, 1367–1381. [Google Scholar] [CrossRef]
- Jin, S.; White, E. Role of autophagy in cancer: Management of metabolic stress. Autophagy 2007, 3, 28–31. [Google Scholar] [CrossRef]
- Lorin, S.; Hamai, A.; Mehrpour, M.; Codogno, P. Autophagy regulation and its role in cancer. Semin. Cancer Biol. 2013, 23, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef]
- Guo, J.Y.; Chen, H.Y.; Mathew, R.; Fan, J.; Strohecker, A.M.; Karsli-Uzunbas, G.; Kamphorst, J.J.; Chen, G.; Lemons, J.M.S.; Karantza, V.; et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes. Dev. 2011, 25, 460–470. [Google Scholar] [CrossRef]
- White, E.; DiPaola, R.S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef]
- Nakazawa, M.; Paller, C.; Kyprianou, N. Mechanisms of Therapeutic Resistance in Prostate Cancer. Curr. Oncol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A basic physiologic process in wound healing. Int. J. Low. Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Koenig, U.; Eckhart, L.; Tschachler, E. Evidence that caspase-13 is not a human but a bovine gene. Biochem. Biophys. Res. Commun. 2001, 285, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Wang, S.; Kuida, K.; Yuan, J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 2002, 9, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.-A.; Aschie, M.; Matei, E.; Cozaru, G.C.; Deacu, M.; Mitroi, A.F.; Baltatescu, G.I.; Nicolau, A.-A.; Mazilu, L.; Tuta, L.A.; et al. Characterization of the Tumor Microenvironment and the Biological Processes with a Role in Prostatic Tumorigenesis. Biomedicines 2022, 10, 1672. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Anwar, S.; Ambros, R.A.; Jennings, T.A.; Ross, J.S.; Beza, A.; Mian, B.; Nazeer, T. Expression of cysteine protease protein 32 in prostatic adenocarcinoma correlates with tumor grade. Arch. Pathol. Lab. Med. 2004, 128, 649–652. [Google Scholar] [CrossRef]
- O’Donovan, N.; Crown, J.; Stunell, H.; Hill, A.D.; McDermott, E.; O’Higgins, N.; Duffy, M.J. Caspase 3 in breast cancer. Clin. Cancer Res. 2003, 9, 738–742. [Google Scholar]
- O’Neill, A.J.; Boran, S.A.; O’Keane, C.; Coffey, R.N.; Hegarty, N.J.; Hegarty, P.; Gaffney, E.F.; Fitzpatrick, J.M.; Watson, R.W. Caspase 3 expression in benign prostatic hyperplasia and prostate carcinoma. Prostate 2001, 47, 183–188. [Google Scholar] [CrossRef]
- Sohn, J.H.; Kim, D.H.; Choi, N.G.; Park, Y.E.; Ro, J.Y. Caspase-3/CPP32 immunoreactivity and its correlation with frequency of apoptotic bodies in human prostatic carcinomas and benign nodular hyperplasias. Histopathology 2000, 37, 555–560. [Google Scholar]
- Winter, R.N.; Kramer, A.; Borkowski, A.; Kyprianou, N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001, 61, 1227–1232. [Google Scholar]
- Soung, Y.H.; Lee, J.W.; Kim, H.S.; Park, W.S.; Kim, S.Y.; Lee, J.H.; Park, J.Y.; Cho, Y.G.; Kim, C.J.; Park, Y.G.; et al. Inactivating mutations of CASPASE-7 gene in human cancers. Oncogene 2003, 22, 8048–8052. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.J.; Soung, Y.H.; Lee, S.H.; Kim, K.M.; Lee, S.H. Absence of CASP7 and CASP8 mutation in gastrointestinal lymphomas. Eur. J. Haematol. 2007, 79, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.J.; Kim, H.S.; Kim, S.Y.; Park, W.S.; Kim, S.H.; Lee, J.Y.; Lee, S.H. Stomach cancer highly expresses both initiator and effector caspases; an immunohistochemical study. Apmis 2002, 110, 825–832. [Google Scholar] [CrossRef]
- Yoo, N.J.; Lee, J.W.; Kim, Y.J.; Soung, Y.H.; Kim, S.Y.; Nam, S.W.; Park, W.S.; Lee, J.Y.; Lee, S.H. Loss of caspase-2, -6 and -7 expression in gastric cancers. Apmis 2004, 112, 330–335. [Google Scholar]
- Jäger, R.; Zwacka, M.R. The Enigmatic Roles of Caspases in Tumor Development. Cancers 2010, 2, 1952–1979. [Google Scholar] [CrossRef]
- Lin, E.Y.; Li, J.F.; Gnatovskiy, L.; Deng, Y.; Zhu, L.; Grzesik, D.A.; Qian, H.; Xue, X.N.; Pollard, J.W. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006, 66, 11238–11246. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Caraban, B.M.; Matei, E.; Cozaru, G.C.; Aşchie, M.; Deacu, M.; Enciu, M.; Bălţătescu, G.I.; Chisoi, A.; Dobrin, N.; Petcu, L.; et al. PD-L1, CD4+, and CD8+ Tumor-Infiltrating Lymphocytes (TILs) Expression Profiles in Melanoma Tumor Microenvironment Cells. J. Pers. Med. 2023, 13, 221. [Google Scholar] [CrossRef]
- Enderling, H.; Anderson, A.R.; Chaplain, M.A.; Beheshti, A.; Hlatky, L.; Hahnfeldt, P. Paradoxical dependencies of tumor dormancy and progression on basic cell kinetics. Cancer Res. 2009, 69, 8814–8821. [Google Scholar] [CrossRef]
- Gurova, K.V.; Gudkov, A.V. Paradoxical role of apoptosis in tumor progression. J. Cell Biochem. 2003, 88, 128–137. [Google Scholar] [CrossRef]
- Lauber, K.; Bohn, E.; Krober, S.M.; Xiao, Y.J.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.L.; Yu, C.T.; Yin, S.C.; Tang, M.J.; Tien, A.C.; Wu, Y.M.; Huang, C.Y. Caspase 3, periodically expressed and activated at G2/M transition, is required for nocodazole-induced mitotic checkpoint. Apoptosis 2006, 11, 765–771. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamauchi, L.; Hunter, T.; Kikkawa, U.; Kamada, S. Possible involvement of caspase-7 in cell cycle progression at mitosis. Genes. Cells 2008, 13, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, A.; Schroer, S.A.; Choi, D.; Tajmir, P.; Radulovich, N.; Ho, J.C.; Wang, L.; Liadis, N.; Hakem, R.; Tsao, M.S.; et al. Absence of caspase-3 protects pancreatic {beta}-cells from c-Myc-induced apoptosis without leading to tumor formation. J. Biol. Chem. 2009, 284, 10947–10956. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qi, F.; Li, L.; Qin, Z.; Li, X.; Wang, X. Autophagy-related genes are potential diagnostic and prognostic biomarkers in prostate cancer. Transl. Androl. Urol. 2020, 9, 2616–2628. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Choi, J.J.; Reich, C.F.; Pisetsky, D.S. Release of DNA from dead and dying lymphocyte and monocyte cell lines in vitro. Scand. J. Immunol. 2004, 60, 159–166. [Google Scholar] [CrossRef]
- Kang, R.; Zeng, L.; Zhu, S.; Xie, Y.; Liu, J.; Wen, Q.; Cao, L.; Xie, M.; Ran, Q.; Kroemer, G.; et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 2018, 24, 97–108.e104. [Google Scholar] [CrossRef]
- Canli, Ö.; Alankuş, Y.B.; Grootjans, S.; Vegi, N.; Hültner, L.; Hoppe, P.S.; Schroeder, T.; Vandenabeele, P.; Bornkamm, G.W.; Greten, F.R. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood J. Am. Soc. Hematol. 2016, 127, 139–148. [Google Scholar] [CrossRef]
- Haberzettl, P.; Hill, B.G. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013, 1, 56–64. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, N.; Jiang, N. Autophagy provides a conceptual therapeutic framework for bone metastasis from prostate cancer. Cell Death Dis. 2021, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, N.; Beretta, G.L. Nanoparticles for Ferroptosis Therapy in Cancer. Pharmaceutics 2021, 13, 1785. [Google Scholar] [CrossRef] [PubMed]
- Goodall, M.L.; Cramer, S.D.; Thorburn, A. Autophagy RIPs into cell death. Cell Cycle 2016, 15, 3014–3015. [Google Scholar] [CrossRef] [PubMed]
- Heidaryan, F.; Bamehr, H.; Babaabasi, B.; Emamvirdizadeh, A.; Mohammadzadeh, N.; Khalili, A. The Trend of ripk1/ripk3 and mlkl Mediated Necroptosis Pathway in Patients with Different Stages of Prostate Cancer as Promising Progression Biomarkers. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Zucker, R.M.; Hunter, E.S.; Rogers, J.M. Confocal laser scanning microscopy of morphology and apoptosis in organogenesis-stage mouse embryos. Methods Mol. Biol. 2000, 135, 191–202. [Google Scholar]
- Proskuryakov, S.Y.; Konoplyannikov, A.G.; Gabai, V.L. Necrosis: A specific form of programmed cell death? Exp. Cell Res. 2003, 283, 1–16. [Google Scholar] [CrossRef]
- Formigli, L.; Papucci, L.; Tani, A.; Schiavone, N.; Tempestini, A.; Orlandini, G.E.; Capaccioli, S.; Orlandini, S.Z. Aponecrosis: Morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J. Cell Physiol. 2000, 182, 41–49. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathology. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed Cell Death, from a Cancer Perspective: An Overview. Mol. Diagn. Ther. 2018, 22, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Halicka, H.D.; Zhao, H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv. Exp. Med. Biol. 2010, 676, 137–147. [Google Scholar] [PubMed]
- Duesberg, P. Does aneuploidy or mutation start cancer? Science 2005, 307, 41. [Google Scholar] [CrossRef]
- Ganem, N.J.; Storchova, Z.; Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 2007, 17, 57–162. [Google Scholar] [CrossRef]
- Zimmet, J.; Ravid, K. Polyploidy: Occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp. Hematol. 2000, 28, 3–16. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Traganos, F.; Zhao, H.; Halicka, H.D.; Li, J. Cytometry of DNA replication and RNA synthesis: Historical perspective and recent advances based on “click chemistry”. Cytom. Part. A 2011, 79, 328–337. [Google Scholar] [CrossRef]
- López-Otero, A.; Ruiz-Delgado, G.J.; Hernández-Arizpe, A.; Ruiz-Argüelles, A.; Ruiz-Argüelles, G.J. The flow-cytometric DNA content of the plasma cells of patients with multiple myeloma is a prognostic factor: A single institution experience. Hematology 2010, 15, 378–381. [Google Scholar] [CrossRef]
- Gerashchenko, B.I.; Huna, A.; Erenpreisa, J. Characterization of breast cancer DNA content profiles as a prognostic tool. Exp. Oncol. 2014, 36, 219–225. [Google Scholar]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Garcıa-Tunon, I.; Ricote, M.; Ruiz, A.; Fraile, B.; Paniagua, R.; Royuela, M. Role of tumor necrosis factor-alpha and its receptors in human benign breast lesions and tumors (in situ and infiltrative). Cancer Sci. 2006, 97, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Royuela, M.; de Miguel, M.P.; Ruiz, A.; Fraile, B.; Arenas, M.I.; Romo, E.; Paniagua, R. Interferongamma and its functional receptors overexpression in benign prostatic hyperplasia and prostatic carcinoma: Parallelism with c-myc and p53 expression. Eur. Cytokine Netw. 2000, 11, 119–127. [Google Scholar]
- Royuela, M.; de Miguel, M.P.; Bethencourt, F.R.; Sánchez-Chapado, M.; Fraile, B.; Arenas, M.I.; Paniagua, R. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J. Endocrinol. 2001, 168, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Levesque, A.A.; Eastman, A. p53-based cancer therapies: Is defective p53 the Achilles heel of the tumor? Carcinogenesis 2007, 28, 13–20. [Google Scholar] [CrossRef]
- Gartel, A.L. Is p21 an oncogene? Mol. Cancer Ther. 2006, 5, 1385–1386. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Jain, A.K.; Raina, K.; Agarwal, R. Deletion of p21/Cdkn1a confers protective effect against prostate tumorigenesis in transgenic adenocarcinoma of the mouse prostate model. Cell Cycle 2013, 12, 1598–1604. [Google Scholar] [CrossRef]
- Slingerland, J.; Pagano, M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J. Cell Physiol. 2000, 183, 10–17. [Google Scholar] [CrossRef]
- Garrett-Engele, C.M.; Tasch, M.A.; Hwang, H.C.; Fero, M.L.; Perlmutter, R.M.; Clurman, B.E.; Roberts, J.M. A mechanism mis regulating p27 in tumors discovered in a functional genomic screen. PLoS Genet. 2007, 3, e219. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, N.; Rodríguez-Berriguete, G.; Vera, R.; Fraile, B.; Olmedilla, G.; Martínez-Onsurbe, P.; Sánchez-Chapado, M.; Paniagua, R.; Royuela, M. Homeostasis: Apoptosis and cell cycle in normal and pathological prostate. Aging Male 2020, 23, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Svenson, K.B.; Longmate, W.M.; Gkirtzimanaki, K.; Sadej, R.; Wang, X.; Zhao, J.; Eliopoulos, A.G.; Berditchevski, F.; Dipersio, C.M. Suppression of integrin alpha 3 beta 1 in breast cancer cells reduces cyclooxygenase-2 gene expression and inhibits tumorigenesis, invasion, and crosstalk to endothelial cells. Cancer Res. 2010, 70, 6359–6367. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Junior, J.; Reis, S.T.; Dall’Oglio, M.; Neves de Oliveira, L.C.; Cury, J.; Carvalho, P.A.; Ribeiro-Filho, L.A.; Moreira Leite, K.R.; Srougi, M. Evaluation of the expression of integrins and cell adhesion molecules through tissue microarray in lymph node metastases of prostate cancer. J. Carcinog. 2009, 8, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rolli, M.; Fransvea, E.; Pilch, J.; Saven, A.; Felding-Habermann, B. Activated integrin alpha v beta 3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha (V) beta (3) and alpha (V) beta (5) integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar]
- Ramirez, N.E.; Zhang, Z.; Madamanchi, A.; Boyd, K.L.; O’Rear, L.D.; Nashabi, A.; Li, Z.; Dupont, W.D.; Zijlstra, A.; Zutter, M.M. The alpha (2) beta (1) integrin is a metastasis suppressor in mouse models and human cancer. J. Clin. Investig. 2011, 121, 226–237. [Google Scholar] [CrossRef]
- Hall, C.L.; Dubyk, C.W.; Riesenberger, T.A.; Shein, D.; Keller, E.T.; van Golen, K.L. Type I collagen receptor (alpha 2 beta 1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia 2008, 10, 797–803. [Google Scholar] [CrossRef]
- Goel, H.; Li, J. Integrins in Prostate Cancer Progression. Endocr. Relat. Cancer 2008, 15, 657–664. [Google Scholar] [CrossRef]
- Juan-Rivera, M.C.; Martínez-Ferrer, M. Integrin Inhibitors in Prostate Cancer. Cancers 2018, 10, 44. [Google Scholar] [CrossRef]
- Ryu, S.; Park, K.M.; Lee, S.H. Gleditsia Sinensis Thorn Attenuates the Collagen-Based Migration of PC3 Prostate Cancer Cells through the Suppression of Integrin Expression. Int. J. Mol. Sci. 2016, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, R.L.; Ryan, K.M. Autophagy in tumour cell death. Semin. Cancer Biol. 2013, 23, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Abou-Kheir, W.; Hynes, P.G.; Martin, P.; Yin, J.J.; Liu, Y.N.; Seng, V.; Lake, R.; Spurrier, J.; Kelly, K. Self-renewing Pten-/- TP53-/- protospheres produce metastatic adenocarcinoma cell lines with multipotent progenitor activity. PLoS ONE 2011, 6, e26112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J. Cell Sci. 2007, 120, 4155–4166. [Google Scholar] [CrossRef]
- Maiti, K.; Oh, D.Y.; Moon, J.S.; Acharjee, S.; Li, J.H.; Bai, D.G.; Park, H.; Lee, K.; Lee, Y.C.; Jung, N.C.; et al. Differential effects of gonadotropin-releasing hormone (GnRH)-I and GnRH-II on prostate cancer cell signaling and death. J. Clin. Endocrinol. Metab. 2005, 90, 4287–4298. [Google Scholar] [CrossRef]
- Kim, D.K.; Yang, J.S.; Maiti, K.; Hwang, J.I.; Kim, K.; Seen, D.; Ahn, Y.; Lee, C.; Kang, B.C.; Kwon, H.B.; et al. A gonadotropin releasing hormone-II antagonist induces autophagy of prostate cancer cells. Cancer Res. 2009, 69, 923–931. [Google Scholar] [CrossRef]
- Piacentini, M.; Evangelisti, C.; Mastroberardino, P.G.; Nardacci, R.; Kroemer, G. Does prothymosin-alpha act as molecular switch between apoptosis and autophagy? Cell Death Differ. 2003, 10, 937–939. [Google Scholar] [CrossRef]
- Zeiss, C.J. The apoptosis-necrosis continuum: Insights from genetically altered mice. Vet. Pathol. 2003, 40, 481–495. [Google Scholar] [CrossRef]
- Elmore, S.P.; Qian, T.; Grissom, S.F.; Lemasters, J.J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001, 15, 2286–2287. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004, 23, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Plati, J.; Bucur, O.; Khosravi, F.R. Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. 2011, 3, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.J.; Green, D.R. Mitochondria in cell death. Essays Biochem. 2010, 47, 99–114. [Google Scholar]
- Matei, E.; Aschie, M.; Mitroi, A.F.; Ghinea, M.M.; Gheorghe, E.; Petcu, L.; Dobrin, N.; Chisoi, A.; Manea, M. Biomarkers involved in evaluation of platelets function in South-Eastern Romanian patients with hematological malignancies subtypes. Medicine 2021, 100, e25944. [Google Scholar] [CrossRef]
- Popovici, V.; Musuc, A.M.; Matei, E.; Karampelas, O.; Ozon, E.A.; Cozaru, G.C.; Schröder, V.; Bucur, L.; Aricov, L.; Anastasescu, M.; et al. ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 14836. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Mitu, M.A.; Musuc, A.M.; Petrescu, S.; et al. Design, Characterization, and Anticancer and Antimicrobial Activities of Mucoadhesive Oral Patches Loaded with Usnea barbata (L.) F. H. Wigg Ethanol Extract F-UBE-HPMC. Antioxidants 2022, 11, 1801. [Google Scholar] [CrossRef]
- Dumitru, A.; Matei, E.; Cozaru, G.C.; Chisoi, A.; Alexandrescu, L.; Popescu, R.C.; Pundiche Butcaru, M.; Dumitru, E.; Rugină, S.; Tocia, C. Endotoxin Inflammatory Action on Cells by Dysregulated Immunological-Barrier-Linked ROS-Apoptosis Mechanisms in Gut–Liver Axis. Int. J. Mol. Sci. 2024, 25, 2472. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, E.; Enciu, M.; Roșu, M.C.; Voinea, F.; Mitroi, A.F.; Deacu, M.; Băltățescu, G.I.; Nicolau, A.-A.; Chisoi, A.; Aşchie, M.; et al. Apoptosis–Cell Cycle–Autophagy Molecular Mechanisms Network in Heterogeneous Aggressive Phenotype Prostate Hyperplasia Primary Cell Cultures Have a Prognostic Role. Int. J. Mol. Sci. 2024, 25, 9329. https://doi.org/10.3390/ijms25179329
Matei E, Enciu M, Roșu MC, Voinea F, Mitroi AF, Deacu M, Băltățescu GI, Nicolau A-A, Chisoi A, Aşchie M, et al. Apoptosis–Cell Cycle–Autophagy Molecular Mechanisms Network in Heterogeneous Aggressive Phenotype Prostate Hyperplasia Primary Cell Cultures Have a Prognostic Role. International Journal of Molecular Sciences. 2024; 25(17):9329. https://doi.org/10.3390/ijms25179329
Chicago/Turabian StyleMatei, Elena, Manuela Enciu, Mihai Cătălin Roșu, Felix Voinea, Anca Florentina Mitroi, Mariana Deacu, Gabriela Isabela Băltățescu, Antonela-Anca Nicolau, Anca Chisoi, Mariana Aşchie, and et al. 2024. "Apoptosis–Cell Cycle–Autophagy Molecular Mechanisms Network in Heterogeneous Aggressive Phenotype Prostate Hyperplasia Primary Cell Cultures Have a Prognostic Role" International Journal of Molecular Sciences 25, no. 17: 9329. https://doi.org/10.3390/ijms25179329





