Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration
Abstract
1. Introduction
2. Folate-Dependent One-Carbon Metabolism: An Overview
3. Sources of Dietary Folate and Its Bioavailability
| Natural Food Sources of Folate | Folate Content (µg/100 g) | % of Recommended Daily Intake (per 100 g) 1 | Reference | |
|---|---|---|---|---|
| Leafy green vegetables | Spinach | 194 | 48.5 | [41] |
| Collard greens | 129 | 32.2 | [42] | |
| Kale | 62 | 15.5 | [43] | |
| Swiss chard | 14 | 3.5 | [44] | |
| Legumes | Lentils | 181 | 45.2 | [45] |
| Chickpeas | 172 | 43.0 | [46] | |
| Beans | 149 | 37.2 | [47] | |
| Peas | 65 | 16.2 | [48] | |
| Fruits | Avocado | 81 | 20.2 | [49] |
| Strawberries | 50 | 12.5 | [50] | |
| Papaya | 37 | 9.2 | [51] | |
| Oranges | 30 | 7.5 | [52] | |
| Whole grains | Quinoa | 42 | 10.5 | [53] |
| Oats | 32 | 8.0 | [54] | |
| Barley | 19 | 4.8 | [55] | |
| Brown rice | 9 | 2.2 | [56] | |
4. Molecular Regulation of Folate Metabolism
4.1. Transcriptional, Translational, and Post-Transcriptional Regulation of Key Enzymes
4.2. Influence of Genetic Polymorphisms and Epigenetic Modifications
5. Folate Metabolism and Cancer
5.1. The Importance of Folate Metabolism in Cell Division and Its Dysfunctional Regulation in Cancer
5.2. Pharmacological Agents Targeting Folate Metabolism in Cancer
| Antifolate Drug | Mechanisms of Action | Clinical Applications | Observations | References |
|---|---|---|---|---|
| Methotrexate | Inhibits DHFR, TYMS, AICART, and amido phosphoribosylltransferase | Various types of cancer | Rapid development of resistance mechanisms by cancer cells | [194] |
| Pralatrexate | Inhibits DHFR | Relapsed or refractory peripheral T-cell lymphoma | Patients should receive folic acid vitamin B12 supplementation | [195,196] |
| Pemetrexed | Inhibits TYMS, THF reductase, and glycinamide ribonucleotide formyltransferase | Non-squamous non-small cell lung cancer | Effective treatment | [197] |
| Raltitrexed | Inhibits TYMS | Advanced colorectal cancer | Effective substitute for 5-FU in metastatic gastric cancer | [198,215] |
| 5-FU | Inhibits TYMS | Colorectal cancer | Response rates of 60-65% | [199,200] |
| 6-MP | Inhibits de novo purine synthesis | Various types of cancer | Used in combination with methotrexate | [201] |
5.3. Preclinical and Clinical Studies Evaluating the Efficacy and Safety of Folate-Targeted Therapies in Cancer
5.4. Impact of Dietary Modifications on Cancer Risk
6. Folate Metabolism and Neurodegeneration
6.1. Roles of Folate Metabolism in Neural Tube Formation, Neuronal Function, and Neurotransmitter Synthesis
6.2. Folate Metabolism in Neurodegeneration
6.3. The Role of Folic Acid Supplementation in Neurodegeneration
6.4. The Role of Combined Supplementation with Folic Acid and Other B Vitamins in Neurodegeneration
6.5. Folate Supplementation Can Reduce Heart Damage Induced by Alzheimer’s Disease
6.6. Pharmacological Agents Targeting Folate Metabolism in Neurodegeneration
7. Concluding Remarks
8. Future Perspectives and Current Therapeutic Challenges
8.1. Resistance Mechanisms to Antifolate Compounds
8.2. Side Effects of Folate-Targeted Therapies
8.3. Targeted Therapies and Delivery Systems
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- Petrova, B.; Maynard, A.G.; Wang, P.; Kanarek, N. Regulatory mechanisms of one-carbon metabolism enzymes. J. Biol. Chem. 2023, 299, 105457. [Google Scholar] [CrossRef]
- Lionaki, E.; Ploumi, C.; Tavernarakis, N. One-carbon metabolism: Pulling the strings behind aging and neurodegeneration. Cells 2022, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Silva, J.A.; Scarpellini, F.; Capucci, R.; Marci, R.; Gallo, I.; Salvatori, F.; D’Aversa, E.; Secchiero, P.; Serino, M.L.; et al. Epigenetic role of LINE-1 methylation and key genes in pregnancy maintenance. Sci. Rep. 2024, 14, 3275. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.L.; Menelaou, K.; Rakoczy, J.; Tan, X.S.; Watson, E.D. Disruption of folate metabolism causes poor alignment and spacing of mouse conceptuses for multiple generations. Front. Cell Dev. Biol. 2021, 9, 723978. [Google Scholar] [CrossRef]
- Li, X.; Pei, P.; Shen, J.; Yu, J.; Wang, F.; Wang, L.; Liu, C.; Wang, S. Folate deficiency reduced aberrant level of DOT1L-mediated histone H3K79 methylation causes disruptive SHH gene expression involved in neural tube defects. Epigenet. Chromatin 2023, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Ren, B.; Fang, Y.; Ren, J.; Liu, X.; Wang, X.; Zhou, F.; Xiao, R.; Luo, X.; You, L.; et al. Epigenetic regulation in cancer. MedComm 2024, 5, e495. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet 2021, 37, 1012–1027. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Chen, Y.; Oyang, L.; Lin, J.; et al. Metabolic reprogramming and epigenetic modifications in cancer: From the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 2023, 55, 1357–1370. [Google Scholar] [CrossRef]
- Wijerathne, C.U.B.; Au-Yeung, K.K.W.; Siow, Y.L.; O, K. 5-Methyltetrahydrofolate attenuates oxidative stress and improves kidney function in acute kidney injury through activation of Nrf2 and antioxidant defense. Antioxidants 2022, 11, 1046. [Google Scholar] [CrossRef]
- Bahmani, F.; Karamali, M.; Shakeri, H.; Asemi, Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Clin. Endocrinol. 2014, 81, 582–587. [Google Scholar] [CrossRef]
- Asemi, Z.; Vahedpoor, Z.; Jamilian, M.; Bahmani, F.; Esmaillzadeh, A. Effects of long-term folate supplementation on metabolic status and regression of cervical intraepithelial neoplasia: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 681–686. [Google Scholar] [CrossRef]
- Quevedo-Ocampo, J.; Escobedo-Calvario, A.; Souza-Arroyo, V.; Miranda-Labra, R.U.; Bucio-Ortiz, L.; Gutiérrez-Ruiz, M.C.; Chávez-Rodríguez, L.; Gomez-Quiroz, L.E. Folate metabolism in hepatocellular carcinoma. What do we know so far? Technol. Cancer Res. Treat. 2022, 21, 15330338221144446. [Google Scholar] [CrossRef]
- He, Y.; Gao, M.; Tang, H.; Cao, Y.; Liu, S.; Tao, Y. Metabolic intermediates in tumorigenesis and progression. Int. J. Biol. Sci. 2019, 15, 1187–1199. [Google Scholar] [CrossRef]
- Tarragó-Celada, J.; Foguet, C.; Tarrado-Castellarnau, M.; Marin, S.; Hernández-Alias, X.; Perarnau, J.; Morrish, F.; Hockenbery, D.; Gomis, R.R.; Ruppin, E.; et al. Cysteine and folate metabolism are targetable vulnerabilities of metastatic colorectal cancer. Cancers 2021, 13, 725. [Google Scholar] [CrossRef]
- Mahmood, K.; Emadi, A. 1-C metabolism-serine, glycine, folates-in acute myeloid leukemia. Pharmaceuticals 2021, 14, 190. [Google Scholar] [CrossRef]
- Delhey, L.M.; Nur Kilinc, E.; Yin, L.; Slattery, J.C.; Tippett, M.L.; Rose, S.; Bennuri, S.C.; Kahler, S.G.; Damle, S.; Legido, A.; et al. The Effect of mitochondrial supplements on mitochondrial activity in children with autism spectrum disorder. J. Clin. Med. 2017, 6, 18. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Chou, L.S.; Lin, C.H.; Wu, H.C.; Li, D.J.; Tseng, P.T. Serum folate levels in bipolar disorder: A systematic review and meta-analysis. BMC Psychiatry 2019, 19, 305. [Google Scholar] [CrossRef]
- Zheng, W.; Li, W.; Qi, H.; Xiao, L.; Sim, K.; Ungvari, G.S.; Lu, X.B.; Huang, X.; Ning, Y.P.; Xiang, Y.T. Adjunctive folate for major mental disorders: A systematic review. J. Affect. Disord. 2020, 267, 123–130. [Google Scholar] [CrossRef]
- Wang, F.; Wu, K.; Li, Y.; Song, R.; Wu, Y.; Zhang, X.; Song, M.; Liang, L.; Smith-Warner, S.A.; Giovannucci, E.L.; et al. Association of folate intake and colorectal cancer risk in the postfortification era in US women. Am. J. Clin. Nutr. 2021, 114, 49–58. [Google Scholar] [CrossRef]
- Chen, P.; Li, C.; Li, X.; Li, J.; Chu, R.; Wang, H. Higher dietary folate intake reduces the breast cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2327–2338. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Hebert, L.E.; Scherr, P.A.; Schneider, J.A. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch. Neurol. 2005, 62, 641–645. [Google Scholar] [CrossRef]
- Kronenberg, G.; Harms, C.; Sobol, R.W.; Cardozo-Pelaez, F.; Linhart, H.; Winter, B.; Balkaya, M.; Gertz, K.; Gay, S.B.; Cox, D.; et al. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J. Neurosci. 2008, 28, 7219–7230. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, X.; Li, Q.; Zhao, J.; Song, A.; An, P.; Du, Y.; Xu, W.; Huang, G. Effects of folic acid and vitamin B12, alone and in combination on cognitive function and inflammatory factors in the elderly with mild cognitive impairment: A single-blind experimental design. Curr. Alzheimer Res. 2019, 16, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Bou Ghanem, A.; Hussayni, Y.; Kadbey, R.; Ratel, Y.; Yehya, S.; Khouzami, L.; Ghadieh, H.E.; Kanaan, A.; Azar, S.; Harb, F. Exploring the complexities of 1C metabolism: Implications in aging and neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1322419. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed]
- Zarou, M.M.; Vazquez, A.; Vignir Helgason, G. Folate metabolism: A re-emerging therapeutic target in haematological cancers. Leukemia 2021, 35, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, H.; Kobayashi, T.; Brenner, D. The emerging role of one-carbon metabolism in T cells. Curr. Opin. Biotechnol. 2021, 68, 193–201. [Google Scholar] [CrossRef]
- Suh, E.; Choi, S.-W.; Friso, S. Chapter 36—One-Carbon Metabolism: An Unsung Hero for Healthy Aging. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 513–522. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- ChemSketch, Version 2023.2.4, Advanced Chemistry Development, Inc. (ACD/Labs), Toronto, ON, Canada. Available online: www.acdlabs.com (accessed on 16 August 2024).
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Liu, Y.; Liu, L.; Li, J.; Du, G.; Chen, J. Synthetic biology-driven microbial production of folates: Advances and perspectives. Bioresour. Technol. 2021, 324, 124624. [Google Scholar] [CrossRef]
- Herrera-Araujo, D. Folic acid advisories: A public health challenge? Health Econ. 2016, 25, 1104–1122. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.M.; Guyot, J.-P.; Humblot, C. Lactic acid fermentation as a tool for increasing the folate content of foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Eljazzar, S.; Ganji, V. Intended and unintended benefits of folic acid fortification—A narrative review. Foods 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Lavriša, Ž.; Hribar, M.; Hristov, H.; Kvarantan, N.; Seljak, B.K.; Gregorič, M.; Blaznik, U.; Gregorič, N.; Zaletel, K.; et al. Dietary intake of folate and assessment of the folate deficiency prevalence in slovenia using serum biomarkers. Nutrients 2021, 13, 3860. [Google Scholar] [CrossRef]
- Castenmiller, J.J.; van de Poll, C.J.; West, C.E.; Brouwer, I.A.; Thomas, C.M.; van Dusseldorp, M. Bioavailability of folate from processed spinach in humans. Effect of food matrix and interaction with carotenoids. Ann. Nutr. Metab. 2000, 44, 163–169. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- The United States Department of Agriculture. Spinach, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168462/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Collards, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170406/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Kale, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/323505/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Chard, Swiss, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169991/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Lentils, Mature Seeds, Cooked, Boiled, without Salt. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/172421/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Chickpeas (Garbanzo Beans, Bengal Gram), Mature Seeds, Cooked, Boiled, with Salt. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173799/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Beans, Black, Mature Seeds, Cooked, Boiled, without Salt. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173735/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Peas, Green, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170419/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Avocado, Raw. 2020. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1102652/nutrients (accessed on 15 August 2024).
- Strålsjö, L.M.; Witthöft, C.M.; Sjöholm, I.M.; Jägerstad, M.I. Folate content in strawberries (Fragaria x ananassa): Effects of cultivar, ripeness, year of harvest, storage, and commercial processing. J. Agric. Food Chem. 2003, 51, 128–133. [Google Scholar] [CrossRef]
- Striegel, L.; Weber, N.; Dumler, C.; Chebib, S.; Netzel, M.E.; Sultanbawa, Y.; Rychlik, M. Promising tropical fruits high in folates. Foods 2019, 8, 363. [Google Scholar] [CrossRef]
- The United States Department of Agriculture. Oranges, Raw, All Commercial Varieties. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169097/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Quinoa, Cooked. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168917/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Oats, Raw. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1101825/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Barley, Hulled. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170283/nutrients (accessed on 15 August 2024).
- The United States Department of Agriculture. Rice, Brown, Cooked, No Added Fat. 2020. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1101631/nutrients (accessed on 15 August 2024).
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, USA, 1998; p. 592. [CrossRef]
- Netzel, M.E.; Dumler, C.; Weber, N.; Striegel, L.; Rychlik, M.; Hong, H.T.; O’Hare, T. The inside and out of folate in strawberries and avocados. Proceedings 2019, 36, 86. [Google Scholar]
- Jones, J.M.; Engleson, J. Whole grains: Benefits and challenges. Annu. Rev. Food Sci. Technol. 2010, 1, 19–40. [Google Scholar] [CrossRef]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef]
- Martinez, H.; Pachón, H.; Kancherla, V.; Oakley, G.P. Food fortification with folic acid for prevention of spina bifida and anencephaly: The need for a paradigm shift in evidence evaluation for policy-making. Am. J. Epidemiol. 2021, 190, 1972–1976. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The concept of folic acid in health and disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- van het Hof, K.H.; Tijburg, L.B.; Pietrzik, K.; Weststrate, J.A. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br. J. Nutr. 1999, 82, 203–212. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Starowicz, M. Effect of different cooking methods on the folate content, organoleptic and functional properties of broccoli and spinach. LWT 2022, 167, 113825. [Google Scholar] [CrossRef]
- Devlin, A.M.; Ling, E.H.; Peerson, J.M.; Fernando, S.; Clarke, R.; Smith, A.D.; Halsted, C.H. Glutamate carboxypeptidase II: A polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum. Mol. Genet 2000, 9, 2837–2844. [Google Scholar] [CrossRef]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef]
- Afman, L.A.; Trijbels, F.J.; Blom, H.J. The H475Y polymorphism in the glutamate carboxypeptidase II gene increases plasma folate without affecting the risk for neural tube defects in humans. J. Nutr. 2003, 133, 75–77. [Google Scholar] [CrossRef]
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12—Multifaceted in vivo functions and in vitro applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef] [PubMed]
- Shere, M.; Bapat, P.; Nickel, C.; Kapur, B.; Koren, G. Association between use of oral contraceptives and folate status: A systematic review and Meta-Analysis. J. Obstet. Gynaecol. Can. 2015, 37, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hiilesmaa, V.K.; Teramo, K.; Granström, M.L.; Bardy, A.H. Serum folate concentrations during pregnancy in women with epilepsy: Relation to antiepileptic drug concentrations, number of seizures, and fetal outcome. Br. Med. J. 1983, 287, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.P.; Van Dyke, D.C.; Willhite, L.A.; Stumbo, P.J.; Berg, M.J. Phenytoin-folic acid interaction. Ann. Pharmacother. 1995, 29, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Linnebank, M.; Moskau, S.; Semmler, A.; Widman, G.; Stoffel-Wagner, B.; Weller, M.; Elger, C.E. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann. Neurol. 2011, 69, 352–359. [Google Scholar] [CrossRef]
- Dansky, L.V.; Andermann, E.; Rosenblatt, D.; Sherwin, A.L.; Andermann, F. Anticonvulsants, folate levels, and pregnancy outcome: A prospective study. Ann. Neurol. 1987, 21, 176–182. [Google Scholar] [CrossRef]
- McIntyre, R.L.; Molenaars, M.; Schomakers, B.V.; Gao, A.W.; Kamble, R.; Jongejan, A.; van Weeghel, M.; van Kuilenburg, A.B.P.; Possemato, R.; Houtkooper, R.H.; et al. Anti-retroviral treatment with zidovudine alters pyrimidine metabolism, reduces translation, and extends healthy longevity via ATF-4. Cell Rep. 2023, 42, 111928. [Google Scholar] [CrossRef]
- Singh, R.K.; van Haandel, L.; Kiptoo, P.; Becker, M.L.; Siahaan, T.J.; Funk, R.S. Methotrexate disposition, anti-folate activity and efficacy in the collagen-induced arthritis mouse model. Eur. J. Pharmacol. 2019, 853, 264–274. [Google Scholar] [CrossRef]
- Franklin, J.L.; Rosenberg, H.H. Impaired folic acid absorption in inflammatory bowel disease: Effects of salicylazosulfapyridine (Azulfidine). Gastroenterology 1973, 64, 517–525. [Google Scholar] [CrossRef]
- Selhub, J.; Dhar, G.J.; Rosenberg, I.H. Inhibition of folate enzymes by sulfasalazine. J. Clin. Investig. 1978, 61, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, K.; Zhu, Y.; Chan, W.; Aguilar, D.; Deshmukh, A.A.; Suarez-Almazor, M.E. Association of serum folate levels with cardiovascular mortality among adults with rheumatoid arthritis. JAMA Netw. Open 2020, 3, e200100. [Google Scholar] [CrossRef] [PubMed]
- Meidahl Petersen, K.; Eplov, K.; Kjær Nielsen, T.; Jimenez-Solem, E.; Petersen, M.; Broedbaek, K.; Daugaard Popik, S.; Kallehave Hansen, L.; Enghusen Poulsen, H.; Trærup Andersen, J. The effect of trimethoprim on serum folate levels in humans: A randomized, double-blind, llacebo-controlled trial. Am. J. Ther. 2016, 23, e382–e387. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Powell, T.L.; Jansson, T. mTOR folate sensing links folate availability to trophoblast cell function. J. Physiol. 2017, 595, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H.; Asara, J.M.; Manning, B.D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016, 351, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Higa, R.; Rosario, F.J.; Powell, T.L.; Jansson, T.; Jawerbaum, A. Inhibition of mTOR signaling impairs rat embryo organogenesis by affecting folate availability. Reproduction 2021, 161, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Good, L.; Dimri, G.P.; Campisi, J.; Chen, K.Y. Regulation of dihydrofolate reductase gene expression and E2F components in human diploid fibroblasts during growth and senescence. J. Cell Physiol. 1996, 168, 580–588. [Google Scholar] [CrossRef]
- Slansky, J.E.; Farnham, P.J. Transcriptional regulation of the dihydrofolate reductase gene. Bioessays 1996, 18, 55–62. [Google Scholar] [CrossRef]
- DeGregori, J.; Kowalik, T.; Nevins, J.R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell Biol. 1995, 15, 4215–4224. [Google Scholar] [CrossRef]
- Ye, J.; Fan, J.; Venneti, S.; Wan, Y.W.; Pawel, B.R.; Zhang, J.; Finley, L.W.; Lu, C.; Lindsten, T.; Cross, J.R.; et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014, 4, 1406–1417. [Google Scholar] [CrossRef]
- Filippone, M.G.; Gaglio, D.; Bonfanti, R.; Tucci, F.A.; Ceccacci, E.; Pennisi, R.; Bonanomi, M.; Jodice, G.; Tillhon, M.; Montani, F.; et al. CDK12 promotes tumorigenesis but induces vulnerability to therapies inhibiting folate one-carbon metabolism in breast cancer. Nat. Commun. 2022, 13, 2642. [Google Scholar] [CrossRef]
- Kovatcheva, M.; Melendez, E.; Chondronasiou, D.; Pietrocola, F.; Bernad, R.; Caballe, A.; Junza, A.; Capellades, J.; Holguín-Horcajo, A.; Prats, N.; et al. Vitamin B(12) is a limiting factor for induced cellular plasticity and tissue repair. Nat. Metab. 2023, 5, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Field, M.S.; Stover, P.J. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1426. [Google Scholar] [CrossRef] [PubMed]
- Ercikan-Abali, E.A.; Banerjee, D.; Waltham, M.C.; Skacel, N.; Scotto, K.W.; Bertino, J.R. Dihydrofolate reductase protein inhibits its own translation by binding to dihydrofolate reductase mRNA sequences within the coding region. Biochemistry 1997, 36, 12317–12322. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.; Allegra, C.J. The role of thymidylate synthase as an RNA binding protein. Bioessays 1996, 18, 191–198. [Google Scholar] [CrossRef]
- Gulati, S.; Brody, L.C.; Banerjee, R. Posttranscriptional regulation of mammalian methionine synthase by B12. Biochem. Biophys. Res. Commun. 1999, 259, 436–442. [Google Scholar] [CrossRef][Green Version]
- Oltean, S.; Banerjee, R. Nutritional modulation of gene expression and homocysteine utilization by vitamin B12. J. Biol. Chem. 2003, 278, 20778–20784. [Google Scholar] [CrossRef]
- Yamada, K.; Strahler, J.R.; Andrews, P.C.; Matthews, R.G. Regulation of human methylenetetrahydrofolate reductase by phosphorylation. Proc. Natl. Acad. Sci. USA 2005, 102, 10454–10459. [Google Scholar] [CrossRef]
- Froese, D.S.; Kopec, J.; Rembeza, E.; Bezerra, G.A.; Oberholzer, A.E.; Suormala, T.; Lutz, S.; Chalk, R.; Borkowska, O.; Baumgartner, M.R.; et al. Structural basis for the regulation of human 5,10-methylenetetrahydrofolate reductase by phosphorylation and S-adenosylmethionine inhibition. Nat. Commun. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Zhu, B.; Xiahou, Z.; Zhao, H.; Peng, B.; Zhao, H.; Xu, X. MTHFR promotes heterochromatin maintenance. Biochem. Biophys. Res. Commun. 2014, 447, 702–706. [Google Scholar] [CrossRef]
- Zheng, Y.; Ramsamooj, S.; Li, Q.; Johnson, J.L.; Yaron, T.M.; Sharra, K.; Cantley, L.C. Regulation of folate and methionine metabolism by multisite phosphorylation of human methylenetetrahydrofolate reductase. Sci. Rep. 2019, 9, 4190. [Google Scholar] [CrossRef]
- Becker, W.; Sippl, W. Activation, regulation, and inhibition of DYRK1A. FEBS J. 2011, 278, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Foukas, L.C.; Berenjeno, I.M.; Gray, A.; Khwaja, A.; Vanhaesebroeck, B. Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc. Natl. Acad. Sci. USA 2010, 107, 11381–11386. [Google Scholar] [CrossRef] [PubMed]
- Kutzbach, C.; Stokstad, E.L. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim. Biophys. Acta 1971, 250, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.; Jencks, D.A.; Khani, S.; Matthews, R.G. Photoaffinity labeling of methylenetetrahydrofolate reductase with 8-azido-S-adenosylmethionine. J. Biol. Chem. 1986, 261, 7697–7700. [Google Scholar] [CrossRef]
- Field, M.S.; Szebenyi, D.M.; Stover, P.J. Regulation of de novo purine biosynthesis by methenyltetrahydrofolate synthetase in neuroblastoma. J. Biol. Chem. 2006, 281, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.D.; Eom, J.Y.; Stover, P.J. Competition between sumoylation and ubiquitination of serine hydroxymethyltransferase 1 determines its nuclear localization and its accumulation in the nucleus. J. Biol. Chem. 2012, 287, 4790–4799. [Google Scholar] [CrossRef]
- Peña, M.M.; Xing, Y.Y.; Koli, S.; Berger, F.G. Role of N-terminal residues in the ubiquitin-independent degradation of human thymidylate synthase. Biochem. J. 2006, 394, 355–363. [Google Scholar] [CrossRef]
- Schneider, J.A.; Rees, D.C.; Liu, Y.T.; Clegg, J.B. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am. J. Hum. Genet. 1998, 62, 1258–1260. [Google Scholar] [CrossRef]
- Pepe, G.; Camacho Vanegas, O.; Giusti, B.; Brunelli, T.; Marcucci, R.; Attanasio, M.; Rickards, O.; De Stefano, G.F.; Prisco, D.; Gensini, G.F.; et al. Heterogeneity in world distribution of the thermolabile C677T mutation in 5,10-methylenetetrahydrofolate reductase. Am. J. Hum. Genet. 1998, 63, 917–920. [Google Scholar] [CrossRef][Green Version]
- Yamada, K.; Chen, Z.; Rozen, R.; Matthews, R.G. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc. Natl. Acad. Sci. USA 2001, 98, 14853–14858. [Google Scholar] [CrossRef]
- Kang, S.S.; Passen, E.L.; Ruggie, N.; Wong, P.W.; Sora, H. Thermolabile defect of methylenetetrahydrofolate reductase in coronary artery disease. Circulation 1993, 88, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, G.; Forti, P.; Maioli, F.; Martelli, M.; Servadei, L.; Brunetti, N.; Porcellini, E.; Licastro, F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am. J. Clin. Nutr. 2005, 82, 636–643. [Google Scholar] [CrossRef]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef]
- Tsang, B.L.; Devine, O.J.; Cordero, A.M.; Marchetta, C.M.; Mulinare, J.; Mersereau, P.; Guo, J.; Qi, Y.P.; Berry, R.J.; Rosenthal, J.; et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: A systematic review and meta-analysis of trials and observational studies. Am. J. Clin. Nutr. 2015, 101, 1286–1294. [Google Scholar] [CrossRef]
- Cabo, R.; Hernes, S.; Slettan, A.; Haugen, M.; Ye, S.; Blomhoff, R.; Mansoor, M.A. Effect of genetic polymorphisms involved in folate metabolism on the concentration of serum folate and plasma total homocysteine (p-tHcy) in healthy subjects after short-term folic acid supplementation: A randomized, double blind, crossover study. Genes Nutr. 2015, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.H.; Botto, L.D.; Gallagher, M.; Friedman, J.M.; Sanders, C.L.; Koontz, D.; Nikolova, S.; Erickson, J.D.; Steinberg, K. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: Findings from the third National Health and Nutrition Examination Survey DNA Bank. Am. J. Clin. Nutr. 2008, 88, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, B.; Graziano, M.; Persichilli, S.; Di Castelnuovo, A.; Mastroiacovo, P.; Iacoviello, L. 5,10-Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms: Genotype frequency and association with homocysteine and folate levels in middle-southern Italian adults. Cell Biochem. Funct. 2014, 32, 1–4. [Google Scholar] [CrossRef]
- Devlin, A.M.; Clarke, R.; Birks, J.; Evans, J.G.; Halsted, C.H. Interactions among polymorphisms in folate-metabolizing genes and serum total homocysteine concentrations in a healthy elderly population. Am. J. Clin. Nutr. 2006, 83, 708–713. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kagawa, Y. Genetic polymorphisms and folate status. Congenit. Anom. 2017, 57, 142–149. [Google Scholar] [CrossRef]
- Pangilinan, F.; Molloy, A.M.; Mills, J.L.; Troendle, J.F.; Parle-McDermott, A.; Signore, C.; O’Leary, V.B.; Chines, P.; Seay, J.M.; Geiler-Samerotte, K.; et al. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med. Genet. 2012, 13, 62. [Google Scholar] [CrossRef]
- Martinelli, M.; Scapoli, L.; Palmieri, A.; Pezzetti, F.; Baciliero, U.; Padula, E.; Carinci, P.; Morselli, P.G.; Carinci, F. Study of four genes belonging to the folate pathway: Transcobalamin 2 is involved in the onset of non-syndromic cleft lip with or without cleft palate. Hum. Mutat. 2006, 27, 294. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Silva, J.N.; Galbiatti, A.L.; Succi, M.; Ruiz, M.T.; Raposo, L.S.; Maniglia, J.V.; Pavarino-Bertelli, E.C.; Goloni-Bertollo, E.M. Head and neck carconogenesis: Impact of MTHFD1 G1958A polymorphism. Rev. Assoc. Med. Bras. 2011, 57, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Dinger, M.E.; Mercer, T.R.; Mehler, M.F. RNA regulation of epigenetic processes. Bioessays 2009, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res. 2009, 667, 82–97. [Google Scholar] [CrossRef]
- Kwok, J.B. Role of epigenetics in Alzheimer’s and Parkinson’s disease. Epigenomics 2010, 2, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Hogg, S.J.; Beavis, P.A.; Dawson, M.A.; Johnstone, R.W. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 2020, 19, 776–800. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, Y.T.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Gurugubelli, K.R.; Ballambattu, V.B. Perspectives on folate with special reference to epigenetics and neural tube defects. Reprod. Toxicol. 2024, 125, 108576. [Google Scholar] [CrossRef] [PubMed]
- Fuso, A.; Nicolia, V.; Cavallaro, R.A.; Ricceri, L.; D’Anselmi, F.; Coluccia, P.; Calamandrei, G.; Scarpa, S. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol. Cell Neurosci. 2008, 37, 731–746. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, S.M.; Zhang, Y.Z. Maternal folic acid supplementation mediates offspring health via DNA methylation. Reprod. Sci. 2020, 27, 963–976. [Google Scholar] [CrossRef]
- Alata Jimenez, N.; Strobl-Mazzulla, P.H. Folate carrier deficiency drives differential methylation and enhanced cellular potency in the neural plate border. Front. Cell Dev. Biol. 2022, 10, 834625. [Google Scholar] [CrossRef]
- Geng, Y.; Gao, R.; Liu, X.; Chen, X.; Liu, S.; Ding, Y.; Mu, X.; Wang, Y.; He, J. Folate deficiency inhibits the PCP pathway and alters genomic methylation levels during embryonic development. J. Cell. Physiol. 2018, 233, 7333–7342. [Google Scholar] [CrossRef]
- Friso, S.; Choi, S.W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef]
- Cooney, C.A.; Dave, A.A.; Wolff, G.L. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 2002, 132, 2393s–2400s. [Google Scholar] [CrossRef]
- Socha, M.W.; Flis, W.; Wartęga, M. Epigenetic genome modifications during pregnancy: The impact of essential nutritional supplements on DNA methylation. Nutrients 2024, 16, 678. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; den Dekker, H.T.; Felix, J.F.; Bohlin, J.; Ligthart, S.; Beckett, E.; Tiemeier, H.; van Meurs, J.B.; Uitterlinden, A.G.; Hofman, A.; et al. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat. Commun. 2016, 7, 10577. [Google Scholar] [CrossRef] [PubMed]
- Kok, D.E.; Richmond, R.C.; Adriaens, M.; Evelo, C.T.; Ford, D.; Mathers, J.C.; Robinson, N.; McKay, J.A. Impact of in utero folate exposure on DNA methylation and its potential relevance for later-life health-evidence from mouse models translated to human cohorts. Mol. Nutr. Food Res. 2022, 66, e2100789. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Binder, A.M. Impact of folic acid supplementation on the epigenetic profile in healthy unfortified individuals—A randomized intervention trial. Epigenetics 2024, 19, 2293410. [Google Scholar] [CrossRef] [PubMed]
- Pufulete, M.; Al-Ghnaniem, R.; Khushal, A.; Appleby, P.; Harris, N.; Gout, S.; Emery, P.W.; Sanders, T.A. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut 2005, 54, 648–653. [Google Scholar] [CrossRef]
- Kok, D.E.; Dhonukshe-Rutten, R.A.; Lute, C.; Heil, S.G.; Uitterlinden, A.G.; van der Velde, N.; van Meurs, J.B.; van Schoor, N.M.; Hooiveld, G.J.; de Groot, L.C.; et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin. Epigenet. 2015, 7, 121. [Google Scholar] [CrossRef]
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699. [Google Scholar] [CrossRef]
- Piñuñuri, R.; Castaño-Moreno, E.; Llanos, M.N.; Ronco, A.M. Epigenetic regulation of folate receptor-α (FOLR1) in human placenta of preterm newborns. Placenta 2020, 94, 20–25. [Google Scholar] [CrossRef]
- Diniz, T.G.; Silva, A.S.; Dos Santos Nunes, M.K.; Ribeiro, M.D.; Filho, J.M.; do Nascimento, R.A.F.; Gomes, C.; Evangelista, I.W.Q.; de Oliveira, N.F.P.; Persuhn, D.C. Physical activity level influences MTHFR gene methylation profile in diabetic patients. Front. Physiol. 2020, 11, 618672. [Google Scholar] [CrossRef]
- Dos Santos Nunes, M.K.; Silva, A.S.; de Queiroga Evangelista, I.W.; Filho, J.M.; Gomes, C.; do Nascimento, R.A.F.; Luna, R.C.P.; de Carvalho Costa, M.J.; de Oliveira, N.F.P.; Persuhn, D.C. Hypermethylation in the promoter of the MTHFR gene is associated with diabetic complications and biochemical indicators. Diabetol. Metab. Syndr. 2017, 9, 84. [Google Scholar] [CrossRef]
- Osunkalu, V.O.; Taiwo, I.A.; Makwe, C.C.; Abiola, A.A.; Quao, R.A.; Anorlu, R.I. Epigenetic modification in methylene tetrahydrofolate reductase (MTHFR) gene of women with pre-eclampsia. J. Obstet. Gynaecol. India 2021, 71, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Oelze, B.; Schumacher, A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS ONE 2008, 3, e2698. [Google Scholar] [CrossRef] [PubMed]
- Serefidou, M.; Venkatasubramani, A.V.; Imhof, A. The impact of one carbon metabolism on histone methylation. Front. Genet. 2019, 10, 764. [Google Scholar] [CrossRef]
- Luka, Z.; Moss, F.; Loukachevitch, L.V.; Bornhop, D.J.; Wagner, C. Histone demethylase LSD1 is a folate-binding protein. Biochemistry 2011, 50, 4750–4756. [Google Scholar] [CrossRef]
- Garcia, B.A.; Luka, Z.; Loukachevitch, L.V.; Bhanu, N.V.; Wagner, C. Folate deficiency affects histone methylation. Med. Hypotheses 2016, 88, 63–67. [Google Scholar] [CrossRef]
- Abali, E.E.; Skacel, N.E.; Celikkaya, H.; Hsieh, Y.C. Regulation of human dihydrofolate reductase activity and expression. Vitam. Horm. 2008, 79, 267–292. [Google Scholar] [CrossRef]
- Nicolas, E.; Roumillac, C.; Trouche, D. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol. Cell. Biol. 2003, 23, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Nimmo-Smith, R.H. The Function of Folic Acid in the Biosynthesis of Purine and Pyrimidine Derivatives. In Ciba Foundation Symposium-Chemistry and Biology of Pteridines; John Wiley & Sons, Ltd.: Chichester, UK, 1954; pp. 237–252. [Google Scholar] [CrossRef]
- Webb, M. The effects of folic acid analogues on the growth and cell division of micro-organisms. In Ciba Foundation Symposium-Chemistry and Biology of Pteridines; John Wiley & Sons, Ltd.: Chichester, UK, 1954; pp. 253–271. [Google Scholar] [CrossRef]
- Wagner, C. Biochemical role of folate in cellular metabolism. Clin. Res. Regul. Aff. 2001, 18, 161–180. [Google Scholar] [CrossRef]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef]
- Choi, S.W.; Mason, J.B. Folate and carcinogenesis: An integrated scheme. J. Nutr. 2000, 130, 129–132. [Google Scholar] [CrossRef]
- Wickremasinghe, R.G.; Hoffbrand, A.V. Reduced rate of DNA replication fork movement in megaloblastic anemia. J. Clin. Investig. 1980, 65, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bills, N.D.; Koury, M.J.; Clifford, A.J.; Dessypris, E.N. Ineffective hematopoiesis in folate-deficient mice. Blood 1992, 79, 2273–2280. [Google Scholar] [CrossRef]
- Cordero, A.M.; Crider, K.S.; Rogers, L.M.; Cannon, M.J.; Berry, R.J. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 421–423. [Google Scholar] [PubMed]
- World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations; World Health Organization: Geneva, Switzerland, 2012; Available online: https://iris.who.int/bitstream/handle/10665/75584/WHO_NMH_NHD_EPG_12.1_eng.pdf?sequence=1 (accessed on 8 July 2024).
- Stanislawska-Sachadyn, A.; Borzyszkowska, J.; Krzeminski, M.; Janowicz, A.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Limon, J. Folate/homocysteine metabolism and lung cancer risk among smokers. PLoS ONE 2019, 14, e0214462. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Dickstein, A.; Jacques, P.F.; Haggarty, P.; Selhub, J.; Dallal, G.; Rosenberg, I.H. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: A hypothesis. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.J.; Woodson, K.; Stolzenberg-Solomon, R.; Virtamo, J.; Selhub, J.; Barrett, M.J.; Albanes, D. Association of the B-vitamins pyridoxal 5′-phosphate (B(6)), B(12), and folate with lung cancer risk in older men. Am. J. Epidemiol. 2001, 153, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Yu, S.; Zhang, S.; Ding, X.; Su, J.; Cheng, Z. Association between folate intake and risk of head and neck squamous cell carcinoma: An overall and dose-response PRISMA meta-analysis. Medicine 2017, 96, e8182. [Google Scholar] [CrossRef]
- Tio, M.; Andrici, J.; Cox, M.R.; Eslick, G.D. Folate intake and the risk of upper gastrointestinal cancers: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2014, 29, 250–258. [Google Scholar] [CrossRef]
- Farber, S.; Diamond, L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 1948, 238, 787–793. [Google Scholar] [CrossRef]
- Kim, Y.I. Does a high folate intake increase the risk of breast cancer? Nutr. Rev. 2006, 64, 468–475. [Google Scholar] [CrossRef]
- Cole, B.F.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Ahnen, D.J.; Bresalier, R.S.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.I.; Burke, C.A.; et al. Folic acid for the prevention of colorectal adenomas: A randomized clinical trial. JAMA 2007, 297, 2351–2359. [Google Scholar] [CrossRef]
- Oliai Araghi, S.; Kiefte-de Jong, J.C.; van Dijk, S.C.; Swart, K.M.A.; van Laarhoven, H.W.; van Schoor, N.M.; de Groot, L.; Lemmens, V.; Stricker, B.H.; Uitterlinden, A.G.; et al. Folic Acid and Vitamin B12 Supplementation and the Risk of Cancer: Long-term Follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol. Biomark. Prev. 2019, 28, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Zhu, Y.; Tao, Y.; Li, X.; Zhai, D.; Bu, Y.; Wan, Z.; Wang, L.; Wang, Y.; Yu, Z. Association between Folate and Health Outcomes: An Umbrella Review of Meta-Analyses. Front. Public Health 2020, 8, 550753. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ali, T.; Kaur, J. Folic acid depletion as well as oversupplementation helps in the progression of hepatocarcinogenesis in HepG2 cells. Sci. Rep. 2022, 12, 16617. [Google Scholar] [CrossRef] [PubMed]
- Bistulfi, G.; Vandette, E.; Matsui, S.; Smiraglia, D.J. Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells. BMC Biol. 2010, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Pitstick, L.; Goral, J.; Ciancio, M.J.; Meyer, A.; Pytynia, M.; Bychek, S.; Zidan, S.; Shuey, J.; Jham, B.C.; Green, J.M. Effects of folate deficiency and sex on carcinogenesis in a mouse model of oral cancer. Oral Dis. 2024, 30, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Narayanan, S.; Blum, S.; Pirie, L.; Brand, G.M. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr. Cancer 2000, 37, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Yu, C.C.; Lu, H.T.; Chou, Y.F.; Huang, R.F. Folate deprivation promotes mitochondrial oxidative decay: DNA large deletions, cytochrome c oxidase dysfunction, membrane depolarization and superoxide overproduction in rat liver. Br. J. Nutr. 2007, 97, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Latteri, S.; Malaguarnera, G.; Catania, V.E.; La Greca, G.; Bertino, G.; Borzi, A.M.; Drago, F.; Malaguarnera, M. Homocysteine serum levels as prognostic marker of hepatocellular carcinoma with portal vein thrombosis. Curr. Mol. Med. 2019, 19, 532–538. [Google Scholar] [CrossRef]
- Cui, L.H.; Song, Y.; Si, H.; Shen, F.; Shin, M.H.; Kim, H.N.; Choi, J.S. Folate metabolism-related gene polymorphisms and susceptibility to primary liver cancer in North China. Med. Oncol. 2012, 29, 1837–1842. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Gagliano, C.; Giordano, M.; Salomone, S.; Vacante, M.; Bucolo, C.; Caraci, F.; Reibaldi, M.; Drago, F.; Avitabile, T.; et al. Homocysteine serum levels in diabetic patients with non proliferative, proliferative and without retinopathy. Biomed. Res. Int. 2014, 2014, 191497. [Google Scholar] [CrossRef] [PubMed]
- Vahed, M.M.; Bahramian, A.; Katebi, K.; Salehnia, F. The relationship between serum folate and homocysteine with head and neck cancer: A systematic review and meta-analysis. Shiraz E-Med. J. 2024, 25, e140961. [Google Scholar]
- Kim, Y.I. Folate and DNA methylation: A mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol. Biomark. Prev. 2004, 13, 511–519. [Google Scholar] [CrossRef]
- Nilsson, R.; Nicolaidou, V.; Koufaris, C. Mitochondrial MTHFD isozymes display distinct expression, regulation, and association with cancer. Gene 2019, 716, 144032. [Google Scholar] [CrossRef]
- Sullivan, M.R.; Darnell, A.M.; Reilly, M.F.; Kunchok, T.; Joesch-Cohen, L.; Rosenberg, D.; Ali, A.; Rees, M.G.; Roth, J.A.; Lewis, C.A.; et al. Methionine synthase is essential for cancer cell proliferation in physiological folate environments. Nat. Metab. 2021, 3, 1500–1511. [Google Scholar] [CrossRef]
- Zhang, W.; Braun, A.; Bauman, Z.; Olteanu, H.; Madzelan, P.; Banerjee, R. Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res. 2005, 65, 1554–1560. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Ghergurovich, J.M.; Xu, X.; Wang, J.Z.; Yang, L.; Ryseck, R.P.; Wang, L.; Rabinowitz, J.D. Methionine synthase supports tumour tetrahydrofolate pools. Nat. Metab. 2021, 3, 1512–1520. [Google Scholar] [CrossRef]
- Krupenko, S.A.; Krupenko, N.I. Loss of ALDH1L1 folate enzyme confers a selective metabolic advantage for tumor progression. Chem. Biol. Interact. 2019, 302, 149–155. [Google Scholar] [CrossRef]
- Krupenko, N.I.; Sharma, J.; Fogle, H.M.; Pediaditakis, P.; Strickland, K.C.; Du, X.; Helke, K.L.; Sumner, S.; Krupenko, S.A. Knockout of putative tumor suppressor Aldh1l1 in mice reprograms metabolism to accelerate growth of tumors in a diethylnitrosamine (DEN) model of liver carcinogenesis. Cancers 2021, 13, 3219. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamamoto, K.; Ueda, T.; Irokawa, H.; Takeda, K.; Sekine, R.; Itoh, F.; Tanaka, Y.; Kuge, S.; Shibata, N. One-carbon metabolizing enzyme ALDH1L1 influences mitochondrial metabolism through 5-aminoimidazole-4-carboxamide ribonucleotide accumulation and serine depletion, contributing to tumor suppression. Sci. Rep. 2023, 13, 13486. [Google Scholar] [CrossRef]
- Oleinik, N.V.; Krupenko, N.I.; Krupenko, S.A. Epigenetic silencing of ALDH1L1, a metabolic regulator of cellular proliferation, in cancers. Genes Cancer 2011, 2, 130–139. [Google Scholar] [CrossRef]
- Qu, Y.; He, Y.; Ruan, H.; Qin, L.; Han, Z. Abnormal downregulation of 10-formyltetrahydrofolate dehydrogenase promotes the progression of oral squamous cell carcinoma by activating PI3K/Akt/Rb pathway. Cancer Med. 2023, 12, 5781–5797. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G. Mechanisms of methotrexate resistance in osteosarcoma cell lines and strategies for overcoming this resistance. Oncol. Lett. 2015, 9, 940–944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broccoli, A.; Argnani, L.; Zinzani, P.L. Peripheral T-cell lymphomas: Focusing on novel agents in relapsed and refractory disease. Cancer Treat. Rev. 2017, 60, 120–129. [Google Scholar] [CrossRef]
- Hui, J.; Przespo, E.; Elefante, A. Pralatrexate: A novel synthetic antifolate for relapsed or refractory peripheral T-cell lymphoma and other potential uses. J. Oncol. Pharm. Pract. 2012, 18, 275–283. [Google Scholar] [CrossRef]
- Li, X.; Wei, S.; Chen, J. Critical appraisal of pemetrexed in the treatment of NSCLC and metastatic pulmonary nodules. OncoTargets Ther. 2014, 7, 937–945. [Google Scholar] [CrossRef][Green Version]
- Barni, S.; Ghidini, A.; Coinu, A.; Borgonovo, K.; Petrelli, F. A systematic review of raltitrexed-based first-line chemotherapy in advanced colorectal cancer. Anticancer Drugs 2014, 25, 1122–1128. [Google Scholar] [CrossRef]
- Ser, Z.; Gao, X.; Johnson, C.; Mehrmohamadi, M.; Liu, X.; Li, S.; Locasale, J.W. Targeting one carbon metabolism with an antimetabolite disrupts pyrimidine homeostasis and induces nucleotide overflow. Cell Rep. 2016, 15, 2367–2376. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Schmiegelow, K.; Nielsen, S.N.; Frandsen, T.L.; Nersting, J. Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: Clinical facts and fiction. J. Pediatr. Hematol. Oncol. 2014, 36, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor alpha in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef]
- Reddy, J.A.; Nelson, M.; Dircksen, C.; Vetzel, M.; Johnson, T.; Cross, V.; Westrick, E.; Qi, L.; Hahn, S.; Santhapuram, H.K.; et al. Pre-clinical studies of EC2629, a highly potent folate- receptor-targeted DNA crosslinking agent. Sci. Rep. 2020, 10, 12772. [Google Scholar] [CrossRef]
- Ibrahim, M.A.I.; Othman, R.; Chee, C.F.; Ahmad Fisol, F. Evaluation of folate-functionalized nanoparticle drug delivery systems-effectiveness and concerns. Biomedicines 2023, 11, 2080. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted radionuclide therapy of human tumors. Int. J. Mol. Sci. 2015, 17, 33. [Google Scholar] [CrossRef]
- Nitipir, C.; Niculae, D.; Orlov, C.; Barbu, M.A.; Popescu, B.; Popa, A.M.; Pantea, A.M.S.; Stanciu, A.E.; Galateanu, B.; Ginghina, O.; et al. Update on radionuclide therapy in oncology. Oncol. Lett. 2017, 14, 7011–7015. [Google Scholar] [CrossRef]
- Siegel, B.A.; Dehdashti, F.; Mutch, D.G.; Podoloff, D.A.; Wendt, R.; Sutton, G.P.; Burt, R.W.; Ellis, P.R.; Mathias, C.J.; Green, M.A.; et al. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: Initial clinical results. J. Nucl. Med. 2003, 44, 700–707. [Google Scholar]
- Fisher, R.E.; Siegel, B.A.; Edell, S.L.; Oyesiku, N.M.; Morgenstern, D.E.; Messmann, R.A.; Amato, R.J. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J. Nucl. Med. 2008, 49, 899–906. [Google Scholar] [CrossRef]
- Muller, C.; Reddy, J.A.; Leamon, C.P.; Schibli, R. Effects of the antifolates pemetrexed and CB3717 on the tissue distribution of (99m)Tc-EC20 in xenografted and syngeneic tumor-bearing mice. Mol. Pharm. 2010, 7, 597–604. [Google Scholar] [CrossRef]
- Reber, J.; Haller, S.; Leamon, C.P.; Muller, C. 177Lu-EC0800 combined with the antifolate pemetrexed: Preclinical pilot study of folate receptor targeted radionuclide tumor therapy. Mol. Cancer Ther. 2013, 12, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Siwowska, K.; Haller, S.; Bortoli, F.; Benesova, M.; Groehn, V.; Bernhardt, P.; Schibli, R.; Muller, C. Preclinical Comparison of Albumin-Binding Radiofolates: Impact of Linker Entities on the In Vitro and In Vivo Properties. Mol. Pharm. 2017, 14, 523–532. [Google Scholar] [CrossRef]
- Ladak, I.; Preti, B.; Dias, B.; Breadner, D.A. Raltitrexed as a substitute for capecitabine in metastatic gastric cancer: A case report and literature review. Ann. Transl. Med. 2022, 10, 1285. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Shi, H.; Guo, J.; Li, C.; Wang, Z. A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des. Devel. Ther. 2015, 9, 4989–4996. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Leamon, C.P. Vintafolide: A novel targeted therapy for the treatment of folate receptor expressing tumors. Ther. Adv. Med. Oncol. 2015, 7, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Bhatnagar, S. Advancements in folate receptor targeting for anti-cancer therapy: A small molecule-drug conjugate approach. Bioorg. Chem. 2021, 112, 104946. [Google Scholar] [CrossRef]
- Kennedy, G.T.; Azari, F.S.; Chang, A.; Nadeem, B.; Bernstein, E.; Segil, A.; Din, A.; Marfatia, I.; Desphande, C.; Okusanya, O.; et al. Comparative experience of short-wavelength versus long-wavelength fluorophores for intraoperative molecular imaging of lung cancer. Ann. Surg. 2022, 276, 711–719. [Google Scholar] [CrossRef]
- Sato, S.; Itamochi, H. Profile of farletuzumab and its potential in the treatment of solid tumors. Onco Targets Ther. 2016, 9, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Sato, J.; Tamura, K.; Shimomura, A.; Ikezawa, H.; Nomoto, M.; Furuuchi, K.; et al. First-in-human phase 1 study of MORAb-202, an antibody-drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-α-positive advanced solid tumors. Clin. Cancer Res. 2021, 27, 3905–3915. [Google Scholar] [CrossRef]
- Heo, Y.A. Mirvetuximab soravtansine: First approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Cristea, M. Antibody-drug conjugates for ovarian cancer: Current clinical development. Curr. Opin. Obstet. Gynecol. 2019, 31, 18–23. [Google Scholar] [CrossRef]
- Daigre, J.; Martinez-Osuna, M.; Bethke, M.; Steiner, L.; Dittmer, V.; Krischer, K.; Bleilevens, C.; Brauner, J.; Kopatz, J.; Grundmann, M.D.; et al. Preclinical evaluation of novel folate receptor 1-directed CAR T cells for ovarian cancer. Cancers 2024, 16, 333. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian cancer immunotherapy and personalized medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef]
- Guzik, P.; Benesova, M.; Ratz, M.; Monne Rodriguez, J.M.; Deberle, L.M.; Schibli, R.; Muller, C. Preclinical evaluation of 5-methyltetrahydrofolate-based radioconjugates-new perspectives for folate receptor-targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 972–983. [Google Scholar] [CrossRef]
- Naumann, R.W.; Coleman, R.L.; Burger, R.A.; Sausville, E.A.; Kutarska, E.; Ghamande, S.A.; Gabrail, N.Y.; Depasquale, S.E.; Nowara, E.; Gilbert, L.; et al. PRECEDENT: A randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2013, 31, 4400–4406. [Google Scholar] [CrossRef]
- Oza, A.M.; Vergote, I.B.; Gilbert, L.G.; Ghatage, P.; Lisyankaya, A.; Ghamande, S.; Chambers, S.K.; Arranz, J.A.; Provencher, D.M.; Bessette, P.; et al. A randomized double-blind phase III trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD/Doxil®/Caelyx®) in combination versus PLD in participants with platinum-resistant ovarian cancer (PROCEED) (NCT01170650). Gynecol. Oncol. 2015, 137, 5–6. [Google Scholar] [CrossRef]
- Edelman, M.J.; Harb, W.A.; Pal, S.E.; Boccia, R.V.; Kraut, M.J.; Bonomi, P.; Conley, B.A.; Rogers, J.S.; Messmann, R.A.; Garon, E.B. Multicenter trial of EC145 in advanced, folate-receptor positive adenocarcinoma of the lung. J. Thorac. Oncol. 2012, 7, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Ma, H.; Perez, W.; Adjei, A.A.; Hanna, N. A randomized, open-label phase II study of single-agent vintafolide versus vintafolide plus docetaxel versus docetaxel alone in second-line NSCLC patients with all target lesions expressing folate-receptor (TARGET). J. Clin. Oncol. 2013, 31, TPS8125. [Google Scholar] [CrossRef]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K.; et al. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J. Clin. Oncol. 2016, 34, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- NCT01218516. A Safety and Efficacy Study of Farletuzumab in Participants with Adenocarcinoma of the Lung. 2013. Available online: https://clinicaltrials.gov/study/NCT01218516 (accessed on 20 August 2024).
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: Results from the SORAYA study. J. Clin. Oncol. 2023, 41, 2436–2445. [Google Scholar] [CrossRef]
- Moore, K.N.; Oza, A.M.; Colombo, N.; Oaknin, A.; Scambia, G.; Lorusso, D.; Konecny, G.E.; Banerjee, S.; Murphy, C.G.; Tanyi, J.L.; et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: Primary analysis of FORWARD I. Ann. Oncol. 2021, 32, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Abu-Remaileh, M.; Kanarek, N.; Freinkman, E.; Gao, X.; Louissaint, A., Jr.; Lewis, C.A.; Sabatini, D.M. Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell 2017, 169, 258–272.e17. [Google Scholar] [CrossRef]
- Tardito, S.; Oudin, A.; Ahmed, S.U.; Fack, F.; Keunen, O.; Zheng, L.; Miletic, H.; Sakariassen, P.O.; Weinstock, A.; Wagner, A.; et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 2015, 17, 1556–1568. [Google Scholar] [CrossRef]
- Cunha, A.; Rocha, A.C.; Barbosa, F.; Baiao, A.; Silva, P.; Sarmento, B.; Queiros, O. Glycolytic inhibitors potentiated the activity of paclitaxel and their nanoencapsulation increased their delivery in a lung cancer model. Pharmaceutics 2022, 14, 2021. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef]
- Gravel, S.P.; Hulea, L.; Toban, N.; Birman, E.; Blouin, M.J.; Zakikhani, M.; Zhao, Y.; Topisirovic, I.; St-Pierre, J.; Pollak, M. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 2014, 74, 7521–7533. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Hoffman, R.M.; Bertino, J.R. Exploiting methionine restriction for cancer treatment. Biochem. Pharmacol. 2018, 154, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, B.M. The role of folic acid in pregnancy. BJOG 1964, 71, 529–542. [Google Scholar] [CrossRef]
- Blom, H.J.; Shaw, G.M.; den Heijer, M.; Finnell, R.H. Neural tube defects and folate: Case far from closed. Nat. Rev. Neurosci. 2006, 7, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, V. Neural tube defects: A review of global prevalence, causes, and primary prevention. Childs Nerv. Syst. 2023, 39, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Rofail, D.; Maguire, L.; Kissner, M.; Colligs, A.; Abetz-Webb, L. A review of the social, psychological, and economic burdens experienced by people with spina bifida and their caregivers. Neurol. Ther. 2013, 2, 1–12. [Google Scholar] [CrossRef]
- Tang, K.F.; Li, Y.L.; Wang, H.Y. Quantitative assessment of maternal biomarkers related to one-carbon metabolism and neural tube defects. Sci. Rep. 2015, 5, 8510. [Google Scholar] [CrossRef]
- Yadav, U.; Kumar, P.; Rai, V. Maternal biomarkers for early prediction of the neural tube defects pregnancies. Birth Defects Res. 2021, 113, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sadhukhan, S.; Munian, D.; Bankura, B.; Das, M. Association of FOLH1, DHFR, and MTHFR gene polymorphisms with susceptibility of neural tube defects: A case control study from Eastern India. Birth Defects Res. 2018, 110, 1129–1138. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Stover, P.J. Insights into metabolic mechanisms underlying folate-responsive neural tube defects: A minireview. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 274–284. [Google Scholar] [CrossRef]
- Pei, P.; Cheng, X.; Yu, J.; Shen, J.; Li, X.; Wu, J.; Wang, S.; Zhang, T. Folate deficiency induced H2A ubiquitination to lead to downregulated expression of genes involved in neural tube defects. Epigenet. Chromatin 2019, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Alata Jimenez, N.; Torres Pérez, S.A.; Sánchez-Vásquez, E.; Fernandino, J.I.; Strobl-Mazzulla, P.H. Folate deficiency prevents neural crest fate by disturbing the epigenetic Sox2 repression on the dorsal neural tube. Dev. Biol. 2018, 444 (Suppl. S1), S193–S201. [Google Scholar] [CrossRef]
- O’Byrne, M.R.; Au, K.S.; Morrison, A.C.; Lin, J.I.; Fletcher, J.M.; Ostermaier, K.K.; Tyerman, G.H.; Doebel, S.; Northrup, H. Association of folate receptor (FOLR1, FOLR2, FOLR3) and reduced folate carrier (SLC19A1) genes with meningomyelocele. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, J.A.; Oetama, B.; Bennett, G.D.; van Waes, J.; Kamen, B.A.; Richardson, J.; Lacey, S.W.; Anderson, R.G.; Finnell, R.H. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat. Genet. 1999, 23, 228–232. [Google Scholar] [CrossRef]
- Toriyama, M.; Toriyama, M.; Wallingford, J.B.; Finnell, R.H. Folate-dependent methylation of septins governs ciliogenesis during neural tube closure. FASEB J. 2017, 31, 3622–3635. [Google Scholar] [CrossRef]
- Yue, Y.; Ren, L.; Zhang, C.; Miao, K.; Tan, K.; Yang, Q.; Hu, Y.; Xi, G.; Luo, G.; Yang, M.; et al. Mitochondrial genome undergoes de novo DNA methylation that protects mtDNA against oxidative damage during the peri-implantation window. Proc. Natl. Acad. Sci. USA 2022, 119, e2201168119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Zhao, H.; Wang, F.; Bao, Y.; Guo, J.; Chang, S.; Wu, L.; Cheng, H.; Chen, S.; et al. Low folate concentration impacts mismatch repair deficiency in neural tube defects. Epigenomics 2020, 12, 5–18. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, 2015, Cd007950. [Google Scholar] [CrossRef]
- Castillo-Lancellotti, C.; Tur, J.A.; Uauy, R. Impact of folic acid fortification of flour on neural tube defects: A systematic review. Public Health Nutr. 2013, 16, 901–911. [Google Scholar] [CrossRef]
- Shlobin, N.A.; LoPresti, M.A.; Du, R.Y.; Lam, S. Folate fortification and supplementation in prevention of folate-sensitive neural tube defects: A systematic review of policy. J. Neurosurg. Pediatr. 2020, 27, 294–310. [Google Scholar] [CrossRef]
- Martinez, H.; Benavides-Lara, A.; Arynchyna-Smith, A.; Ghotme, K.A.; Arabi, M.; Arynchyn, A. Global strategies for the prevention of neural tube defects through the improvement of folate status in women of reproductive age. Childs Nerv. Syst. 2023, 39, 1719–1736. [Google Scholar] [CrossRef]
- Viswanathan, M.; Urrutia, R.P. Folic acid supplementation to prevent neural tube defects: A limited systematic review update for the U.S. In Preventive Services Task Force. Evidence Synthesis No. 230; AHRQ Publication No. 22-05302-EF-1; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2023. [Google Scholar]
- Stahl, S.M. L-methylfolate: A vitamin for your monoamines. J. Clin. Psychiatry 2008, 69, 1352–1353. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Bottiglieri, T.; Hyland, K.; Laundy, M.; Godfrey, P.; Carney, M.W.; Toone, B.K.; Reynolds, E.H. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol. Med. 1992, 22, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Bender, A.; Hagan, K.E.; Kingston, N. The association of folate and depression: A meta-analysis. J. Psychiatry Res. 2017, 95, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Sotoudeh, G.; Amini, M.; Raisi, F.; Mansoori, A.; Hosseinzadeh, M. The relationship between dietary patterns and depression mediated by serum levels of folate and vitamin B12. BMC Psychiatry 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Liwinski, T.; Lang, U.E. Folate and its significance in depressive disorders and suicidality: A comprehensive narrative review. Nutrients 2023, 15, 3859. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Maruf, A.A.; Poweleit, E.A.; Brown, L.C.; Strawn, J.R.; Bousman, C.A. Systematic review and meta-analysis of L-methylfolate augmentation in depressive disorders. Pharmacopsychiatry 2022, 55, 139–147. [Google Scholar] [CrossRef]
- Roberts, E.; Carter, B.; Young, A.H. Caveat emptor: Folate in unipolar depressive illness, a systematic review and meta-analysis. J. Psychopharmacol. 2018, 32, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Schefft, C.; Kilarski, L.L.; Bschor, T.; Köhler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Thöny, B.; Sequeira, J.M.; Ansseau, M.; Philippe, P.; Boemer, F.; Bours, V.; Quadros, E.V. Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. Mol. Genet. Metab. 2014, 113, 307–314. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Cerebral folate deficiency, folate receptor alpha autoantibodies and leucovorin (folinic acid) treatment in autism spectrum disorders: A systematic review and meta-analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef]
- Lewis, S.J.; Lawlor, D.A.; Davey Smith, G.; Araya, R.; Timpson, N.; Day, I.N.; Ebrahim, S. The thermolabile variant of MTHFR is associated with depression in the British Women’s Heart and Health Study and a meta-analysis. Mol. Psychiatry 2006, 11, 352–360. [Google Scholar] [CrossRef][Green Version]
- Gilbody, S.; Lewis, S.; Lightfoot, T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: A HuGE review. Am. J. Epidemiol. 2007, 165, 1–13. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Hadipour, E.; Shabani, S.; Salehi, Z. Folate receptor alpha autoantibodies in the serum of patients with relapsing-remitting multiple sclerosis (RRMS). Clin. Neurol. Neurosurg. 2024, 237, 108161. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kim, W.K.; Choi, Y.B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Huang, X.; Jobling, M.F.; Barrow, C.J.; Beyreuther, K.; Masters, C.L.; Bush, A.I.; Cappai, R. Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: Possible risk factors in the Alzheimer’s-type neurodegenerative pathways. J. Neurochem. 2001, 76, 1509–1520. [Google Scholar] [CrossRef]
- Ho, P.I.; Ashline, D.; Dhitavat, S.; Ortiz, D.; Collins, S.C.; Shea, T.B.; Rogers, E. Folate deprivation induces neurodegeneration: Roles of oxidative stress and increased homocysteine. Neurobiol. Dis. 2003, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Arning, E.; Bottiglieri, T.; Böger, R.H.; Sigmund, C.D.; Faraci, F.M.; Lentz, S.R. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 2004, 35, 1957–1962. [Google Scholar] [CrossRef]
- Elens, I.; Dekeyster, E.; Moons, L.; D’Hooge, R. Methotrexate affects cerebrospinal fluid folate and tau levels and induces late cognitive deficits in mice. Neuroscience 2019, 404, 62–70. [Google Scholar] [CrossRef]
- Shaikh, A.; Roy, H. Folate deprivation induced neuroinflammation impairs cognition. Neurosci. Lett. 2023, 807, 137264. [Google Scholar] [CrossRef]
- Cantone, M.; Sacco, L. Editorial: Highlights in Alzheimer’s and Parkinson’s disease. Front. Hum. Neurosci. 2023, 17, 1238525. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, F.; Zhang, S.; Xin, R.; Sun, Y. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Parkinsons. Dis. 2021, 7, 70. [Google Scholar] [CrossRef]
- Charbit, J.; Vidal, J.S.; Hanon, O. The role of nutrition in the prevention of cognitive decline. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 9–16. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef] [PubMed]
- Waly, M.I. Nutritional Management and Metabolic Aspects of Hyperhomocysteinemia; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ma, F.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Huang, G. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: A case-control study. Curr. Alzheimer Res. 2015, 12, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.; Milan, G.; Ruocco, A.; Gallotta, G.; Guiotto, G.; Di Minno, G. Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer’s dementia. A case-control study. Gerontology 2001, 47, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.C.; Brentani, H.P.; Forlenza, O.V.; Diniz, B.S. Serum folic acid is reduced in patients with Alzheimer’s disease. Arch. Clin. 2012, 39, 90–93. [Google Scholar]
- Yukawa, M.; Naka, H.; Murata, Y.; Katayama, S.; Kohriyama, T.; Mimori, Y.; Nakamura, S. Folic acid-responsive neurological diseases in Japan. J. Nutr. Sci. Vitaminol. 2001, 47, 181–187. [Google Scholar] [CrossRef]
- dos Santos, E.F.; Busanello, E.N.; Miglioranza, A.; Zanatta, A.; Barchak, A.G.; Vargas, C.R.; Saute, J.; Rosa, C.; Carrion, M.J.; Camargo, D.; et al. Evidence that folic acid deficiency is a major determinant of hyperhomocysteinemia in Parkinson’s disease. Metab. Brain Dis. 2009, 24, 257–269. [Google Scholar] [CrossRef]
- Periñán, M.T.; Macías-García, D.; Jesús, S.; Martín-Rodríguez, J.F.; Muñoz-Delgado, L.; Jimenez-Jaraba, M.V.; Buiza-Rueda, D.; Bonilla-Toribio, M.; Adarmes-Gómez, A.D.; Gómez-Garre, P.; et al. Homocysteine levels, genetic background, and cognitive impairment in Parkinson’s disease. J. Neurol. 2023, 270, 477–485. [Google Scholar] [CrossRef]
- Tomic, S.; Pekic, V.; Popijac, Z.; Pucic, T.; Vinkovic, M.P.; Kuric, T.G.; Popovic, Z. Hyperhomocysteinemia influenced malnutrition in Parkinson’s disease patients. Neurol. Sci. 2018, 39, 1691–1695. [Google Scholar] [CrossRef]
- Ma, F.; Li, Q.; Zhou, X.; Zhao, J.; Song, A.; Li, W.; Liu, H.; Xu, W.; Huang, G. Effects of folic acid supplementation on cognitive function and Abeta-related biomarkers in mild cognitive impairment: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 345–356. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Zhao, J.; Han, F.; Marseglia, A.; Liu, H.; Huang, G. Effects of 6-Month Folic Acid Supplementation on Cognitive Function and Blood Biomarkers in Mild Cognitive Impairment: A Randomized Controlled Trial in China. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Song, A.; Liu, H.; Xu, W.; Huang, G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci. Rep. 2016, 6, 37486. [Google Scholar] [CrossRef]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, X.; Wang, Y.; Zhou, D.; Li, W.; Wilson, J.X.; Chang, H.; Huang, G. Folic acid delays age-related cognitive decline in senescence-accelerated mouse prone 8: Alleviating telomere attrition as a potential mechanism. Aging 2019, 11, 10356–10373. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.R.; Hoff, A.L.; Costa, M. Folic acid supplementation in dementia: A preliminary report. J. Geriatr. Psychiatry Neurol. 2003, 16, 156–159. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: A randomized controlled trial. Mediators Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Connelly, P.J.; Prentice, N.P.; Cousland, G.; Bonham, J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2008, 23, 155–160. [Google Scholar] [CrossRef]
- Hama, Y.; Hamano, T.; Shirafuji, N.; Hayashi, K.; Ueno, A.; Enomoto, S.; Nagata, M.; Kimura, H.; Matsunaga, A.; Ikawa, M.; et al. Influences of folate supplementation on homocysteine and cognition in patients with folate deficiency and cognitive impairment. Nutrients 2020, 12, 3138. [Google Scholar] [CrossRef]
- Ibrahimagic, O.C.; Smajlovic, D.; Dostovic, Z.; Pasic, Z.; Kunic, S.; Iljazovic, A.; Hajdarevic, D.S. Hyperhomocysteinemia and Its Treatment in Patients with Parkinson’s Disease. Mater. Sociomed. 2016, 28, 303–306. [Google Scholar] [CrossRef]
- Ling, Y.; Yuan, S.; Huang, X.; Tan, S.; Cheng, H.; Xu, A.; Lyu, J. Associations of folate/folic acid supplementation alone and in combination with other B vitamins on dementia risk and brain structure: Evidence from 466 224 UK Biobank participants. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad266. [Google Scholar] [CrossRef]
- Muller, T.; Woitalla, D.; Kuhn, W. Benefit of folic acid supplementation in parkinsonian patients treated with levodopa. J. Neurol. Neurosurg. Psychiatry 2003, 74, 549. [Google Scholar] [CrossRef][Green Version]
- Hu, X.W.; Qin, S.M.; Li, D.; Hu, L.F.; Liu, C.F. Elevated homocysteine levels in levodopa-treated idiopathic Parkinson’s disease: A meta-analysis. Acta Neurol. Scand. 2013, 128, 73–82. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Wang, Y.; Ma, Y.; Chen, L.; Zhang, H.; Xue, M.; Liang, H. Folic acid rescues dopaminergic neurons in MPTP-induced mice by inhibiting the NLRP3 inflammasome and ameliorating mitochondrial impairment. J. Agric. Food Chem. 2024, 72, 5734–5745. [Google Scholar] [CrossRef] [PubMed]
- Haghdoost-Yazdi, H.; Fraidouni, N.; Faraji, A.; Jahanihashemi, H.; Sarookhani, M. High intake of folic acid or complex of B vitamins provides anti-Parkinsonism effect: No role for serum level of homocysteine. Behav. Brain Res. 2012, 233, 375–381. [Google Scholar] [CrossRef]
- Srivastav, S.; Singh, S.K.; Yadav, A.K.; Srikrishna, S. Folic Acid Supplementation Ameliorates Oxidative Stress, Metabolic Functions and Developmental Anomalies in a Novel Fly Model of Parkinson’s Disease. Neurochem. Res. 2015, 40, 1350–1359. [Google Scholar] [CrossRef]
- Hu, N.; Wang, X. The level of homocysteine in amyotrophic lateral sclerosis: A systematic review and meta-analysis. Neurol. Sci. 2023, 44, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, Z.; Sun, W.; Yuan, Y.; Jiao, B.; Zhang, X.; Shen, L.; Jiang, H.; Xia, K.; Tang, B.; et al. Association between vitamins and amyotrophic lateral sclerosis: A center-based survey in Mainland China. Front. Neurol. 2020, 11, 488. [Google Scholar] [CrossRef]
- Wang, H.; Fan, D. The relation between plasma homocysteine level and amyotrophic lateral sclerosis. Zhonghua Nei Ke Za Zhi 2012, 51, 308–310. [Google Scholar] [PubMed]
- Zoccolella, S.; Simone, I.L.; Lamberti, P.; Samarelli, V.; Tortelli, R.; Serlenga, L.; Logroscino, G. Elevated plasma homocysteine levels in patients with amyotrophic lateral sclerosis. Neurology 2008, 70, 222–225. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, L.; Wang, Q.; Le, W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1 G93A transgenic mice. Neuropharmacology 2008, 54, 1112–1119. [Google Scholar] [CrossRef]
- An, Y.; Feng, L.; Zhang, X.; Wang, Y.; Wang, Y.; Tao, L.; Qin, Z.; Xiao, R. Dietary intakes and biomarker patterns of folate, vitamin B(6), and vitamin B(12) can be associated with cognitive impairment by hypermethylation of redox-related genes NUDT15 and TXNRD1. Clin. Epigenet. 2019, 11, 139. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with Alzheimer’s disease: A randomized, single-blinded, placebo-controlled trial. J. Prev. Alzheimers Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kong, H.; Pang, W.; Yang, H.; Lu, H.; Huang, C.; Jiang, Y. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr. Neurosci. 2016, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, C.J.; Chien, K.L.; Chen, S.T.; Chen, R.C. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: A 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 2007, 29, 2204–2214. [Google Scholar] [CrossRef]
- Kretzschmar, G.C.; Targa, A.D.S.; Soares-Lima, S.C.; Dos Santos, P.I.; Rodrigues, L.S.; Macedo, D.A.; Ribeiro Pinto, L.F.; Lima, M.M.S.; Boldt, A.B.W. Folic acid and vitamin B12 prevent deleterious effects of rotenone on object novelty recognition memory and kynu expression in an animal model of Parkinson’s disease. Genes 2022, 13, 2397. [Google Scholar] [CrossRef]
- Boston, P.F.; McKirdy, S.J.; Al-Turki, M.A.; Barker, M.E.; Russell, J.M. Vitamin B12 and folate levels in progression of Alzheimer’s disease—A short report. Int. J. Psychiatry Clin. Pract. 2020, 24, 68–70. [Google Scholar] [CrossRef]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G.; et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. JAMA 2008, 300, 1774–1783. [Google Scholar] [CrossRef]
- Ford, A.H.; Flicker, L.; Alfonso, H.; Thomas, J.; Clarnette, R.; Martins, R.; Almeida, O.P. Vitamins B(12), B(6), and folic acid for cognition in older men. Neurology 2010, 75, 1540–1547. [Google Scholar] [CrossRef]
- De Marchi, F.; Vignaroli, F.; Mazzini, L.; Comi, C.; Tondo, G. New Insights into the Relationship between Nutrition and Neuroinflammation in Alzheimer’s Disease: Preventive and Therapeutic Perspectives. CNS Neurol. Disord. Drug Targets 2024, 23, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J. The LipiDiDiet trial: What does it add to the current evidence for Fortasyn Connect in early Alzheimer’s disease? Clin. Interv. Aging 2019, 14, 1481–1492. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Sakamoto, T.; Ulus, I.H. Use of phosphatide precursors to promote synaptogenesis. Annu. Rev. Nutr. 2009, 29, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Scheltens, P.; McKeith, I.; Blesa, R.; Harrison, J.E.; Bertolucci, P.H.; Rockwood, K.; Wilkinson, D.; Wijker, W.; Bennett, D.A.; et al. Effect size analyses of souvenaid in patients with Alzheimer’s disease. J. Alzheimers Dis. 2017, 55, 1131–1139. [Google Scholar] [CrossRef]
- Sijben, J.W.C.; de Wilde, M.C.; Wieggers, R.; Groenendijk, M.; Kamphuis, P.J.G.H. A multi nutrient concept to enhance synapse formation and function: Science behind a medical food for Alzheimer’s disease. Oléagineux Corp. Gras Lipides 2011, 18, 267–270. [Google Scholar] [CrossRef]
- Olde Rikkert, M.G.; Verhey, F.R.; Blesa, R.; von Arnim, C.A.; Bongers, A.; Harrison, J.; Sijben, J.; Scarpini, E.; Vandewoude, M.F.; Vellas, B.; et al. Tolerability and safety of Souvenaid in patients with mild Alzheimer’s disease: Results of multi-center, 24-week, open-label extension study. J. Alzheimers Dis. 2015, 44, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Kamphuis, P.J.; Verhey, F.R.; Olde Rikkert, M.G.; Wurtman, R.J.; Wilkinson, D.; Twisk, J.W.; Kurz, A. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement. 2010, 6, 1.e11–10.e11. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Twisk, J.W.; Blesa, R.; Scarpini, E.; von Arnim, C.A.; Bongers, A.; Harrison, J.; Swinkels, S.H.; Stam, C.J.; de Waal, H.; et al. Efficacy of Souvenaid in mild Alzheimer’s disease: Results from a randomized, controlled trial. J. Alzheimers Dis. 2012, 31, 225–236. [Google Scholar] [CrossRef]
- de Waal, H.; Stam, C.J.; Lansbergen, M.M.; Wieggers, R.L.; Kamphuis, P.J.; Scheltens, P.; Maestu, F.; van Straaten, E.C. The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: A randomised controlled study. PLoS ONE 2014, 9, e86558. [Google Scholar] [CrossRef]
- Shah, R.C.; Kamphuis, P.J.; Leurgans, S.; Swinkels, S.H.; Sadowsky, C.H.; Bongers, A.; Rappaport, S.A.; Quinn, J.F.; Wieggers, R.L.; Scheltens, P.; et al. The S-Connect study: Results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 59. [Google Scholar] [CrossRef]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A Phase II Randomized Clinical Trial of a Nutritional Formulation for Cognition and Mood in Alzheimer’s Disease. J. Alzheimers Dis. 2015, 45, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Chiu, C.H.; Kuo, W.W.; Ju, D.T.; Shen, C.Y.; Chen, R.J.; Lin, C.C.; Viswanadha, V.P.; Liu, J.S.; Huang, R.S.; et al. The preventive effects of edible folic acid on cardiomyocyte apoptosis and survival in early onset triple-transgenic Alzheimer’s disease model mice. Environ. Toxicol. 2018, 33, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.T.; Huang, R.S.; Tsai, B.C.; Su, Y.C.; Chiu, P.L.; Chang, Y.M.; Padma, V.V.; Ho, T.J.; Yao, C.H.; Kuo, W.W.; et al. Folic Acid and Folinic Acid Protect Hearts of Aging Triple-transgenic Alzheimer’s Disease mice via IGF1R/PI3K/AKT and SIRT1/AMPK Pathways. Neurotox. Res. 2023, 41, 648–659. [Google Scholar] [CrossRef]
- Mattson, M.P.; Haberman, F. Folate and homocysteine metabolism: Therapeutic targets in cardiovascular and neurodegenerative disorders. Curr. Med. Chem. 2003, 10, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.M.; Song, Z.F.; Xu, H.D. Association between MTHFR genetic polymorphism and Parkinson’s disease susceptibility: A meta-analysis. Open Med. 2019, 14, 613–624. [Google Scholar] [CrossRef]
- Matherly, L.H.; Hou, Z. Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam. Horm. 2008, 79, 145–184. [Google Scholar] [CrossRef]
- Zhao, R.; Diop-Bove, N.; Visentin, M.; Goldman, I.D. Mechanisms of membrane transport of folates into cells and across epithelia. Annu. Rev. Nutr. 2011, 31, 177–201. [Google Scholar] [CrossRef]
- Qiu, A.; Jansen, M.; Sakaris, A.; Min, S.H.; Chattopadhyay, S.; Tsai, E.; Sandoval, C.; Zhao, R.; Akabas, M.H.; Goldman, I.D. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006, 127, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Rothem, L.; Aronheim, A.; Assaraf, Y.G. Alterations in the expression of transcription factors and the reduced folate carrier as a novel mechanism of antifolate resistance in human lLeukemia cells. J. Biol. Chem. 2003, 278, 8935–8941. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Dell’Oro, S.; Capello, D.; Ponzoni, M.; Iuzzolino, P.; Rossi, D.; Pasini, F.; Ambrosetti, A.; Orvieto, E.; Ferrarese, F.; et al. Aberrant methylation in the promoter region of the reduced folate carrier gene is a potential mechanism of resistance to methotrexate in primary central nervous system lymphomas. Br. J. Haematol. 2004, 126, 657–664. [Google Scholar] [CrossRef]
- Liu, T.; Singh, R.; Rios, Z.; Bhushan, A.; Li, M.; Sheridan, P.P.; Bearden, S.E.; Lai, J.C.K.; Agbenowu, S.; Cao, S.; et al. Tyrosine phosphorylation of HSC70 and its interaction with RFC mediates methotrexate resistance in murine L1210 leukemia cells. Cancer Lett. 2015, 357, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.; Ifergan, I.; Rothem, L.; Jansen, G.; Assaraf, Y.G. Coexistence of multiple mechanisms of PT523 resistance in human leukemia cells harboring 3 reduced folate carrier alleles: Transcriptional silencing, inactivating mutations, and allele loss. Blood 2006, 107, 3288–3294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matherly, L.H.; Hou, Z.; Gangjee, A. The promise and challenges of exploiting the proton-coupled folate transporter for selective therapeutic targeting of cancer. Cancer Chemother. Pharmacol. 2018, 81, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Raz, S.; Stark, M.; Assaraf, Y.G. Folylpoly-γ-glutamate synthetase: A key determinant of folate homeostasis and antifolate resistance in cancer. Drug Resist. Updat. 2016, 28, 43–64. [Google Scholar] [CrossRef]
- Wong, Y.C.; Bernal, S.D. Reversal of methotrexate resistance in human squamous carcinoma cells by SQM1-liposome. Anticancer Res. 1999, 19, 251–254. [Google Scholar]
- Scionti, I.; Michelacci, F.; Pasello, M.; Hattinger, C.M.; Alberghini, M.; Manara, M.C.; Bacci, G.; Ferrari, S.; Scotlandi, K.; Picci, P.; et al. Clinical impact of the methotrexate resistance-associated genes C-MYC and dihydrofolate reductase (DHFR) in high-grade osteosarcoma. Ann. Oncol. 2008, 19, 1500–1508. [Google Scholar] [CrossRef]
- Organista-Nava, J.; Gómez-Gómez, Y.; Illades-Aguiar, B.; Rivera-Ramírez, A.B.; Saavedra-Herrera, M.V.; Leyva-Vázquez, M.A. Overexpression of dihydrofolate reductase is a factor of poor survival in acute lymphoblastic leukemia. Oncol. Lett. 2018, 15, 8405–8411. [Google Scholar] [CrossRef]
- Göker, E.; Waltham, M.; Kheradpour, A.; Trippett, T.; Mazumdar, M.; Elisseyeff, Y.; Schnieders, B.; Steinherz, P.; Tan, C.; Berman, E.; et al. Amplification of the dihydrofolate reductase gene Is a mechanism of acquired resistance to methotrexate in patients with acute lymphoblastic leukemia and is correlated with p53 gene mutations. Blood 1995, 86, 677–684. [Google Scholar] [CrossRef]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of methotrexate toxicity: A comprehensive literature review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Huang, L.; Li, G.; Chen, L.; Ma, J.; Li, M.; Wei, M.; Zhou, W.; Zhou, C.; et al. Discovery of novel biomarkers of therapeutic responses in Han Chinese pemetrexed-based treated advanced NSCLC patients. Front. Pharmacol. 2019, 10, 944. [Google Scholar] [CrossRef]
- Masuda, S. Functional characteristics and pharmacokinetic significance of kidney-specific organic anion transporters, OAT-K1 and OAT-K2, in the urinary excretion of anionic drugs. Drug Metab. Pharmacokinet. 2003, 18, 91–103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse effects of low-dose methotrexate: A randomized trial. Ann. Intern. Med. 2020, 172, 369–380. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Al-Abd, A.M.; El-Dine, R.S.; El-Halawany, A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J. Adv. Res. 2015, 6, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Manautou, J.E. Regulation of hepatic ABCC transporters by xenobiotics and in disease states. Drug Metab. Rev. 2010, 42, 482–538. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, A.A.; Moiseeva, E.V.; Onishchenko, N.R.; Boldyrev, I.A.; Singin, A.S.; Budko, A.P.; Shprakh, Z.S.; Molotkovsky, J.G.; Vodovozova, E.L. Liposomal formulation of a methotrexate lipophilic prodrug: Assessment in tumor cells and mouse T-cell leukemic lymphoma. Int. J. Nanomed. 2017, 12, 3735–3749. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.S.; Khalid, F.; Khan, M.T.; Samra, Z.Q.; Muhammad, S.; Zhang, Y.J.; Mou, K. A novel method of magnetic nanoparticles functionalized with anti-folate receptor antibody and methotrexate for antibody mediated targeted drug delivery. Molecules 2022, 27, 261. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug delivery challenges in brain disorders across the blood-brain barrier: Novel methods and future considerations for improved therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-enhanced delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Zheng, S.; Guo, Y.; Yan, F.; Yan, F.; Qi, R.; Shen, J. Chances and challenges in intranasal administration delivery for brain disease treatment. Clin. Transl. Discov. 2023, 3, e253. [Google Scholar] [CrossRef]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for enhancing the permeation of CNS-active drugs through the blood-brain barrier: A review. Molecules 2018, 23, 1289. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K. Non-invasive drug delivery across the blood-brain barrier: A prospective analysis. Pharmaceutics 2023, 15, 2599. [Google Scholar] [CrossRef] [PubMed]
- Saucier-Sawyer, J.K.; Deng, Y.; Seo, Y.E.; Cheng, C.J.; Zhang, J.; Quijano, E.; Saltzman, W.M. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J. Drug Target 2015, 23, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for targeted brain drug delivery: What do we know? Int. J. Mol. Sci. 2021, 22, 11654. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Hynynen, K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert. Opin. Drug Deliv. 2014, 11, 711–721. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
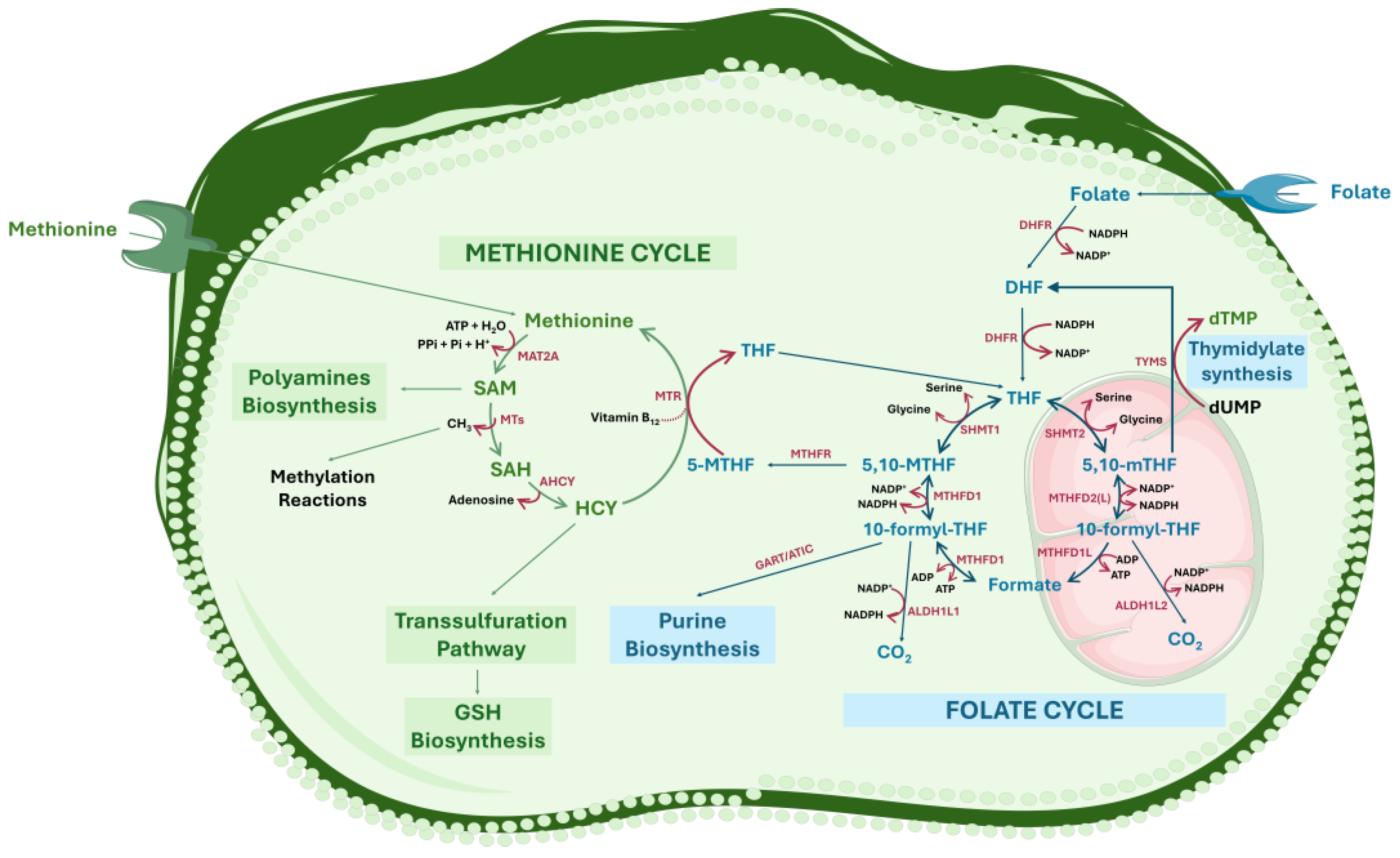
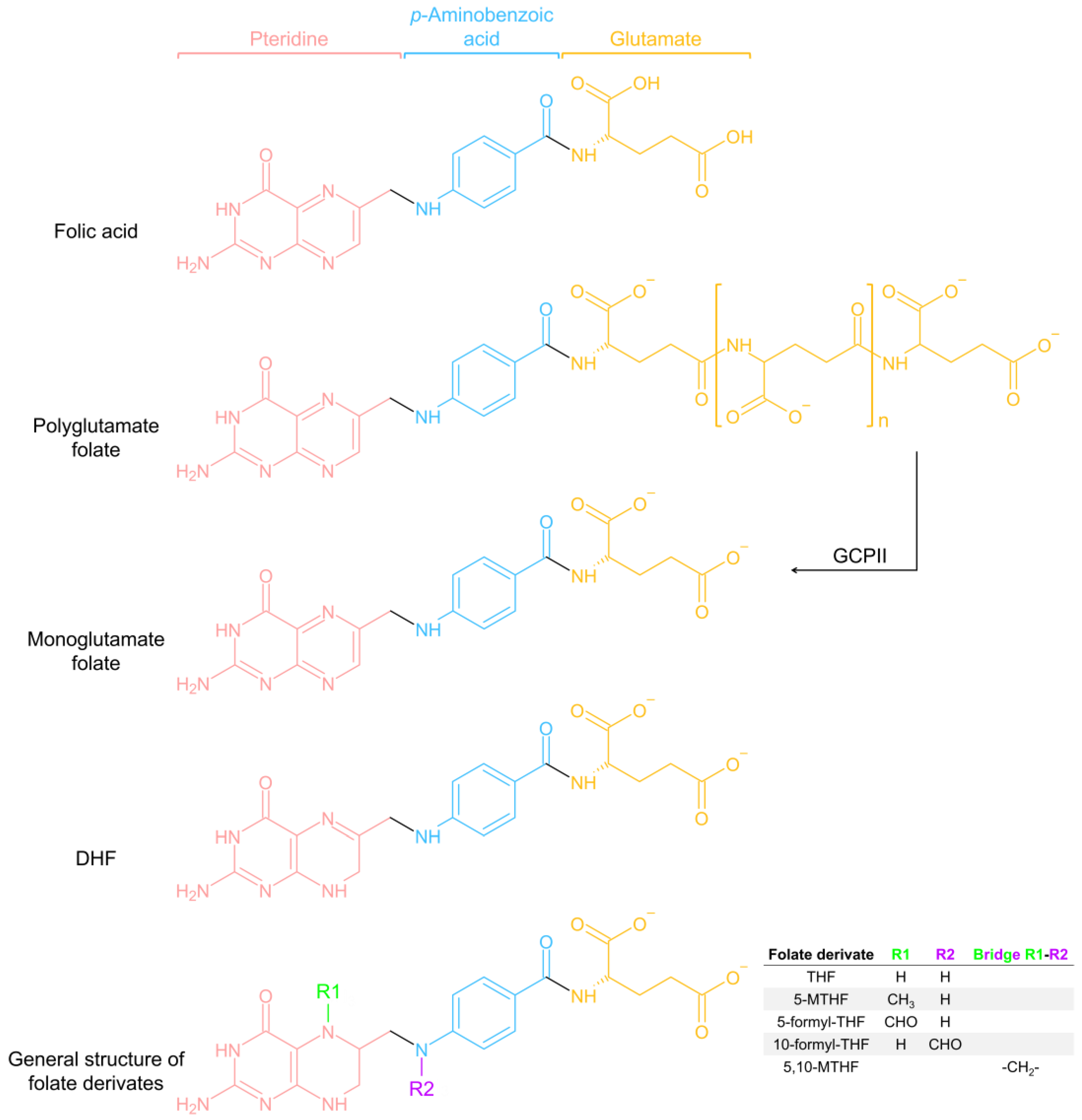
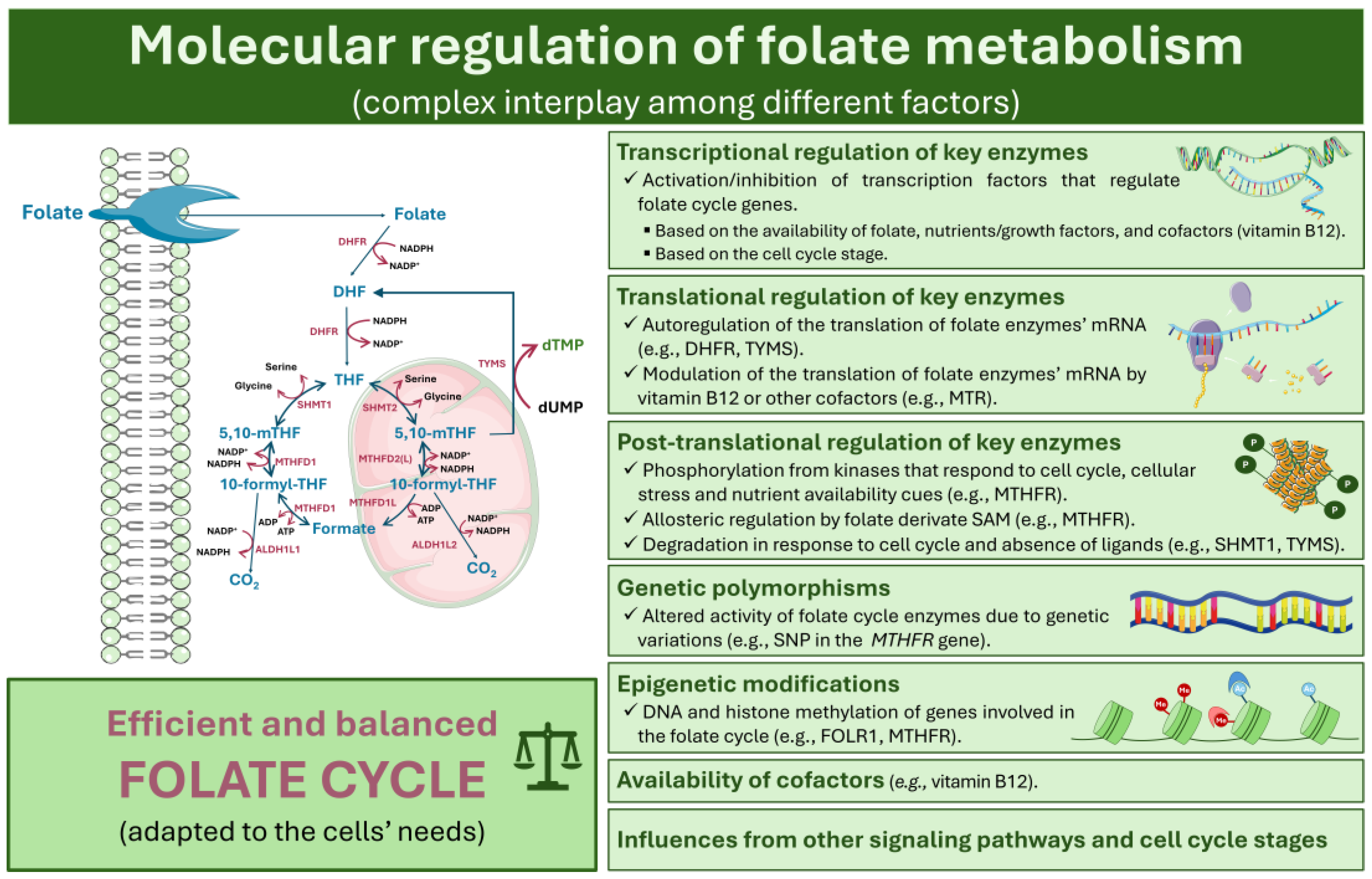


| Antifolate Drug | Description | Clinical Applications | Other Considerations | References |
|---|---|---|---|---|
| Vintafolide (EC145) | Folic acid conjugate with vinca alkaloid | Solid tumors (ovarian and non-small cell lung cancer) |
| [229,230,231,232] |
| Pafolacianine (OTL38) | Conjugate between folate and NIR fluorescent dye | Lung cancer |
| [220] |
| EC2629 | Folate conjugate of a DNA crosslinking agent | In combination with FOLR-positive KB human xenografts in mice |
| [204] |
| Farletuzumab (MORAb003) | Monoclonal antibody therapy | Solid tumors |
| [233,234] |
| MORAb202 | ADC with FOLR binding antibody | Advanced solid tumors |
| [222] |
| Mirvetuximab soravtansine (IMGB853) or Elahere™ | ADC targeting FOLR1 | FOLRα-positive ovarian, fallopian tube, and peritoneal cancers |
| [235,236] |
| CAR T-cell therapies | Cellular immunotherapy | Ovarian cancer |
| [225] |
| 5-MTHF | Albumin-binding radioconjugates | Cervical carcinoma |
| [228] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobral, A.F.; Cunha, A.; Silva, V.; Gil-Martins, E.; Silva, R.; Barbosa, D.J. Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 9339. https://doi.org/10.3390/ijms25179339
Sobral AF, Cunha A, Silva V, Gil-Martins E, Silva R, Barbosa DJ. Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration. International Journal of Molecular Sciences. 2024; 25(17):9339. https://doi.org/10.3390/ijms25179339
Chicago/Turabian StyleSobral, Ana Filipa, Andrea Cunha, Vera Silva, Eva Gil-Martins, Renata Silva, and Daniel José Barbosa. 2024. "Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration" International Journal of Molecular Sciences 25, no. 17: 9339. https://doi.org/10.3390/ijms25179339
APA StyleSobral, A. F., Cunha, A., Silva, V., Gil-Martins, E., Silva, R., & Barbosa, D. J. (2024). Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration. International Journal of Molecular Sciences, 25(17), 9339. https://doi.org/10.3390/ijms25179339








