Biological Properties of the Mucus and Eggs of Helix aspersa Müller as a Potential Cosmetic and Pharmaceutical Raw Material: A Preliminary Study
Abstract
:1. Introduction
2. Results
2.1. The Basic Composition of SM and SEs
2.2. The Fatty Acids Profile of SM and SE
2.3. The Amino Acids Content of SM and SE
2.4. Metabolites Identified in Fresh SE, Freeze-Dried SE, and Freeze-Dried SM
2.5. Antimicrobial Activity of SM and SE
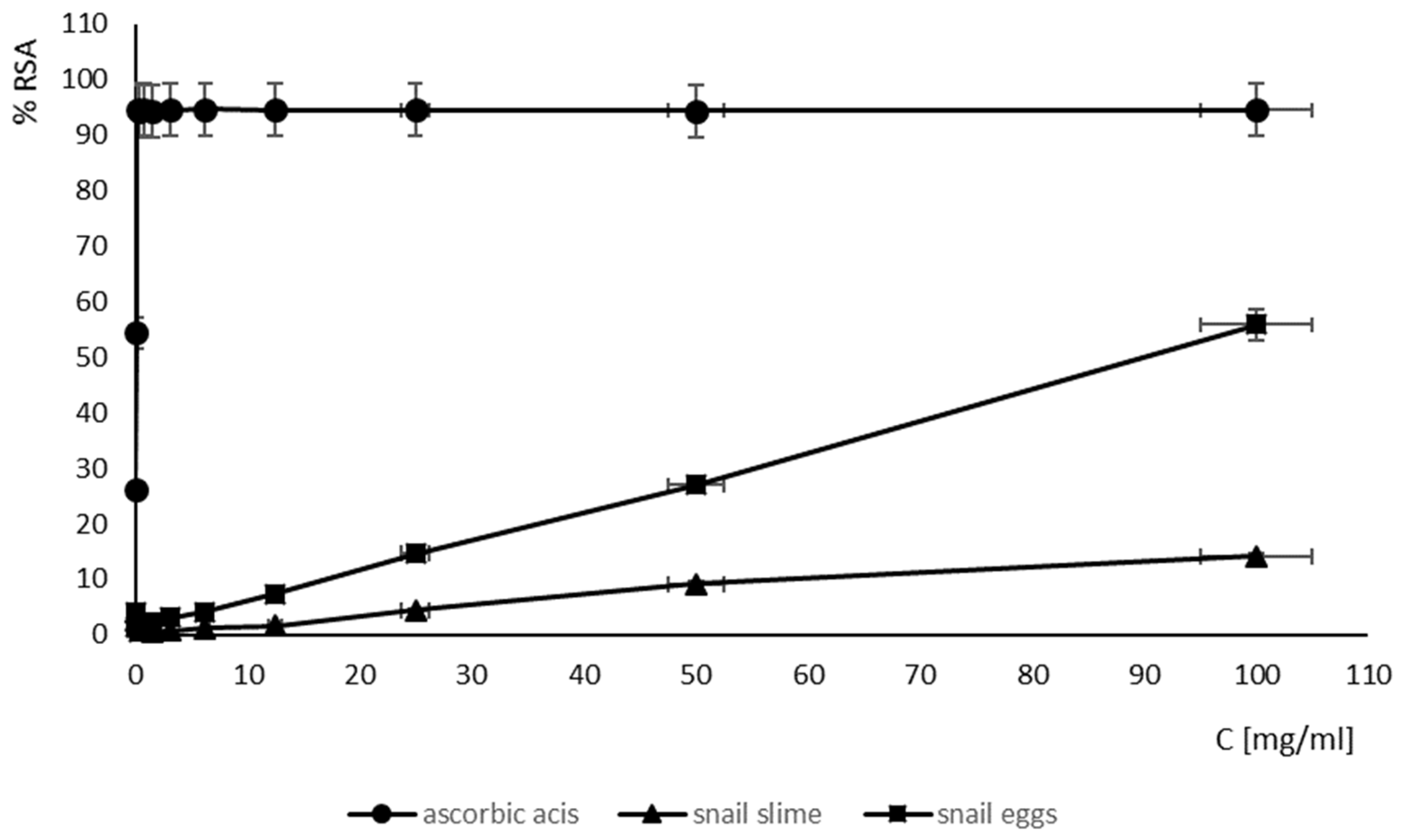
2.6. Antioxidant Activity of SM and SE
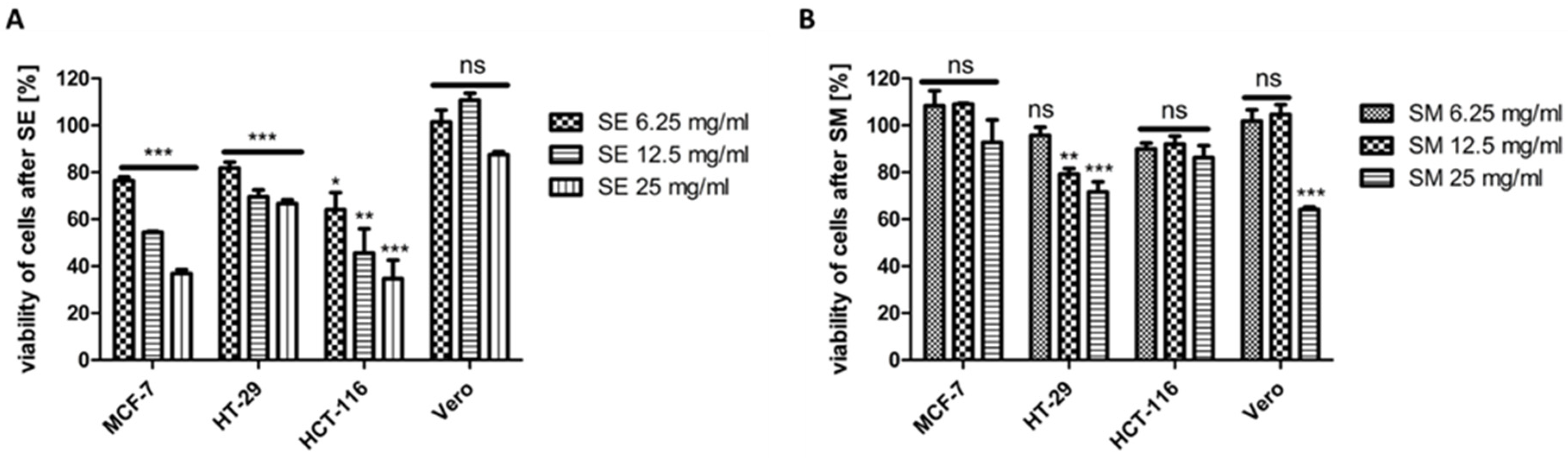
2.7. Effect of SEs and SM on the Viability of MCF-7, HCT-116, HT-29, and Vero Cell Lines
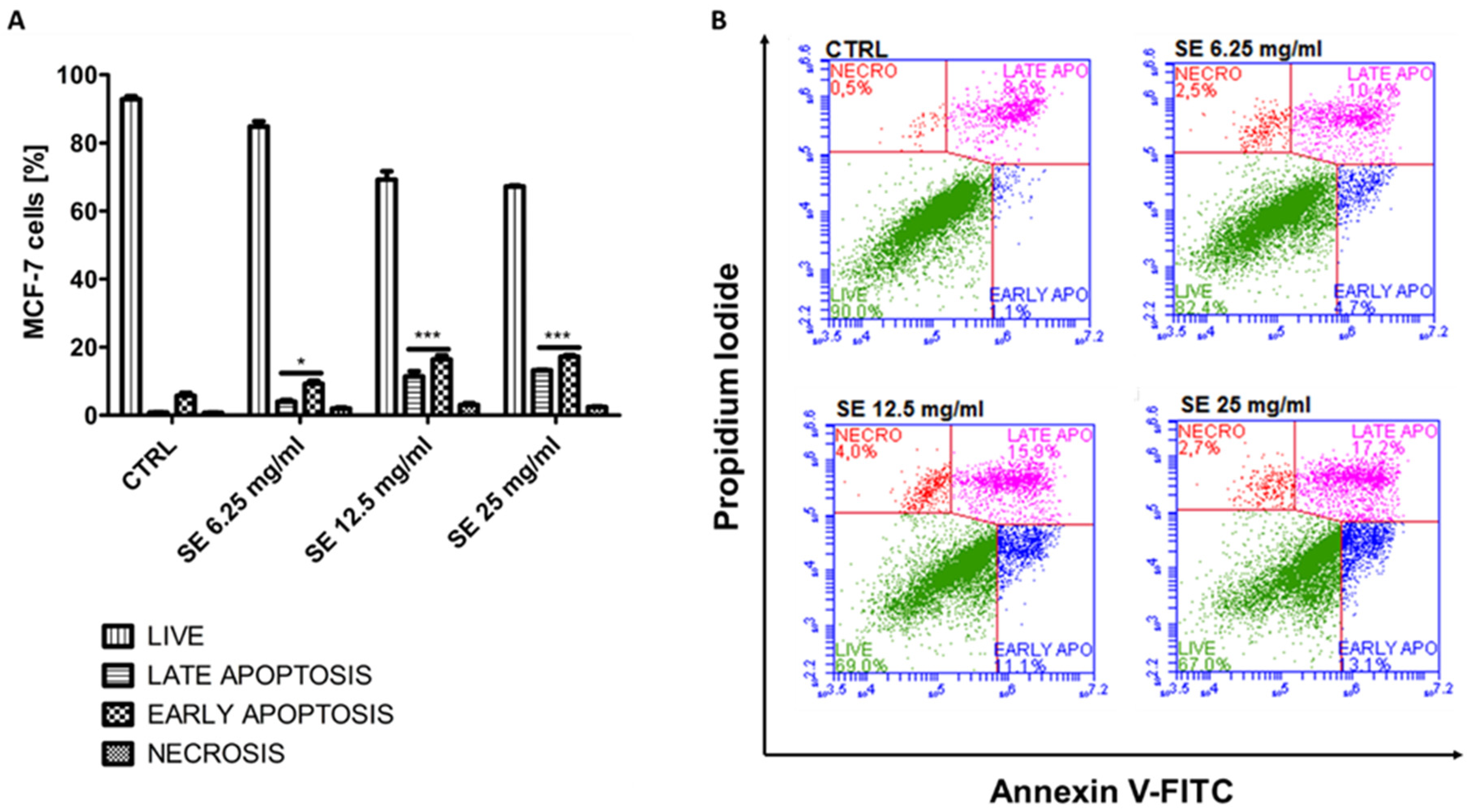
2.8. Induction of Apoptosis in MCF-7 and HCT-116 Cells
3. Discussion
4. Materials and Methods
4.1. SM and SEs from H. aspersa
4.2. Microorganisms
4.3. Cell Cultures
4.4. Chemicals
4.5. Analysis of the Basic Composition of SM and SEs
4.6. Analysis of Fatty Acids Profile of SM and SEs
4.7. Analysis of Amino Acids Content of SM and SEs
4.8. Untargeted Metabolomic Analysis by LC–MS Technique
4.9. Determination of the Antimicrobial Activity of SM and SEs—Agar Diffusion Method
4.10. Determination of Minimum Inhibition Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) of SM and SE by Broth Microdilution Methods
4.11. Determination of the Antioxidant Activity of Lyophilized SM and SEs—ABTS Assay
4.12. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)—Based Viability Assay
4.13. Detection of Apoptosis by Annexin V/Propidium Iodide (PI) Labeling
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Brown, A.N.; Gold, M.H. Snail extract for skin: A review of uses, projections, and limitations. J. Cosmet. Dermatol. 2024, 23, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.G.; Simova, S.D.; Dangalov, M.; Velkova, L.; Atanasov, V.; Dolashki, A.; Dolashka, P. An 1H NMR-and MS-based study of metabolites profiling of garden snail Helix aspersa mucus. Metabolites 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Dolashki, A.; Velkova, L.; Stevanovic, S.; Molin, L.; Traldi, P.; Velikova, R.; Voelter, W. Bioactive compounds isolated from garden snails. J. BioSci. Biotechnol. 2015, 147–155. [Google Scholar]
- Aouji, M.; Rkhaila, A.; Bouhaddioui, B.; Zirari, M.; Harifi, H.; Taboz, Y.; Lrhorfi, L.A.; Bengueddour, R. Chemical composition, mineral profile, anti-bacterial, and wound healing properties of snail slime of Helix aspersa Müller. BioMed. 2023, 13, 10–19. [Google Scholar] [CrossRef]
- Onzo, A.; Pascale, R.; Acquavia, M.A.; Cosma, P.; Gubitosa, J.; Gaeta, C.; Iannece, P.; Tsybin, Y.; Rizzi, V.; Guerrieri, A.; et al. Untargeted analysis of pure snail slime and snail slime-induced Au nanoparticles metabolome with MALDI FT-ICR MS. J. Mass Spectrometry. 2021, 56, e4722. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Siracusa, R.; Genovese, T.; D’Amico, R.; Impellizzeri, D.; Di Paola, R.; Cuzzocrea, S.; et al. Protective effect of snail secretion filtrate against ethanol-induced gastric ulcer in mice. Sci. Rep. 2021, 11, 3638. [Google Scholar] [CrossRef]
- Cerullo, A.R.; McDermott, M.B.; Pepi, L.E.; Liu, Z.L.; Barry, D.; Zhang, S.; Yang, X.; Chen, X.; Azadi, P.; Holford, M.; et al. Comparative mucomic analysis of three functionally distinct Cornu aspersum secretions. Nat. Commun. 2023, 14, 5361. [Google Scholar] [CrossRef]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- Laneri, S.; Lorenzo, R.D.; Sacchi, A.; Dini, I. Dosage of bioactive molecules in the nutricosmeceutical Helix aspersa muller mucus and formulation of new cosmetic cream with moisturizing effect. Nat. Prod. Commun. 2019, 14, 1934578X19868606. [Google Scholar] [CrossRef]
- Kostadinova, N.; Voynikov, Y.; Dolashki, A.; Krumova, E.; Abrashev, R.; Kowalewski, D.; Stevanovic, S.; Velkova, L.; Velikova, R.; Dolashka, P. Antioxidative screening of fractions from the mucus of garden snail Cornu aspersum. Bulg. Chem. Commun. 2018, 50, 176–183. [Google Scholar]
- Kim, Y.; Sim, W.J.; Lee, J.S.; Lim, T.G. Snail mucin is a functional food ingredient for skin. J. Funct. Foods 2022, 92, 105053. [Google Scholar] [CrossRef]
- Rosanto, Y.B.; Hasan, C.Y.; Rahardjo, R.; Pangestiningsih, T.W. Effect of snail mucus on angiogenesis during wound healing. F1000Research 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Zizioli, D.; Mastinu, A.; Muscò, A.; Bonini, S.A.; Finazzi, D.; Avisani, R.; Morelli, G.B.K.; Pecorelli, S.; Memo, M. Pro-angiogenetic effects of purified extracts from Helix aspersa during zebrafish development. Curr. Issues Mol. Biol. 2022, 44, 3364–3377. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Z.; Luo, L.; Tao, M.; Chang, X.; Yang, L.; Huang, X.; Hu, L.; Wu, M. A non-anticoagulant heparin-like snail glycosaminoglycan promotes healing of diabetic wound. Carbohydr. Polym. 2020, 247, 116682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Deng, T.; Tao, M.; Lin, L.; Sun, L.; Song, X.; Gao, D.; Li, J.; Wang, Z.; Wang, X.; et al. Snail-inspired AFG/GelMA hydrogel accelerates diabetic wound healing via inflammatory cytokines suppression and macrophage polarization. Biomater. 2023, 299, 122141. [Google Scholar] [CrossRef]
- Tancheva, L.; Lazarova, M.; Velkova, L.; Dolashki, A.; Uzunova, D.; Borislava, M.; Polinaa, P.-K.; Yozljama, H.; Petjaa, G.; Krasimirad, T.; et al. Beneficial effects of snail Helix aspersa extract in an experimental model of Alzheimer’s type dementia. J. Alzheimer’s Dis. 2022, 88, 155–175. [Google Scholar] [CrossRef]
- Tsvetanova, E.; Alexandrova, A.; Georgieva, A.; Tancheva, L.; Lazarova, M.; Dolashka, P.; Velkova, L.; Dolashki, A.; Atanasov, V.; Kalfin, R. Effect of mucus extract of Helix aspersa on scopolamine-induced cognitive impairment and oxidative stress in rat’s brain. Bulg. Chem. Commun. 2020, 52, 107–111. [Google Scholar]
- Mencucci, R.; Strazzabosco, G.; Cristofori, V.; Alogna, A.; Bortolotti, D.; Gafà, R.; Cennamo, M.; Favuzza, E.; Trapella, C.; Gentili, V.; et al. GlicoPro, novel standardized and sterile snail mucus extract for multi-modulative ocular formulations: New perspective in dry eye disease management. Pharmaceutics 2021, 13, 2139. [Google Scholar] [CrossRef]
- Cilia, G.; Fratini, F. Antimicrobial properties of terrestrial snail and slug mucus. J. Complement. Integr. Med. 2018, 15, 20170168. [Google Scholar] [CrossRef]
- Ellijimi, C.; Ben Hammouda, M.; Othman, H.; Moslah, W.; Jebali, J.; Mabrouk, H.B.; Morjen, M.; Haoues, M.; Luis, J.; Marrakchi, N.; et al. Helix Aspersa Maxima mucus exhibits antimelanogenic and antitumoral effects against melanoma cells. Biomed. Pharmacother. 2018, 101, 871–880. [Google Scholar] [CrossRef]
- El-Ouar, I.; Braicu, C.; Naimi, D.; Irimie, A.L.E.X.E.N.D.R.U.; Berindan-Neagoe, I. Anti tumour effect of aqueous extract from Helix aspersa. Pharmacogn. Mag. 2013, 13, 281. [Google Scholar]
- Teerasak, E.; Thongararm, P.; Roytrakul, S.; Meesuk, L.; Chumnanpuen, P. Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput. Struct. Biotech. J. 2016, 14, 49–57. [Google Scholar]
- Smith, B.N.; Odero-Marah, V.A. The role of snail in prostate cancer. Cell Adhes. Migr. 2012, 6, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Toshkova, R.; Velkova, L.; Dolashki, A.; Dolashka, P. Hemocyanins from Helix and Rapana snails exhibit in vitro antitumor effects in human colorectal adenocarcinoma. Biomedicines 2020, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Dolashki, A.; Velkova, L.; Dolashka, P.; Toshkova, R. Assessment of the in vitro and in vivo antitumor activity of hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi myeloid tumor model. Biomedicines 2023, 11, 1545. [Google Scholar] [CrossRef]
- Stabili, L.; Schirosi, R.; Parisi, M.G.; Piraino, S.; Cammarata, M. The mucus of Actinia equina (Anthozoa, Cnidaria): An unexplored resource for potential applicative purposes. Mar. Drugs 2015, 13, 5276–5296. [Google Scholar] [CrossRef]
- Jänicke, R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009, 117, 219–221. [Google Scholar] [CrossRef]
- EL-Zawawy, N.A.; Mona, M.M. Evaluation and comparison of antimicrobial efficacy of snail mucus of Egyptian Eremina desertorum and Helix aspersa with novel approach of their anti-inflammatory and wound healing potencies. Res. Square 2021, 1–21. [Google Scholar]
- Dolashki, A.; Velkova, L.; Daskalova, E.; Zheleva, N.; Topalova, Y.; Atanasov, V.; Voelter, W.; Dolashka, P. Antimicrobial compounds from the mucus of garden snail Cornu aspersa. Biomedicine 2020, 8, 315. [Google Scholar]
- Matusiewicz, M.; Marczak, K.; Kwiecińska, B.; Kupis, J.; Zglińska, K.; Niemiec, T.; Kosieradzka, I. Effect of extracts from eggs of Helix aspersa maxima and Helix aspersa aspersa snails on Caco-2 colon cancer cells. PeerJ 2022, 10, e13217. [Google Scholar] [CrossRef]
- Kanjilal, S.; Kaki, S.S. Antimicrobial Materials for Biomedical Applications; Domb, A.J., Kunduru, K.R., Farah, S., Eds.; The Royal Society of Chemistry: London, UK, 2019; Volume 16, pp. 457–480. [Google Scholar]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D.C.; Galli, G.R.; Curcio, R.; Malaguarnera, R.; Belfiore, A.; Cappello, A.R.; Maggiolini, M. The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Dis. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Fauser, J.K.; Matthews, G.M.; Cummins, A.G.; Howarth, G.S. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 2014, 59, 214–224. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Gu, J.; Yan, W.; Zhang, J. Steric configuration-enabled selective antimicrobial activity of chiral cysteine. Biochem. Biophys. Res. Commun. 2019, 512, 505–510. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, W.; Yang, X.; Yan, X.; Liu, R. A novel cysteine-rich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides 2013, 39, 1–5. [Google Scholar] [CrossRef]
- Banerji, B.; Pramanik, S.K.; Pal, U.; Maiti, N.C. Potent anticancer activity of cystine-based dipeptides and their interaction with serum albumins. Chem. Cent. J. 2013, 7, 91. [Google Scholar] [CrossRef]
- Chu, P.Y.; Liu, M.Y. Amino acid cysteine induces senescence and decelerates cell growth in melanoma. J. Funct. Foods 2015, 18, 455–462. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix Aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef]
- Pitt, S.J.; Hawthorne, J.A.; Garcia-Maya, M.; Alexandrovich, A.; Symonds, R.C.; Gunn, A. Identification and characterisation of anti-Pseudomonas aeruginosa proteins in mucus of the brown garden snail, Cornu aspersum. Br. J. Biomed. Sci. 2019, 76, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Velkova, L.; Dolashki, A.; Petrova, V.; Pisareva, E.; Kaynarov, D.; Kermedchiev, M.; Todorova, M.; Dolashka, P. Antibacterial properties of peptide and protein fractions from Cornu aspersum mucus. Molecules 2024, 29, 2886. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Nissimova, A.; Daskalova, E.; Velkova, L.; Topalova, Y.; Hristova, P.; Traldi, P.; Voelter, W.; Dolashka, P. Structure and antibacterial activity of isolated peptides from the mucus of garden snail Cornu aspersum. Bulg. Chem. Commun. C 2018, 50, 195–200. [Google Scholar]
- Velkova, L.; Nissimova, A.; Dolashki, A.; Daskalova, E.; Dolashka, P.; Topalova, Y. Glycine-rich peptides from Cornu aspersum snail with antibacterial activity. Bulg. Chem. Commun. 2018, 50, 169–175. [Google Scholar]
- Abimbola Okeniyi, F.; Oghenebrorhie Mavis, O.; Oyewale Olawoye, S.; Adekunle Animashahun, R.; Gbemisola Adeyonu, A. Antimicrobial potentials of mucus mucin from different species of giant African land snails on some typed culture pathogenic bacteria. Asian J. Agric. Biol. 2022, 4, 1–12. [Google Scholar]
- Etim, L.; Aleruchi, C.; Obande, G. Antibacterial properties of snail mucus on bacteria isolated from patients with wound infection. Br. Microbiol. Res. J. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Harti, A.S.; Sutanto, Y.; Putriningrum, R.; Umarianti, T.; Windyastuti, E.; Irdianty, M.S. The effectiveness of chitosan and snail seromucous as anti tuberculosis drugs. Open Access Maced. J. Med. Sci. 2021, 9, 510–514. [Google Scholar] [CrossRef]
- Gugu, T.H.; Onwusoba, R.C.; Onyi, P.N.; Ozioko, A.C. Synergistic interaction of natural snail mucin and lincomycin for immuno-chemotherapy against Streptopneumococcal infection: Checkerboard evaluations. Int. J. Pharm. Investig. 2020, 10, 379–383. [Google Scholar] [CrossRef]
- Dolashka, P.; Dolashki, A.; Voelter, W.; Van Beeumen, J.; Stevanovic, S. Antimicrobial activity of peptides from the hemolymph of Helix lucorum snails. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 1061–1071. [Google Scholar]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Kosieradzka, I.; Niemiec, T.; Grodzik, M.; Antushevich, H.; Strojny, B.; Gołębiewska, M. In vitro influence of extracts from snail Helix aspersa Müller on the colon cancer cell line Caco-2. Int. J. Mol. Sci. 2018, 19, 1064. [Google Scholar] [CrossRef] [PubMed]
- Vlahova, Z.; Tzintzarov, A.; Petrova, M.; Dushkov, A.; Velkova, L.; Dolashki, A.; Dolashka, P.; Ugrinova, I. Additive effects of Helix aspersa mucus fractions with chemotherapy drugs in breast cancer: Exploring anti-proliferative potential. Proceed. Bulg. Acad. Sci. 2023, 76, 1505–1516. [Google Scholar] [CrossRef]
- Petrova, M.; Vlahova, Z.; Schröder, M.; Tzintzarov, A.; Velkova, L.; Kaynarov, D.; Dolashki, A.; Dolashka, P.; Ugrinova, I. Anti-tumour activity of bioactive compounds isolated from the hemolymph and mucus of the garden snail Helix aspersa against a panel of human cancer cell lines. Proceed. Bulg. Acad. Sci. 2023, 76, 1350–1359. [Google Scholar] [CrossRef]
- Ho, C.Y.; Hu, D.W.; Chen, B.R.; Yang, C.C.; Yao, C.H.; Ni, T.; Chen, Y.S.; Tu, C.Y.; Chang, W.C.; Wu, Y.C.; et al. Snail mucus induces cytotoxicity and chemosensitivity of triple-negative breast cancer cells via activation of Fas signaling pathway. Anticancer Res. 2022, 42, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Ziada, M.; Mona, M.; Basyony, M.; Salem, M. In vivo and in vitro antitumor effects of Helix desertorum hemolymph by inducing cell cycle arrest and apoptosis. Egypt. J. Cancer Biomed. Res. 2021, 5, 155–164. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaitherburg, Sweden, 2011; p. 2590. [Google Scholar]
- Białek, M.; Czauderna, M.; Białek, A. Partial replacement of rapeseed oil with fish oil, and dietary antioxidants supplementation affects concentrations of biohydrogenation products and conjugated fatty acids in rumen and selected lamb tissues. Anim. Feed Sci. Technol. 2018, 241, 63–74. [Google Scholar] [CrossRef]
- Czauderna, M.; Karpińska, M.; Woliński, J.; Zaworski, K.; Białek, M.; Pierzynowski, S.; Wojtak, W.; Pierzynowska, K. Improved lipid saponification for chromatographic quantification of fatty acids in porcine erythrocytes—An important lipidomic biomarker of the effectiveness of dietary fat supplementation in pigs as a large animal model for human studies. J. Anim. Feed Sci. 2023, 32, 385–399. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Niedźwiedzka, K.M.; Wąsowska, I. Determination of free- and protein primary amino acids in biological materials by high-performance liquid chromatography and photodiode array detection. J. Anim. Feed Sci. 2002, 11, 143–167. [Google Scholar] [CrossRef]
- Ivanova, L.; Tartor, H.; Grove, S.; Kristoffersen, A.; Uhlig, S. Workflow for the targeted and untargeted detection of small metabolites in fish skin mucus. Fishes 2018, 3, 21. [Google Scholar] [CrossRef]
- EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing. Version 11.0 Valid from January 2023. Available online: www.eucast.org (accessed on 1 January 2023).
- EUCAST Broth Microdilution. Version 5.0 Valid from January 2024. Available online: www.eucast.org (accessed on 1 January 2024).
- EUCAST Method for Susceptibility Testing of Yeasts. Version 7.4 Valid from October 2023. Available online: www.eucast.org (accessed on 13 October 2023).
- EUCAST Method for Susceptibility Testing of Moulds. Version 9.4 Valid from April 2022. Available online: www.eucast.org (accessed on 1 April 2022).
- Chojnacki, K.; Wińska, P.; Skierka, K.; Wielechowska, M.; Bretner, M. Synthesis, in vitro antiproliferative activity and kinase profile of new benzimidazole and benzotriazole derivatives. Bioorg. Chem. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Wińska, P.; Wielechowska, M.; Koronkiewicz, M.; Borowiecki, P. Synthesis and anticancer activity of novel dual inhibitors of human protein kinases CK2 and PIM-1. Pharmaceutical 2023, 15, 1991. [Google Scholar] [CrossRef] [PubMed]

| Sample | SE (Fresh) | SE (Freeze-Dried) | SM (Freeze-Dried) | |
|---|---|---|---|---|
| Variables | ||||
| Nitrogen [%] | 0.74 ± 0.06 | 3.63 ± 0.07 | 0.29 ± 0.03 | |
| Crude protein [%] | 4.63 ± 0.38 | 22.7 ± 0.40 | 1.81 ± 0.16 | |
| Dry matter [%] | 15.6 ± 0.28 | 95.8 ± 0.05 | 97.4 ± 0.11 | |
| Ash [%] | 3.60 ± 0.05 | 25.8 ± 0.04 | 1.98 ± 0.04 | |
| Crude fat [%] | 0.23 ± 0.03 | 0.38 ± 0.02 | 1.86 ± 0.06 | |
| Energy [cal/g] | 3758 ± 6.43 | 4224 ± 3.06 | 7071 ± 6.66 | |
| Sample | SE (Fresh) | SE (Freeze-Dried) | SM (Freeze-Dried) | |
|---|---|---|---|---|
| Fatty Acid | ||||
| ƩFAs | 325 ± 68.1 | 911 ± 272 | 564 ± 149 | |
| C10:0 | 2.63 ± 1.54 | 4.63 ± 1.84 | 4.35 ± 1.34 | |
| C12:0 | 5.78 ± 2.89 | 24.2 ± 2.2 | 7.05 ± 1.14 | |
| C14:0 | 15.2 ± 5.37 | 42.6 ± 11.2 | 24.7 ± 6.29 | |
| C15:0 | 3.22 ± 1.09 | 3.52 ± 1.90 | 0.00 ± 0.00 | |
| C16:0 | 102 ± 34.1 | 318 ± 140 | 193 ± 19.8 | |
| C17:0 | 3.48 ± 0.91 | 3.45 ± 1.37 | 0.65 ± 0.17 | |
| C18:0 | 136 ± 30.2 | 437 ± 101 | 237 ± 43.5 | |
| C20:0 | 1.13 ± 0.31 | 3.77 ± 1.32 | 2.32 ± 0.57 | |
| Ʃ SFA | 269 ± 66.6 | 837 ± 326 | 469 ± 80.2 | |
| c7 C16:1 | 7.40 ± 2.52 | 2.02 ± 3.21 | 5.05 ± 5.03 | |
| c9 C16:1 | 4.94 ± 2.91 | 4.09 ± 6.91 | 4.64 ± 2.79 | |
| c9 C18:1 | 27.0 ± 11.2 | 59.6 ± 39.1 | 51.9 ± 26.8 | |
| c11 C18:1 | 1.88 ± 1.79 | 8.57 ± 11.9 | 3.23 ± 3.51 | |
| Ʃ MUFA | 41.2 ± 13.9 | 74.3 ± 48.1 | 64.8 ± 15.6 | |
| c9c12 C18:2 (LA) | 13.1 ± 1.8 | 0.00 ± 0.00 | 27.7 ± 5.14 | |
| c5c8c11c14 C20:4 (AA) | 0.80 ± 1.96 | 0.00 ± 0.00 | 1.95 ± 0.21 | |
| Ʃ PUFA | 13.9 ± 1.32 | 0.00 ± 0.00 | 29.7 ± 8.11 | |
| Sample | SE (Fresh) | SE (Freeze-Dried) | SM (Freeze-Dried) | |
|---|---|---|---|---|
| Amino Acid | ||||
| Cysteine | 4.83 ± 0.06 | 5.19 ± 0.04 | 5.30 ± 0.04 | |
| Aspartic acid | 1.85 ± 0.02 | 3.91 ± 0.04 | 0.44 ± 0.02 | |
| Glutamic acid | 0.72 ± 0.04 | 0.62 ± 0.03 | 0.26 ± 0.02 | |
| Asparagine | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Glutamine | 0.67 ± 0.02 | 1.83 ± 0.04 | 0.09 ± 0.02 | |
| Histidine | 0.84 ± 0.04 | 1.83 ± 0.02 | 0.20 ± 0.02 | |
| Serine | 0.99 ± 0.03 | 2.27 ± 0.04 | 0.70 ± 0.05 | |
| Arginine | 1.48 ± 0.02 | 3.48 ± 0.07 | 0.00 ± 0.00 | |
| Glycine | 0.26 ± 0.03 | 0.56 ± 0.04 | 0.00 ± 0.00 | |
| Threonine | 0.29 ± 0.04 | 0.52 ± 0.02 | 0.00 ± 0.00 | |
| Tyrosine | 0.69 ± 0.04 | 1.53 ± 0.03 | 0.00 ± 0.00 | |
| Alanine | 0.38 ± 0.01 | 0.88 ± 0.03 | 0.16 ± 0.03 | |
| Taurine | 0.00 ± 0.00 | 0.62 ± 0.06 | 0.00 ± 0.00 | |
| Methionine | 0.00 ± 0.00 | 2.48 ± 0.06 | 0.00 ± 0.00 | |
| Valine | 0.00 ± 0.00 | 0.19 ± 0.03 | 0.06 ± 0.01 | |
| Phenylalanine | 1.01 ± 0.06 | 0.00 ± 0.00 | 0.19 ± 0.02 | |
| Isoleucine | 0.41 ± 0.03 | 0.47 ± 0.03 | 0.10 ± 0.01 | |
| Leucine | 0.41 ± 0.04 | 1.12 ± 0.03 | 0.00 ± 0.00 | |
| Cysteine | 29.4 ± 0.10 | 70.3 ± 0.10 | 7.99 ± 0.06 | |
| Homocysteine | 0.14 ± 0.03 | 0.33 ± 0.04 | 0.00 ± 0.00 | |
| Lysine | 1.51 ± 0.02 | 2.93 ± 0.03 | 0.20 ± 0.01 | |
| ƩAA | 45.9 ± 0.19 | 101 ± 0.17 | 15.7 ± 0.14 | |
| ƩexoAA | 5.96 ± 0.08 | 13.0 ± 0.09 | 0.75 ± 0.07 | |
| ƩsulfuricAA | 34.2 ± 0.16 | 78.0 ± 0.06 | 13.3 ± 0.05 | |
| Diameter inhibition Zone [mm] | |||||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | E. coli | C. albicans | A. brasiliensis | |
| SM | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| SE | 12 ± 0.9 | 3 ± 0.8 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| ofloxacin 5 μg | 29 ± 0.5 | 25 ± 0.5 | 35 ± 0 | - | - |
| nystatin 100 IU | - | - | - | 21 ± 0.4 | 15 ± 0 |
| Microorganism | SM | SE | Novobiocin | Fluconazol | ||||
|---|---|---|---|---|---|---|---|---|
| MIC [mg/mL] | MBC/MFC [mg/mL] | MIC [mg/mL] | MBC/MFC [mg/mL] | MIC [μg/mL] | MBC [μg/mL] | MIC [μg/mL] | MFC [μg/mL] | |
| S. aureus | >50.00 | >50.00 | 12.50 | >50.00 | 0.49 | 0.98 | - | - |
| P. aeruginosa | >50.00 | >50.00 | 3.12 | >50.00 | 15.6 | 31.25 | - | - |
| E. coli | >50.00 | >50.00 | >50.00 | >50.00 | 125 | 250 | - | - |
| C. albicans | >50.00 | >50.00 | >50.00 | >50.00 | - | - | 0.49 | 0.98 |
| EC50 [mg/mL] | |
|---|---|
| snail mucus | ↑100 |
| snail eggs | 89.64 |
| ascorbic acid | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, A.; Wińska, P.; Białek, M.; Herman, A.P. Biological Properties of the Mucus and Eggs of Helix aspersa Müller as a Potential Cosmetic and Pharmaceutical Raw Material: A Preliminary Study. Int. J. Mol. Sci. 2024, 25, 9958. https://doi.org/10.3390/ijms25189958
Herman A, Wińska P, Białek M, Herman AP. Biological Properties of the Mucus and Eggs of Helix aspersa Müller as a Potential Cosmetic and Pharmaceutical Raw Material: A Preliminary Study. International Journal of Molecular Sciences. 2024; 25(18):9958. https://doi.org/10.3390/ijms25189958
Chicago/Turabian StyleHerman, Anna, Patrycja Wińska, Małgorzata Białek, and Andrzej P. Herman. 2024. "Biological Properties of the Mucus and Eggs of Helix aspersa Müller as a Potential Cosmetic and Pharmaceutical Raw Material: A Preliminary Study" International Journal of Molecular Sciences 25, no. 18: 9958. https://doi.org/10.3390/ijms25189958
APA StyleHerman, A., Wińska, P., Białek, M., & Herman, A. P. (2024). Biological Properties of the Mucus and Eggs of Helix aspersa Müller as a Potential Cosmetic and Pharmaceutical Raw Material: A Preliminary Study. International Journal of Molecular Sciences, 25(18), 9958. https://doi.org/10.3390/ijms25189958






