Cytokines and Pancreatic Ductal Adenocarcinoma: Exploring Their Relationship with Molecular Subtypes and Prognosis
Abstract
1. Introduction
2. Results
2.1. Descriptive Analysis of Clinical and Analytical Variables
2.2. Analysis of the Relationship between the Analyzed Cytokines and Other Analytical Parameters with Molecular Subtypes
2.3. Survival Analysis of the Different Treatment Regimens Received and Relationship between the Molecular Subtype and Prognosis
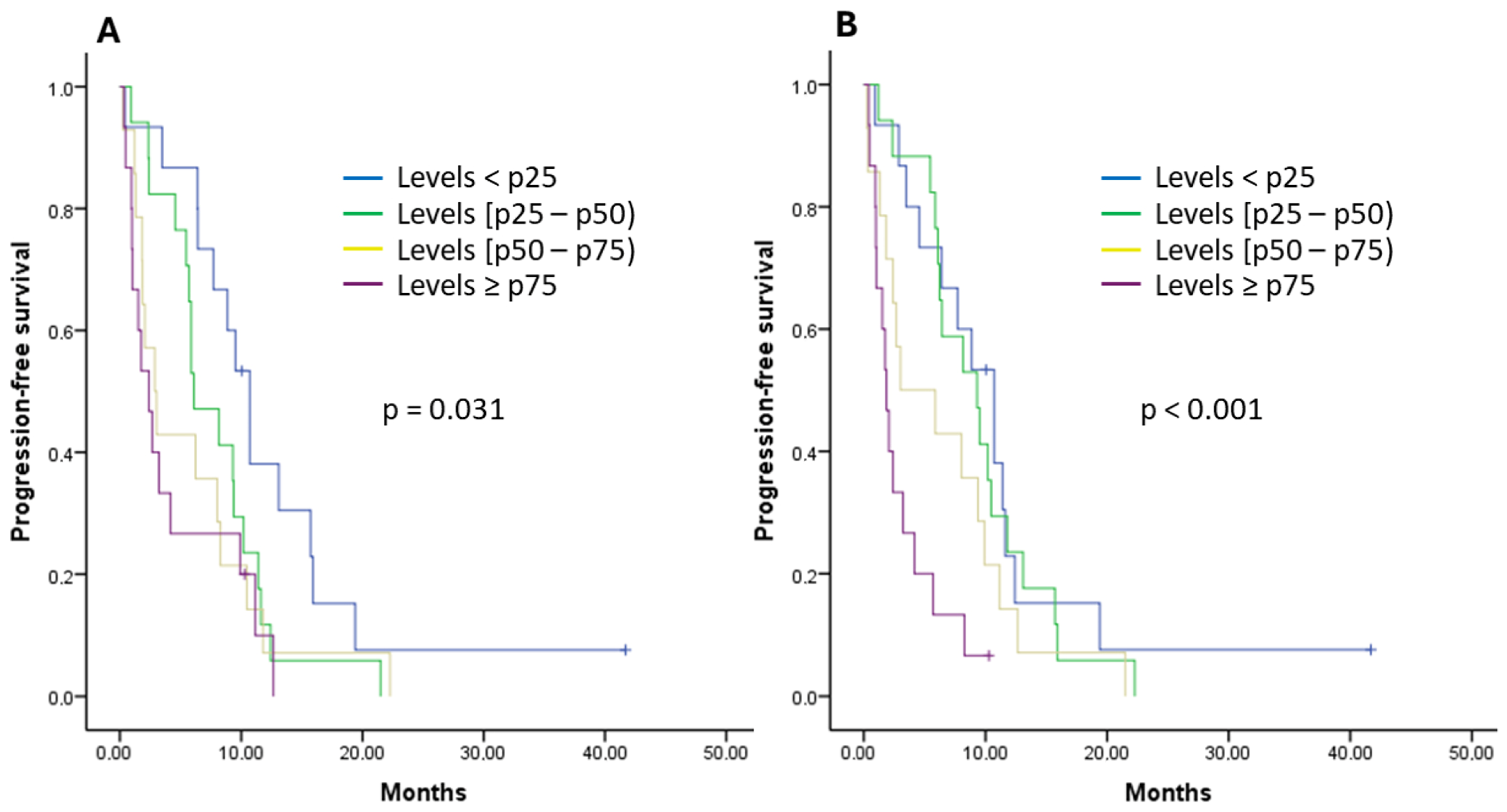
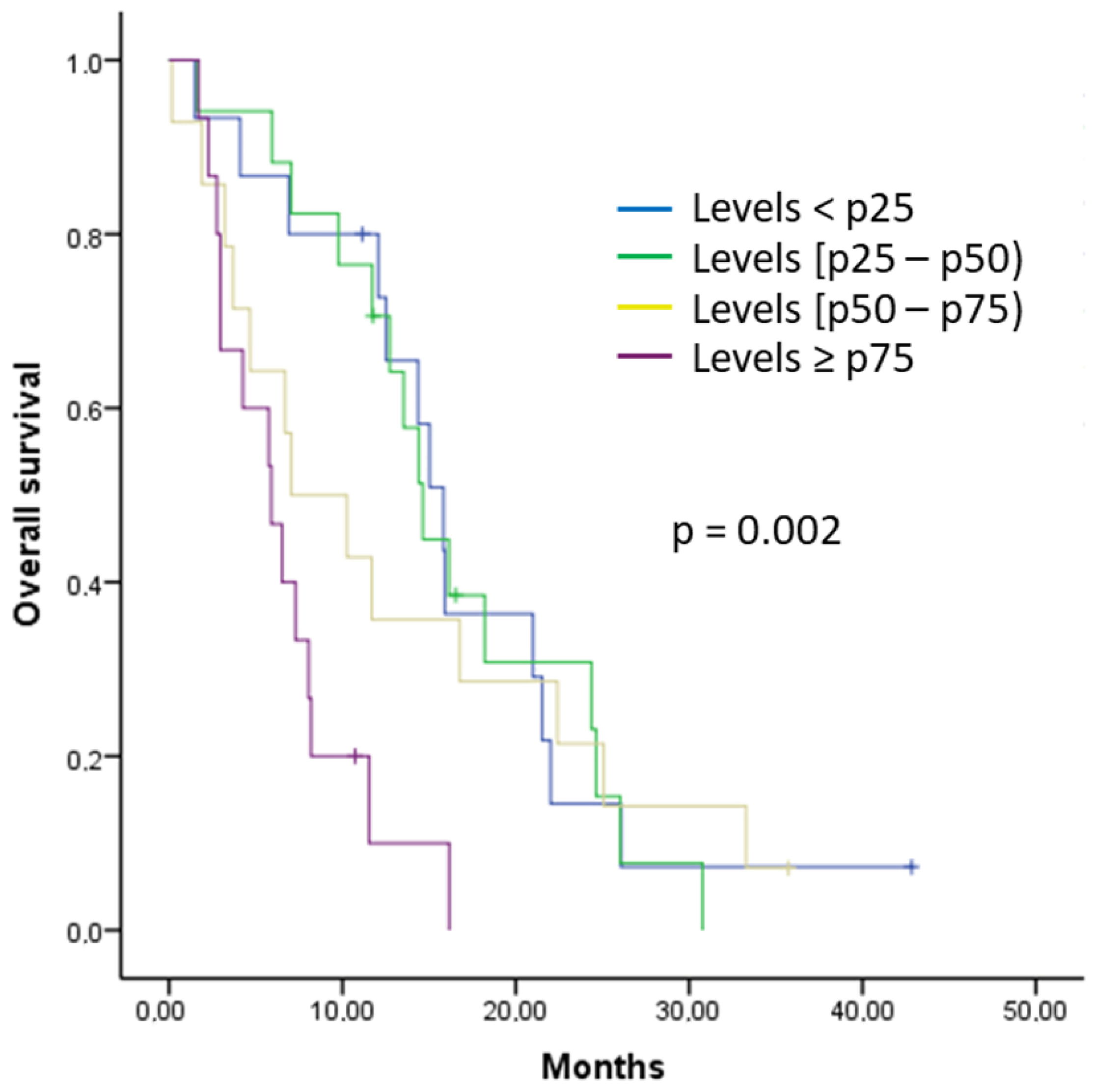
2.4. Analysis of the Relationship between the Analyzed Cytokines and Survival
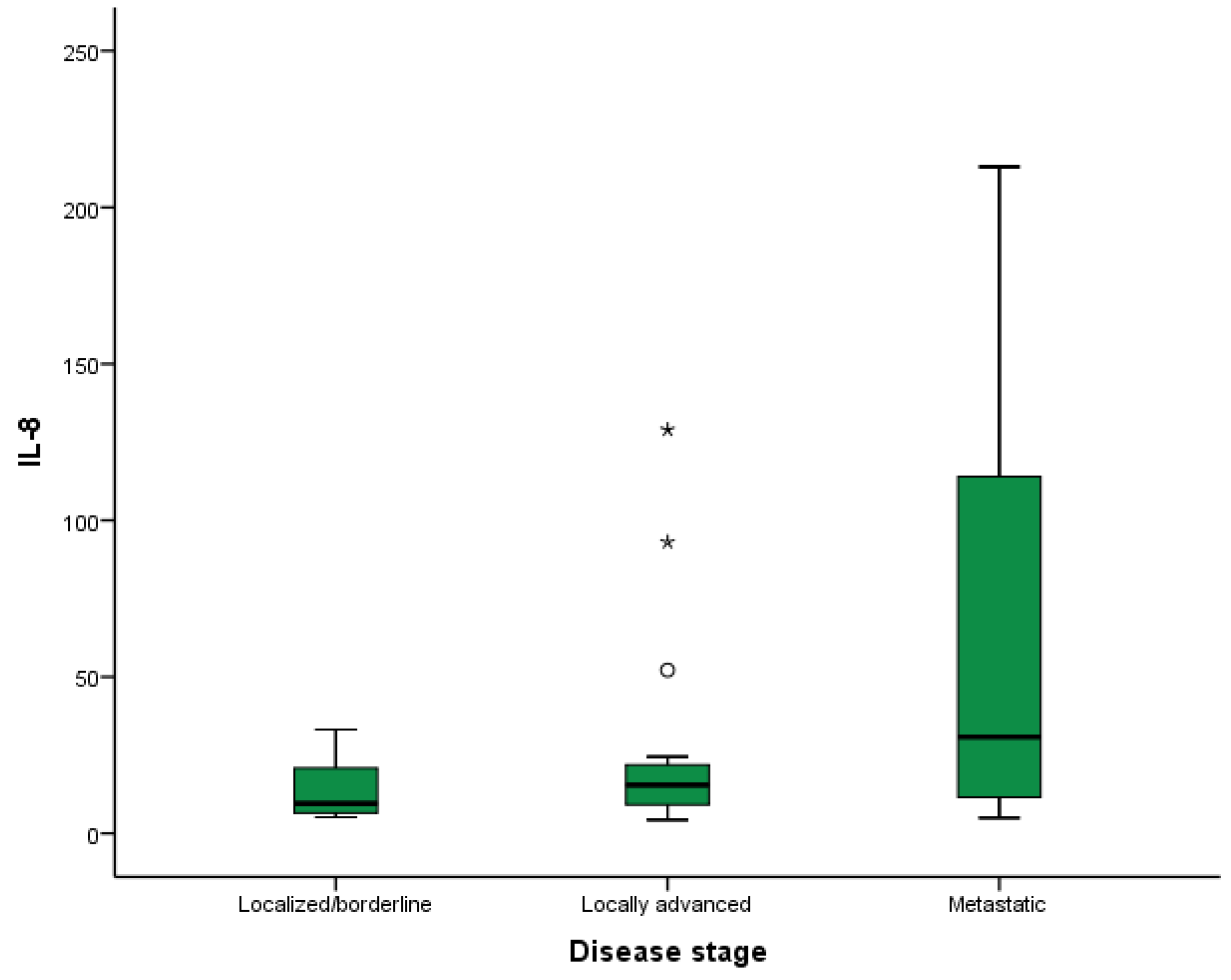
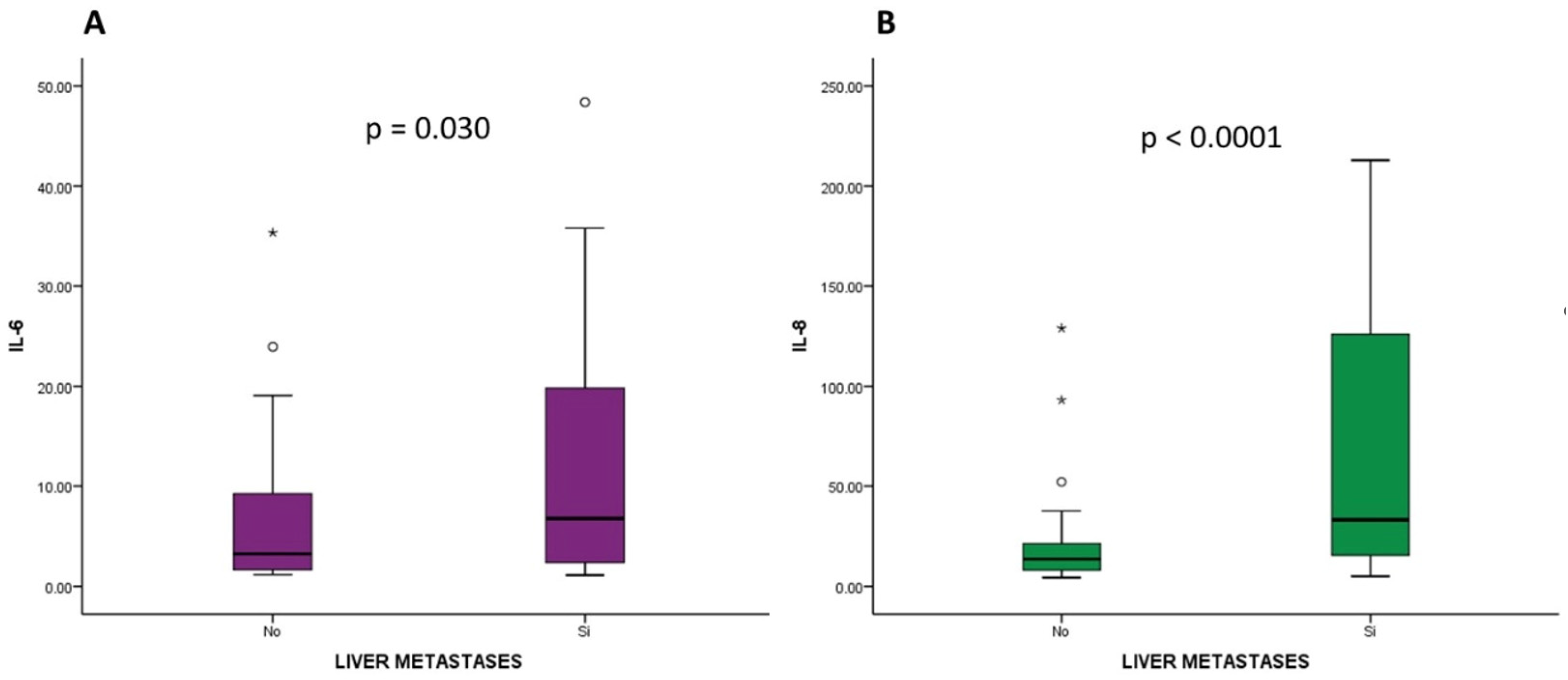
2.5. Analysis of the Relationship between the Analyzed Cytokines and Disease Stage at Diagnosis and Location of Metastases
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Clinical and Analytical Variables Included
4.3. Processing of Cytology Samples
4.4. Processing of Plasma Samples and Cytokine Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Las Cifras del Cancer en España; Sociedad Española de Oncología Médica (SEOM): Madrid, Spain, 2024; ISBN 978-84-09-58445-1.
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Pazo Cid, R.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef]
- Carrato, A.; Pazo-Cid, R.; Macarulla, T.; Gallego, J.; Jiménez-Fonseca, P.; Rivera, F.; Cano, M.T.; Rodriguez-Garrote, M.; Pericay, C.; Alés, I.; et al. Nab-Paclitaxel plus Gemcitabine and FOLFOX in Metastatic Pancreatic Cancer. NEJM Evid. 2024, 3, EVIDoa2300144. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H.; et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; de Santiago, I.; Chlon, L.; Markowetz, F. Master Regulators of Oncogenic KRAS Response in Pancreatic Cancer: An Integrative Network Biology Analysis. PLoS Med. 2017, 14, e1002223. [Google Scholar] [CrossRef] [PubMed]
- Puleo, F.; Nicolle, R.; Blum, Y.; Cros, J.; Marisa, L.; Demetter, P.; Quertinmont, E.; Svrcek, M.; Elarouci, N.; Iovanna, J.; et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology 2018, 155, 1999–2013.e3. [Google Scholar] [CrossRef]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Flores-Figueroa, E.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J.; et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, G.M.; Grünwald, B.T.; Jang, G.-H.; Masoomian, M.; Picardo, S.; Grant, R.C.; Denroche, R.E.; Zhang, A.; Wang, Y.; Lam, B.; et al. GATA6 Expression Distinguishes Classical and Basal-like Subtypes in Advanced Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4901–4910. [Google Scholar] [CrossRef]
- Shi, Z.D.; Lee, K.; Yang, D.; Amin, S.; Verma, N.; Li, Q.V.; Zhu, Z.; Soh, C.L.; Kumar, R.; Evans, T.; et al. Genome Editing in hPSCs Reveals GATA6 Haploinsufficiency and a Genetic Interaction with GATA4 in Human Pancreatic Development. Cell Stem. Cell. 2017, 20, 675–688.e6. [Google Scholar] [CrossRef]
- Aung, K.L.; Fischer, S.E.; Denroche, R.E.; Jang, G.-H.; Dodd, A.; Creighton, S.; Southwood, B.; Liang, S.-B.; Chadwick, D.; Zhang, A.; et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin. Cancer Res. 2018, 24, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Ionasz, V.; Paliwal, S.; Hruschka, N.; de Villarreal, J.M.; Öllinger, R.; Mueller, S.; Dienes, H.P.; Schindl, M.; Gruber, E.S.; et al. A GATA6-centred gene regulatory network involving HNFs and ΔNp63 controls plasticity and immune escape in pancreatic cancer. Gut 2022, 71, 766–777. [Google Scholar] [CrossRef]
- Kokumai, T.; Omori, Y.; Ishida, M.; Ohtsuka, H.; Mizuma, M.; Nakagawa, K.; Maeda, C.; Ono, Y.; Mizukami, Y.; Miura, S.; et al. GATA6 and CK5 Stratify the Survival of Patients With Pancreatic Cancer Undergoing Neoadjuvant Chemotherapy. Mod. Pathol. 2023, 36, 100102. [Google Scholar] [CrossRef]
- Zhou, X.; An, J.; Kurilov, R.; Brors, B.; Hu, K.; Peccerella, T.; Roessler, S.; Pfütze, K.; Schulz, A.; Wolf, S.; et al. Persister cell phenotypes contribute to poor patient outcomes after neoadjuvant chemotherapy in PDAC. Nat. Cancer 2023, 4, 1362–1381. [Google Scholar] [CrossRef]
- Martinelli, P.; Carrillo-de Santa Pau, E.; Cox, T.; Sainz, B., Jr.; Dusetti, N.; Greenhalf, W.; Rinaldi, L.; Costello, E.; Ghaneh, P.; Malats, N.; et al. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut 2017, 66, 1665–1676. [Google Scholar] [CrossRef]
- Duan, K.; Jang, G.H.; Grant, R.C.; Wilson, J.M.; Notta, F.; O’Kane, G.M.; Knox, J.J.; Gallinger, S.; Fischer, S. The value of GATA6 immunohistochemistry and computer-assisted diagnosis to predict clinical outcome in advanced pancreatic cancer. Sci. Rep. 2021, 11, 14951. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Beatty, G.L. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu. Rev. Pathol. 2023, 18, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Van Duijneveldt, G.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for the IL-6 family of cytokines in pancreatic cancer. Clin Sci. 2020, 134, 2091–2115. [Google Scholar] [CrossRef] [PubMed]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef]
- Kuwada, Y.; Sasaki, T.; Morinaka, K.; Kitadai, Y.; Mukaida, N.; Chayama, K. Potential involvement of IL-8 and its receptors in the invasiveness of pancreatic cancer cells. Int. J. Oncol. 2003, 22, 765–771. [Google Scholar] [CrossRef]
- von Bernstorff, W.; Voss, M.; Freichel, S.; Schmid, A.; Vogel, I.; Jöhnk, C.; Henne-Bruns, D.; Kremer, B.; Kalthoff, H. Systemic and local immunosuppression in pancreatic cancer patients. Clin. Cancer Res. 2001, 7 (Suppl. S3), 925s–932s. [Google Scholar]
- Ebrahimi, B.; Tucker, S.L.; Li, D.; Abbruzzese, J.L.; Kurzrock, R. Cytokines in pancreatic carcinoma: Correlation with phenotypic characteristics and prognosis. Cancer 2004, 101, 2727–2736. [Google Scholar] [CrossRef]
- Dima, S.O.; Tanase, C.; Albulescu, R.; Herlea, V.; Chivu-Economescu, M.; Purnichescu-Purtan, R.; Dumitrascu, T.; Duda, D.G.; Popescu, I. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas 2012, 41, 1001–1007. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.W.; Pan, H.C.; Hsu, Y.H.; Chang, K.C.; Wu, L.W.; Chen, W.Y.; Chang, M.S. IL-20 antagonist suppresses PD-L1 expression and prolongs survival in pancreatic cancer models. Nat. Commun. 2020, 11, 4611. [Google Scholar] [CrossRef] [PubMed]
- Lesina, M.; Kurkowski, M.U.; Ludes, K.; Rose-John, S.; Treiber, M.; Klöppel, G.; Yoshimura, A.; Reindl, W.; Sipos, B.; Akira, S.; et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011, 19, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Feurino, L.W.; Wang, H.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008, 99, 733–737. [Google Scholar] [CrossRef]
- Egberts, J.H.; Cloosters, V.; Noack, A.; Schniewind, B.; Thon, L.; Klose, S.; Kettler, B.; von Forstner, C.; Kneitz, C.; Tepel, J.; et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008, 68, 1443–1450. [Google Scholar] [CrossRef]
- Zhao, C.; Pu, Y.; Zhang, H.; Hu, X.; Zhang, R.; He, S.; Zhao, Q.; Mu, B. IL10-modified Human Mesenchymal Stem Cells inhibit Pancreatic Cancer growth through Angiogenesis Inhibition. J. Cancer 2020, 11, 5345–5352. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, Z.; Zhao, H.; Fan, Y.; Tu, X.; Wang, Y.; Liu, X. The role of TGF-β in the tumor microenvironment of pancreatic cancer. Genes Dis. 2022, 10, 1513–1524. [Google Scholar] [CrossRef]
- Hu, B.; Qiu-Lan, H.; Lei, R.E.; Shi, C.; Jiang, H.X.; Qin, S.Y. Interleukin-9 Promotes Pancreatic Cancer Cells Proliferation and Migration via the miR-200a/Beta-Catenin Axis. BioMed Res. Int. 2017, 2017, 2831056. [Google Scholar] [CrossRef]
- Picard, F.S.R.; Lutz, V.; Brichkina, A.; Neuhaus, F.; Ruckenbrod, T.; Hupfer, A.; Raifer, H.; Klein, M.; Bopp, T.; Pfefferle, P.I.; et al. IL-17A-producing CD8+ T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts. Gut 2023, 72, 1510–1522. [Google Scholar] [CrossRef]
- Chen, Z.; Qiao, S.; Yang, L.; Sun, M.; Li, B.; Lu, A.; Li, F. Mechanistic Insights into the Roles of the IL-17/IL-17R Families in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 13539. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, C.; Liu, Q.; Zhu, X. CXCL10 is a prognostic marker for pancreatic adenocarcinoma and tumor microenvironment remodeling. BMC Cancer 2023, 23, 150. [Google Scholar] [CrossRef] [PubMed]
- Herremans, K.M.; Szymkiewicz, D.D.; Riner, A.N.; Bohan, R.P.; Tushoski, G.W.; Davidson, A.M.; Lou, X.; Leong, M.C.; Dean, B.D.; Gerber, M.; et al. The interleukin-1 axis and the tumor immune microenvironment in pancreatic ductal adenocarcinoma. Neoplasia 2022, 28, 100789. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.B.; Phillips, M.; McQuinn, C.; Zaidi, M.Y.; Knochelmann, H.M.; Greene, E.; Robinson, B.; Herting, C.J.; Mace, T.A.; Chen, Z.; et al. Dual IL-6 and CTLA-4 blockade regresses pancreatic tumors in a T cell- and CXCR3-dependent manner. JCI Insight 2023, 8, e155006. [Google Scholar] [CrossRef] [PubMed]
- Pourali, G.; Zafari, N.; Velayati, M.; Mehrabadi, S.; Maftooh, M.; Hassanian, S.M.; Mobarhan, M.G.; Ferns, G.A.; Avan, A.; Khazaei, M. Therapeutic Potential of Targeting Transforming Growth Factor-beta (TGF-β) and Programmed Death-ligand 1 (PD-L1) in Pancreatic Cancer. Curr. Drug Targets 2023, 24, 1335–1345. [Google Scholar] [CrossRef]
- Yuan, S.; Miao, Y.; Ruan, X.; Chen, J.; Li, X.; Larsson, S.C. Therapeutic role of interleukin-1 receptor antagonist in pancreatic diseases: Mendelian randomization study. Front. Immunol. 2023, 14, 1240754. [Google Scholar] [CrossRef]
- Fan, Y.C.; Fong, Y.C.; Kuo, C.T.; Li, C.W.; Chen, W.Y.; Lin, J.D.; Bürtin, F.; Linnebacher, M.; Bui, Q.T.; Lee, K.D.; et al. Tumor-derived interleukin-1 receptor antagonist exhibits immunosuppressive functions and promotes pancreatic cancer. Cell Biosci. 2023, 13, 147. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Shen, W.; Tao, G.Q.; Zhang, Y.; Cai, B.; Sun, J.; Tian, Z.Q. TGF-β in pancreatic cancer initiation and progression: Two sides of the same coin. Cell Biosci. 2017, 7, 39. [Google Scholar] [CrossRef]
- Imafuji, H.; Matsuo, Y.; Ueda, G.; Omi, K.; Hayashi, Y.; Saito, K.; Tsuboi, K.; Morimoto, M.; Koide, S.; Ogawa, R.; et al. Acquisition of gemcitabine resistance enhances angiogenesis via upregulation of IL-8 production in pancreatic cancer. Oncol. Rep. 2019, 41, 3508–3516. [Google Scholar] [CrossRef]
- Merz, V.; Zecchetto, C.; Santoro, R.; Simionato, F.; Sabbadini, F.; Mangiameli, D.; Piro, G.; Cavaliere, A.; Deiana, M.; Valenti, M.T.; et al. Plasma IL8 Is a Biomarker for TAK1 Activation and Predicts Resistance to Nanoliposomal Irinotecan in Patients with Gemcitabine-Refractory Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4661–4669. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; Terao, K.; Ochiai, A. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 2063–2069. [Google Scholar] [CrossRef]
- Feng, L.; Qi, Q.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J. Int. Med. Res. 2018, 46, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour necrosis factor and cancer. J. Pathol. 2013, 230, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fan, W.; Xu, Z.; Chen, H.; He, Y.; Yang, G.; Yang, G.; Hu, H.; Tang, S.; Wang, P.; et al. Inhibiting tumor necrosis factor-alpha diminishes desmoplasia and inflammation to overcome chemoresistance in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 81110–81122. [Google Scholar] [CrossRef] [PubMed]
- Xie, K. Interleukin-8 and human cancer biology. Cytokine Growth Factor. Rev. 2001, 12, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Turker, S.; Cilbir, E.; Guven, D.C.; Karacin, C.; Yalcin, S. The relation between inflammation-based parameters and survival in metastatic pancreatic cancer. J. Cancer Res. Ther. 2021, 17, 510–515. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Wang, X.; Shi, Y.; Dai, G.; Li, X. Pretreatment platelet to lymphocyte ratio is predictive of overall survival in metastatic pancreatic ductal adenocarcinoma. Transl. Cancer Res. 2019, 8, 17–22. [Google Scholar] [CrossRef]
- Karafin, M.S.; Cummings, C.T.; Fu, B.; Iacobuzio-Donahue, C.A. The developmental transcription factor Gata4 is overexpressed in pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2009, 3, 47–55. [Google Scholar] [PubMed]
- Heredia-Soto, V.; Gutiérrez-Sainz, L.; Ghanem, I.; Guerra, L.; Palacios, E.; de Uribe, M.; Trilla-Fuertes, L.; de Miguel, M.; Cejas, P.; Medina, L.; et al. The Relationship between the Expression of GATA4 and GATA6 with the Clinical Characteristics and Prognosis of Resectable Pancreatic Adenocarcinoma. Biomedicines 2023, 11, 252. [Google Scholar] [CrossRef]

| Variables | Patients (%) |
|---|---|
| Sex | n 73 |
| Male | 33 (45) |
| Female | 40 (55) |
| ECOG performance status at diagnosis | |
| 0 | 24 (33) |
| 1 | 45 (62) |
| 2 | 4 (5) |
| Smoking history | |
| Smoker | 21 (29) |
| Former smoker | 25 (34) |
| Never smoker | 27 (37) |
| Diabetes mellitus | 26 (36) |
| Chronic pancreatitis | 6 (8) |
| Primary tumor location | |
| Head | 41 (56) |
| Body and tail | 32 (44) |
| Stage | |
| Localized/borderline | 12 (16) |
| Locally advanced | 21 (29) |
| Metastatic | 40 (55) |
| Hemoglobin | |
| Normal hemoglobin (≥12.5 in males and ≥11.8 in females) | 43 (58.9) |
| Anemia grade 1 (Normal hemoglobin—Hb 10 gr/dL) | 28 (38.3) |
| Anemia grade 2 (Hb 9.9 gr/dL—Hb 8 gr/dL) | 1 (1.4) |
| Anemia grade 3 (Hb 7.9 gr/dL—Hb 6.5 gr/dL) | 1 (1.4) |
| Platelets | |
| <150.000/µL | 5 (6.8) |
| ≥150.000/µL | 68 (93.2) |
| C-reactive protein (CRP) | |
| >5 mg/L | 54 (74.0) |
| ≤5 mg/L | 19 (26.0) |
| Neutrophil-to-lymphocyte ratio (NLR) | |
| >Median | 37 (50.7) |
| ≤Median | 36 (49.3) |
| Platelet-to-lymphocyte ratio (PLR) | |
| >Median | 36 (49.3) |
| ≤Median | 37 (50.7) |
| CA19-9 | |
| >36 UI/mL | 49 (67.1) |
| ≤36 UI/mL | 24 (32.9) |
| Molecular subtype | n 34 |
| Classical | 17 (50) |
| Basal-like | 9 (26) |
| Mixed | 5 (15) |
| Null | 3 (9) |
| Cytokines | Univariate Analysis for PFS during First-Line | ||
|---|---|---|---|
| p-Value | HR | 95% CI | |
| IL-1α | 0.011 | 1.004 | 1.001–1.006 |
| IL-1β | 0.001 | 1.439 | 1.150–1.801 |
| IL-6 | <0.001 | 1.016 | 1.008–1.023 |
| IL-8 | 0.045 | 1.001 | 1.00003–1.003 |
| IL-9 | 0.004 | 1.010 | 1.003–1.017 |
| IL-10 | 0.880 | 0.997 | 0.954–1.041 |
| IP-10 | 0.169 | 1.0001 | 0.999–1.0004 |
| IL-15 | <0.001 | 1.210 | 1.110–1.318 |
| IL-17A | 0.377 | 1.093 | 0.897–1.333 |
| TGF-β1 | 0.305 | 1.00001 | 0.999–1.00004 |
| TGF-β2 | 0.719 | 1.00005 | 0.999–1.0003 |
| TGF-β3 | 0.486 | 0.998 | 0.993–1.003 |
| TNF-α | 0.192 | 1.007 | 0.996–1.018 |
| Cytokines | Univariate Analysis for OS | ||
| p-Value | HR | 95% CI | |
| IL-1α | 0.096 | 1.002 | 0.999–1.005 |
| IL-1β | 0.023 | 1.298 | 1.036–1.626 |
| IL-6 | 0.001 | 1.012 | 1.005–1.019 |
| IL-8 | 0.058 | 1.001 | 0.999–1.003 |
| IL-9 | 0.054 | 1.007 | 0.999–1.014 |
| IL-10 | 0.717 | 1.008 | 0.967–1.050 |
| IP-10 | 0.231 | 1.0001 | 0.999–1.0003 |
| IL-15 | <0.001 | 1.189 | 1.086–1.302 |
| IL-17A | 0.478 | 1.075 | 0.881–1.311 |
| TGF-β1 | 0.146 | 1.00002 | 0.999–1.00005 |
| TGF-β2 | 0.730 | 1.00005 | 0.999–1.0003 |
| TGF-β3 | 0.320 | 0.997 | 0.992–1.003 |
| TNF-α | 0.052 | 1.012 | 0.999–1.023 |
| Multivariate Analysis for PFS during First-Line | |||
|---|---|---|---|
| Variables | p-Value | HR | 95% CI |
| Initial model | |||
| ECOG performance status | 0.200 | 1.62 | 0.77–3.41 |
| Stage | 0.786 | 1.09 | 0.57–2.07 |
| Chemotherapy regimen used during first-line treatment * | 0.406 | 1.31 | 0.68–2.51 |
| CA19-9 levels above the upper limit of normal | 0.180 | 1.55 | 0.81–2.98 |
| IL-6 (reference group < p25) | 0.676 | ||
| 0.276 | 1.65 | 0.67–4.07 |
| 0.289 | 1.67 | 0.64–4.35 |
| 0.322 | 1.81 | 0.55–5.92 |
| IL-8 (reference group < p25) | 0.025 | ||
| 0.967 | 1.01 | 0.45–2.29 |
| 0.431 | 1.52 | 0.53–4.33 |
| 0.009 | 4.95 | 1.48–16.54 |
| Final model | |||
| ECOG performance status | 0.038 | 1.97 | 1.03–3.74 |
| IL-8 (reference group < p25) | <0.001 | ||
| 0.848 | 1.07 | 0.51–2.23 |
| 0.130 | 1.80 | 0.84–3.85 |
| <0.001 | 7.92 | 3.22–19.48 |
| Multivariate analysis for OS | |||
| Variables | p-Value | HR | 95% CI |
| Initial model | |||
| ECOG performance status | 0.082 | 1.82 | 0.92–3.58 |
| Stage | 0.721 | 0.89 | 0.48–1.65 |
| Chemotherapy regimen used during first-line treatment * | 0.955 | 0.98 | 0.53–1.79 |
| CA19-9 levels above the upper limit of normal | 0.071 | 1.83 | 0.94–3.56 |
| IL-8 (reference group < p25) | 0.008 | ||
| 0.901 | 0.95 | 0.44–2.04 |
| 0.663 | 1.20 | 0.52–2.76 |
| 0.003 | 3.84 | 1.58–9.34 |
| Final model | |||
| ECOG performance status | 0.080 | 1.78 | 0.93–3.41 |
| CA19-9 levels above the upper limit of normal | 0.064 | 1.86 | 0.96–3.59 |
| IL-8 (reference group < p25) | 0.007 | ||
| 0.872 | 0.93 | 0.43–2.01 |
| 0.721 | 1.15 | 0.51–2.59 |
| 0.003 | 3.68 | 1.57–8.61 |
| Cytokines | Localized/Borderline Disease | Locally Advanced Disease | Metastatic Disease | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p25 | p50 | p75 | p25 | p50 | p75 | p25 | p50 | p75 | ||
| IL-1α | 0.80 | 1.67 | 3.15 | 0.45 | 0.76 | 1.03 | 0.58 | 1.00 | 3.43 | 0.128 |
| IL-1β | 1.25 | 1.79 | 2.25 | 1.21 | 1.51 | 1.99 | 1.12 | 1.37 | 1.80 | 0.481 |
| IL-6 | 1.35 | 2.24 | 4.56 | 1.95 | 5.48 | 11.30 | 2.29 | 6.29 | 20.20 | 0.112 |
| IL-8 | 6.45 | 9.58 | 20.97 | 8.91 | 15.40 | 22.92 | 11.41 | 30.81 | 117 | 0.008 |
| IL-9 | 1.35 | 3.45 | 6.98 | 1.73 | 2.73 | 6.15 | 1.53 | 2.03 | 3.18 | 0.557 |
| IL-10 | 3.50 | 5.56 | 6.76 | 3.33 | 4.27 | 5.41 | 2.74 | 3.86 | 6.85 | 0.737 |
| IP-10 | 369.00 | 437.50 | 958.25 | 444 | 637 | 1502 | 415 | 661 | 1087.25 | 0.444 |
| IL-15 | 3.16 | 4.95 | 5.75 | 2.73 | 3.62 | 4.59 | 2.90 | 4.09 | 5.45 | 0.432 |
| IL-17A | 1.42 | 2.16 | 2.89 | 1.55 | 2.17 | 2.90 | 1.33 | 1.55 | 2.02 | 0.057 |
| TGF-β1 | 8102.75 | 12,553.50 | 32,262 | 6581.50 | 15,285 | 21,542 | 8243 | 14,496.50 | 21,854 | 0.791 |
| TGF-β2 | 409.00 | 566.00 | 1629.75 | 330 | 559 | 1573.50 | 428 | 639 | 1099.25 | 0.944 |
| TGF-β3 | 30.92 | 39.94 | 87.89 | 36.62 | 72.36 | 132 | 33.30 | 63.71 | 72.36 | 0.417 |
| TNF-α | 8.67 | 12.09 | 25.18 | 8.16 | 17.69 | 23.52 | 10.70 | 16.31 | 30.21 | 0.475 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Sainz, L.; Heredia-Soto, V.; Rodríguez-García, A.M.; Crespo Sánchez, M.G.; Serrano-Olmedo, M.G.; Molero-Luis, M.; Losantos-García, I.; Ghanem, I.; Pérez-Wert, P.; Custodio, A.; et al. Cytokines and Pancreatic Ductal Adenocarcinoma: Exploring Their Relationship with Molecular Subtypes and Prognosis. Int. J. Mol. Sci. 2024, 25, 9368. https://doi.org/10.3390/ijms25179368
Gutierrez-Sainz L, Heredia-Soto V, Rodríguez-García AM, Crespo Sánchez MG, Serrano-Olmedo MG, Molero-Luis M, Losantos-García I, Ghanem I, Pérez-Wert P, Custodio A, et al. Cytokines and Pancreatic Ductal Adenocarcinoma: Exploring Their Relationship with Molecular Subtypes and Prognosis. International Journal of Molecular Sciences. 2024; 25(17):9368. https://doi.org/10.3390/ijms25179368
Chicago/Turabian StyleGutierrez-Sainz, Laura, Victoria Heredia-Soto, Ana Margarita Rodríguez-García, María Gema Crespo Sánchez, María Gemma Serrano-Olmedo, Marta Molero-Luis, Itsaso Losantos-García, Ismael Ghanem, Pablo Pérez-Wert, Ana Custodio, and et al. 2024. "Cytokines and Pancreatic Ductal Adenocarcinoma: Exploring Their Relationship with Molecular Subtypes and Prognosis" International Journal of Molecular Sciences 25, no. 17: 9368. https://doi.org/10.3390/ijms25179368
APA StyleGutierrez-Sainz, L., Heredia-Soto, V., Rodríguez-García, A. M., Crespo Sánchez, M. G., Serrano-Olmedo, M. G., Molero-Luis, M., Losantos-García, I., Ghanem, I., Pérez-Wert, P., Custodio, A., Mendiola, M., & Feliu, J. (2024). Cytokines and Pancreatic Ductal Adenocarcinoma: Exploring Their Relationship with Molecular Subtypes and Prognosis. International Journal of Molecular Sciences, 25(17), 9368. https://doi.org/10.3390/ijms25179368







