Exploring Evolutionary Pathways and Abiotic Stress Responses through Genome-Wide Identification and Analysis of the Alternative Oxidase (AOX) Gene Family in Common Oat (Avena sativa)
Abstract
:1. Introduction
2. Results
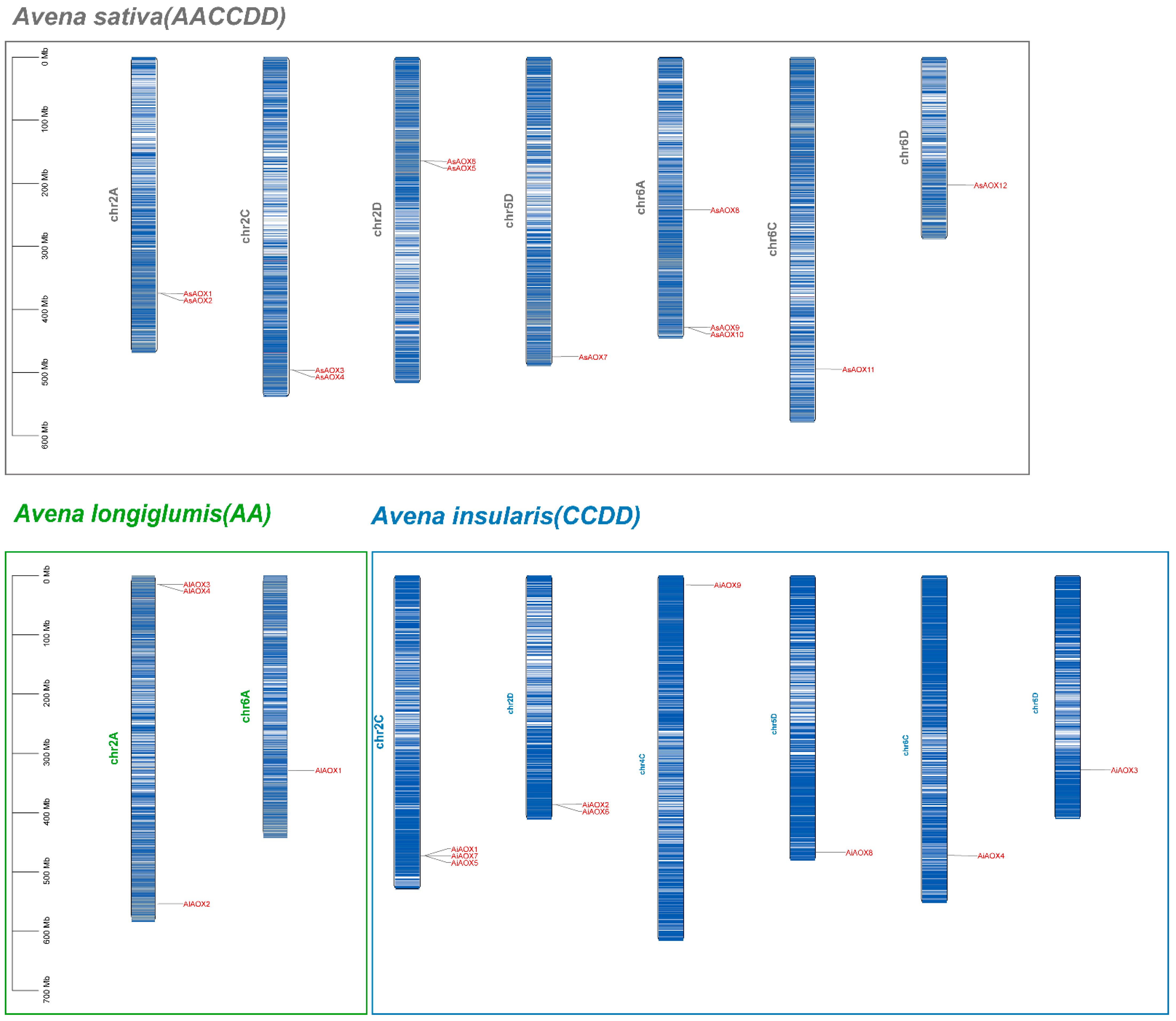
2.1. Identification and Chromosomal Location of AsAOXs, AlAOXs and AiAOXs
2.2. Physicochemical Properties and Subcellular Localization Prediction of AsAOXs, AlAOXs, and AiAOXs
2.3. Phylogenetic Analysis of AOX Genes in Oat and 10 Plants
2.4. Evolutionary Conservation Analysis of AsAOXs, AiAOXs, and AlAOXs
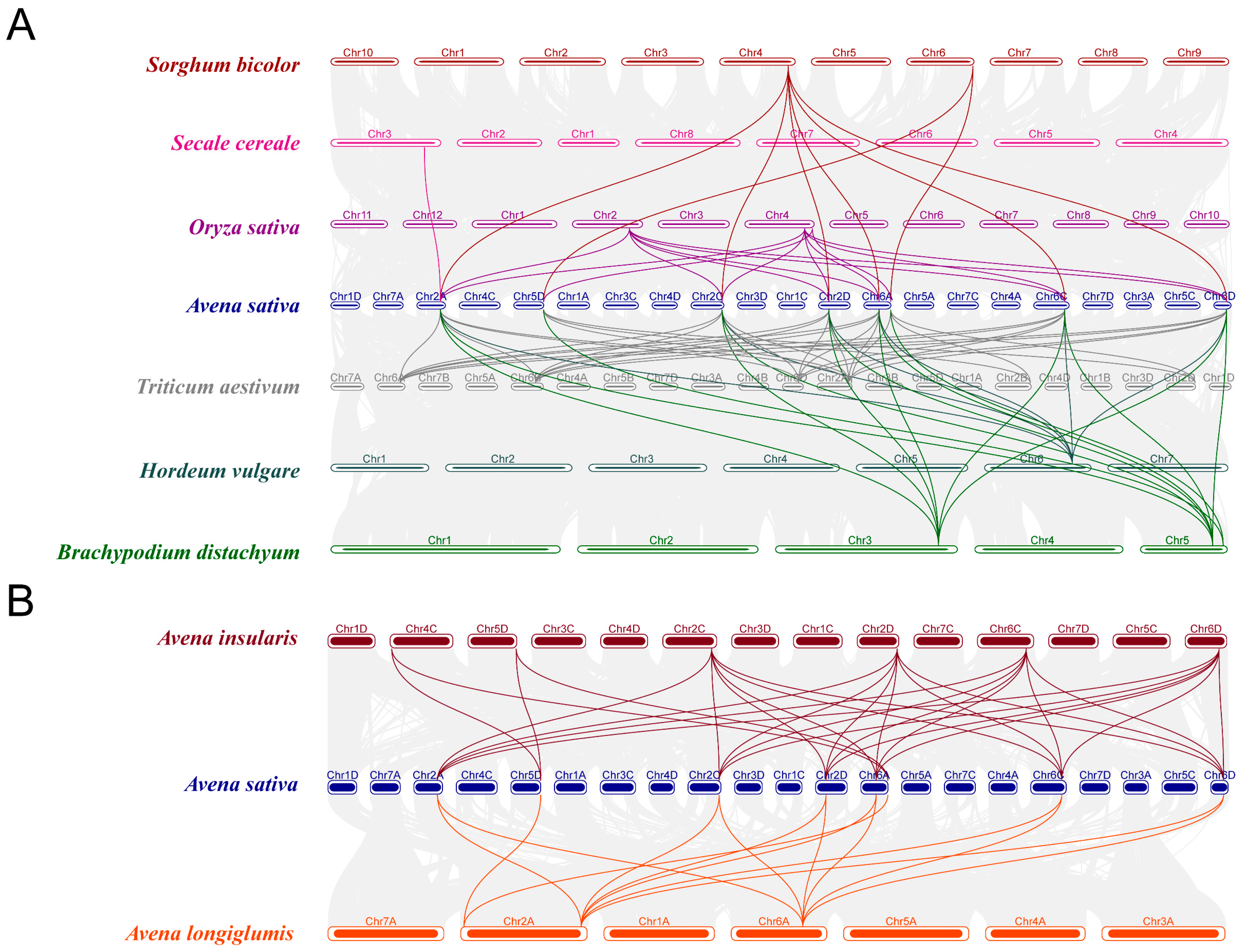
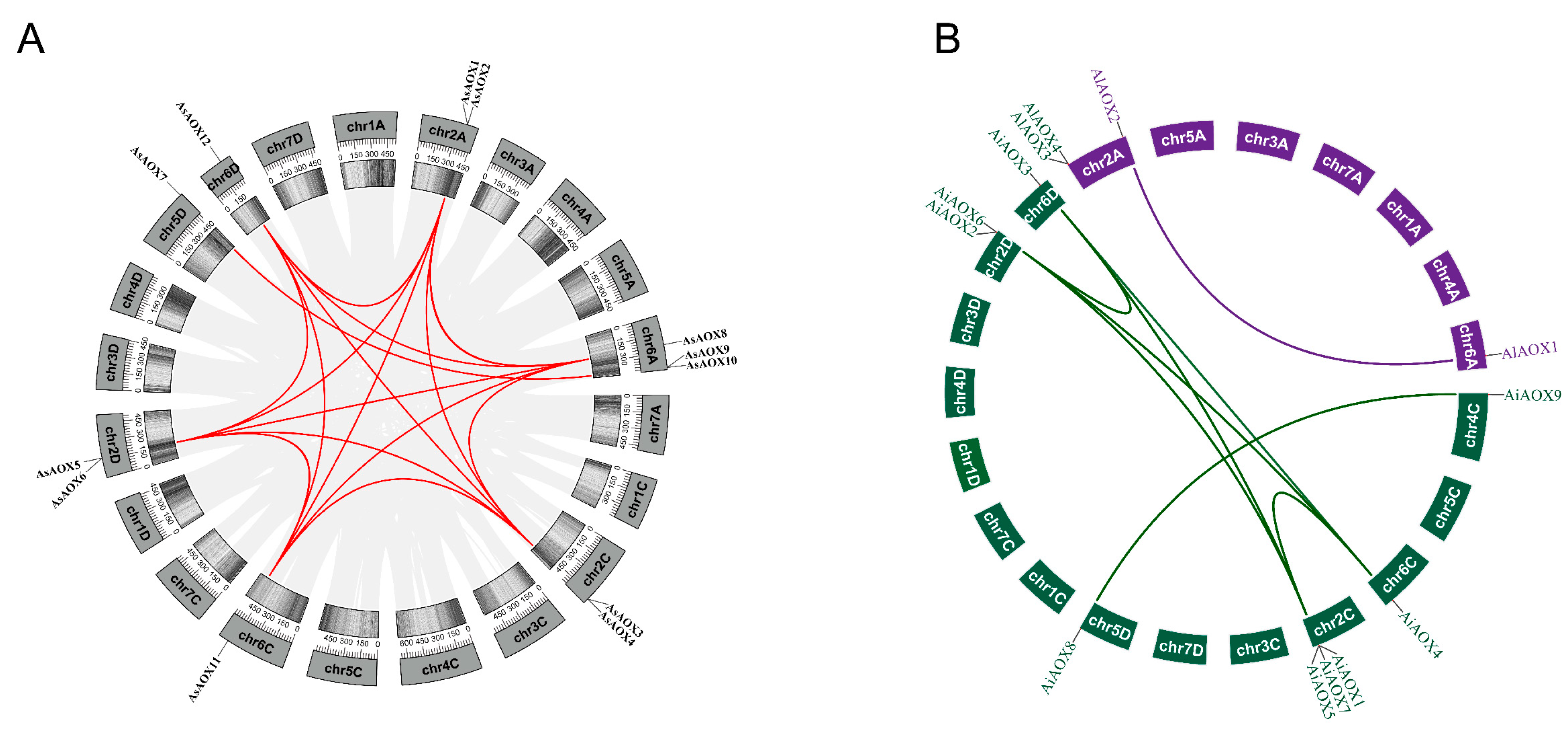
2.5. Synteny and Orthologous Genes Analysis of AOXs Genes
2.6. Analysis of Gene Duplication and Selection Pressure of AsAOX, AiAOX, and AlAOX
2.7. Analysis of Cis-Regulatory Elements of AsAOXs
2.8. Analysis of 3D Structure of AsAOXs Proteins
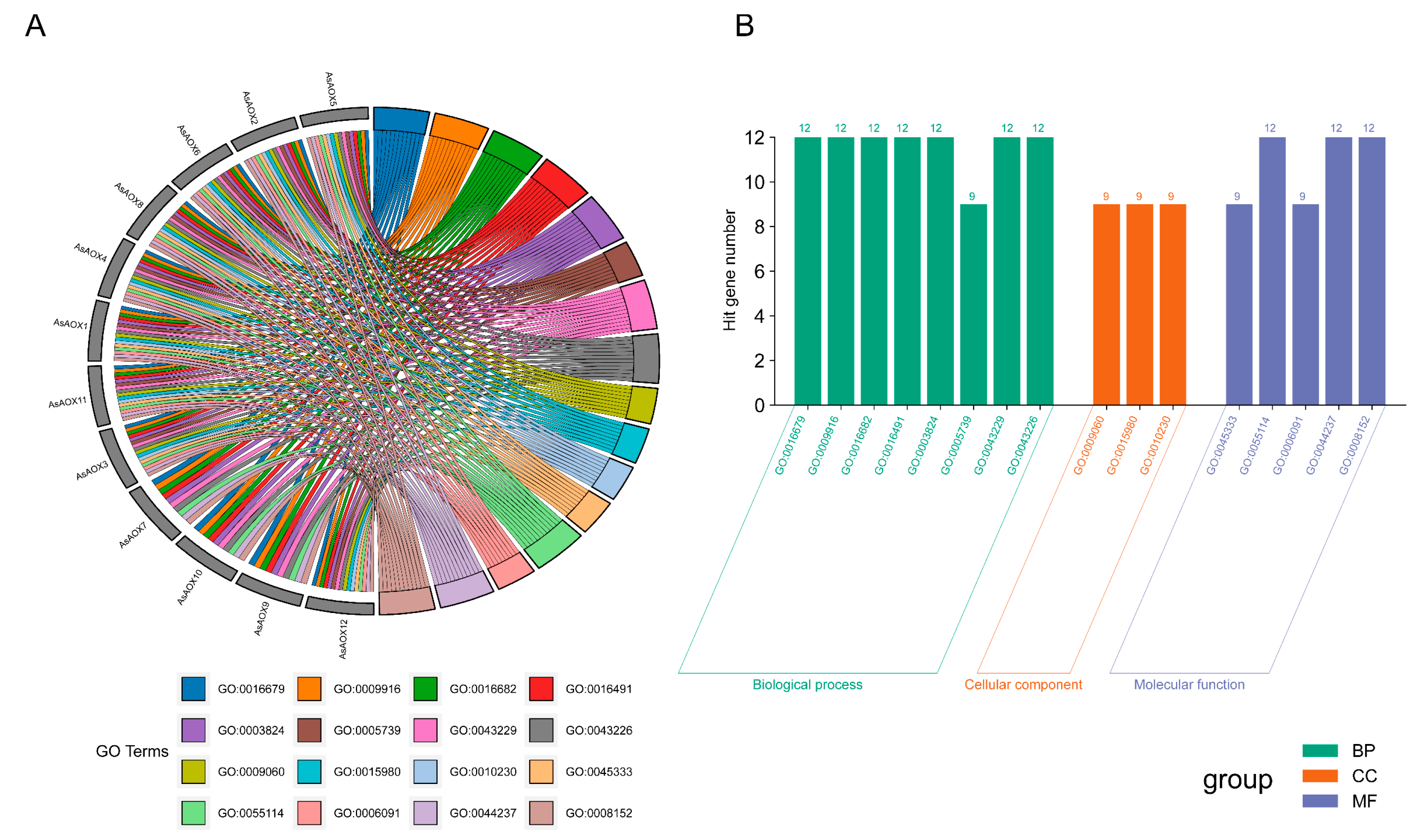
2.9. Analysis of Gene Ontology (GO) Enrichment and Protein–Protein Interaction Network
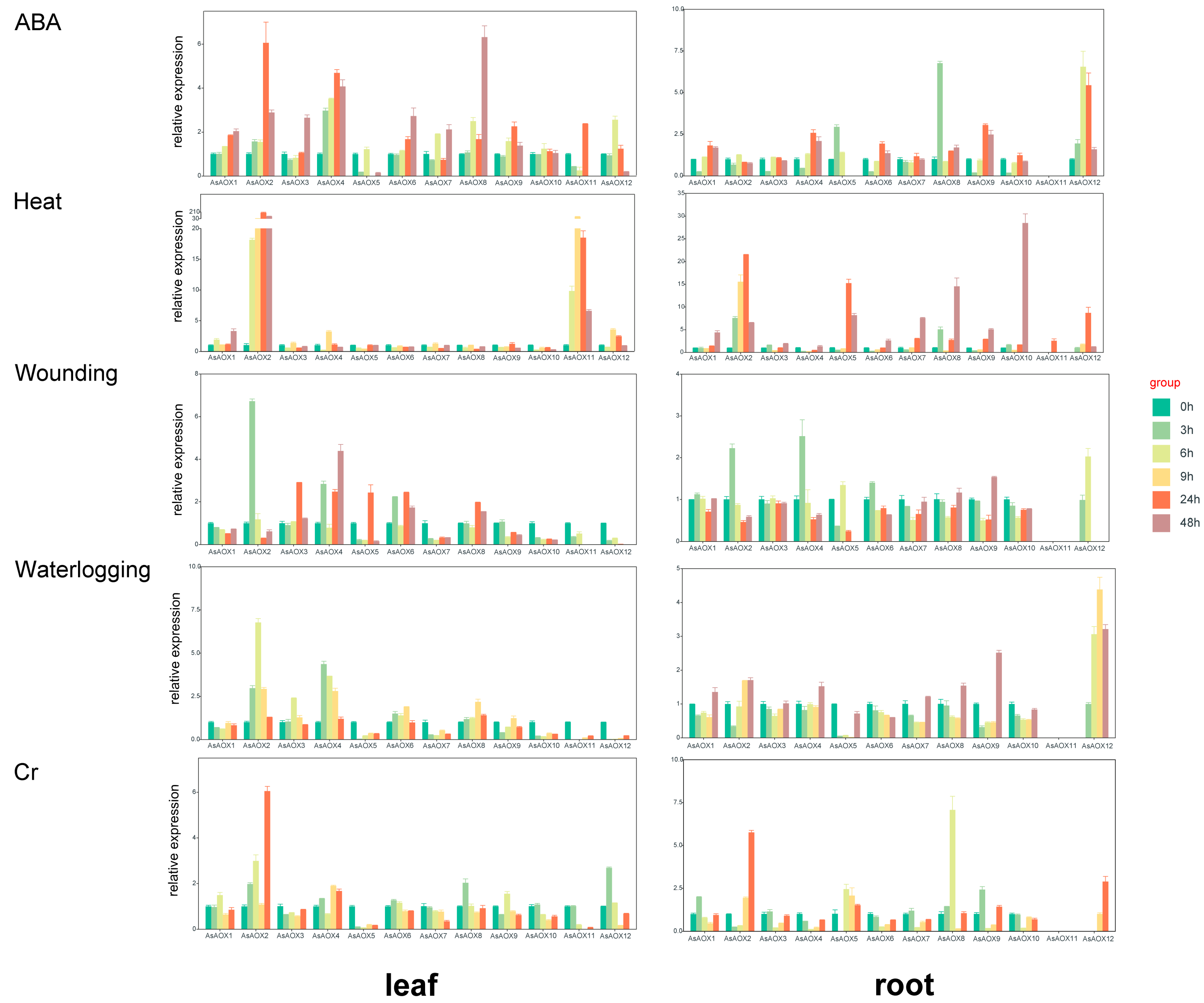
2.10. Expression Analysis and Tissue Specificity of Leaf and Root by RT-qPCR
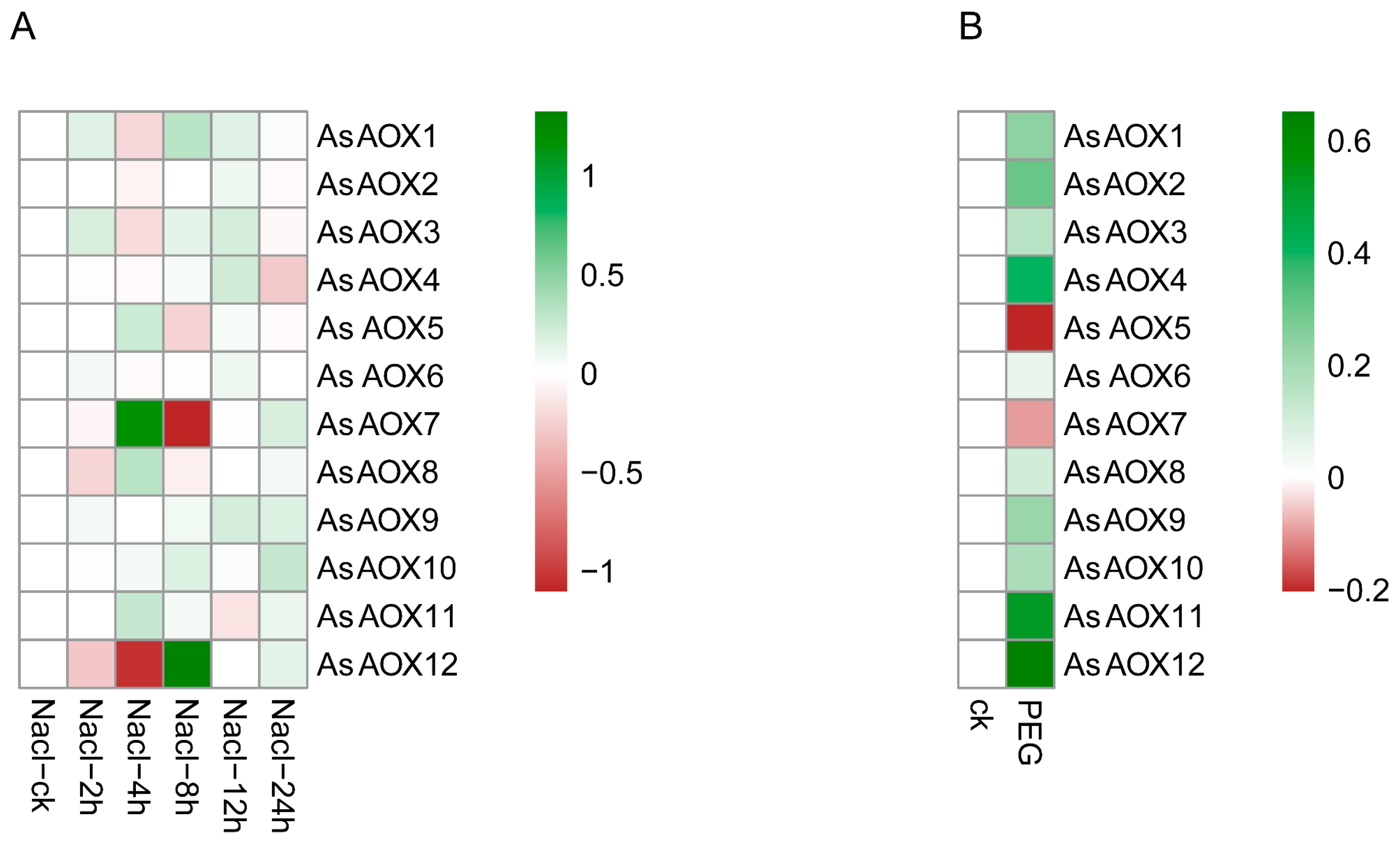
2.11. Oat Expression Analysis of Leaf by RNA-Seq
3. Discussion
3.1. Phylogenetic Analysis
3.2. Exploration of Evolutionary Pathways
3.3. Exploration of Evolutionary Conservation and Divergence
3.4. Preservation Mechanism of AsAOX Duplicated Genes
3.5. Gene Expression and Tissue Specificity
3.6. Gene Expression Regulation by CREs
3.7. Exploration of the Potential Functions of Some AsAOXs
3.8. Prospects for AsAOX Gene Family
4. Materials and Methods
4.1. Identification and Classification of AsAOXs Gene Family in A. sativa (AsAOXs), A. longiglumis (AlAOXs), and A. insularis (AiAOXs)
4.2. Analysis of Conserved Motif, Structural Domain, and Gene Structure of AsAOXs, AlAOXs, and AiAOXs Gene Family
4.3. Gene Location, Protein Characteristics, and Subcellular Localization Prediction of AsAOXs, AlAOXs, and AiAOXs Gene Family
4.4. Analysis of Gene Duplication, Selection Pressure, and Gene Synteny of AOXs Genes
4.5. Identification of Cis-Regulatory Elements in Promoter Region of AsAOXs Gene Family
4.6. Multi-Sequence Alignment and Phylogenetic Analysis of AOXs Gene Family
4.7. Prediction of Protein 3D Structure and Protein–Protein Interaction
4.8. Analysis of Gene Ontology (GO) Enrichment of AOXs Genes in A. sativa
4.9. Information, Culture Environment, and Treatment of Oat Plant Material
4.10. RT-qPCR and RNA-Seq Analysis of Oat
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, Y.; Chen, D.; Zang, Y.; Zhang, Q.; Yi, Y.; Qu, G. Genome-Wide Identification, Classification, Characterization, and Expression Analysis of the Wall-Associated Kinase Family during Fruit Development and under Wound Stress in Tomato (Solanum lycopersicum L.). Genes 2020, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, R.; Gu, T.; Han, J.; Qiu, D.; Su, P.; Feng, J.; Chang, J.; Yang, G.; He, G. Genome-wide identification and expression profiling of trihelix gene family under abiotic stresses in wheat. BMC Genom. 2019, 20, 287. [Google Scholar] [CrossRef]
- He, F.; Shi, Y.J.; Li, J.L.; Lin, T.T.; Zhao, K.J.; Chen, L.H.; Mi, J.X.; Zhang, F.; Zhong, Y.; Lu, M.M.; et al. Genome-wide analysis and expression profiling of Cation/H(+) exchanger (CAX) family genes reveal likely functions in cadmium stress responses in poplar. Int. J. Biol. Macromol. 2022, 204, 76–88. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant Responses to Herbivory, Wounding, and Infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, S.; Wu, J.; Liu, T.; Liu, B.; Xiong, Y.; Zhao, J.; You, M.; Lei, X.; Ma, X. Genome-Wide Analysis of the Oat (Avena sativa) HSP90 Gene Family Reveals Its Identification, Evolution, and Response to Abiotic Stress. Int. J. Mol. Sci. 2024, 25, 2305. [Google Scholar] [CrossRef]
- Sun, M.; Sun, S.; Jia, Z.; Zhang, H.; Ou, C.; Ma, W.; Wang, J.; Li, M.; Mao, P. Genome-wide analysis and expression profiling of glyoxalase gene families in oat (Avena sativa) indicate their responses to abiotic stress during seed germination. Front. Plant Sci. 2023, 14, 1215084. [Google Scholar] [CrossRef]
- Wang, X.; Geng, X.; Bi, X.; Li, R.; Chen, Y.; Lu, C. Genome-wide identification of AOX family genes in Moso bamboo and functional analysis of PeAOX1b_2 in drought and salinity stress tolerance. Plant Cell Rep. 2022, 41, 2321–2339. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R.; Day, D.A.; Whelan, J. Alternative Oxidase Is Positive for Plant Performance. Trends Plant Sci. 2018, 23, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Padmasree, K.; Raghavendra, A.S. Consequence of restricted mitochondrial oxidative metabolism on photosynthetic carbon assimilation in mesophyll protoplasts: Decrease in light activation of four chloroplastic enzymes. Physiol. Plant 2001, 112, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.E. Unique opportunities for future research on the alternative oxidase of plants. Plant Physiol. 2023, 191, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Guan, D.; Sun, K.; Wang, Y.; Zhang, T.; Wang, R. Expression and signal regulation of the alternative oxidase genes under abiotic stresses. Acta Biochim. Biophys. Sin. 2013, 45, 985–994. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Dahal, K.; Alber, N.A.; Chadee, A. Photosynthesis, respiration and growth: A carbon and energy balancing act for alternative oxidase. Mitochondrion 2020, 52, 197–211. [Google Scholar] [CrossRef]
- Ding, C.Q.; Ng, S.; Wang, L.; Wang, Y.C.; Li, N.N.; Hao, X.Y.; Zeng, J.M.; Wang, X.C.; Yang, Y.J. Genome-wide identification and characterization of ALTERNATIVE OXIDASE genes and their response under abiotic stresses in Camellia sinensis (L.) O. Kuntze. Planta 2018, 248, 1231–1247. [Google Scholar] [CrossRef]
- Yang, H.; Deng, L.; Liu, H.; Fan, S.; Hua, W.; Liu, J. Overexpression of BnaAOX1b Confers Tolerance to Osmotic and Salt Stress in Rapeseed. G3 Genes Genomes Genet. 2019, 9, 3501–3511. [Google Scholar] [CrossRef]
- Pham, H.M.; Kebede, H.; Ritchie, G.; Trolinder, N.; Wright, R.J. Alternative oxidase (AOX) over-expression improves cell expansion and elongation in cotton seedling exposed to cool temperatures. Theor. Appl. Genet. 2018, 131, 2287–2298. [Google Scholar] [CrossRef]
- Oh, G.G.K.; O’Leary, B.M.; Signorelli, S.; Millar, A.H. Alternative oxidase (AOX) 1a and 1d limit proline-induced oxidative stress and aid salinity recovery in Arabidopsis. Plant Physiol. 2022, 188, 1521–1536. [Google Scholar] [CrossRef]
- Campos, M.D.; Nogales, A.; Cardoso, H.G.; Kumar, S.R.; Nobre, T.; Sathishkumar, R.; Arnholdt-Schmitt, B. Stress-Induced Accumulation of DcAOX1 and DcAOX2a Transcripts Coincides with Critical Time Point for Structural Biomass Prediction in Carrot Primary Cultures (Daucus carota L.). Front. Genet. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.J.; Lv, X.; Lin, H.H. Unraveling the evolution and regulation of the alternative oxidase gene family in plants. Dev. Genes. Evol. 2015, 225, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Arnholdt-Schmitt, B.; Costa, J.H.; de Melo, D.F. AOX—A functional marker for efficient cell reprogramming under stress? Trends Plant Sci. 2006, 11, 281–287. [Google Scholar] [CrossRef]
- Considine, M.J.; Holtzapffel, R.C.; Day, D.A.; Whelan, J.; Millar, A.H. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol. 2002, 129, 949–953. [Google Scholar] [CrossRef]
- Costa, J.H.; Santos, C.P.; de Sousa, E.L.B.; Moreira Netto, A.N.; Saraiva, K.D.; Arnholdt-Schmitt, B. In silico identification of alternative oxidase 2 (AOX2) in monocots: A new evolutionary scenario. J. Plant Physiol. 2017, 210, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.H.; de Melo, D.F.; Gouveia, Z.; Cardoso, H.G.; Peixe, A.; Arnholdt-Schmitt, B. The alternative oxidase family of Vitis vinifera reveals an attractive model to study the importance of genomic design. Physiol. Plant 2009, 137, 553–565. [Google Scholar] [CrossRef]
- Costa, J.H.; McDonald, A.E.; Arnholdt-Schmitt, B.; Fernandes de Melo, D. A classification scheme for alternative oxidases reveals the taxonomic distribution and evolutionary history of the enzyme in angiosperms. Mitochondrion 2014, 19 Pt B, 172–183. [Google Scholar] [CrossRef]
- Guo, Y.L. Gene family evolution in green plants with emphasis on the origination and evolution of Arabidopsis thaliana genes. Plant J. 2013, 73, 941–951. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Cui, S.; Yan, L.; Wu, B.; Wei, S. Genome-wide identification and functional analysis of the cellulose synthase-like gene superfamily in common oat (Avena sativa L.). Phytochemistry 2024, 218, 113940. [Google Scholar] [CrossRef]
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.E.; Vanlerberghe, G.C.; Staples, J.F. Alternative oxidase in animals: Unique characteristics and taxonomic distribution. J. Exp. Biol. 2009, 212 Pt 16, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.K.; Kang, S.O. Characterization of the gene family encoding alternative oxidase from Candida albicans. Biochem. J. 2001, 356 Pt 2, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Saika, H.; Ohtsu, K.; Hamanaka, S.; Nakazono, M.; Tsutsumi, N.; Hirai, A. AOX1c, a novel rice gene for alternative oxidase; comparison with rice AOX1a and AOX1b. Genes. Genet. Syst. 2002, 77, 31–38. [Google Scholar] [CrossRef]
- Djajanegara, I.; Finnegan, P.M.; Mathieu, C.; McCabe, T.; Whelan, J.; Day, D.A. Regulation of alternative oxidase gene expression in soybean. Plant Mol. Biol. 2002, 50, 735–742. [Google Scholar] [CrossRef]
- Karpova, O.V.; Kuzmin, E.V.; Elthon, T.E.; Newton, K.J. Differential expression of alternative oxidase genes in maize mitochondrial mutants. Plant Cell 2002, 14, 3271–3284. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; McIntosh, L. Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol. 1992, 100, 115–119. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, C.; Lu, T.; Fan, Y.; Ren, Y.; Zhao, J.; Shan, X.; Guan, Y.; Song, P.; Li, D.; et al. New insights into molecular features of the genome-wide AOX family and their responses to various stresses in common wheat (Triticum aestivum L.). Gene 2023, 888, 147756. [Google Scholar] [CrossRef]
- Brew-Appiah, R.A.T.; York, Z.B.; Krishnan, V.; Roalson, E.H.; Sanguinet, K.A. Genome-wide identification and analysis of the ALTERNATIVE OXIDASE gene family in diploid and hexaploid wheat. PLoS ONE 2018, 13, e0201439. [Google Scholar] [CrossRef]
- Saisho, D.; Nambara, E.; Naito, S.; Tsutsumi, N.; Hirai, A.; Nakazono, M. Characterization of the gene family for alternative oxidase from Arabidopsis thaliana. Plant Mol. Biol. 1997, 35, 585–596. [Google Scholar] [CrossRef]
- Velada, I.; Cardoso, H.G.; Ragonezi, C.; Nogales, A.; Ferreira, A.; Valadas, V.; Arnholdt-Schmitt, B. Alternative Oxidase Gene Family in Hypericum perforatum L.: Characterization and Expression at the Post-germinative Phase. Front. Plant Sci. 2016, 7, 1043. [Google Scholar] [CrossRef] [PubMed]
- Araujo Castro, J.; Gomes Ferreira, M.D.; Santana Silva, R.J.; Andrade, B.S.; Micheli, F. Alternative oxidase (AOX) constitutes a small family of proteins in Citrus clementina and Citrus sinensis L. Osb. PLoS ONE 2017, 12, e0176878. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, H.; Guo, L.; Deng, C.; Wang, C.; Wang, Y.; Kang, L.; Zhou, P.; Yu, K.; Dong, X.; et al. Reference genome assemblies reveal the origin and evolution of allohexaploid oat. Nat. Genet. 2022, 54, 1248–1258. [Google Scholar] [CrossRef]
- Sun, M.; Sun, S.; Jia, Z.; Ma, W.; Mao, C.; Ou, C.; Wang, J.; Zhang, H.; Hong, L.; Li, M.; et al. Genome-Wide Analysis and Expression Profiling of Glutathione Reductase Gene Family in Oat (Avena sativa) Indicate Their Responses to Abiotic Stress during Seed Imbibition. Int. J. Mol. Sci. 2022, 23, 11650. [Google Scholar] [CrossRef]
- Rashid, A.; Khajuria, M.; Ali, V.; Faiz, S.; Jamwal, S.; Vyas, D. Genome-wide identification and expression profiling of WRKY family suggest their potential role in cannabinoid regulation in Cannabis sativa L. Ind. Crops Prod. 2023, 206, 117706. [Google Scholar] [CrossRef]
- Wei, Y.L.; Jin, J.P.; Liang, D.; Gao, J.; Li, J.; Xie, Q.; Lu, C.Q.; Yang, F.X.; Zhu, G.F. Genome-wide identification of Cymbidium sinense WRKY gene family and the importance of its Group III members in response to abiotic stress. Front. Plant Sci. 2022, 13, 969010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Zhao, X.Q.; Xue, H.; Zheng, Y.; Meng, H.; Jia, Y.; Bo, S.L. The evolution mechanism of intron length. Genomics 2016, 108, 47–55. [Google Scholar] [CrossRef]
- Li, J.L.; Li, H.; Zhao, J.J.; Yang, P.; Xiang, X.; Wei, S.Y.; Wang, T.; Shi, Y.J.; Huang, J.; He, F. Genome-wide identification and characterization of the RZFP gene family and analysis of its expression pattern under stress in Populus trichocarpa. Int. J. Biol. Macromol. 2024, 255, 128108. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhang, M.; Yang, Z.; Chen, X.; Wu, X. Evolution and expression analysis of carotenoid cleavage oxygenase gene family in Chinese mitten crab Eriocheir sinensis. Int. J. Biol. Macromol. 2024, 257 Pt 1, 128475. [Google Scholar] [CrossRef]
- Mengarelli, D.A.; Zanor, M.I. Genome-wide characterization and analysis of the CCT motif family genes in soybean (Glycine max). Planta 2021, 253, 15. [Google Scholar] [CrossRef]
- Xiao, G.; He, P.; Zhao, P.; Liu, H.; Zhang, L.; Pang, C.; Yu, J. Genome-wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J. Exp. Bot. 2018, 69, 4323–4337. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.X.; Wang, Q.G.; Zhang, H.; Zhou, N.N.; Yan, H.J.; Jian, H.Y.; Li, S.B.; Xiang, G.S.; Tang, K.X.; Qiu, X.Q. Genome-wide identification and evolutionary analysis of MLO gene family in Rosaceae plants. Hortic. Plant J. 2022, 8, 110–122. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.A.; Rouze, P.; Van de Peer, Y. Higher intron loss rate in Arabidopsis thaliana than A. lyrata is consistent with stronger selection for a smaller genome. Mol. Biol. Evol. 2012, 29, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; VanKuren, N.W.; Chen, S.; Vibranovski, M.D. New gene evolution: Little did we know. Annu. Rev. Genet. 2013, 47, 307–333. [Google Scholar] [CrossRef]
- Noble, J.A.; Bielski, N.V.; Liu, M.J.; DeFalco, T.A.; Stegmann, M.; Nelson, A.D.L.; McNamara, K.; Sullivan, B.; Dinh, K.K.; Khuu, N.; et al. Evolutionary analysis of the LORELEI gene family in plants reveals regulatory subfunctionalization. Plant Physiol. 2022, 190, 2539–2556. [Google Scholar] [CrossRef]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef]
- Kuzmin, E.; Taylor, J.S.; Boone, C. Retention of duplicated genes in evolution. Trends Genet. 2022, 38, 59–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.B.; Sun, S.F.; Liu, S.Y.; Liu, M.J.; Lin, J. Phylogenetic and ion-response analyses reveal a relationship between gene expansion and functional divergence in the Ca(2+)/cation antiporter family in Angiosperms. Plant Mol. Biol. 2021, 105, 303–320. [Google Scholar] [CrossRef]
- Hamala, T.; Moore, C.; Cowan, L.; Carlile, M.; Gopaulchan, D.; Brandrud, M.K.; Birkeland, S.; Loose, M.; Kolar, F.; Koch, M.A.; et al. Impact of whole-genome duplications on structural variant evolution in Cochlearia. Nat. Commun. 2024, 15, 5377. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Ming, R.; Alam, M.; Paterson, A.H. Synteny and collinearity in plant genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Tran, L.S.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Konieczna, W.; Mierek-Adamska, A.; Chojnacka, N.; Antoszewski, M.; Szydlowska-Czerniak, A.; Dabrowska, G.B. Characterization of the Metallothionein Gene Family in Avena sativa L. and the Gene Expression during Seed Germination and Heavy Metal Stress. Antioxidants 2023, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Marand, A.P.; Eveland, A.L.; Kaufmann, K.; Springer, N.M. cis-Regulatory Elements in Plant Development, Adaptation, and Evolution. Annu. Rev. Plant Biol. 2023, 74, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Van Aken, O.; Schwarzlander, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Zou, C.; Sun, K.; Mackaluso, J.D.; Seddon, A.E.; Jin, R.; Thomashow, M.F.; Shiu, S.H. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 14992–14997. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Ji, Y.; Wang, C.; Wang, S.; Shi, Y.; Le, J.; Zhang, M. The heat shock factor 20-HSF4-cellulose synthase A2 module regulates heat stress tolerance in maize. Plant Cell 2024, 36, 2652–2667. [Google Scholar] [CrossRef]
- Li, Y.T.; Liu, M.J.; Li, Y.; Liu, P.; Zhao, S.J.; Gao, H.Y.; Zhang, Z.S. Photoprotection by mitochondrial alternative pathway is enhanced at heat but disabled at chilling. Plant J. 2020, 104, 403–415. [Google Scholar] [CrossRef]
- Sun, W.; Xia, L.; Deng, J.; Sun, S.; Yue, D.; You, J.; Wang, M.; Jin, S.; Zhu, L.; Lindsey, K.; et al. Evolution and subfunctionalization of CIPK6 homologous genes in regulating cotton drought resistance. Nat. Commun. 2024, 15, 5733. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Guo, C.; Sun, J.; Li, Z.; Wang, Y.; Yang, A.; Pu, W.; Guo, Y.; Gao, J.; et al. NtNAC053, A Novel NAC Transcription Factor, Confers Drought and Salt Tolerances in Tobacco. Front. Plant Sci. 2022, 13, 817106. [Google Scholar] [CrossRef]
- Yao, E.; Blake, V.C.; Cooper, L.; Wight, C.P.; Michel, S.; Cagirici, H.B.; Lazo, G.R.; Birkett, C.L.; Waring, D.J.; Jannink, J.L.; et al. GrainGenes: A data-rich repository for small grains genetics and genomics. Database 2022, 2022, baac030. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: An integrated platform for exploring and visualizing orthologous data across genomes. Nucleic Acids Res. 2023, 51, W397–W403. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Miao, B.; Wang, S.; Dong, W.; Xu, H.; Si, C.; Wang, W.; Duan, S.; Lou, J.; Bao, Z.; et al. Hiplot: A comprehensive and easy-to-use web service for boosting publication-ready biomedical data visualization. Brief. Bioinform. 2022, 23, bbac261. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.-H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.; Aziz, U.; Zhao, C.; Zhang, M. Evaluation of duplicated reference genes for quantitative real-time PCR analysis in genome unknown hexaploid oat (Avena sativa L.). Plant Methods 2020, 16, 138. [Google Scholar] [CrossRef]
- Wu, B.; Hu, Y.; Huo, P.; Zhang, Q.; Chen, X.; Zhang, Z. Transcriptome analysis of hexaploid hulless oat in response to salinity stress. PLoS ONE 2017, 12, e0171451. [Google Scholar] [CrossRef]




| Gene Name | AA | MW | PI | II | AI | GRAVY | SP |
|---|---|---|---|---|---|---|---|
| AsAOX1 | 334 | 37,413.71 | 8.39 | 42.64 | 77.78 | −0.237 | Mitochondrion |
| AsAOX2 | 416 | 46,772.25 | 8.88 | 55.29 | 77.26 | −0.344 | Nucleus/Mitochondrion |
| AsAOX3 | 334 | 37,384.71 | 8.68 | 40.08 | 77.78 | −0.236 | Mitochondrion |
| AsAOX4 | 326 | 36,735.96 | 6.96 | 47.5 | 80.61 | −0.24 | Chloroplast |
| AsAOX5 | 328 | 36,800.07 | 7.3 | 44.86 | 82.53 | −0.21 | Chloroplast |
| AsAOX6 | 334 | 37,397.71 | 8.39 | 42.64 | 78.08 | −0.23 | Mitochondrion |
| AsAOX7 | 353 | 40,196.47 | 5.18 | 68.02 | 73.03 | −0.305 | Chloroplast |
| AsAOX8 | 384 | 42,425.61 | 9.28 | 38.98 | 86.69 | −0.131 | Chloroplast |
| AsAOX9 | 352 | 40,143.26 | 5.01 | 61.49 | 71.31 | −0.339 | Chloroplast |
| AsAOX10 | 319 | 36,735.62 | 5.47 | 61.89 | 76.52 | −0.251 | Mitochondrion |
| AsAOX11 | 385 | 42,535.73 | 9.3 | 39.05 | 86.75 | −0.135 | Chloroplast |
| AsAOX12 | 336 | 37,379.95 | 7.83 | 32.25 | 88.33 | −0.115 | Chloroplast |
| AiAOX1 | 334 | 37,384.71 | 8.68 | 40.08 | 77.78 | −0.236 | Mitochondrion |
| AiAOX2 | 334 | 37,427.74 | 8.39 | 42.64 | 77.78 | −0.237 | Mitochondrion |
| AiAOX3 | 336 | 37,335.94 | 8.4 | 32.5 | 88.63 | −0.099 | Chloroplast |
| AiAOX4 | 385 | 42,550.74 | 9.39 | 39.83 | 86.49 | −0.141 | Mitochondrion |
| AiAOX5 | 327 | 36,770.99 | 6.96 | 44.56 | 82.45 | −0.233 | Chloroplast |
| AiAOX6 | 328 | 36,800.07 | 7.3 | 44.86 | 82.53 | −0.21 | Chloroplast |
| AiAOX7 | 330 | 37,123.33 | 6.74 | 44.06 | 79.09 | −0.25 | Chloroplast |
| AiAOX8 | 350 | 40,088.34 | 5.24 | 65.94 | 70.57 | −0.346 | Chloroplast |
| AiAOX9 | 312 | 36,018.92 | 5.69 | 63.06 | 76.67 | −0.239 | Chloroplast |
| AlAOX1 | 353 | 39,421.42 | 8.84 | 32.72 | 88.78 | −0.102 | Chloroplast |
| AlAOX2 | 333 | 37,268.59 | 8.69 | 43.48 | 78.02 | −0.226 | Mitochondrion |
| AlAOX3 | 320 | 36,567.26 | 5.27 | 58.71 | 76.28 | −0.221 | Chloroplast |
| AlAOX4 | 353 | 40,197.49 | 4.96 | 62.99 | 73.03 | −0.295 | Chloroplast |
| Seq1 | Seq2 | Ka | Ks | Ka/Ks | Duplication Type |
|---|---|---|---|---|---|
| AsAOX1 | AsAOX3 | 0.006618 | 0.101104 | 0.065459 | Segmental |
| AsAOX1 | AsAOX6 | 0.00132 | 0.105669 | 0.012488 | Segmental |
| AsAOX1 | AsAOX8 | 0.130136 | 0.530657 | 0.245235 | Segmental |
| AsAOX1 | AsAOX11 | 0.133032 | 0.510456 | 0.260614 | Segmental |
| AsAOX1 | AsAOX12 | 0.130136 | 0.522285 | 0.249166 | Segmental |
| AsAOX3 | AsAOX6 | 0.005291 | 0.139623 | 0.037895 | Segmental |
| AsAOX4 | AsAOX8 | 0.252513 | 0.52896 | 0.477377 | Segmental |
| AsAOX3 | AsAOX11 | 0.140535 | 0.50901 | 0.276094 | Segmental |
| AsAOX3 | AsAOX12 | 0.137656 | 0.52901 | 0.260215 | Segmental |
| AsAOX6 | AsAOX8 | 0.129355 | 0.595988 | 0.217042 | Segmental |
| AsAOX6 | AsAOX11 | 0.132251 | 0.582591 | 0.227004 | Segmental |
| AsAOX6 | AsAOX12 | 0.129355 | 0.595988 | 0.217042 | Segmental |
| AsAOX7 | AsAOX9 | 0.033025 | 0.237917 | 0.138808 | Segmental |
| AsAOX8 | AsAOX11 | 0.015335 | 0.097405 | 0.157438 | Segmental |
| AsAOX8 | AsAOX12 | 0.001329 | 0.052867 | 0.025131 | Segmental |
| AsAOX11 | AsAOX12 | 0.006671 | 0.082786 | 0.080584 | Segmental |
| AiAOX1 | AiAOX2 | 0.00662 | 0.139623 | 0.047411 | Segmental |
| AiAOX1 | AiAOX4 | 0.142912 | 0.514069 | 0.278002 | Segmental |
| AiAOX1 | AiAOX3 | 0.139355 | 0.527957 | 0.263951 | Segmental |
| AiAOX2 | AiAOX4 | 0.139859 | 0.587538 | 0.238042 | Segmental |
| AiAOX2 | AiAOX3 | 0.131048 | 0.594732 | 0.220347 | Segmental |
| AiAOX9 | AiAOX8 | 0.088943 | 0.318747 | 0.27904 | Segmental |
| AiAOX4 | AiAOX3 | 0.010707 | 0.087054 | 0.122996 | Segmental |
| AlAOX2 | AlAOX1 | 0.13146 | 0.49955 | 0.263157 | Segmental |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, Z.; Peng, J.; Mou, H.; Wang, Z.; Dao, Y.; Liu, T.; Kong, D.; Liu, S.; Xiong, Y.; et al. Exploring Evolutionary Pathways and Abiotic Stress Responses through Genome-Wide Identification and Analysis of the Alternative Oxidase (AOX) Gene Family in Common Oat (Avena sativa). Int. J. Mol. Sci. 2024, 25, 9383. https://doi.org/10.3390/ijms25179383
Liu B, Zhang Z, Peng J, Mou H, Wang Z, Dao Y, Liu T, Kong D, Liu S, Xiong Y, et al. Exploring Evolutionary Pathways and Abiotic Stress Responses through Genome-Wide Identification and Analysis of the Alternative Oxidase (AOX) Gene Family in Common Oat (Avena sativa). International Journal of Molecular Sciences. 2024; 25(17):9383. https://doi.org/10.3390/ijms25179383
Chicago/Turabian StyleLiu, Boyang, Zecheng Zhang, Jinghan Peng, Haipeng Mou, Zhaoting Wang, Yixin Dao, Tianqi Liu, Dandan Kong, Siyu Liu, Yanli Xiong, and et al. 2024. "Exploring Evolutionary Pathways and Abiotic Stress Responses through Genome-Wide Identification and Analysis of the Alternative Oxidase (AOX) Gene Family in Common Oat (Avena sativa)" International Journal of Molecular Sciences 25, no. 17: 9383. https://doi.org/10.3390/ijms25179383





