A Butyrate-Yielding Dietary Supplement Prevents Acute Alcoholic Liver Injury by Modulating Nrf2-Mediated Hepatic Oxidative Stress and Gut Microbiota
Abstract
1. Introduction
2. Results
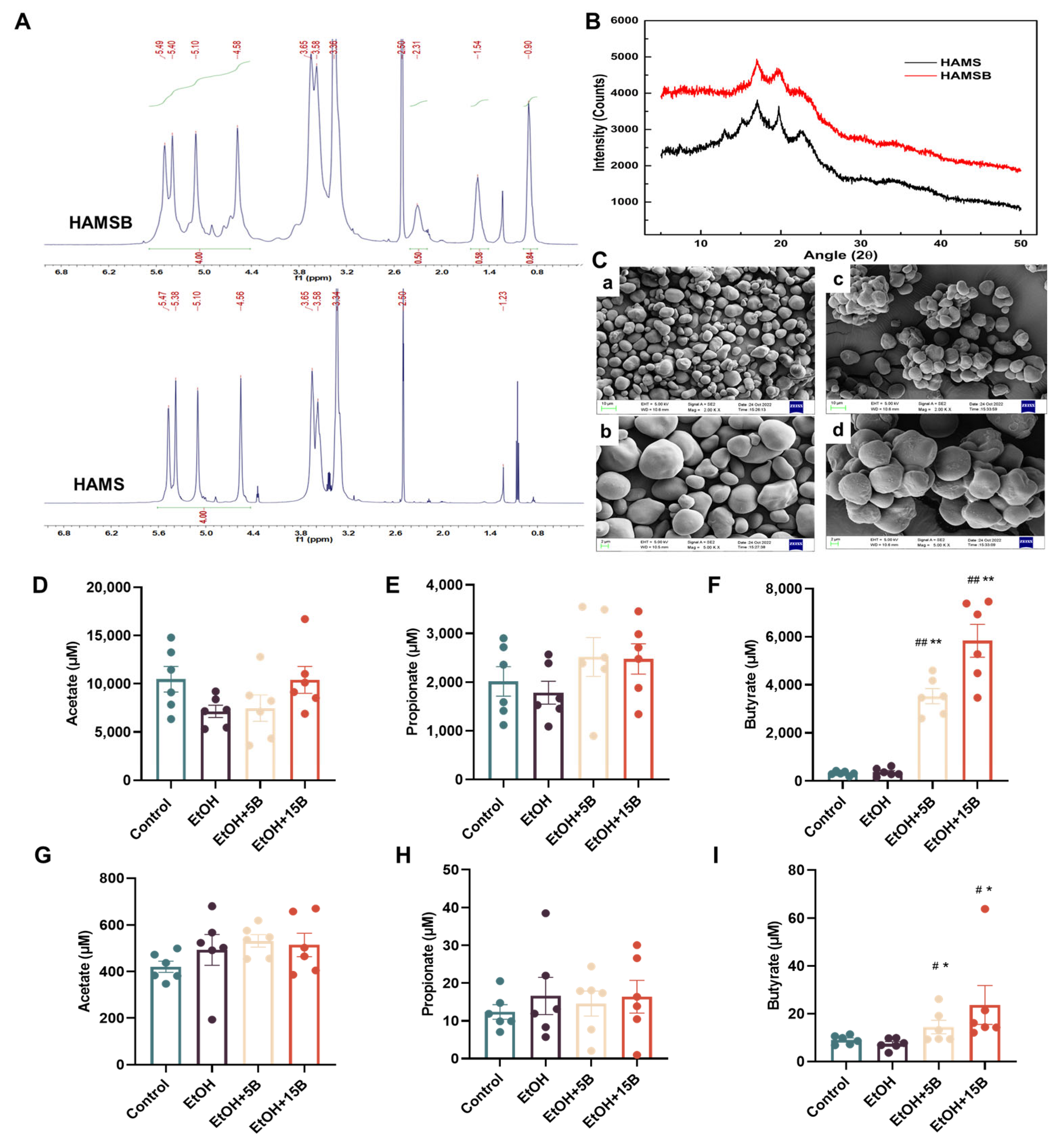
2.1. Characterization and SCFAs Delivery Effect of HAMSB
2.2. Effect of HAMSB on Liver Function in Acute ALD Mice
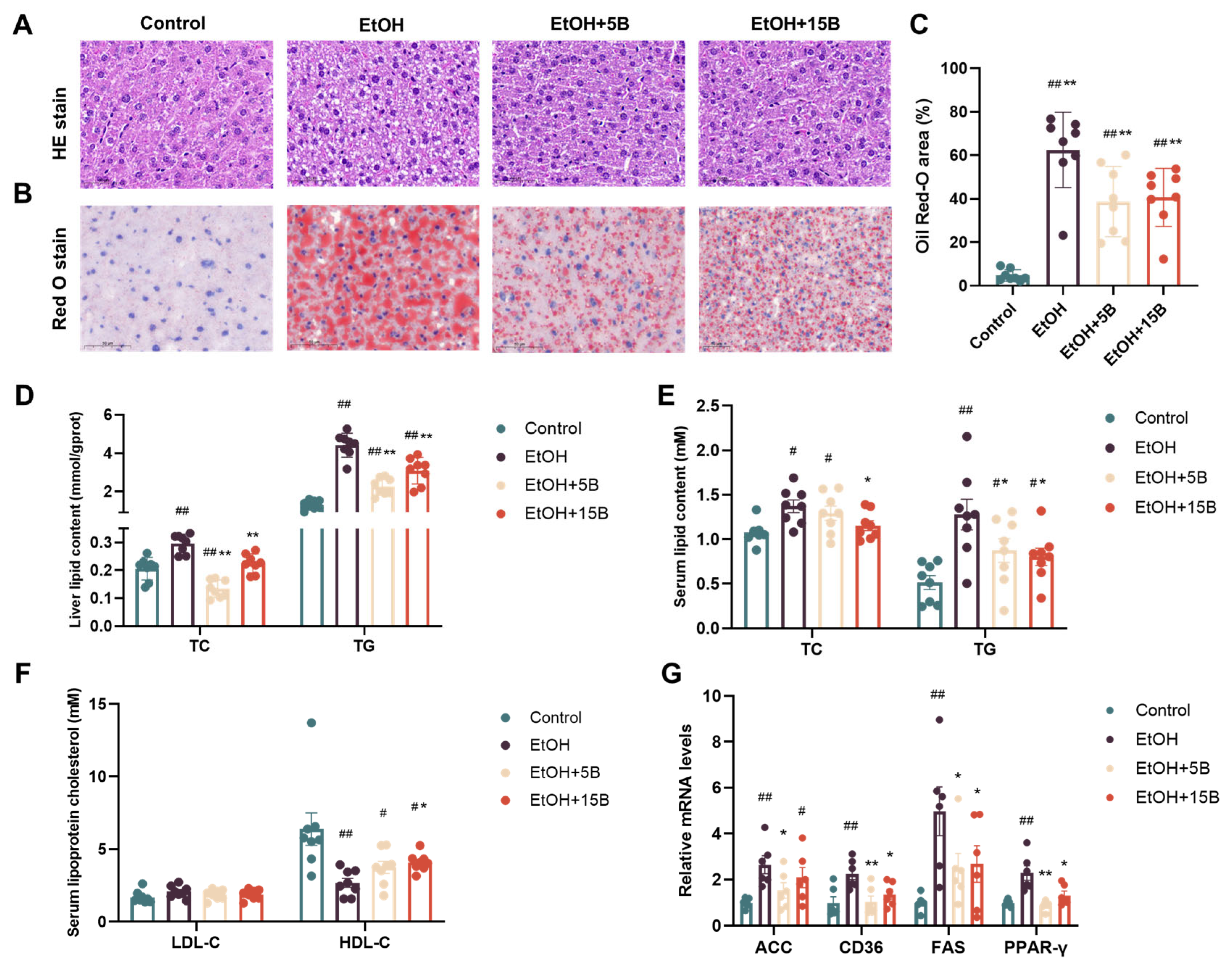
2.3. Effect of HAMSB on Liver Steatosis in Acute ALD Mice
2.4. Comparison of the Effects of HAMSB and Butyrate in Alleviating ALD
2.5. Effect of HAMSB on Oxidative Stress in the Liver of Acute ALD Mice
2.6. Effect of HAMSB on Nrf2-Mediated Antioxidant Signaling in the Livers of Acute ALD Mice
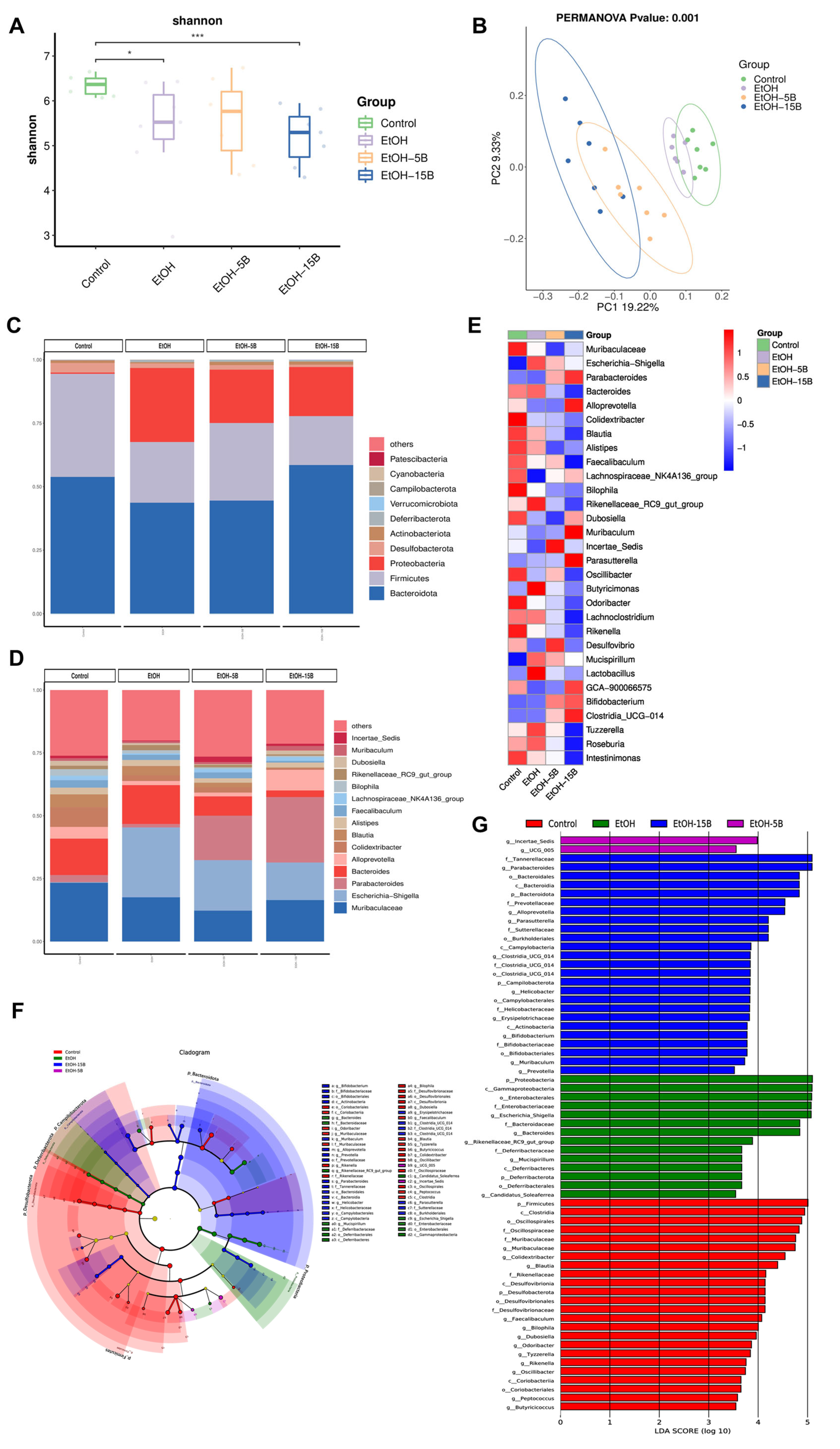
2.7. Effect of HAMSB on Diversity and Structure of Intestinal Microbiota in Acute ALD Mice
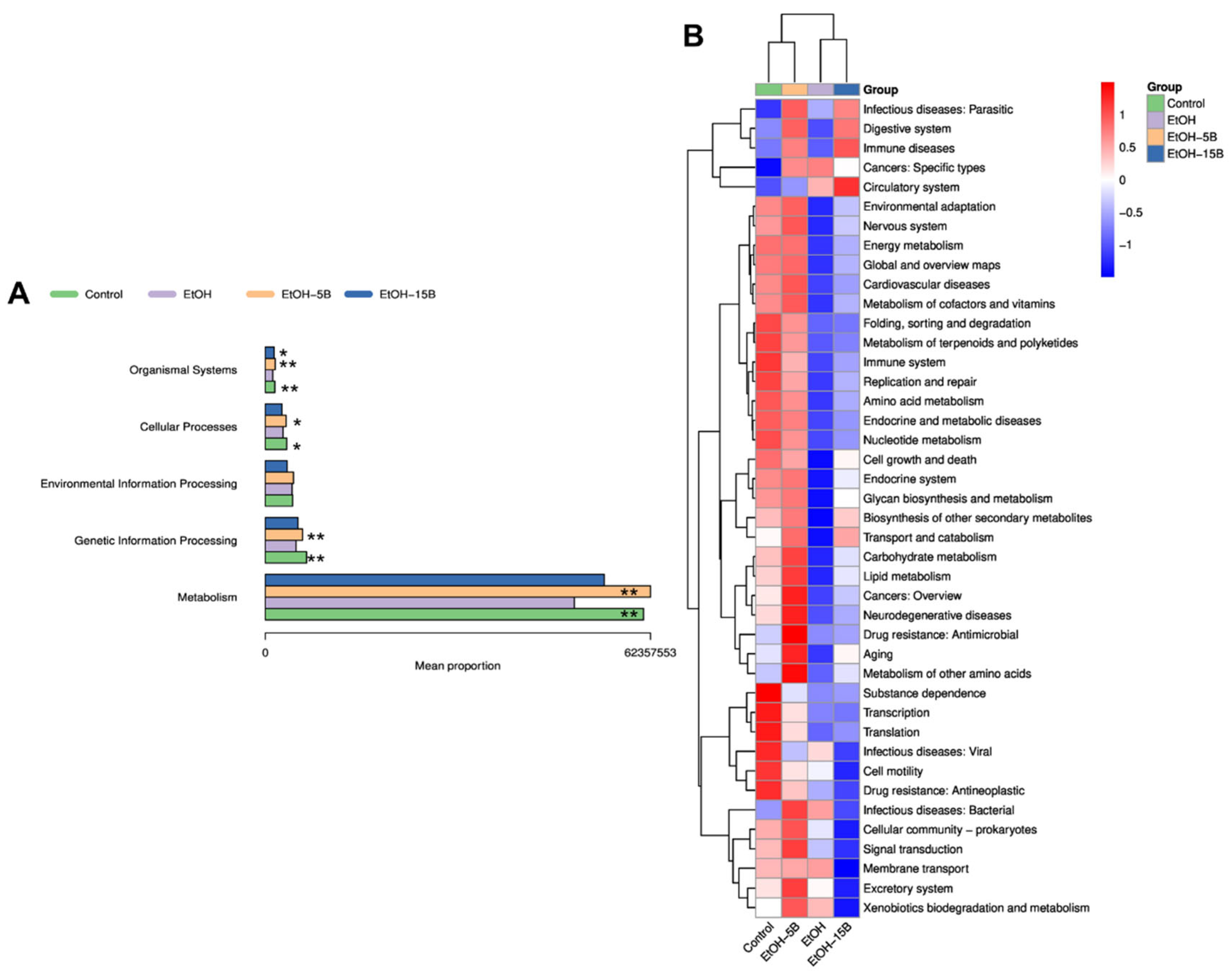
2.8. Effect of HAMSB on Functional Profiles of Gut Microbiota in Acute ALD Mice
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. HAMSB Preparation and Characterization
4.2.1. Preparation of HAMSB
4.2.2. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
4.2.3. Scanning Electron Microscopy (SEM) Observation
4.2.4. X-ray Diffraction (XRD) Measurement
4.3. Animals and Treatments
4.4. Biochemical Analysis
4.5. Histological Analysis
4.6. Quantification of Short-Chain Fatty Acids (SCFAs)
4.7. Western Blot Analysis
4.8. Real-Time Quantitative PCR
4.9. Sequencing to Determine Gut Microbiota Diversity
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Masson, S.; Anstee, Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Marino, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yu, H.R.; Lin, I.C.; Tiao, M.M.; Huang, L.T.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schroder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Marino, E.; Degli-Esposti, M.A.; Marques, F.Z.; et al. Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef]
- Smirnova, E.; Puri, P.; Muthiah, M.D.; Daitya, K.; Brown, R.; Chalasani, N.; Liangpunsakul, S.; Shah, V.H.; Gelow, K.; Siddiqui, M.S.; et al. Fecal Microbiome Distinguishes Alcohol Consumption from Alcoholic Hepatitis But Does Not Discriminate Disease Severity. Hepatology 2020, 72, 271–286. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Ma, Y.; Zhang, J.; Xu, C.; Ma, J.; Hussain, M.A.; Hou, J.; Qian, S. Alleviating Effect of Lacticaseibacillus rhamnosus 1.0320 Combined with Dihydromyricetin on Acute Alcohol Exposure-Induced Hepatic Impairment: Based on Short-Chain Fatty Acids and Adenosine 5’-Monophosphate-Activated Protein Kinase-Mediated Lipid Metabolism Signaling Pathway. J. Agric. Food Chem. 2023, 71, 4837–4850. [Google Scholar]
- Ganesan, R.; Gupta, H.; Jeong, J.J.; Sharma, S.P.; Won, S.M.; Oh, K.K.; Yoon, S.J.; Han, S.H.; Yang, Y.J.; Baik, G.H.; et al. Characteristics of microbiome-derived metabolomics according to the progression of alcoholic liver disease. Hepatol. Int. 2024, 18, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef] [PubMed]
- Donde, H.; Ghare, S.; Joshi-Barve, S.; Zhang, J.; Vadhanam, M.V.; Gobejishvili, L.; Lorkiewicz, P.; Srivastava, S.; McClain, C.J.; Barve, S. Tributyrin Inhibits Ethanol-Induced Epigenetic Repression of CPT-1A and Attenuates Hepatic Steatosis and Injury. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, L.; Li, Z.; Li, C.; Hong, Y.; Gu, Z. Butyrylated starch protects mice from DSS-induced colitis: Combined effects of butyrate release and prebiotic supply. Food Funct. 2021, 12, 11290–11302. [Google Scholar] [CrossRef]
- Annison, G.; Illman, R.J.; Topping, D.L. Acetylated, Propionylated or Butyrylated Starches Raise Large Bowel Short-Chain Fatty Acids Preferentially When Fed to Rats. J. Nutr. 2003, 133, 3523–3528. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- Liu, L.; Xie, P.; Li, W.; Wu, Y.; An, W. Augmenter of Liver Regeneration Protects against Ethanol-Induced Acute Liver Injury by Promoting Autophagy. Am. J. Pathol. 2019, 189, 552–567. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, R.; Mu, Y.; Song, Y.; Hao, N.; Wei, Y.; Wang, Q.; Mackay, C.R. Propionate Ameliorates Alcohol-Induced Liver Injury in Mice via the Gut-Liver Axis: Focus on the Improvement of Intestinal Permeability. J. Agric. Food Chem. 2022, 70, 6084–6096. [Google Scholar] [CrossRef]
- Yang, C.; Liao, A.M.; Cui, Y.; Yu, G.; Hou, Y.; Pan, L.; Chen, W.; Zheng, S.; Li, X.; Ma, J.; et al. Wheat embryo globulin protects against acute alcohol-induced liver injury in mice. Food Chem. Toxicol. 2021, 153, 112240. [Google Scholar] [CrossRef]
- Anurag, L.; Aniket, S.; Shalik, J.; Amarja, L.; Dhananjay, R.; Sachin, J. Non-alcoholic fatty liver disease prevalence and associated risk factors—A study from rural sector of Maharashtra. Trop. Gastroenterol. 2015, 36, 25–30. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2013, 2013, 781050. [Google Scholar] [CrossRef]
- Hao, L.; Zhong, W.; Sun, X.; Zhou, Z. TLR9 Signaling Protects Alcohol-Induced Hepatic Oxidative Stress but Worsens Liver Inflammation in Mice. Front. Pharmacol. 2021, 12, 709002. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Sun, J.; Fu, J.; Li, L.; Chen, C.; Wang, H.; Hou, Y.; Xu, Y.; Pi, J. Nrf2 in alcoholic liver disease. Toxicol. Appl. Pharmacol. 2018, 357, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.; Song, G.Y.; Eom, C.B.; Ahn, J.H.; Kim, S.M.; Shim, A.; Han, Y.H.; Roh, Y.S.; Han, C.Y.; Bae, E.J.; et al. Loss of ERdj5 exacerbates oxidative stress in mice with alcoholic liver disease via suppressing Nrf2. Free Radic. Biol. Med. 2022, 184, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, S.; Yang, Z.; Luan, C.; Lu, W.; Hao, F.; Tang, Y.; Han, X.; Wang, D. 4-Methylguaiacol alleviated alcoholic liver injury by increasing antioxidant capacity and enhancing autophagy through the Nrf2-Keap1 pathway. Food Biosci. 2023, 51, 102160. [Google Scholar] [CrossRef]
- Sun, J.; Hong, Z.; Shao, S.; Li, L.; Yang, B.; Hou, Y.; Wang, H.; Xu, Y.; Zhang, Q.; Pi, J.; et al. Liver-specific Nrf2 deficiency accelerates ethanol-induced lethality and hepatic injury in vivo. Toxicol. Appl. Pharmacol. 2021, 426, 115617. [Google Scholar]
- Rabelo, A.C.S.; de Padua Lucio, K.; Araujo, C.M.; de Araujo, G.R.; de Amorim Miranda, P.H.; Carneiro, A.C.A.; de Castro Ribeiro, E.M.; de Melo Silva, B.; de Lima, W.G.; Costa, D.C. Baccharis trimera protects against ethanol induced hepatotoxicity in vitro and in vivo. J. Ethnopharmacol. 2018, 215, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Addolorato, G.; Ponziani, F.R.; Dionisi, T.; Mosoni, C.; Vassallo, G.A.; Sestito, L.; Petito, V.; Picca, A.; Marzetti, E.; Tarli, C.; et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. 2020, 40, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, W.; Chen, G.; Yang, Z.; Lv, X.; Zheng, L.; Sun, J.; Ai, L.; Sun, B.; Ni, L. Ameliorative effects of monascin from red mold rice on alcoholic liver injury and intestinal microbiota dysbiosis in mice. Food Biosci. 2022, 50, 102079. [Google Scholar] [CrossRef]
- Quigley, E.M.; Stanton, C.; Murphy, E.F. The gut microbiota and the liver. Pathophysiological and clinical implications. J. Hepatol. 2013, 58, 1020–1027. [Google Scholar]
- Wen, Y.; Zhou, Y.; Tian, L.; He, Y. Ethanol extracts of Isochrysis zhanjiangensis alleviate acute alcoholic liver injury and modulate intestinal bacteria dysbiosis in mice. J. Sci. Food Agric. 2024, 104, 4354–4362. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2020, 18, 4–17. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. In-depth investigation of the mechanisms of Echinacea purpurea polysaccharide mitigating alcoholic liver injury in mice via gut microbiota informatics and liver metabolomics. Int. J. Biol. Macromol. 2022, 209 Pt A, 1327–1338. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, S.; Wang, Z.; Fu, X.; Huang, Q.; Yuan, Y.; Wang, K.; Zhang, B. In vitro fecal fermentation of propionylated high-amylose maize starch and its impact on gut microbiota. Carbohydr. Polym. 2019, 223, 115069. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, X.; Guo, M.; Hu, Q.; Mu, Y.; Hao, N.; Wei, Y.; Wang, Q.; Mackay, C.R. Indole acetylated high-amylose maize starch: Synthesis, characterization and application for amelioration of colitis. Carbohydr. Polym. 2023, 302, 120425. [Google Scholar] [CrossRef]




| Ingredients (g) | Control Diet | 5% HAMSB Diet | 15% HAMSB Diet | |||
|---|---|---|---|---|---|---|
| g | kcal | g | kcal | g | kcal | |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 |
| L-cystine | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 444 | 1776 | 422.5 | 1690 | 379.5 | 1518 |
| HAMSB | 0 | 0 | 50 | 86 | 150 | 258 |
| Sucrose | 100 | 400 | 100 | 400 | 100 | 400 |
| Maltodextrin | 45.725 | 182.9 | 50 | 200 | 50 | 200 |
| Cellulose | 50 | 0 | 50 | 0 | 50 | 0 |
| Sunflower seed oil | 70 | 630 | 70 | 630 | 70 | 630 |
| Mineral mix S10022G | 0 | 12 | 0 | 12 | 0 | 12 |
| Vitamin mix V10037 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2.5 | 0 | 2.5 | 0 | 2.5 | 0 |
| Total | 960.225 | 3840.9 | 993 | 3858 | 1050 | 3858 |
| gm% | kcal% | gm% | kcal% | gm% | kcal% | |
| Protein | 21.1 | 21.1 | 20.4 | 21 | 19.3 | 21 |
| Carbohydrate | 62.6 | 62.5 | 63.7 | 62.6 | 65.7 | 62.6 |
| Fat | 7.9 | 16.4 | 7 | 16.3 | 6.7 | 16.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Guo, M.; Wang, H.; Liu, H.; Wei, Y.; Wang, X.; Mackay, C.R.; Wang, Q. A Butyrate-Yielding Dietary Supplement Prevents Acute Alcoholic Liver Injury by Modulating Nrf2-Mediated Hepatic Oxidative Stress and Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 9420. https://doi.org/10.3390/ijms25179420
Xu Q, Guo M, Wang H, Liu H, Wei Y, Wang X, Mackay CR, Wang Q. A Butyrate-Yielding Dietary Supplement Prevents Acute Alcoholic Liver Injury by Modulating Nrf2-Mediated Hepatic Oxidative Stress and Gut Microbiota. International Journal of Molecular Sciences. 2024; 25(17):9420. https://doi.org/10.3390/ijms25179420
Chicago/Turabian StyleXu, Qi, Mei Guo, Haidi Wang, Haitao Liu, Yunbo Wei, Xiao Wang, Charles R. Mackay, and Quanbo Wang. 2024. "A Butyrate-Yielding Dietary Supplement Prevents Acute Alcoholic Liver Injury by Modulating Nrf2-Mediated Hepatic Oxidative Stress and Gut Microbiota" International Journal of Molecular Sciences 25, no. 17: 9420. https://doi.org/10.3390/ijms25179420
APA StyleXu, Q., Guo, M., Wang, H., Liu, H., Wei, Y., Wang, X., Mackay, C. R., & Wang, Q. (2024). A Butyrate-Yielding Dietary Supplement Prevents Acute Alcoholic Liver Injury by Modulating Nrf2-Mediated Hepatic Oxidative Stress and Gut Microbiota. International Journal of Molecular Sciences, 25(17), 9420. https://doi.org/10.3390/ijms25179420






