Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
3. Serotonergic Pathway and Its Key Role in Physiology and Pathophysiology of the Human Organism
4. The Role of 5-HT in OSA: Genetics, Pathogenesis, Physiological Functions, and Correlations with Coexisting Pathologies
4.1. Genetics
4.2. Digestion
4.3. Circulation
| Genetic Implications | Associations and Manifestations |
|---|---|
| 5-HTTLPR l allele | Associations with OSA severity in older patients; manifested by the increase of AHI by 4.46 per hour of sleep in comparison to alleles [28]. |
| 5-HT2A receptor polymorphism -1438G/A | Positive risk factor for OSA especially in men [29,30]. |
| 5-HT2A receptor polymorphism -rs2770304 | Co-occurrence between sleep bruxism and OSA [31]. |
| 5-HTR2A polymorphism -1438G/A | Greater susceptibility to OSA development [33]. |
| 5-HT2A rs6313 and rs6311 polymorphisms | Associations between vasoconstriction, hypertension, and atherosclerosis and its relationship with OSA within the Kurdish population [57]. |
| 5-HT2C receptor knockout in an animal model | Predominant links between weight gain and the pathogenesis of OSA [44]. |
4.4. Sleep
4.5. Respiration
4.6. Muscle Tone and Genioglossal Muscle Activity
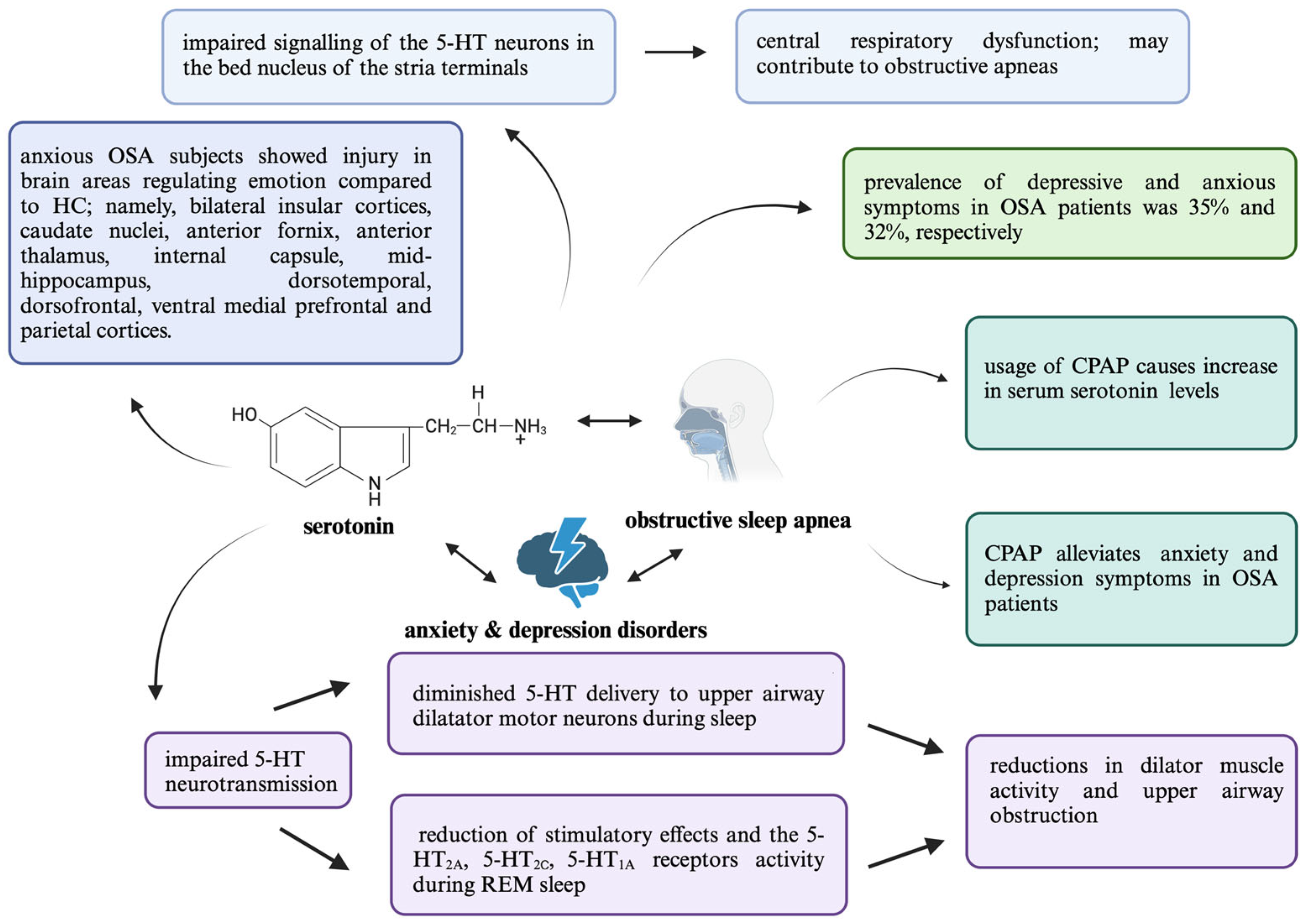
5. 5-HT in the Context of OSA and Its Correlation with Psychiatric Comorbidities
5.1. Depression
5.2. Anxiety Disorders
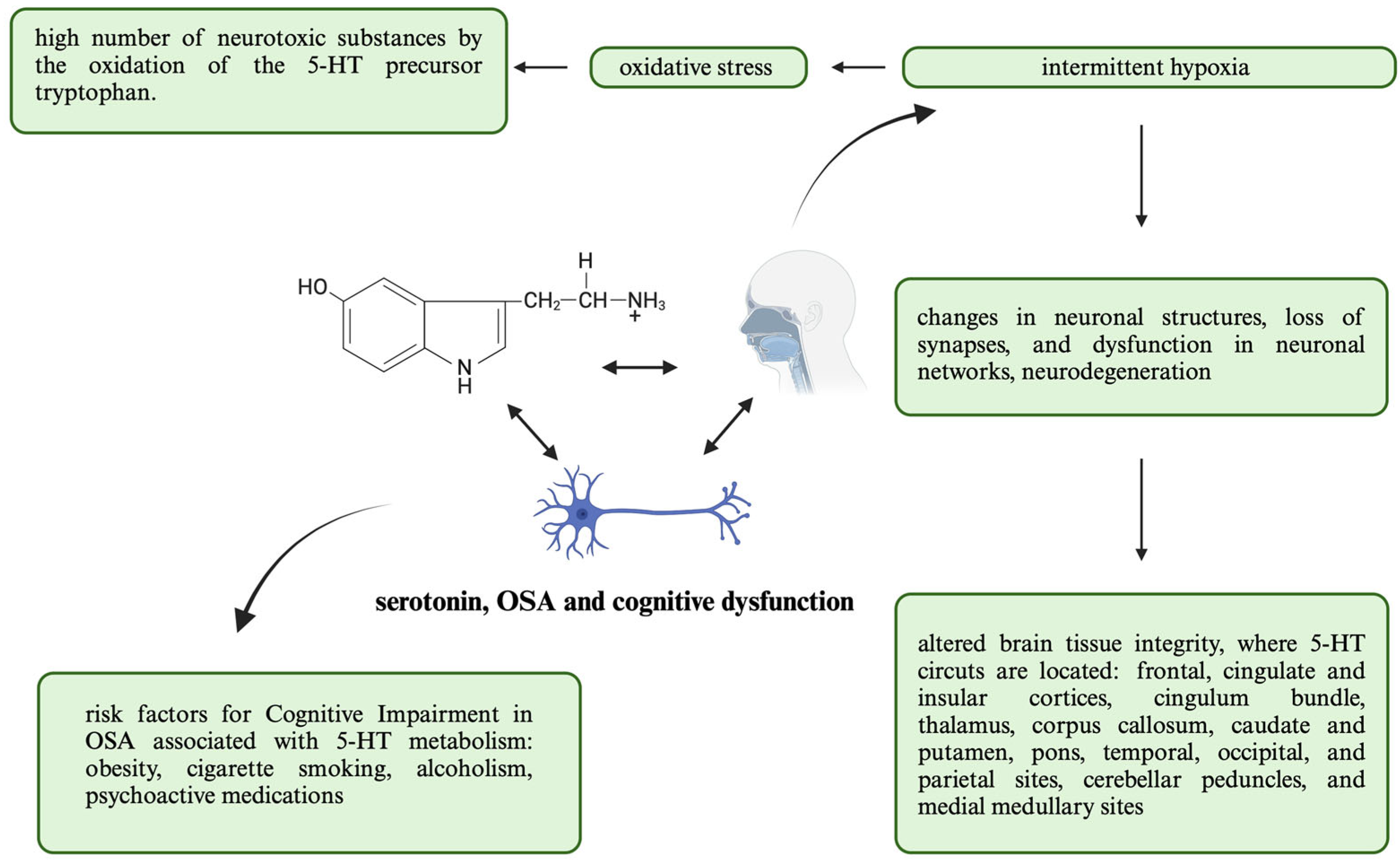
5.3. Cognitive Dysfunction
6. Clinical Implications
6.1. Antidepressants
6.2. Different Medications
6.3. Drugs Combinations
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bonsignore, M.R. Obesity and Obstructive Sleep Apnea. In From Obesity to Diabetes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 181–201. [Google Scholar]
- Bouzerda, A. Risque Cardiovasculaire et Syndrome d’apnées Obstructives Du Sommeil. Pan Afr. Med. J. 2018, 29, 47. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Mokhlesi, B. Obstructive Sleep Apnea and Diabetes. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive Sleep Apnea, Depression and Cognitive Impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive Sleep Apnea. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef]
- Lorenzi-Filho, G.; Almeida, F.R.; Strollo, P.J. Treating OSA: Current and Emerging Therapies beyond CPAP. Respirology 2017, 22, 1500–1507. [Google Scholar] [CrossRef]
- Zinchuk, A.; Yaggi, H.K. Phenotypic Subtypes of OSA. Chest 2020, 157, 403–420. [Google Scholar] [CrossRef]
- Jenkins, T.; Nguyen, J.; Polglaze, K.; Bertrand, P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Di Giovanni, G. Serotonin in Health and Disease. Int. J. Mol. Sci. 2020, 21, 3500. [Google Scholar] [CrossRef]
- Lipford, M.C.; Ramar, K.; Liang, Y.J.; Lin, C.W.; Chao, Y.T.; An, J.; Chiu, C.H.; Tsai, Y.J.; Shu, C.H.; Lee, F.P.; et al. Serotnin as a possible biomarker in obstructive sleep apnea. Sleep Med. Rev. 2016, 28, 125–132. [Google Scholar] [CrossRef]
- Yang, D.; Gouaux, E. Illumination of Serotonin Transporter Mechanism and Role of the Allosteric Site. Sci. Adv. 2021, 7, eabl3857. [Google Scholar] [CrossRef]
- Andersen, J.; Kristensen, A.S.; Bang-Andersen, B.; Strømgaard, K. Recent Advances in the Understanding of the Interaction of Antidepressant Drugs with Serotonin and Norepinephrine Transporters. Chem. Commun. 2009, 25, 3677. [Google Scholar] [CrossRef]
- Gharsalli, H.; Harizi, C.; Zaouche, R.; Sahnoun, I.; Saffar, F.; Maalej, S.; Douik El Gharbi, L. Prevalence of Depression and Anxiety in Obstructive Sleep Apnea. Tunis. Med. 2022, 100, 525–533. [Google Scholar] [PubMed]
- Maierean, A.D.; Bordea, I.R.; Salagean, T.; Hanna, R.; Alexescu, T.G.; Chis, A.; Todea, D.A. Polymorphism of the Serotonin Transporter Gene and the Peripheral 5-Hydroxytryptamine in Obstructive Sleep Apnea: What Do We Know and What Are We Looking for? A Systematic Review of the Literature. Nat. Sci. Sleep 2021, 13, 125–139. [Google Scholar] [CrossRef]
- Stipica Safic, I.; Pecotic, R.; Pavlinac Dodig, I.; Dogas, Z.; Valic, Z.; Valic, M. Phrenic Long-Term Depression Evoked by Intermittent Hypercapnia Is Modulated by Serotonergic and Adrenergic Receptors in Raphe Nuclei. J. Neurophysiol. 2018, 120, 321–329. [Google Scholar] [CrossRef] [PubMed]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The Roles of Peripheral Serotonin in Metabolic Homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Savelieva, K.V.; Zhao, S.; Pogorelov, V.M.; Rajan, I.; Yang, Q.; Cullinan, E.; Lanthorn, T.H. Genetic Disruption of Both Tryptophan Hydroxylase Genes Dramatically Reduces Serotonin and Affects Behavior in Models Sensitive to Antidepressants. PLoS ONE 2008, 3, e3301. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Sreeja, V.; Jose, A.; Patel, S.; Menon, B.; Athira, K.V.; Chakravarty, S. Pharmacogenetics of Selective Serotonin Reuptake Inhibitors (SSRI): A Serotonin Reuptake Transporter (SERT)-Based Approach. Neurochem. Int. 2024, 173, 105672. [Google Scholar] [CrossRef]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The Serotonin-N-Acetylserotonin–Melatonin Pathway as a Biomarker for Autism Spectrum Disorders. Transl. Psychiatry 2014, 4, e479. [Google Scholar] [CrossRef]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocr. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Craven, R.M.; Grahame-Smith, D.G.; Newberry, N.R. 5-HT1A and 5-HT2 Receptors Differentially Regulate the Excitability of 5-HT-Containing Neurones of the Guinea Pig Dorsal Raphe Nucleus in Vitro. Brain Res. 2001, 899, 159–168. [Google Scholar] [CrossRef]
- Smith, H.R.; Leibold, N.K.; Rappoport, D.A.; Ginapp, C.M.; Purnell, B.S.; Bode, N.M.; Alberico, S.L.; Kim, Y.-C.; Audero, E.; Gross, C.T.; et al. Dorsal Raphe Serotonin Neurons Mediate CO2-Induced Arousal from Sleep. J. Neurosci. 2018, 38, 1915–1925. [Google Scholar] [CrossRef]
- Paterson, D.S.; Hilaire, G.; Weese-Mayer, D.E. Medullary Serotonin Defects and Respiratory Dysfunction in Sudden Infant Death Syndrome. Respir. Physiol. Neurobiol. 2009, 168, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, H.; Hu, L.; Gong, W.; Wang, J.; Fu, C.; Liu, Z.; Li, S. Chronic Intermittent Hypoxia Affects Endogenous Serotonergic Inputs and Expression of Synaptic Proteins in Rat Hypoglossal Nucleus. Am. J. Transl. Res. 2017, 9, 546–557. [Google Scholar]
- Jagannathan, R.; Seixas, A.; St-Jules, D.; Jagannathan, L.; Rogers, A.; Hu, L.; Jean-Louis, G.; Sevick, M.A. Systems Biology Genetic Approach Identifies Serotonin Pathway as a Possible Target for Obstructive Sleep Apnea: Results from a Literature Search Review. Sleep Disord. 2017, 2017, 6768323. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.M.; Primeau, M.M.; Hallmayer, J.F.; Lazzeroni, L.C.; Hubbard, J.T.; O’Hara, R. Serotonin Transporter Polymorphism Is Associated with Increased Apnea–Hypopnea Index in Older Adults. Int. J. Geriatr. Psychiatry 2014, 29, 227–235. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.-B.; Ding, M.; Liu, J.-N.; Zhu, X.-F.; Gu, J.-H.; Lu, G. Association between the −1438G/A and T102C Polymorphisms of 5-HT2A Receptor Gene and Obstructive Sleep Apnea: A Meta-Analysis. Mol. Biol. Rep. 2013, 40, 6223–6231. [Google Scholar] [CrossRef]
- Piatto, V.B.; de Carvalho, T.B.O.; De Marchi, N.S.A.; Molina, F.D.; Maniglia, J.V. Polymorphisms in the 5-HTR2A Gene Related to Obstructive Sleep Apnea Syndrome. Braz. J. Otorhinolaryngol. 2011, 77, 348–355. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic Basis of Sleep Bruxism and Sleep Apnea—Response to a Medical Puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef]
- Duarte, J.; Pauletto, P.; Polmann, H.; Réus, J.C.; de Souza, J.F.; Gaio, D.C.; Brancher, J.A.; Vieira, A.; Machado-Souza, C.; de Souza Melo, G.; et al. Is There an Association of Genetic Polymorphisms of the Catechol-O-Methyltransferase Gene (Rs165656 and Rs174675) and the 5-Hydroxytryptamine Receptor 2A Gene (Rs4941573 and Rs6313) with Sleep Bruxism in Individuals with Obstructive Sleep Apnea? Arch. Oral Biol. 2022, 133, 105315. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guan, J.; Yi, H.; Yin, S. A Systematic Review and Meta-Analysis of the Association between Serotonergic Gene Polymorphisms and Obstructive Sleep Apnea Syndrome. PLoS ONE 2014, 9, e86460. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The Serotonin Signaling System: From Basic Understanding To Drug Development for Functional GI Disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Darch, H.T.; Collins, M.K.; O’Riordan, K.J.; Cryan, J.F. Microbial Memories: Sex-Dependent Impact of the Gut Microbiome on Hippocampal Plasticity. Eur. J. Neurosci. 2021, 54, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Sochal, M.; Bialasiewicz, P.; Gabryelska, A.; Talar-Wojnarowska, R.; Fichna, J.; Szmyd, B.; Malecka-Panas, E. The Evaluation of Serum Serotonin Level among Patients with Crohn’s Disease and Its Impact on Quality of Life. Gastroenterology 2021, 160, S75. [Google Scholar] [CrossRef]
- Barra, N.G.; Kwon, Y.H.; Morrison, K.M.; Steinberg, G.R.; Wade, M.G.; Khan, W.I.; Vijayan, M.M.; Schertzer, J.D.; Holloway, A.C. Increased Gut Serotonin Production in Response to Bisphenol A Structural Analogs May Contribute to Their Obesogenic Effects. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E80–E91. [Google Scholar] [CrossRef]
- da Cruz Moreira-Junior, E. Hyper-Serotonergic State Determines Onset and Progression of Idiopathic Parkinson’s Disease. Med. Hypotheses 2019, 133, 109399. [Google Scholar] [CrossRef]
- Pynnönen, P.A.; Isometsä, E.T.; Aronen, E.T.; Verkasalo, M.A.; Savilahti, E.; Aalberg, V.A. Mental Disorders in Adolescents with Celiac Disease. Psychosomatics 2004, 45, 325–335. [Google Scholar] [CrossRef]
- Hoffman, K.; Mansoor, E.; Panhwar, M.S.; Regueiro, M.; Cooper, G.; Qazi, T. Prevalence of Obstructive Sleep Apnea Is Increased in Patients with Inflammatory Bowel Disease: A Large, Multi-Network Study. Crohn’s Colitis 360 2022, 4, otac026. [Google Scholar] [CrossRef]
- Barnes, A.; Andrews, J.M.; Mukherjee, S.; Bryant, R.V.; Bampton, P.; Spizzo, P.; Fraser, R.J.; Mountifield, R. Simple Novel Screening Tool for Obstructive Sleep Apnea in Inflammatory Bowel Disease. Crohn’s Colitis 360 2023, 5, otad016. [Google Scholar] [CrossRef]
- El Hage Chehade, N.; Fu, Y.; Ghoneim, S.; Shah, S.; Song, G.; Fass, R. Association between Obstructive Sleep Apnea and Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2023, 38, 1244–1251. [Google Scholar] [CrossRef]
- Sareli, A.E.; Cantor, C.R.; Williams, N.N.; Korus, G.; Raper, S.E.; Pien, G.; Hurley, S.; Maislin, G.; Schwab, R.J. Obstructive Sleep Apnea in Patients Undergoing Bariatric Surgery—A Tertiary Center Experience. Obes. Surg. 2011, 21, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Tecott, L.H.; Sun, L.M.; Akana, S.F.; Strack, A.M.; Lowenstein, D.H.; Dallman, M.F.; Julius, D. Eating Disorder and Epilepsy in Mice Lacking 5-HT2C Serotonin Receptors. Nature 1995, 374, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Przegaliński, E.; Witek, K.; Wydra, K.; Kotlińska, J.H.; Filip, M. 5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators. Nutrients 2023, 15, 1449. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, J. Sleep and Obesity. Sleep Med. Clin. 2022, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Higashi, Y. Obesity and Endothelial Function. Biomedicines 2022, 10, 1745. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 4, 851–863. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Tse, H.-F.; Lam, B.; Tsang, K.W.T.; Lam, W.-K. Endothelial Function in Obstructive Sleep Apnea and Response to Treatment. Am. J. Respir. Crit. Care Med. 2004, 169, 348–353. [Google Scholar] [CrossRef]
- Hollenberg, N.K. Large and Small Vessel Responses to Serotonin in the Peripheral Circulation. J. Cardiovasc. Pharmacol. 1985, 7, 89–91. [Google Scholar] [CrossRef]
- van Zwieten, P.A.; Chalmers, J.P. Different Types of Centrally Acting Antihypertensives and Their Targets in the Central Nervous System. Cardiovasc. Drugs Ther. 1994, 8, 787–799. [Google Scholar] [CrossRef]
- Benjamin, J.; Greenberg, B.D.; Murphy, D.L. Daily Administration Ofm-Chlorophenylpiperazine to Healthy Human Volunteers Rapidly Attenuates Many of Its Behavioral, Hormonal, Cardiovascular and Temperature Effects. Psychopharmacology 1996, 127, 140–149. [Google Scholar] [CrossRef]
- Ferreira, H.S.; de Castro e Silva, E.; Cointeiro, C.; Oliveira, E.; Faustino, T.N.; Fregoneze, J.B. Role of central 5-HT3 receptors in the control of blood pressure in stressed and non-stressed rats. Brain Res. 2004, 1028, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Hofmann, B.; Dhein, S.; Gergs, U. Cardiac Roles of Serotonin (5-HT) and 5-HT-Receptors in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4765. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, Sleep Deprivation, Autonomic Nervous System and Cardiovascular Diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Abdolsamadi, M.; Rasouli, S.; Alizadeh Severi, A.; Khirehgesh, M.R.; Safari, F.; Mahdieh, N.; Khazaie, H.; Soleymani, B.; Akbari, B. The Association Between the 5-Hydroxytryptamine Receptor 2A Gene Variants Rs6311 and Rs6313 and Obstructive Sleep Apnea in the Iranian Kurdish Population. Genet. Test Mol. Biomark. 2024, 28, 159–164. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.J.; Harper, R.M. Dorsal Raphe Neurons: Depression of Firing during Sleep in Cats. Brain Res. 1976, 101, 569–575. [Google Scholar] [CrossRef]
- Trulson, M.E.; Jacobs, B.L. Raphe Unit Activity in Freely Moving Cats: Correlation with Level of Behavioral Arousal. Brain Res. 1979, 163, 135–150. [Google Scholar] [CrossRef]
- Siegel, J.M. The Neurotransmitters of Sleep. J. Clin. Psychiatry 2004, 65 (Suppl. S16), 4–7. [Google Scholar]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of Sleep Deprivation on the Human Metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of Serotonergic and Noradrenergic Systems in the Pathophysiology of Depression and Anxiety Disorders. Depress. Anxiety 2000, 12 (Suppl. S1), 2–19. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.G.; Lancel, M.; Flachskamm, C.; Reul, J.M.H.M.; Holsboer, F.; Linthorst, A.C.E. Effect of Sleep and Sleep Deprivation on Serotonergic Neurotransmission in the Hippocampus: A Combined in Vivo Microdialysis/EEG Study in Rats. Eur. J. Neurosci. 2003, 17, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Rodríguez, A.; González-Piña, R.; González-Maciel, A.; Arch-Tirado, E. Serotonin and 5-Hydroxy-Indole-Acetic Acid Contents in Dorsal Raphe and Suprachiasmatic Nuclei in Normal, Malnourished and Rehabilitated Rats under 24 h of Sleep Deprivation. Brain Res. 2006, 1110, 95–101. [Google Scholar] [CrossRef]
- Mogilnicka, E. REM Sleep Deprivation Changes Behavioral Response to Catecholaminergic and Serotonergic Receptor Activation in Rats. Pharmacol. Biochem. Behav. 1981, 15, 149–151. [Google Scholar] [CrossRef]
- Eydipour, Z.; Nasehi, M.; Vaseghi, S.; Jamaldini, S.H.; Zarrindast, M.-R. The Role of 5-HT4 Serotonin Receptors in the CA1 Hippocampal Region on Memory Acquisition Impairment Induced by Total (TSD) and REM Sleep Deprivation (RSD). Physiol. Behav. 2020, 215, 112788. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.R.; Tattersall, G.J.; Harris, M.B.; McEvoy, S.D.; Richerson, D.N.; Deneris, E.S.; Johnson, R.L.; Chen, Z.-F.; Richerson, G.B. Defects in Breathing and Thermoregulation in Mice with Near-Complete Absence of Central Serotonin Neurons. J. Neurosci. 2008, 28, 2495–2505. [Google Scholar] [CrossRef]
- Carley, D.W.; Radulovacki, M. Role of Peripheral Serotonin in the Regulation of Central Sleep Apneas in Rats. Chest 1999, 115, 1397–1401. [Google Scholar] [CrossRef]
- Matsumoto, S. Effects of Carotid Body Chemoreceptor Stimulation by 5-HT on Phrenic Nerve Activity and Ventilation in the Rabbit. Arch. Int. Pharmacodyn. Ther. 1981, 254, 282–292. [Google Scholar]
- Hodges, M.R.; Echert, A.E.; Puissant, M.M.; Mouradian, G.C. Fluoxetine Augments Ventilatory CO2 Sensitivity in Brown Norway but Not Sprague Dawley Rats. Respir. Physiol. Neurobiol. 2013, 186, 221–228. [Google Scholar] [CrossRef]
- Pho, H.; Amorim, M.R.; Qiu, Q.; Shin, M.; Kim, L.J.; Anokye-Danso, F.; Jun, J.J.; Ahima, R.S.; Branco, L.G.S.; Kuhn, D.M.; et al. The Effect of Brain Serotonin Deficiency on Breathing Is Magnified by Age. Physiol. Rep. 2022, 10, e15245. [Google Scholar] [CrossRef]
- Behan, M.; Zabka, A.G.; Mitchell, G.S. Age and Gender Effects on Serotonin-Dependent Plasticity in Respiratory Motor Control. Respir. Physiol. Neurobiol. 2002, 131, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.J.; Leiter, J.C. Take a Deep Breath and Wake up: The Protean Role of Serotonin Preventing Sudden Death in Infancy. Exp. Neurol. 2020, 326, 113165. [Google Scholar] [CrossRef]
- Fallert, M.; Böhmer, G.; Dinse, H.R.; Sommer, T.J.; Bittner, A. Microelectrophoretic Application of Putative Neurotransmitters onto Various Types of Bulbar Respiratory Neurons. Arch. Ital. Biol. 1979, 117, 1–12. [Google Scholar]
- Onimaru, H.; Shamoto, A.; Homma, I. Modulation of Respiratory Rhythm by 5-HT in the Brainstem-Spinal Cord Preparation from Newborn Rat. Pflugers. Arch. 1998, 435, 485. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.; Hennequin, S.; Monteau, R.; Hilaire, G. Serotonergic Influences on Central Respiratory Activity: An In Vitro Study in the Newborn Rat. Brain Res. 1990, 535, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Manzke, T.; Dutschmann, M.; Schlaf, G.; Mörschel, M.; Koch, U.R.; Ponimaskin, E.; Bidon, O.; Lalley, P.M.; Richter, D.W. Serotonin Targets Inhibitory Synapses to Induce Modulation of Network Functions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2589–2602. [Google Scholar] [CrossRef]
- Lalley, P.M.; Benacka, R.; Bischoff, A.M.; Richter, D.W. Nucleus Raphe Obscurus Evokes 5-HT-1A Receptor-Mediated Modulation of Respiratory Neurons. Brain Res. 1997, 747, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent Hypoxemia and OSA. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef]
- Sebastiani, J.; Sabatelli, A.; McDonald, M.D. Mild Hypoxia Exposure Impacts Peripheral Serotonin Uptake and Degradation in Gulf Toadfish (Opsanus Beta). J. Exp. Biol. 2022, 225, jeb244064. [Google Scholar] [CrossRef]
- PAVLINAC DODIG, I.; PECOTIC, R.; VALIC, M.; DOGAS, Z. Acute Intermittent Hypoxia Induces Phrenic Long-term Facilitation Which Is Modulated by 5-HT 1A Receptor in the Caudal Raphe Region of the Rat. J. Sleep Res. 2012, 21, 195–203. [Google Scholar] [CrossRef]
- Gozal, E.; Row, B.W.; Schurr, A.; Gozal, D. Developmental Differences in Cortical and Hippocampal Vulnerability to Intermittent Hypoxia in the Rat. Neurosci. Lett. 2001, 305, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Susarla, S.M.; Thomas, R.J.; Abramson, Z.R.; Kaban, L.B. Biomechanics of the Upper Airway: Changing Concepts in the Pathogenesis of Obstructive Sleep Apnea. Int. J. Oral Maxillofac. Surg. 2010, 39, 1149–1159. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Eisele, D.W.; Hari, A.; Testerman, R.; Erickson, D.; Smith, P.L. Electrical Stimulation of the Lingual Musculature in Obstructive Sleep Apnea. J. Appl. Physiol. 1996, 81, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol. Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112, Erratum in Physiol. Rev. 2010, 90, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Morrison, J.L.; Liu, H.; Horner, R.L. Role of Endogenous Serotonin in Modulating Genioglossus Muscle Activity in Awake and Sleeping Rats. Am. J. Respir. Crit. Care Med. 2005, 172, 1338–1347. [Google Scholar] [CrossRef]
- Neuzeret, P.; Sakai, K.; Gormand, F.; Petitjean, T.; Buda, C.; Sastre, J.; Parrot, S.; Guidon, G.; Lin, J. Application of Histamine or Serotonin to the Hypoglossal Nucleus Increases Genioglossus Muscle Activity across the Wake–Sleep Cycle. J. Sleep Res. 2009, 18, 113–121. [Google Scholar] [CrossRef]
- Morin, D.; Di Pasquale, E.; Hilaire, G.; Monteau, R. Possible Involvement of Serotonin in Obstructive Apnea of the Newborn. Neonatology 1994, 65, 176–181. [Google Scholar] [CrossRef]
- El-Ad, B.; Lavie, P. Effect of Sleep Apnea on Cognition and Mood. Int. Rev. Psychiatry 2005, 17, 277–282. [Google Scholar] [CrossRef]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef]
- Chinvararak, C.; Garcia-Borreguero, D. Comorbid Major Depressive Disorder and Obstructive Sleep Apnea. Case Rep. Psychiatry 2022, 2022, 2943059. [Google Scholar] [CrossRef]
- Naji, M.; Komarov, M.; Krishnan, G.P.; Malhotra, A.; Powell, F.L.; Rukhadze, I.; Fenik, V.B.; Bazhenov, M. Computational Model of Brain-Stem Circuit for State-Dependent Control of Hypoglossal Motoneurons. J. Neurophysiol. 2018, 120, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Lacerte, M.; Hays Shapshak, A.; Mesfin, F.B. Hypoxic Brain Injury; StatPearls: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in Sleep Disturbance: A Review on a Bidirectional Relationship, Mechanisms and Treatment. J. Cell Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef]
- Leyton, M.; Young, S.N.; Benkelfat, C. Relapse of Depression after Rapid Depletion of Tryptophan. Lancet 1997, 349, 1840–1841. [Google Scholar] [CrossRef]
- Träskman, L. Monoamine Metabolites in CSF and Suicidal Behavior. Arch. Gen. Psychiatry 1981, 38, 631. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The Role of 5-HT Receptors in Depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Holmes, A.; Murphy, D.L.; Crawley, J.N. Abnormal Behavioral Phenotypes of Serotonin Transporter Knockout Mice: Parallels with Human Anxiety and Depression. Biol. Psychiatry 2003, 54, 953–959. [Google Scholar] [CrossRef]
- Veasey, S.C. Serotonin Agonists and Antagonists in Obstructive Sleep Apnea. Am. J. Respir. Med. 2003, 2, 21–29. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Wellman, A. Targeting Endotypic Traits with Medications for the Pharmacological Treatment of Obstructive Sleep Apnea. A Review of the Current Literature. J. Clin. Med. 2019, 8, 1846. [Google Scholar] [CrossRef]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The Role of Serotonin in Fear Learning and Memory: A Systematic Review of Human Studies. Brain Sci. 2023, 13, 1197. [Google Scholar] [CrossRef]
- Crofford, L.J. Psychological Aspects of Chronic Musculoskeletal Pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 147–155. [Google Scholar] [CrossRef]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The Correlation of Anxiety and Depression with Obstructive Sleep Apnea Syndrome. J. Res. Med. Sci. 2014, 19, 205–210. [Google Scholar] [PubMed]
- Gelenberg, A.J. Psychiatric and Somatic Markers of Anxiety: Identification and Pharmacologic Treatment. Prim. Care Companion J. Clin. Psychiatry 2000, 2, 49–54. [Google Scholar] [CrossRef]
- Švob Štrac, D.; Pivac, N.; Mück-Šeler, D. The Serotonergic System and Cognitive Function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Jaromirska, J.; Kaczmarski, P.; Strzelecki, D.; Sochal, M.; Białasiewicz, P.; Gabryelska, A. Shedding Light on Neurofilament Involvement in Cognitive Decline in Obstructive Sleep Apnea and Its Possible Role as a Biomarker. Front. Psychiatry 2023, 14, 1289367. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, S.G.; Madsen, K.; Svarer, C.; Pinborg, L.H.; Holm, S.; Paulson, O.B.; Waldemar, G.; Knudsen, G.M. Reduced 5-HT2A Receptor Binding in Patients with Mild Cognitive Impairment. Neurobiol. Aging 2008, 29, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.-J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Shobatake, R.; Ota, H.; Takahashi, N.; Ueno, S.; Sugie, K.; Takasawa, S. The Impact of Intermittent Hypoxia on Metabolism and Cognition. Int. J. Mol. Sci. 2022, 23, 12957. [Google Scholar] [CrossRef]
- Benkirane, O.; Delwiche, B.; Mairesse, O.; Peigneux, P. Impact of Sleep Fragmentation on Cognition and Fatigue. Int. J. Environ. Res. Public Health 2022, 19, 15485. [Google Scholar] [CrossRef]
- Gildeh, N.; Drakatos, P.; Higgins, S.; Rosenzweig, I.; Kent, B.D. Emerging Co-Morbidities of Obstructive Sleep Apnea: Cognition, Kidney Disease, and Cancer. J. Thorac. Dis. 2016, 8, E901–E917. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.; Kong, L.; Li, P.; Zeng, Y.; Li, K.; Xie, W.; Shu, Y.; Liu, X.; Peng, D. Frequency-Specific Regional Homogeneity Alterations and Cognitive Function in Obstructive Sleep Apnea Before and After Short-Term Continuous Positive Airway Pressure Treatment. Nat. Sci. Sleep 2021, 13, 2221–2238. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Mari, L.; Chiaravalloti, A.; Paoli, B.; Nuccetelli, M.; Izzi, F.; Giambrone, M.P.; Camedda, R.; Bernardini, S.; Schillaci, O.; et al. 18F-FDG PET, Cognitive Functioning, and CSF Biomarkers in Patients with Obstructive Sleep Apnoea before and after Continuous Positive Airway Pressure Treatment. J. Neurol. 2022, 269, 5356–5367. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Sahib, A.K.; Kang, D.; Aysola, R.S.; Kumar, R. Brain Tissue Integrity Mapping in Adults with Obstructive Sleep Apnea Using T1-Weighted and T2-Weighted Images. Ther. Adv. Neurol. Disord. 2022, 15, 175628642211375. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Ayas, N.; Pépin, J.-L. Pharmacologic Therapy for Obstructive Sleep Apnea: Are We Seeing Some Light at the End of the Tunnel? Am. J. Respir. Crit. Care Med. 2023, 208, 1263–1264. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A Review of Central 5-HT Receptors and Their Function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Heym, J.; Koe, B.K. Pharmacology of Sertraline: A Review. J. Clin. Psychiatry 1988, 49, 40–45. [Google Scholar]
- Zhang, B.; Hao, Y.; Jia, F.; Li, X.; Tang, Y.; Zheng, H.; Liu, W. Effect of Sertraline on Breathing in Depressed Patients without Moderate-to-Severe Sleep-Related Breathing Disorders. Sleep Breath. 2015, 19, 1377–1386. [Google Scholar] [CrossRef]
- Bourin, M.; Chue, P.; Guillon, Y. Paroxetine: A Review. CNS Drug Rev. 2001, 7, 25–47. [Google Scholar] [CrossRef]
- Berry, R.B.; Yamaura, E.M.; Gill, K.; Reist, C. Acute Effects of Paroxetine on Genioglossus Activity in Obstructive Sleep Apnea. Sleep 1999, 22, 1087–1092. [Google Scholar] [CrossRef][Green Version]
- Nevels, R.M.; Gontkovsky, S.T.; Williams, B.E. Paroxetine-The Antidepressant from Hell? Probably Not, But Caution Required. Psychopharmacol. Bull. 2016, 46, 77–104. [Google Scholar]
- Tuomilehto, H.; Seppä, J.; Uusitupa, M. Obesity and Obstructive Sleep Apnea—Clinical Significance of Weight Loss. Sleep Med. Rev. 2013, 17, 321–329. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Tasali, E. Weight Loss Is Integral to Obstructive Sleep Apnea Management. Ten-Year Follow-up in Sleep AHEAD. Am. J. Respir. Crit. Care Med. 2021, 203, 161–162. [Google Scholar] [CrossRef]
- Frazer, A.; Daws, L.C. Serotonin Transporter Function In Vivo: Assessment by Chronoamperometry. Ann. N. Y. Acad. Sci. 1998, 861, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Brownell, L.G.; West, P.; Sweatman, P.; Acres, J.C.; Kryger, M.H. Protriptyline in Obstructive Sleep Apnea. N. Engl. J. Med. 1982, 307, 1037–1042. [Google Scholar] [CrossRef]
- Carley, D.W.; Radulovacki, M. Mirtazapine, a Mixed-Profile Serotonin Agonist/Antagonist, Suppresses Sleep Apnea in the Rat. Am. J. Respir. Crit. Care Med. 1999, 160, 1824–1829. [Google Scholar] [CrossRef]
- Marshall, N.S.; Yee, B.J.; Desai, A.V.; Buchanan, P.R.; Wong, K.K.; Crompton, R.; Melehan, K.L.; Zack, N.; Rao, S.G.; Gendreau, R.M.; et al. Two Randomized Placebo-Controlled Trials to Evaluate the Efficacy and Tolerability of Mirtazapine for the Treatment of Obstructive Sleep Apnea. Sleep 2008, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.; Sharma, G. Obesity and Respiratory Diseases. Chronic Respir. Dis. 2008, 5, 233–242. [Google Scholar] [CrossRef]
- Smales, E.T.; Edwards, B.A.; Deyoung, P.N.; McSharry, D.G.; Wellman, A.; Velasquez, A.; Owens, R.; Orr, J.E.; Malhotra, A. Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold. Ann. Am. Thorac. Soc. 2015, 12, 758–764. [Google Scholar] [CrossRef]
- Veasey, S.C.; Fenik, P.; Panckeri, K.; Pack, A.I.; Hendricks, J.C. The Effects of Trazodone with L-Tryptophan on Sleep-Disordered Breathing in the English Bulldog. Am. J. Respir. Crit. Care Med. 1999, 160, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lv, T.; Wu, J.; Lyu, Y. Trazodone Changed the Polysomnographic Sleep Architecture in Insomnia Disorder: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 14453. [Google Scholar] [CrossRef]
- Loane, C.; Politis, M. Buspirone: What Is It All About? Brain Res. 2012, 1461, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, W.B.; Maczaj, M.; Holt, J. Buspirone Administration to Sleep Apnea Patients. J. Clin. Psychopharmacol. 1991, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zheng, M.; Zhao, W.; Huang, J.; Lao, L.; Li, H.; Lu, J.; Chen, W.; Liu, X.; Deng, H. Associations of Depression, Anxiety, and Life Events With the Risk of Obstructive Sleep Apnea Evaluated by Berlin Questionnaire. Front. Med. 2022, 9, 799792. [Google Scholar] [CrossRef] [PubMed]
- Benatti, B.; Camuri, G.; Dell’Osso, B.; Cremaschi, L.; Sembira, E.; Palazzo, C.; Oldani, L.; Dobrea, C.; Arici, C.; Primavera, D.; et al. Which Factors Influence Onset and Latency to Treatment in Generalized Anxiety Disorder, Panic Disorder, and Obsessive–Compulsive Disorder? Int. Clin. Psychopharmacol. 2016, 31, 347–352. [Google Scholar] [CrossRef]
- Seidel, W.F.; Cohen, S.A.; Bliwise, N.G.; Dement, W.C. Buspirone: An Anxiolytic without Sedative Effect. Psychopharmacology 1985, 87, 371–373. [Google Scholar] [CrossRef]
- Veasey, S.C.; Chachkes, J.; Fenik, P.; Hendricks, J.C. The Effects of Ondansetron on Sleep-Disordered Breathing in the English Bulldog. Sleep 2001, 24, 155–160. [Google Scholar] [CrossRef][Green Version]
- Radulovacki, M.; Trbovic, S.M.; Carley, D.W. Serotonin 5-HT3-Receptor Antagonist GR 38032F Suppresses Sleep Apneas in Rats. Sleep 1998, 21, 131–136. [Google Scholar] [CrossRef][Green Version]
- Bryson, J.C. Clinical Safety of Ondansetron. Semin. Oncol. 1992, 19, 26–32. [Google Scholar]
- Prasad, B.; Radulovacki, M.; Olopade, C.; Herdegen, J.J.; Logan, T.; Carley, D.W. Prospective Trial of Efficacy and Safety of Ondansetron and Fluoxetine in Patients with Obstructive Sleep Apnea Syndrome. Sleep 2010, 33, 982–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanzel, D.A.; Proia, N.G.; Hudgel, D.W. Response of Obstructive Sleep Apnea to Fluoxetine and Protriptyline. Chest 1991, 100, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Michelson, D.; Amsterdam, J.D.; Quitkin, F.M.; Reimherr, F.W.; Rosenbaum, J.F.; Zajecka, J.; Sundell, K.L.; Kim, Y.; Beasley, C.M. Changes in Weight During a 1-Year Trial of Fluoxetine. Am. J. Psychiatry 1999, 156, 1170–1176. [Google Scholar] [CrossRef]
- Robillard, R.; Saad, M.; Ray, L.B.; BuJáki, B.; Douglass, A.; Lee, E.K.; Soucy, L.; Spitale, N.; De Koninck, J.; Kendzerska, T. Selective Serotonin Reuptake Inhibitor Use Is Associated with Worse Sleep-Related Breathing Disturbances in Individuals with Depressive Disorders and Sleep Complaints: A Retrospective Study. J. Clin. Sleep Med. 2021, 17, 505–513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska, A.; Jaromirska, J.; Gabryelska, A.; Sochal, M. Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 9427. https://doi.org/10.3390/ijms25179427
Witkowska A, Jaromirska J, Gabryelska A, Sochal M. Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential. International Journal of Molecular Sciences. 2024; 25(17):9427. https://doi.org/10.3390/ijms25179427
Chicago/Turabian StyleWitkowska, Alicja, Julia Jaromirska, Agata Gabryelska, and Marcin Sochal. 2024. "Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential" International Journal of Molecular Sciences 25, no. 17: 9427. https://doi.org/10.3390/ijms25179427
APA StyleWitkowska, A., Jaromirska, J., Gabryelska, A., & Sochal, M. (2024). Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential. International Journal of Molecular Sciences, 25(17), 9427. https://doi.org/10.3390/ijms25179427







