Cyclic Adenosine Monophosphate Signaling in Chronic Kidney Disease: Molecular Targets and Therapeutic Potentials
Abstract
1. Introduction
2. Methodology
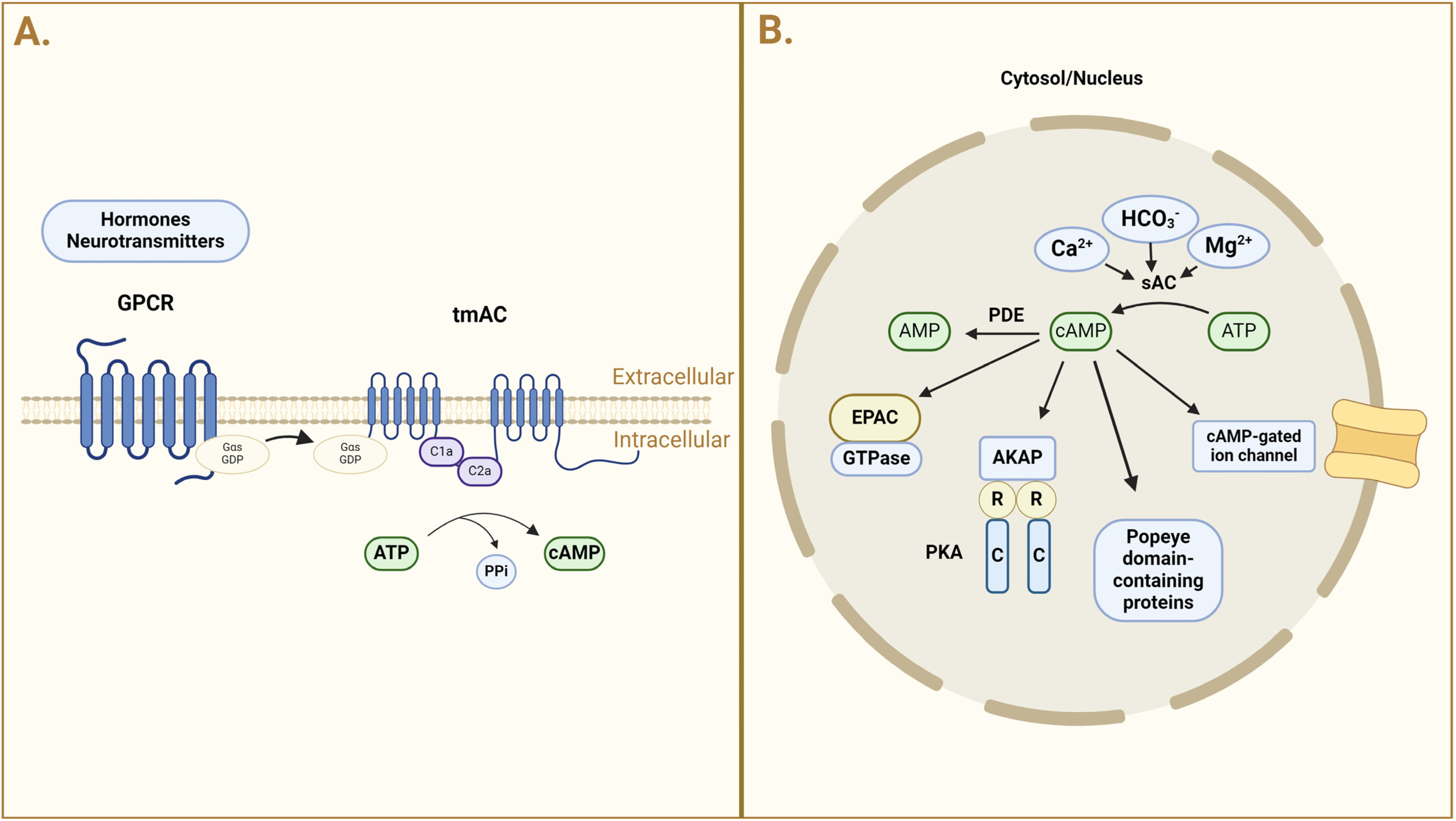
3. cAMP: Formation, Distribution, and Regulation
4. cAMP in Kidney Physiology
4.1. cAMP and AVP in the Collecting Duct
4.2. cAMP and Kidney Fibrosis
4.3. cAMP and Immune Modulation
4.4. cAMP in Nephrotic Syndrome
5. cAMP in Chronic Kidney Disease
5.1. Autosomal Polycystic Kidney Disease
5.2. Diabetic Nephropathy
5.3. cAMP and Bartter Syndrome
5.4. cAMP in Liddle Syndrome
5.5. cAMP in Renal Tubular Acidosis
5.6. cAMP and Nephrogenic Diabetes Insipidus
5.7. cAMP in Fabry Disease
5.8. The Role of cAMP in Renal Cell Carcinoma (RCC) and Implications for CKD
5.9. cAMP in AKI-to-CKD Transition
6. Therapies Targeting cAMP Signaling
6.1. Phosphodiesterase Inhibitors
6.2. Adenylate Cyclase Activators
6.3. cAMP Analogs
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fularski, P.; Czarnik, W.; Frankenstein, H.; Gąsior, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Unveiling Selected Influences on Chronic Kidney Disease Development and Progression. Cells 2024, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ladilov, Y. Editorial: Advances in cAMP Signaling Research: Basic and Translational Aspects. Front. Physiol. 2023, 14, 1266718. [Google Scholar] [CrossRef]
- Halls, M.L.; Cooper, D.M.F. Adenylyl Cyclase Signalling Complexes—Pharmacological Challenges and Opportunities. Pharmacol. Ther. 2017, 172, 171–180. [Google Scholar] [CrossRef]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular Organization of the cAMP Signaling Pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef]
- Zippin, J.H.; Chen, Y.; Nahirney, P.; Kamenetsky, M.; Wuttke, M.S.; Fischman, D.A.; Levin, L.R.; Buck, J. Compartmentalization of Bicarbonate-Sensitive Adenylyl Cyclase in Distinct Signaling Microdomains. FASEB J. 2003, 17, 82–84. [Google Scholar] [CrossRef]
- Appukuttan, A.; Kasseckert, S.A.; Micoogullari, M.; Flacke, J.-P.; Kumar, S.; Woste, A.; Abdallah, Y.; Pott, L.; Reusch, H.P.; Ladilov, Y. Type 10 Adenylyl Cyclase Mediates Mitochondrial Bax Translocation and Apoptosis of Adult Rat Cardiomyocytes under Simulated Ischaemia/Reperfusion. Cardiovasc. Res. 2012, 93, 340–349. [Google Scholar] [CrossRef]
- Mika, D.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. PDEs Create Local Domains of cAMP Signaling. J. Mol. Cell. Cardiol. 2012, 52, 323–329. [Google Scholar] [CrossRef]
- Sholokh, A.; Klussmann, E. Local Cyclic Adenosine Monophosphate Signalling Cascades—Roles and Targets in Chronic Kidney Disease. Acta Physiol. 2021, 232, e13641. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Edemir, B.; Stefan, E.; Wiesner, B.; Lorenz, D.; Theilig, F.; Schmitt, R.; Vossebein, L.; Tamma, G.; Beyermann, M.; et al. Identification of a Novel A-Kinase Anchoring Protein 18 Isoform and Evidence for Its Role in the Vasopressin-Induced Aquaporin-2 Shuttle in Renal Principal Cells. J. Biol. Chem. 2004, 279, 26654–26665. [Google Scholar] [CrossRef] [PubMed]
- Whiting, J.L.; Ogier, L.; Forbush, K.A.; Bucko, P.; Gopalan, J.; Seternes, O.-M.; Langeberg, L.K.; Scott, J.D. AKAP220 Manages Apical Actin Networks That Coordinate Aquaporin-2 Location and Renal Water Reabsorption. Proc. Natl. Acad. Sci. USA 2016, 113, E4328–E4337. [Google Scholar] [CrossRef]
- Okutsu, R.; Rai, T.; Kikuchi, A.; Ohno, M.; Uchida, K.; Sasaki, S.; Uchida, S. AKAP220 Colocalizes with AQP2 in the Inner Medullary Collecting Ducts. Kidney Int. 2008, 74, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Klussmann, E.; Tamma, G.; Lorenz, D.; Wiesner, B.; Maric, K.; Hofmann, F.; Aktories, K.; Valenti, G.; Rosenthal, W. An Inhibitory Role of Rho in the Vasopressin-Mediated Translocation of Aquaporin-2 into Cell Membranes of Renal Principal Cells. J. Biol. Chem. 2001, 276, 20451–20457. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Klussmann, E.; Maric, K.; Aktories, K.; Svelto, M.; Rosenthal, W.; Valenti, G. Rho Inhibits cAMP-Induced Translocation of Aquaporin-2 into the Apical Membrane of Renal Cells. Am. J. Physiol. Ren. Physiol. 2001, 281, F1092–F1101. [Google Scholar] [CrossRef]
- Cheung, P.W.; Bouley, R.; Brown, D. Targeting the Trafficking of Kidney Water Channels for Therapeutic Benefit. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 175–194. [Google Scholar] [CrossRef]
- Battistone, M.A.; Nair, A.V.; Barton, C.R.; Liberman, R.N.; Peralta, M.A.; Capen, D.E.; Brown, D.; Breton, S. Extracellular Adenosine Stimulates Vacuolar ATPase–Dependent Proton Secretion in Medullary Intercalated Cells. J. Am. Soc. Nephrol. 2018, 29, 545–556. [Google Scholar] [CrossRef]
- Dema, A.; Faust, D.; Lazarow, K.; Wippich, M.; Neuenschwander, M.; Zühlke, K.; Geelhaar, A.; Pallien, T.; Hallscheidt, E.; Eichhorst, J.; et al. Cyclin-Dependent Kinase 18 Controls Trafficking of Aquaporin-2 and Its Abundance through Ubiquitin Ligase STUB1, Which Functions as an AKAP. Cells 2020, 9, 673. [Google Scholar] [CrossRef]
- Nedvetsky, P.I.; Tabor, V.; Tamma, G.; Beulshausen, S.; Skroblin, P.; Kirschner, A.; Mutig, K.; Boltzen, M.; Petrucci, O.; Vossenkämper, A.; et al. Reciprocal Regulation of Aquaporin-2 Abundance and Degradation by Protein Kinase A and P38-MAP Kinase. J. Am. Soc. Nephrol. 2010, 21, 1645–1656. [Google Scholar] [CrossRef]
- Erdorf, M.; Seifert, R. Pharmacological Characterization of Adenylyl Cyclase Isoforms in Rabbit Kidney Membranes. Naunyn Schmiedeberg’s Arch. Pharmacol. 2011, 383, 357–372. [Google Scholar] [CrossRef]
- Breyer, M.D.; Breyer, R.M. G Protein-Coupled Prostanoid Receptors and the Kidney. Annu. Rev. Physiol. 2001, 63, 579–605. [Google Scholar] [CrossRef] [PubMed]
- Morath, R.; Klein, T.; Seyberth, H.W.; Nüsing, R.M. Immunolocalization of the Four Prostaglandin E2 Receptor Proteins EP1, EP2, EP3, and EP4 in Human Kidney. J. Am. Soc. Nephrol. 1999, 10, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Chabardés, D.; Imbert-Teboul, M.; Gagnan-Brunette, M.; Morel, F. Different Hormonal Target Sites along the Mouse and Rabbit Nephrons. Curr. Probl. Clin. Biochem. 1977, 8, 447–454. [Google Scholar] [PubMed]
- Chabardès, D.; Imbert-Teboul, M.; Montégut, M.; Clique, A.; Morel, F. Distribution of Calcitonin-Sensitive Adenylate Cyclase Activity along the Rabbit Kidney Tubule. Proc. Natl. Acad. Sci. USA 1976, 73, 3608–3612. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Collier, W.L.; Rossodivita, I.; Amenta, F. Dopamine Receptors Mediating Inhibition of the Cyclic Adenosine Monophosphate Generating System in the Rat Renal Cortex. J. Auton. Pharmacol. 1991, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Schoeppe, W. Effects of Dopamine on Kidney Function. Proc. R. Soc. Med. 1977, 70 (Suppl. S2), 36–42. [Google Scholar] [CrossRef]
- Bailly, C.; Imbert-Teboul, M.; Chabardès, D.; Hus-Citharel, A.; Montégut, M.; Clique, A.; Morel, F. The Distal Nephron of Rat Kidney: A Target Site for Glucagon. Proc. Natl. Acad. Sci. USA 1980, 77, 3422–3424. [Google Scholar] [CrossRef]
- Chabardès, D.; Firsov, D.; Aarab, L.; Clabecq, A.; Bellanger, A.C.; Siaume-Perez, S.; Elalouf, J.M. Localization of mRNAs Encoding Ca2+-Inhibitable Adenylyl Cyclases along the Renal Tubule. Functional Consequences for Regulation of the cAMP Content. J. Biol. Chem. 1996, 271, 19264–19271. [Google Scholar] [CrossRef]
- Carraro-Lacroix, L.R.; Malnic, G.; Girardi, A.C.C. Regulation of Na+/H+ Exchanger NHE3 by Glucagon-like Peptide 1 Receptor Agonist Exendin-4 in Renal Proximal Tubule Cells. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1647–F1655. [Google Scholar] [CrossRef]
- Crajoinas, R.O.; Oricchio, F.T.; Pessoa, T.D.; Pacheco, B.P.M.; Lessa, L.M.A.; Malnic, G.; Girardi, A.C.C. Mechanisms Mediating the Diuretic and Natriuretic Actions of the Incretin Hormone Glucagon-like Peptide-1. Am. J. Physiol.-Ren. Physiol. 2011, 301, F355–F363. [Google Scholar] [CrossRef]
- Chabardès, D.; Imbert-Teboul, M.; Montégut, M.; Clique, A.; Morel, F. Catecholamine Sensitive Adenylate Cyclase Activity in Different Segments of the Rabbit Nephron. Pflug. Arch. 1975, 361, 9–15. [Google Scholar] [CrossRef]
- Chabardès, D.; Gagnan-Brunette, M.; Imbert-Teboul, M.; Gontcharevskaia, O.; Montégut, M.; Clique, A.; Morel, F. Adenylate Cyclase Responsiveness to Hormones in Various Portions of the Human Nephron. J. Clin. Investig. 1980, 65, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Chabardès, D.; Imbert, M.; Clique, A.; Montégut, M.; Morel, F. PTH Sensitive Adenyl Cyclase Activity in Different Segments of the Rabbit Nephron. Pflug. Arch. 1975, 354, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Imbert, M.; Chabardès, D.; Montegut, M.; Clique, A.; Morel, F. Vasopressin Dependent Adenylate Cyclase in Single Segments of Rabbit Kidney Tubule. Pflug. Arch. 1975, 357, 173–186. [Google Scholar] [CrossRef]
- Duggan, K.A.; Hodge, G.; Chen, J.; Trajanovska, S.; Hunter, T. Vasoactive Intestinal Peptide Infusion Reverses Existing Renal Interstitial Fibrosis via a Blood Pressure Independent Mechanism in the Rat. Eur. J. Pharmacol. 2020, 873, 172979. [Google Scholar] [CrossRef]
- Boularan, C.; Gales, C. Cardiac cAMP: Production, Hydrolysis, Modulation and Detection. Front. Pharmacol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Tang, T.; Murray, F.; Schroth, J.; Insel, P.A.; Fenton, R.A.; Hammond, H.K.; Vallon, V. Adenylate Cyclase 6 Determines cAMP Formation and Aquaporin-2 Phosphorylation and Trafficking in Inner Medulla. J. Am. Soc. Nephrol. 2010, 21, 2059–2068. [Google Scholar] [CrossRef]
- Sherpa, R.T.; Mohieldin, A.M.; Pala, R.; Wachten, D.; Ostrom, R.S.; Nauli, S.M. Sensory Primary Cilium Is a Responsive cAMP Microdomain in Renal Epithelia. Sci. Rep. 2019, 9, 6523. [Google Scholar] [CrossRef]
- Strait, K.A.; Stricklett, P.K.; Chapman, M.; Kohan, D.E. Characterization of Vasopressin-Responsive Collecting Duct Adenylyl Cyclases in the Mouse. Am. J. Physiol. Ren. Physiol. 2010, 298, F859–F867. [Google Scholar] [CrossRef][Green Version]
- Salhadar, K.; Matthews, A.; Raghuram, V.; Limbutara, K.; Yang, C.-R.; Datta, A.; Chou, C.-L.; Knepper, M.A. Phosphoproteomic Identification of Vasopressin/cAMP/Protein Kinase A–Dependent Signaling in Kidney. Mol. Pharmacol. 2021, 99, 358–369. [Google Scholar] [CrossRef]
- Isobe, K.; Jung, H.J.; Yang, C.-R.; Claxton, J.; Sandoval, P.; Burg, M.B.; Raghuram, V.; Knepper, M.A. Systems-Level Identification of PKA-Dependent Signaling in Epithelial Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E8875–E8884. [Google Scholar] [CrossRef]
- Raychowdhury, M.K.; Ramos, A.J.; Zhang, P.; McLaughin, M.; Dai, X.-Q.; Chen, X.-Z.; Montalbetti, N.; Del Rocío Cantero, M.; Ausiello, D.A.; Cantiello, H.F. Vasopressin Receptor-Mediated Functional Signaling Pathway in Primary Cilia of Renal Epithelial Cells. Am. J. Physiol. Ren. Physiol. 2009, 296, F87–F97. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S. Cellular and Molecular Mechanisms in Kidney Fibrosis. J. Clin. Investig. 2014, 124, 2299–2306. [Google Scholar] [CrossRef]
- Ding, H.; Bai, F.; Cao, H.; Xu, J.; Fang, L.; Wu, J.; Yuan, Q.; Zhou, Y.; Sun, Q.; He, W.; et al. PDE/cAMP/Epac/C/EBP-β Signaling Cascade Regulates Mitochondria Biogenesis of Tubular Epithelial Cells in Renal Fibrosis. Antioxid. Redox Signal. 2018, 29, 637–652. [Google Scholar] [CrossRef]
- Schinner, E.; Wetzl, V.; Schlossmann, J. Cyclic Nucleotide Signalling in Kidney Fibrosis. Int. J. Mol. Sci. 2015, 16, 2320–2351. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. cAMP and Epac in the Regulation of Tissue Fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Börgeson, E.; Sharma, K. Obesity, Immunomodulation and Chronic Kidney Disease. Curr. Opin. Pharmacol. 2013, 13, 618–624. [Google Scholar] [CrossRef]
- Brennan, E.P.; Cacace, A.; Godson, C. Specialized Pro-Resolving Mediators in Renal Fibrosis. Mol. Asp. Med. 2017, 58, 102–113. [Google Scholar] [CrossRef]
- Perretti, M.; Dalli, J. Exploiting the Annexin A1 Pathway for the Development of Novel Anti-Inflammatory Therapeutics. Br. J. Pharmacol. 2009, 158, 936–946. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection Regulates Pro-Resolving Mediators That Lower Antibiotic Requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Higgins, G.; Buchanan, P.; Perriere, M.; Al-Alawi, M.; Costello, R.W.; Verriere, V.; McNally, P.; Harvey, B.J.; Urbach, V. Activation of P2RY11 and ATP Release by Lipoxin A4 Restores the Airway Surface Liquid Layer and Epithelial Repair in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2014, 51, 178–190. [Google Scholar] [CrossRef]
- Jensen, M.S.; Mutsaers, H.A.M.; Tingskov, S.J.; Christensen, M.; Madsen, M.G.; Olinga, P.; Kwon, T.-H.; Nørregaard, R. Activation of the Prostaglandin E2 EP2 Receptor Attenuates Renal Fibrosis in Unilateral Ureteral Obstructed Mice and Human Kidney Slices. Acta Physiol. 2019, 227, e13291. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.F.; Farrell, F.X.; Morel, D.; Law, W.; Murphy, S. Adenosine Signaling Increases Proinflammatory and Profibrotic Mediators through Activation of a Functional Adenosine 2B Receptor in Renal Fibroblasts. Ann. Clin. Lab. Sci. 2016, 46, 339–345. [Google Scholar] [PubMed]
- Sussman, C.R.; Wang, X.; Chebib, F.T.; Torres, V.E. Modulation of Polycystic Kidney Disease by G-Protein Coupled Receptors and Cyclic AMP Signaling. Cell. Signal. 2020, 72, 109649. [Google Scholar] [CrossRef] [PubMed]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; E Silva, P.M.R.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the Signaling: Role of cAMP for the Resolution of Inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef] [PubMed]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master Regulator of Innate Immune Cell Function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef]
- Wuyts, W.A.; Vanaudenaerde, B.M.; Dupont, L.J.; Demedts, M.G.; Verleden, G.M. Modulation by cAMP of IL-1beta-Induced Eotaxin and MCP-1 Expression and Release in Human Airway Smooth Muscle Cells. Eur. Respir. J. 2003, 22, 220–226. [Google Scholar] [CrossRef]
- Steen, A.; Larsen, O.; Thiele, S.; Rosenkilde, M.M. Biased and g Protein-Independent Signaling of Chemokine Receptors. Front. Immunol. 2014, 5, 277. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on Inflammation in Chronic Kidney Disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Alves, A.C.; Pires, A.L.; Lagente, V.; Cordeiro, R.S.; Martins, M.A.; e Silva, P.M. Effect of Selective Phosphodiesterase Inhibitors on the Rat Eosinophil Chemotactic Response in Vitro. Mem. Inst. Oswaldo Cruz 1997, 92 (Suppl. S2), 201–204. [Google Scholar] [CrossRef][Green Version]
- Ottonello, L.; Morone, M.P.; Dapino, P.; Dallegri, F. Cyclic AMP-Elevating Agents down-Regulate the Oxidative Burst Induced by Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) in Adherent Neutrophils. Clin. Exp. Immunol. 1995, 101, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Hickey, F.B.; Martin, F. Role of the Immune System in Diabetic Kidney Disease. Curr. Diab. Rep. 2018, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.K.; Bao, R.; Li, Y.C. Critical Role of the cAMP-PKA Pathway in Hyperglycemia-induced Epigenetic Activation of Fibrogenic Program in the Kidney. FASEB J. 2017, 31, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Szaszák, M.; Christian, F.; Rosenthal, W.; Klussmann, E. Compartmentalized cAMP Signalling in Regulated Exocytic Processes in Non-Neuronal Cells. Cell. Signal. 2008, 20, 590–601. [Google Scholar] [CrossRef]
- Wernerson, A. Altered Ultrastructural Distribution of Nephrin in Minimal Change Nephrotic Syndrome. Nephrol. Dial. Transplant. 2003, 18, 70–76. [Google Scholar] [CrossRef]
- Birmelé, B.; De Agostini, A.; Girardin, E.P. Role of Cyclic AMP in Idiopathic Nephrotic Syndrome: A Pathway Involving a Decrease in Glomerular Cell Heparan Sulfates? J. Cell. Biochem. 2000, 78, 363–370. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Huang, Z.; Mancini, J.A. Phosphodiesterase 4 Inhibitors for the Treatment of Asthma and COPD. Curr. Med. Chem. 2006, 13, 3253–3262. [Google Scholar] [CrossRef]
- Ansari, M.N.; Aloliet, R.I.; Ganaie, M.A.; Khan, T.H.; Najeeb-Ur-Rehman; Imam, F.; Hamad, A.M. Roflumilast, a Phosphodiesterase 4 Inhibitor, Attenuates Cadmium-Induced Renal Toxicity via Modulation of NF-κB Activation and Induction of NQO1 in Rats. Hum. Exp. Toxicol. 2019, 38, 588–597. [Google Scholar] [CrossRef]
- Wang, L.; Ellis, M.J.; Gomez, J.A.; Eisner, W.; Fennell, W.; Howell, D.N.; Ruiz, P.; Fields, T.A.; Spurney, R.F. Mechanisms of the Proteinuria Induced by Rho GTPases. Kidney Int. 2012, 81, 1075–1085. [Google Scholar] [CrossRef]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Angielski, S.; Piwkowska, A. Extracellular ATP Modulates Podocyte Function through P2Y Purinergic Receptors and Pleiotropic Effects on AMPK and cAMP/PKA Signaling Pathways. Arch. Biochem. Biophys. 2020, 695, 108649. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Torres, V.E. Osmoregulation, Vasopressin, and cAMP Signaling in Autosomal Dominant Polycystic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 459–470. [Google Scholar] [CrossRef]
- Hanaoka, K.; Guggino, W.B. cAMP Regulates Cell Proliferation and Cyst Formation in Autosomal Polycystic Kidney Disease Cells. J. Am. Soc. Nephrol. 2000, 11, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Pelling, J.C.; Ramaswamy, N.T.; Eppler, J.W.; Wallace, D.P.; Nagao, S.; Rome, L.A.; Sullivan, L.P.; Grantham, J.J. cAMP Stimulates the in Vitro Proliferation of Renal Cyst Epithelial Cells by Activating the Extracellular Signal-Regulated Kinase Pathway. Kidney Int. 2000, 57, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Hopp, K.; Hommerding, C.J.; Wang, X.; Ye, H.; Harris, P.C.; Torres, V.E. Tolvaptan plus Pasireotide Shows Enhanced Efficacy in a PKD1 Model. J. Am. Soc. Nephrol. 2015, 26, 39–47. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Yang, Q.; Hu, J.; Feng, J.; Liang, W.; Ding, G. AKAP1 Mediates High Glucose-Induced Mitochondrial Fission through the Phosphorylation of Drp1 in Podocytes. J. Cell. Physiol. 2020, 235, 7433–7448. [Google Scholar] [CrossRef]
- Fujita, H.; Morii, T.; Fujishima, H.; Sato, T.; Shimizu, T.; Hosoba, M.; Tsukiyama, K.; Narita, T.; Takahashi, T.; Drucker, D.J.; et al. The Protective Roles of GLP-1R Signaling in Diabetic Nephropathy: Possible Mechanism and Therapeutic Potential. Kidney Int. 2014, 85, 579–589. [Google Scholar] [CrossRef]
- Ookawara, M.; Nio, Y.; Yamasaki, M.; Kuniyeda, K.; Hanauer, G.; Tohyama, K.; Hazama, M.; Matsuo, T. Protective Effect of a Novel Phosphodiesterase 4 Selective Inhibitor, Compound A, in Diabetic Nephropathy Model Mice. Eur. J. Pharmacol. 2021, 894, 173852. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Zhang, S.; Wang, H.; Liu, Y.; Liu, J.; Li, X.; Zeng, M.; Han, Y.; Liu, F.; et al. Epac Activation Ameliorates Tubulointerstitial Inflammation in Diabetic Nephropathy. Acta Pharmacol. Sin. 2022, 43, 659–671. [Google Scholar] [CrossRef]
- Rieg, T.; Tang, T.; Uchida, S.; Hammond, H.K.; Fenton, R.A.; Vallon, V. Adenylyl Cyclase 6 Enhances NKCC2 Expression and Mediates Vasopressin-Induced Phosphorylation of NKCC2 and NCC. Am. J. Pathol. 2013, 182, 96–106. [Google Scholar] [CrossRef]
- Asteria, C. Molecular Basis of Bartter’s Syndrome: New Insights into the Correlation between Genotype and Phenotype. Eur. J. Endocrinol. 1997, 137, 613–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hebert, S.C. Bartter Syndrome. Curr. Opin. Nephrol. Hypertens. 2003, 12, 527–532. [Google Scholar] [CrossRef]
- Snyder, P.M. Liddle’s Syndrome Mutations Disrupt cAMP-Mediated Translocation of the Epithelial Na+ Channel to the Cell Surface. J. Clin. Investig. 2000, 105, 45–53. [Google Scholar] [CrossRef]
- Bubien, J.K.; Ismailov, I.I.; Berdiev, B.K.; Cornwell, T.; Lifton, R.P.; Fuller, C.M.; Achard, J.M.; Benos, D.J.; Warnock, D.G. Liddle’s Disease: Abnormal Regulation of Amiloride-Sensitive Na+ Channels by Beta-Subunit Mutation. Am. J. Physiol.-Cell Physiol. 1996, 270, C208–C213. [Google Scholar] [CrossRef]
- Lu, C.; Pribanic, S.; Debonneville, A.; Jiang, C.; Rotin, D. The PY Motif of ENaC, Mutated in Liddle Syndrome, Regulates Channel Internalization, Sorting and Mobilization from Subapical Pool. Traffic 2007, 8, 1246–1264. [Google Scholar] [CrossRef]
- Shimkets, R.A.; Warnock, D.G.; Bositis, C.M.; Nelson-Williams, C.; Hansson, J.H.; Schambelan, M.; Gill, J.R.; Ulick, S.; Milora, R.V.; Findling, J.W.; et al. Liddle’s Syndrome: Heritable Human Hypertension Caused by Mutations in the β Subunit of the Epithelial Sodium Channel. Pediatr. Nephrol. 1996, 10, 342. [Google Scholar] [CrossRef]
- Păunescu, T.G.; Ljubojevic, M.; Russo, L.M.; Winter, C.; McLaughlin, M.M.; Wagner, C.A.; Breton, S.; Brown, D. cAMP Stimulates Apical V-ATPase Accumulation, Microvillar Elongation, and Proton Extrusion in Kidney Collecting Duct A-Intercalated Cells. Am. J. Physiol.-Ren. Physiol. 2010, 298, F643–F654. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh Khayyat, N.; Tomilin, V.; Pyrshev, K.; Zaika, O.; Mei, F.; Cheng, X.; Pochynyuk, O. Exchange Protein Directly Activated by cAMP Isoform 2 (Epac2) but Not Isoform 1 (Epac1) Is Critical for Renal Acid-Base Handling. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Jean-Alphonse, F.; Perkovska, S.; Frantz, M.-C.; Durroux, T.; Méjean, C.; Morin, D.; Loison, S.; Bonnet, D.; Hibert, M.; Mouillac, B.; et al. Biased Agonist Pharmacochaperones of the AVP V2 Receptor May Treat Congenital Nephrogenic Diabetes Insipidus. J. Am. Soc. Nephrol. 2009, 20, 2190–2203. [Google Scholar] [CrossRef]
- Takeda, S.; Lin, C.-T.; Morgano, P.G.; McINTYRE, S.J.; Dousa, T.P. High Activity of Low-Michaelis-Menten Constant 3′,5′-Cyclic Adenosine Monophosphate-Phosphodiesterase Isozymes in Renal Inner Medulla of Mice With Hereditary Nephrogenic Diabetes Insipidus. Endocrinology 1991, 129, 287–294. [Google Scholar] [CrossRef]
- Rochdi, M.D.; Vargas, G.A.; Carpentier, E.; Oligny-Longpré, G.; Chen, S.; Kovoor, A.; Gitelman, S.E.; Rosenthal, S.M.; Von Zastrow, M.; Bouvier, M. Functional Characterization of Vasopressin Type 2 Receptor Substitutions (R137H/C/L) Leading to Nephrogenic Diabetes Insipidus and Nephrogenic Syndrome of Inappropriate Antidiuresis: Implications for Treatments. Mol. Pharmacol. 2010, 77, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J. Fabry Disease, an Under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338. [Google Scholar] [CrossRef]
- Wilcox, W.R.; Oliveira, J.P.; Hopkin, R.J.; Ortiz, A.; Banikazemi, M.; Feldt-Rasmussen, U.; Sims, K.; Waldek, S.; Pastores, G.M.; Lee, P.; et al. Females with Fabry Disease Frequently Have Major Organ Involvement: Lessons from the Fabry Registry. Mol. Genet. Metab. 2008, 93, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Das, A.M.; Naim, H.Y. Chapter 3 Biochemical Basis of Fabry Disease with Emphasis on Mitochondrial Function and Protein Trafficking. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2009; Volume 49, pp. 57–71. ISBN 978-0-12-374798-3. [Google Scholar]
- Li, Y.; Chen, D.; Li, Y.; Jin, L.; Liu, J.; Su, Z.; Qi, Z.; Shi, M.; Jiang, Z.; Ni, L.; et al. Oncogenic cAMP Responsive Element Binding Protein 1 Is Overexpressed upon Loss of Tumor Suppressive miR-10b-5p and miR-363-3p in Renal Cancer. Oncol. Rep. 2016, 35, 1967–1978. [Google Scholar] [CrossRef]
- Cao, M.; Ajay, A.K.; Gasser, M.; Hsiao, L.-L.; Waaga-Gasser, A.M. Abstract 967: Phosphodiesterase 4D Depletion Enhances Anti-Tumor Effects of Tyrosine Kinase Inhibitor in Renal Cell Carcinoma Involving CRAF-ERK Pathway. Cancer Res. 2021, 81, 967. [Google Scholar] [CrossRef]
- Sherwood, J.; Burns, E.; Shouval, D. Stimulation by cAMP of Erythropoietin Secretion by an Established Human Renal Carcinoma Cell Line. Blood 1987, 69, 1053–1057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanomogi, H. Establishment and characterization of a renal carcinoma cell line producing erythropoietin. Jpn. J. Urol. 1997, 88, 778–787. [Google Scholar] [CrossRef]
- Guan, X.; Liu, Y.; Xin, W.; Qin, S.; Gong, S.; Xiao, T.; Zhang, D.; Li, Y.; Xiong, J.; Yang, K.; et al. Activation of EP4 Alleviates AKI-to-CKD Transition through Inducing CPT2-Mediated Lipophagy in Renal Macrophages. Front. Pharmacol. 2022, 13, 1030800. [Google Scholar] [CrossRef]
- Huen, S.C.; Cantley, L.G. Macrophages in Renal Injury and Repair. Annu. Rev. Physiol. 2017, 79, 449–469. [Google Scholar] [CrossRef]
- Cheng, J.; Grande, J.P. Cyclic Nucleotide Phosphodiesterase (PDE) Inhibitors: Novel Therapeutic Agents for Progressive Renal Disease. Exp. Biol. Med. 2007, 232, 38–51. [Google Scholar]
- Wang, F.; Li, M.; Cheng, L.; Zhang, T.; Hu, J.; Cao, M.; Zhao, J.; Guo, R.; Gao, L.; Zhang, X. Intervention with Cilostazol Attenuates Renal Inflammation in Streptozotocin-Induced Diabetic Rats. Life Sci. 2008, 83, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Pofi, R.; Fiore, D.; De Gaetano, R.; Panio, G.; Gianfrilli, D.; Pozza, C.; Barbagallo, F.; Xiang, Y.K.; Giannakakis, K.; Morano, S.; et al. Phosphodiesterase-5 Inhibition Preserves Renal Hemodynamics and Function in Mice with Diabetic Kidney Disease by Modulating miR-22 and BMP7. Sci. Rep. 2017, 7, 44584. [Google Scholar] [CrossRef]
- Degerman, E.; Belfrage, P.; Manganiello, V.C. Structure, Localization, and Regulation of cGMP-Inhibited Phosphodiesterase (PDE3). J. Biol. Chem. 1997, 272, 6823–6826. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.; Findlay, J.E.; Carfray, G.; Allcock, R.W.; Jiang, Z.; Moore, C.; Muir, A.L.; Lannoy, M.; Fertig, B.A.; Mai, D.; et al. Small-Molecule Allosteric Activators of PDE4 Long Form Cyclic AMP Phosphodiesterases. Proc. Natl. Acad. Sci. USA 2019, 116, 13320–13329. [Google Scholar] [CrossRef]
- Hayashi, T.; Maruyama, S.; Nangaku, M.; Narita, I.; Hirakata, H.; Tanabe, K.; Morita, S.; Tsubakihara, Y.; Imai, E.; Akizawa, T.; et al. Darbepoetin Alfa in Patients with Advanced CKD without Diabetes: Randomized, Controlled Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A.; Li, M.; Batuman, V. Potential Protective Action of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP38) on in Vitro and in Vivo Models of Myeloma Kidney Injury. Blood 2006, 107, 661–668. [Google Scholar] [CrossRef][Green Version]
- Gjertsen, B.T.; Mellgren, G.; Otten, A.; Maronde, E.; Genieser, H.-G.; Jastorff, B.; Vintermyr, O.K.; McKnight, G.S.; D⊘skeland, S.O. Novel (Rp)-cAMPS Analogs as Tools for Inhibition of cAMP-Kinase in Cell Culture. J. Biol. Chem. 1995, 270, 20599–20607. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in Targeting Cyclic Nucleotide Phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Chiang, W.-C.; Lin, S.-L.; Tsai, T.-J. Therapeutic Efficacy of Pentoxifylline on Proteinuria and Renal Progression: An Update. J. Biomed. Sci. 2017, 24, 84. [Google Scholar] [CrossRef]
- Mazzon, E.; Esposito, E.; Di Paola, R.; Impellizzeri, D.; Bramanti, P.; Cuzzocrea, S. Olprinone, a Specific Phosphodiesterase (PDE)-III Inhibitor, Reduces the Development of Multiple Organ Dysfunction Syndrome in Mice. Pharmacol. Res. 2011, 64, 68–79. [Google Scholar] [CrossRef]
- Chini, C.C.; Chini, E.N.; Williams, J.M.; Matousovic, K.; Dousa, T.P. Formation of Reactive Oxygen Metabolites in Glomeruli Is Suppressed by Inhibition of cAMP Phosphodiesterase Isozyme Type IV. Kidney Int. 1994, 46, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Dousa, T.P. Cyclic-3′,5′-Nucleotide Phosphodiesterase Isozymes in Cell Biology and Pathophysiology of the Kidney. Kidney Int. 1999, 55, 29–62. [Google Scholar] [CrossRef]
- Xu, M.; Yu, X.; Meng, X.; Huang, S.; Zhang, Y.; Zhang, A.; Jia, Z. Inhibition of PDE4/PDE4B Improves Renal Function and Ameliorates Inflammation in Cisplatin-Induced Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2020, 318, F576–F588. [Google Scholar] [CrossRef]
- Afsar, B.; Ortiz, A.; Covic, A.; Gaipov, A.; Esen, T.; Goldsmith, D.; Kanbay, M. Phosphodiesterase Type 5 Inhibitors and Kidney Disease. Int. Urol. Nephrol. 2015, 47, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Sanchez-Lozada, L.G.; Soto, V.; Manrique, A.M.; Ortiz-Vega, K.M.; Santamaría, J.; Medina-Campos, O.N.; Cristóbal, M.; Avila-Casado, C.; Pedraza-Chaverri, J.; et al. Sildenafil Treatment Prevents Glomerular Hypertension and Hyperfiltration in Rats with Renal Ablation. Kidney Blood Press. Res. 2012, 35, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tzoumas, N.; Farrah, T.E.; Dhaun, N.; Webb, D.J. Established and Emerging Therapeutic Uses of PDE Type 5 Inhibitors in Cardiovascular Disease. Br. J. Pharmacol. 2020, 177, 5467–5488. [Google Scholar] [CrossRef] [PubMed]
- Scheele, W.; Diamond, S.; Gale, J.; Clerin, V.; Tamimi, N.; Le, V.; Walley, R.; Grover-Páez, F.; Perros-Huguet, C.; Rolph, T.; et al. Phosphodiesterase Type 5 Inhibition Reduces Albuminuria in Subjects with Overt Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 3459–3468. [Google Scholar] [CrossRef]
- Maass, P.G.; Aydin, A.; Luft, F.C.; Schächterle, C.; Weise, A.; Stricker, S.; Lindschau, C.; Vaegler, M.; Qadri, F.; Toka, H.R.; et al. PDE3A Mutations Cause Autosomal Dominant Hypertension with Brachydactyly. Nat. Genet. 2015, 47, 647–653. [Google Scholar] [CrossRef]
- Ercu, M.; Markó, L.; Schächterle, C.; Tsvetkov, D.; Cui, Y.; Maghsodi, S.; Bartolomaeus, T.U.P.; Maass, P.G.; Zühlke, K.; Gregersen, N.; et al. Phosphodiesterase 3A and Arterial Hypertension. Circulation 2020, 142, 133–149. [Google Scholar] [CrossRef]
- Schuster, H.; Wienker, T.E.; Bähring, S.; Bilginturan, N.; Toka, H.R.; Neitzel, H.; Jeschke, E.; Toka, O.; Gilbert, D.; Lowe, A.; et al. Severe Autosomal Dominant Hypertension and Brachydactyly in a Unique Turkish Kindred Maps to Human Chromosome 12. Nat. Genet. 1996, 13, 98–100. [Google Scholar] [CrossRef]
- Schuster, H.; Wienker, T.F.; Toka, H.R.; Bähring, S.; Jeschke, E.; Toka, O.; Busjahn, A.; Hempel, A.; Tahlhammer, C.; Oelkers, W.; et al. Autosomal Dominant Hypertension and Brachydactyly in a Turkish Kindred Resembles Essential Hypertension. Hypertension 1996, 28, 1085–1092. [Google Scholar] [CrossRef]
- Toka, O.; Tank, J.; Schächterle, C.; Aydin, A.; Maass, P.G.; Elitok, S.; Bartels-Klein, E.; Hollfinger, I.; Lindschau, C.; Mai, K.; et al. Clinical Effects of Phosphodiesterase 3A Mutations in Inherited Hypertension with Brachydactyly. Hypertension 2015, 66, 800–808. [Google Scholar] [CrossRef]
- Klussmann, E. Protein-Protein Interactions of PDE4 Family Members—Functions, Interactions and Therapeutic Value. Cell. Signal. 2016, 28, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.S.; Raman, A.; Reif, G.A.; Magenheimer, B.S.; White, C.; Calvet, J.P.; Wallace, D.P. Phosphodiesterase Isoform Regulation of Cell Proliferation and Fluid Secretion in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R.; Caceres, P.; Alvarez-Leefmans, F.J.; Ortiz, P.A. cGMP Decreases Surface NKCC2 Levels in the Thick Ascending Limb: Role of Phosphodiesterase 2 (PDE2). Am. J. Physiol.-Ren. Physiol. 2008, 295, F877–F887. [Google Scholar] [CrossRef]
- Cheng, J.; Thompson, M.A.; Walker, H.J.; Gray, C.E.; Diaz Encarnacion, M.M.; Warner, G.M.; Grande, J.P. Differential Regulation of Mesangial Cell Mitogenesis by cAMP Phosphodiesterase Isozymes 3 and 4. Am. J. Physiol.-Ren. Physiol. 2004, 287, F940–F953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chini, C.C.S.; Grande, J.P.; Chini, E.N.; Dousa, T.P. Compartmentalization of cAMP Signaling in Mesangial Cells by Phosphodiesterase Isozymes PDE3 and PDE4 Regulation of Superoxidation and Mitogenesis. J. Biol. Chem. 1997, 272, 9854–9859. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Szilagyi, C.; Allen, J.M.; Bertrand, C.; Lagente, V. Role of PDE4 in Superoxide Anion Generation through P44/42 MAPK Regulation: A cAMP and a PKA-independent Mechanism. Br. J. Pharmacol. 2004, 143, 257–268. [Google Scholar] [CrossRef]
- Ookawara, M.; Nio, Y. Phosphodiesterase 4 Inhibitors in Diabetic Nephropathy. Cell. Signal. 2022, 90, 110185. [Google Scholar] [CrossRef]
- Coskuner, E.R.; Ozkan, B. Reno-Protective Effects of Phosphodiesterase 5 Inhibitors. Clin. Exp. Nephrol. 2021, 25, 585–597. [Google Scholar] [CrossRef]
- Abbad, L.; Detrait, M.; Kavvadas, P.; Melis, L.; Hadji, S.; Bergonnier, D.; Lezoualch, F.; Chatziantoniou, C. #4904 Epac1-Mediated Camp Signalling Promotes Cellular Energy Adaptations In Podocytes To Protect From Glomerulonephritis. Nephrol. Dial. Transplant. 2023, 38, gfad063c_4904. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Cao, X.; Lu, Y.; Mi, Z.; He, C.; Liu, J.; Zheng, Z.; Li, M.J.; Li, T.; et al. Activation of P-TEFb by cAMP-PKA Signaling in Autosomal Dominant Polycystic Kidney Disease. Sci. Adv. 2019, 5, eaaw3593. [Google Scholar] [CrossRef] [PubMed]
- Stefan, E.; Wiesner, B.; Baillie, G.S.; Mollajew, R.; Henn, V.; Lorenz, D.; Furkert, J.; Santamaria, K.; Nedvetsky, P.; Hundsrucker, C.; et al. Compartmentalization of cAMP-Dependent Signaling by Phosphodiesterase-4D Is Involved in the Regulation of Vasopressin-Mediated Water Reabsorption in Renal Principal Cells. J. Am. Soc. Nephrol. 2007, 18, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Chepurny, O.G.; Matsoukas, M.-T.; Liapakis, G.; Leech, C.A.; Milliken, B.T.; Doyle, R.P.; Holz, G.G. Nonconventional Glucagon and GLP-1 Receptor Agonist and Antagonist Interplay at the GLP-1 Receptor Revealed in High-Throughput FRET Assays for cAMP. J. Biol. Chem. 2019, 294, 3514–3531. [Google Scholar] [CrossRef] [PubMed]
| Kidney Disease | Role of cAMP | References |
|---|---|---|
| Nephrotic syndrome | cAMP modulates podocyte function, affecting cytoskeletal dynamics, cell adhesion, and protein expression crucial for maintaining the filtration barrier. | [9,64] |
| Diabetic nephropathy | cAMP influences the CREB-binding protein-mediated hyperacetylation of profibrotic genes, contributing to fibrogenesis and kidney damage. | [63] |
| Autosomal dominant polycystic kidney disease | cAMP promotes cystogenesis by stimulating cell proliferation and fluid secretion in the cyst lumen through V2 receptor activation. | [72] |
| Bartter syndrome | cAMP modulates ion transport and salt reabsorption in the thick ascending limb of the loop of Henle, impacting NKCC2 regulation. | [36] |
| Liddle syndrome | cAMP regulates the ENaC activity and trafficking, with mutations in ENaC disrupting this regulation and causing hypertension. | [55] |
| Renal tubular acidosis | cAMP affects proton pumps and bicarbonate transporters, regulating acid–base balance in distal tubules and influencing V-ATPase activity. | [16] |
| Nephrogenic diabetes insipidus | cAMP mediates vasopressin effects on water reabsorption by promoting AQP2 trafficking to the apical membrane in the collecting ducts. | [15] |
| Fabry disease | cAMP impacts lysosomal function and substrate accumulation, contributing to kidney dysfunction by modulating lysosomal enzyme activity. | [8] |
| Renal cell carcinoma | cAMP influences cell proliferation, migration, and apoptosis through CREB1 regulation, impacting tumor growth and survival. | [35] |
| Therapeutic Agent | Status | Mechanism of Action | Kidney Disease | Effects on Kidney Function | References |
|---|---|---|---|---|---|
| Pentoxifylline | On the market (off label use) | Non-selective PDE inhibitor, increases cAMP levels | Diabetic nephropathy, CKD | Reduces proteinuria, inflammation, and fibrosis; delays progression to ESKD | [101] |
| Cilostazol | On the market (off label use) | PDE3 inhibitor, increases cAMP levels | Diabetic nephropathy, CKD | Prevents glomerular hypertrophy and inflammation; improves blood pressure and vascular function | [102] |
| Sildenafil | On the market (off label use) | PDE5 inhibitor, increases cGMP levels | Diabetic nephropathy | Reduces progression of nephropathy and hypertension; improves hemodynamic parameters | [103] |
| PF-00489791 | Clinical trials | PDE5 inhibitor, increases cGMP levels | Diabetic nephropathy | Decreases albuminuria and urine albumin-to-creatinine ratio | [104] |
| GLP-1 Receptor Agonists | On the market | Activates cAMP production via GLP-1 receptor stimulation | Diabetic nephropathy | Reduces inflammation, oxidative stress, and fibrosis; improves renal function | [77] |
| MR-L2 | Research stage | Activates long forms of PDE4, reduces cAMP levels | Polycystic kidney disease | Suppresses cyst development and progression | [105] |
| Forskolin | On the market | AC activator, increases cAMP levels | CKD | Promotes protective cellular responses, reduces fibrosis | [106] |
| PACAP38 | Research stage | AC activator, reduces off-target effects | CKD | Prevents renal injury by suppressing proinflammatory cytokine production, inhibits p38 MAPK and NF-κB pathways | [107] |
| (Rp)-8-Br-cAMPS, (Rp)-8-Cl-cAMPS | Research stage | cAMP analogs, selectively inhibit cAMP-dependent protein kinase I | CKD | Target specific signaling pathways, reduce off-target actions | [108] |
| Rolipram | Research stage | PDE4 inhibitor, increases cAMP levels | Renal fibrosis | Activates Epac1/Rap1 pathway, reduces tubular epithelial cell damage and fibrosis | [43] |
| PDE Isoform | Localization in the Kidney | Role in CKD | References |
|---|---|---|---|
| PDE1 (cAMP) PDE1 (cGMP) | Cortical tubules + Proximal tubule epithelial cells +/++ Inner medullary collecting duct cells ++ Glomeruli ++++ Mesangial cells +++ Cortical tubules +++ Proximal tubule epithelial cells +/++ Inner medullary collecting duct cells + | Inhibition of PDE1 causes greater stimulation of ERK and proliferation of ADPKD cells. | [113,125] |
| PDE2 | Glomeruli ++++ Proximal tubule epithelial cells + | cGMP-stimulated PDE2 mediates the inhibitory effect of nitric oxide on NaCl absorption by the medullary thick ascending limb of the loop of Henle. | [113,126] |
| PDE3 | Glomeruli ++ Glomerular epithelial cells +/− Mesangial cells +++ Cortical tubules ++ Proximal tubule epithelial cells +/− Inner medullary collecting duct cells + | PDE3-linked cAMP–PKA pathway modulates mitogenesis. | [113,127,128] |
| PDE4 | Glomeruli ++ Glomerular epithelial cells ++++ Mesangial cells ++++ Cortical tubules +++ Proximal tubule epithelial cells ++++ Inner medullary collecting duct cells +++ | PDE4 inhibition suppresses oxidative stress, fibrosis, and inflammation in mesangial cells and podocyte cells, which protect podocyte loss, which leads to albuminuria and glomerulosclerosis. In tubulointerstitial lesions, PDE4 inhibition suppresses inflammation and epithelial–mesenchymal transition, which leads to tubulointerstitial fibrosis. PDE4-linked cAMP–PKA pathway modulates generation of reactive oxygen species. | [113,128,129,130] |
| PDE5 | Glomeruli +++ Mesangial cells +++ Cortical tubules +++ Proximal tubule epithelial cells +/++ Inner medullary collecting duct cells + | PDE5 inhibition regulates the excretory function and hemodynamics of the kidney. PDE5 contributes to the regulation of renal vascular blood flow by limiting the vascular relaxation caused by cGMP. PDE5 contributes to the regulation of natriuresis through the degradation of cGMP. | [113,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delrue, C.; Speeckaert, R.; Moresco, R.N.; Speeckaert, M.M. Cyclic Adenosine Monophosphate Signaling in Chronic Kidney Disease: Molecular Targets and Therapeutic Potentials. Int. J. Mol. Sci. 2024, 25, 9441. https://doi.org/10.3390/ijms25179441
Delrue C, Speeckaert R, Moresco RN, Speeckaert MM. Cyclic Adenosine Monophosphate Signaling in Chronic Kidney Disease: Molecular Targets and Therapeutic Potentials. International Journal of Molecular Sciences. 2024; 25(17):9441. https://doi.org/10.3390/ijms25179441
Chicago/Turabian StyleDelrue, Charlotte, Reinhart Speeckaert, Rafael Noal Moresco, and Marijn M. Speeckaert. 2024. "Cyclic Adenosine Monophosphate Signaling in Chronic Kidney Disease: Molecular Targets and Therapeutic Potentials" International Journal of Molecular Sciences 25, no. 17: 9441. https://doi.org/10.3390/ijms25179441
APA StyleDelrue, C., Speeckaert, R., Moresco, R. N., & Speeckaert, M. M. (2024). Cyclic Adenosine Monophosphate Signaling in Chronic Kidney Disease: Molecular Targets and Therapeutic Potentials. International Journal of Molecular Sciences, 25(17), 9441. https://doi.org/10.3390/ijms25179441







