Identification of Shaker Potassium Channel Family Members and Functional Characterization of SsKAT1.1 in Stenotaphrum secundatum Suggest That SsKAT1.1 Contributes to Cold Resistance
Abstract
:1. Introduction
2. Results
2.1. Gene Identification
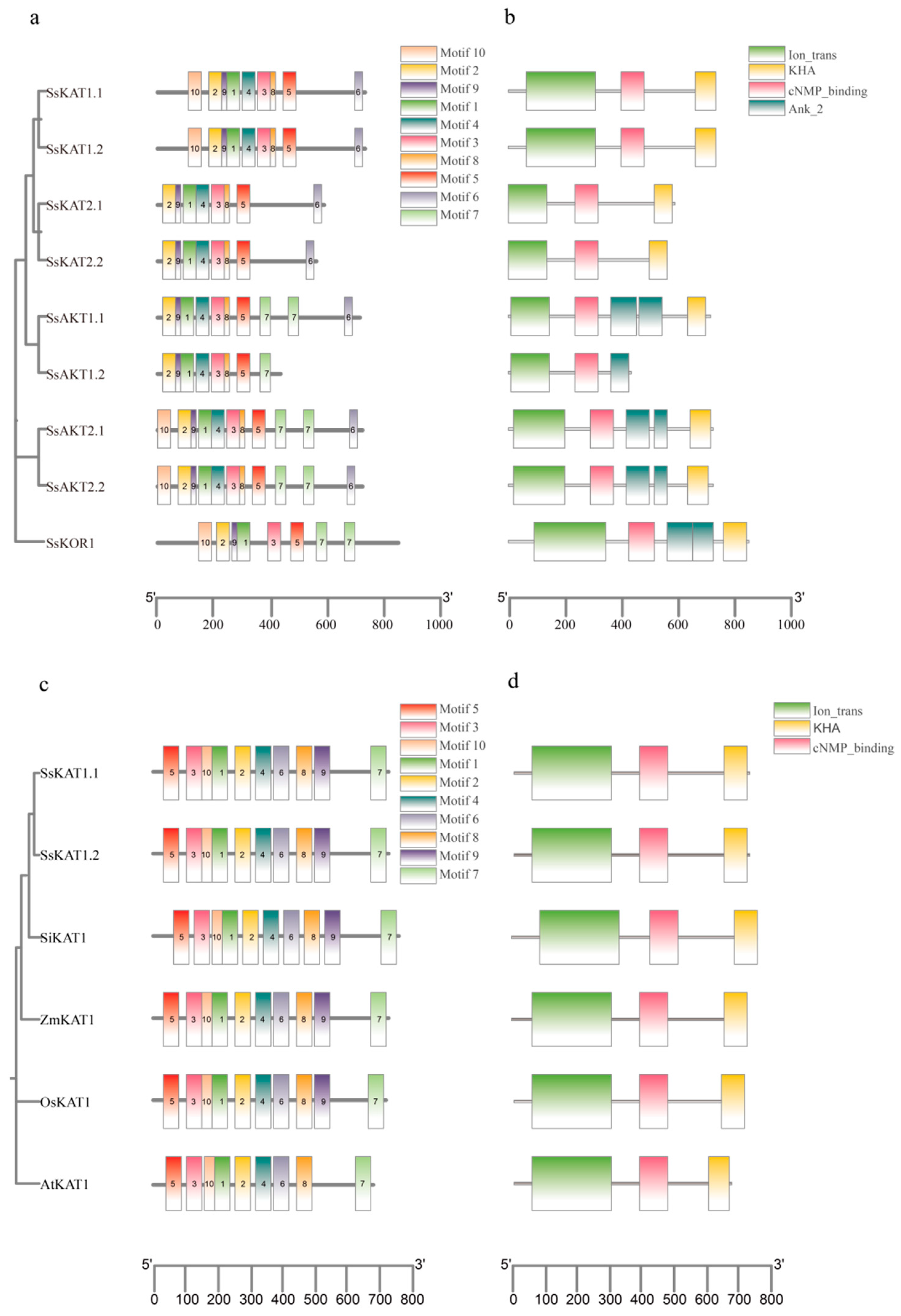
2.2. Motif and Protein Domain Analysis
2.3. Protein Properties
2.4. Protein Secondary Structure and Three-Dimensional Structure Prediction
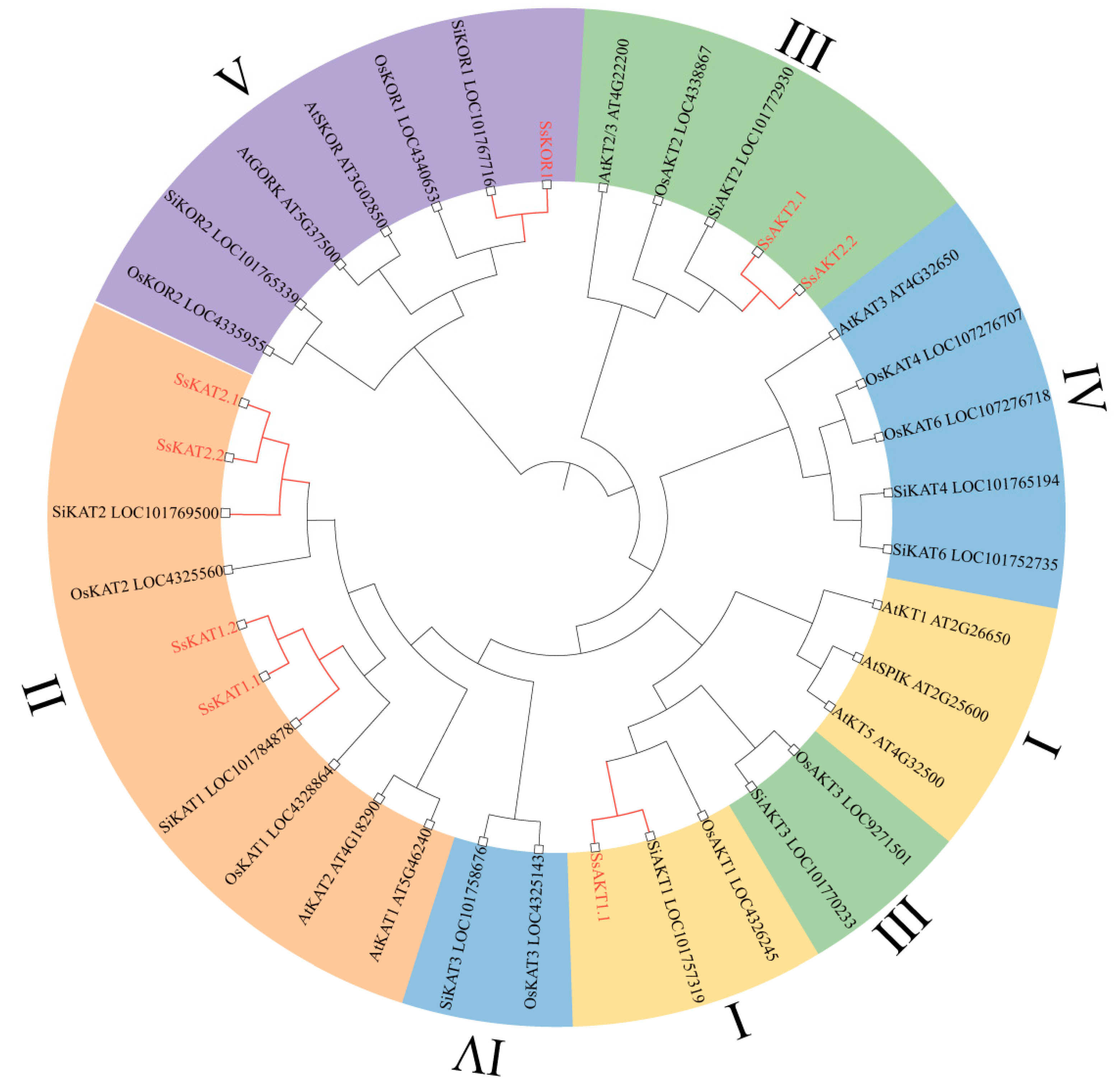
2.5. Analysis of the Phylogenetic Relationship of the Shaker K+ Channel
2.6. Relative Expression Levels of Shaker K+ Channel Family Members in Different Tissues
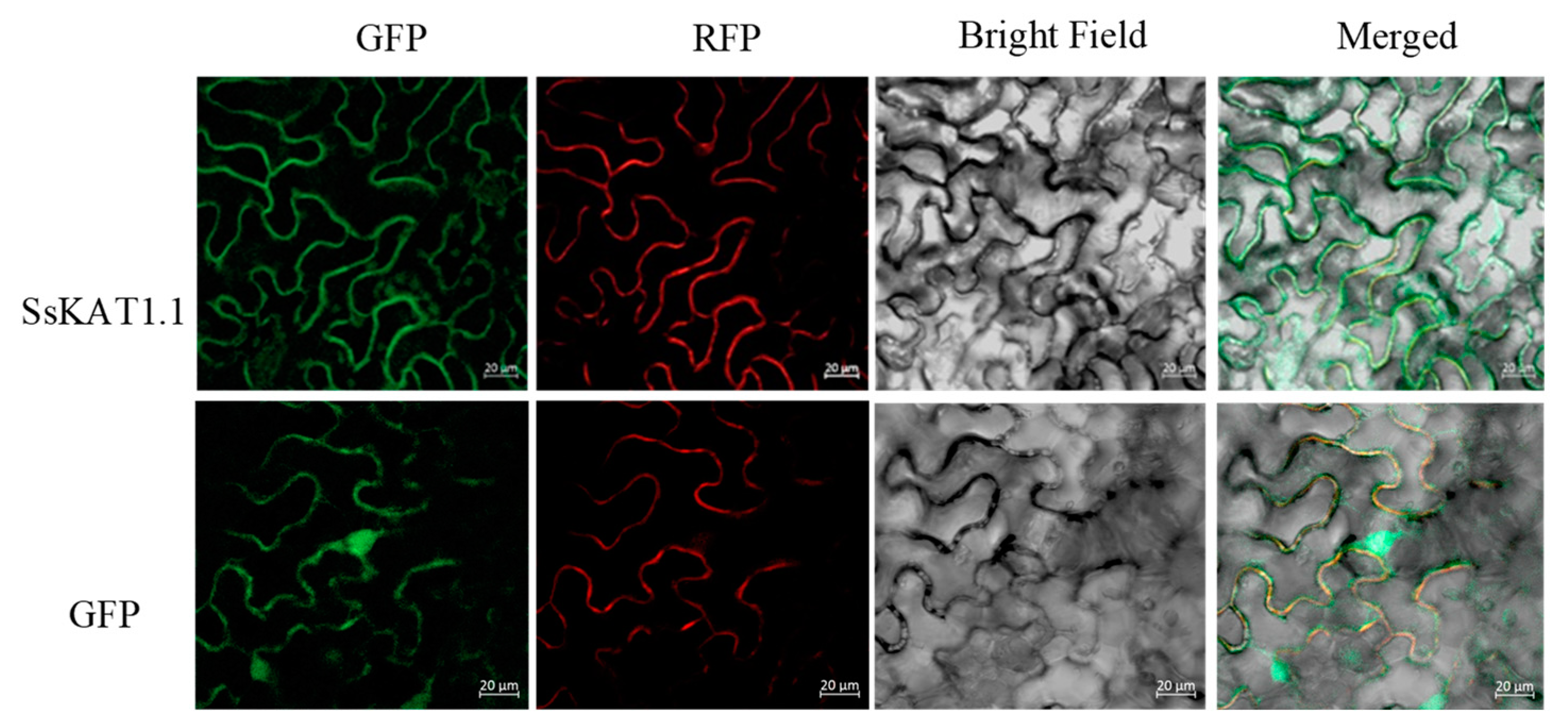
2.7. SsKAT1.1 Is Located to Plasma Membrane
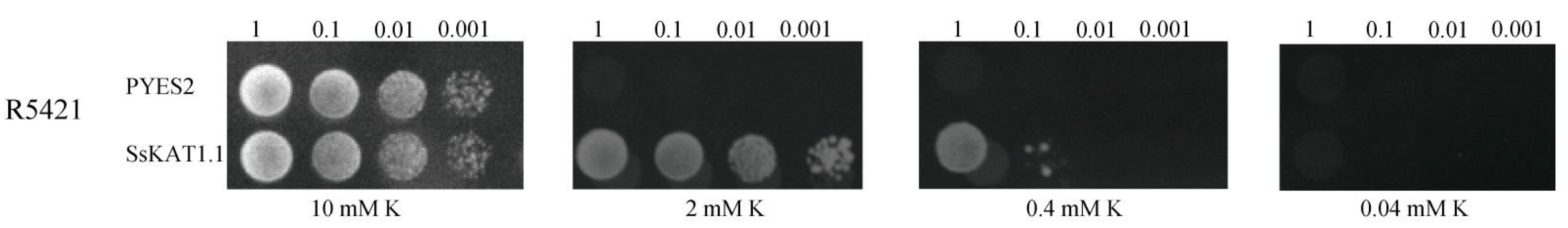
2.8. SsKAT1.1 Has a Potassium Absorption Function
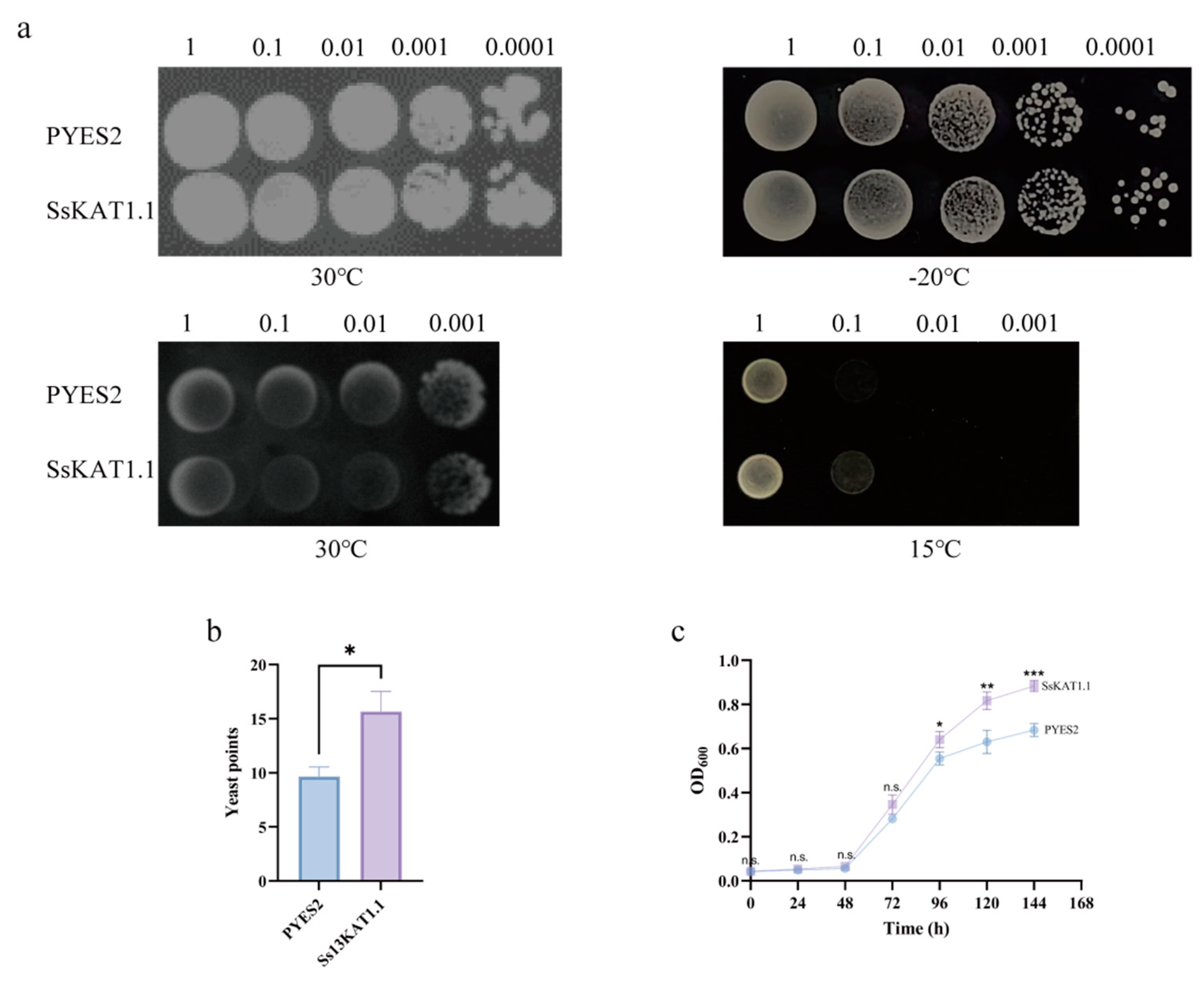
2.9. Expression of SsKAT1.1 in Yeast Enhances Cold Resistance
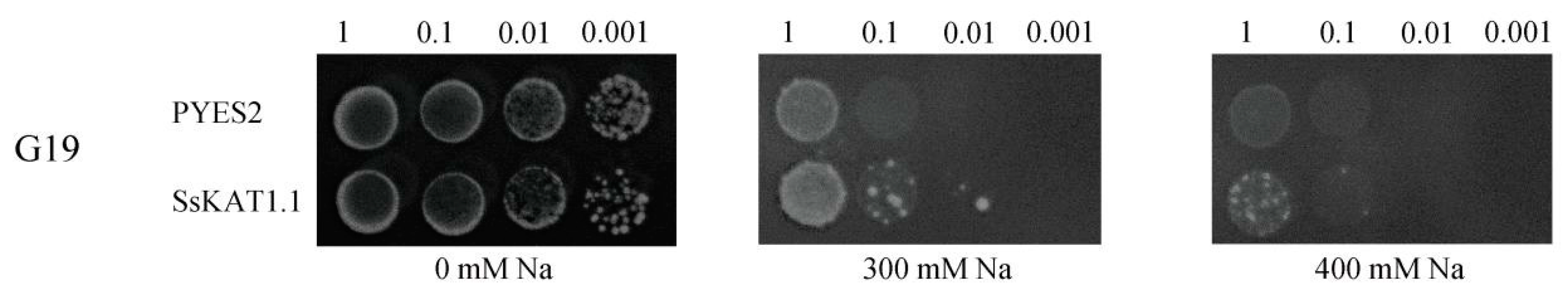
2.10. Expression of SsKAT1.1 in Yeast Enhances Salt Tolerance
3. Discussion
3.1. Stenotaphrum secundatum and Setaria italica Shaker K+ Channels Have a Close Phylogenetic Relationship
3.2. Shaker K+ Channel Members of Stenotaphrum secundatum Have More than One Corresponding Gene
3.3. Several Shaker K+ Channels in Stenotaphrum secundatum Have Not Been Identified
3.4. SsKAT1.1 Has a Potassium Absorption Function, and Its Potassium Absorption Function Depends on Higher Concentrations of K+
3.5. SsKAT1.1 Participates in Cellular Cold Resistance
3.6. SsKAT1.1 Participates in Cell Salt Tolerance
4. Materials and Methods
4.1. Plant Material Culture
4.2. Sequencing and Identification of Shaker K+ Channel Genes in Stenotaphrum secundatum
4.3. Protein Analysis, Structure Prediction, and Three-Dimensional Modelling
4.4. Motif and Protein Domain Analysis
4.5. Evolutionary Tree Construction
4.6. Construction of the SsKAT1.1-PYES2 Yeast Expression Vector and Yeast Transformation
4.7. qRT-PCR
4.8. Subcellular Location Analysis in Tobacco
4.9. Yeast Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Luo, Y.; Zhang, X.; Xu, J.; Zheng, Y.; Pu, S.; Duan, Z.; Li, Z.; Liu, G.; Chen, J.; Wang, Z. Phenotypic and Molecular Marker Analysis Uncovers the Genetic Diversity of the Grass Stenotaphrum secundatum. BMC Genet. 2020, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.J.; Smith, M.A.L.; Knight, S.L. Salinity Effects on St. Augustinegrass: A Novel System to Quantify Stress Response 1. J. Plant Nutr. 1989, 12, 893–908. [Google Scholar] [CrossRef]
- Philley, H.W.; Watson, C.E., Jr.; Krans, J.V.; Goatley, J.M., Jr.; Maddox, V.L.; Tomaso-Peterson, M. Inheritance of Cold Tolerance in St. Augustinegrass. Crop Sci. 1998, 38, 451–454. [Google Scholar] [CrossRef]
- Samarakoon, S.P.; Shelton, H.M.; Wilson, J.R. Voluntary Feed Intake by Sheep and Digestibility of Shaded Stenotaphrum secundatum and Pennisetum Clandestinum Herbage. J. Agric. Sci. 1990, 114, 143–150. [Google Scholar] [CrossRef]
- Samarakoon, S.P.; Wilson, J.R.; Shelton, H.M. Growth, Morphology and Nutritive Quality of Shaded Stenotaphrum secundatum, Axonopus compressus and Pennisetum clandestinum. J. Agric. Sci. 1990, 114, 161–169. [Google Scholar] [CrossRef]
- Singh, B.; Sindhu, S.S.; Arora, A.; Yadav, H. Evaluation of Physiological and Growing Behavior of Warm Season Turfgrass Species against Salinity Stress. Indian. J. Agric. Sci. 2019, 89, 482–488. [Google Scholar] [CrossRef]
- Wang, Z.; Raymer, P.; Chen, Z. Isolation and Characterization of Microsatellite Markers for Stenotaphrum trin. Using. 454 Sequencing Technology. HortScience 2017, 52, 16–19. [Google Scholar] [CrossRef]
- Lebaudy, A.; Véry, A.-A.; Sentenac, H. K+ Channel Activity in Plants: Genes, Regulations and Functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, Y.; Wu, W.-H. Membrane Transporters for Nitrogen, Phosphate and Potassium Uptake in Plants. J. Integr. Plant Biol. 2008, 50, 835–848. [Google Scholar] [CrossRef]
- Raddatz, N.; Morales de los Ríos, L.; Lindahl, M.; Quintero, F.J.; Pardo, J.M. Coordinated Transport of Nitrate, Potassium, and Sodium. Front. Plant Sci. 2020, 11, 247. [Google Scholar] [CrossRef]
- Duby, G.; Hosy, E.; Fizames, C.; Alcon, C.; Costa, A.; Sentenac, H.; Thibaud, J.-B. AtKC1, a Conditionally Targeted Shaker-Type Subunit, Regulates the Activity of Plant K+ Channels. Plant J. Cell Mol. Biol. 2008, 53, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Q.; Wu, W.-H.; Wang, Y. The K+ Channel KZM2 Is Involved in Stomatal Movement by Modulating Inward K+ Currents in Maize Guard Cells. Plant J. 2017, 92, 662–675. [Google Scholar] [CrossRef]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice Shaker Potassium Channel OsKAT1 Confers Tolerance to Salinity Stress on Yeast and Rice Cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, F.; Tian, X.; Li, Z. Identification of Shaker Potassium Channel Family Members in Gossypium hirsutum L. and Characterization of GhKAT1aD. Life 2023, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Wang, H.; Wang, X.; Lv, M.; Yang, P.; Zhang, L. Identification and Characterization of Shaker K+ Channel Gene Family in Foxtail Millet (Setaria italica) and Their Role in Stress Response. Front. Plant Sci. 2022, 13, 907635. [Google Scholar] [CrossRef] [PubMed]
- Pilot, G.; Pratelli, R.; Gaymard, F.; Meyer, Y.; Sentenac, H. Five-Group Distribution of the Shaker-like K+ Channel Family in Higher Plants. J. Mol. Evol. 2003, 56, 418–434. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, A.; Sun, J.; Chen, X.; Liu, M.; Zhao, P.; Jiang, W.; Tang, Z. Identification of Shaker K Channel Family Members in Sweetpotato and Functional Exploration of IbAKT1+. Gene 2021, 768, 145311. [Google Scholar] [CrossRef]
- Huang, Y.-N.; Yang, S.-Y.; Li, J.-L.; Wang, S.-F.; Wang, J.-J.; Hao, D.-L.; Su, Y.-H. The Rectification Control and Physiological Relevance of Potassium Channel OsAKT2. Plant Physiol. 2021, 187, 2296–2310. [Google Scholar] [CrossRef]
- Bock, K.W.; Honys, D.; Ward, J.M.; Padmanaban, S.; Nawrocki, E.P.; Hirschi, K.D.; Twell, D.; Sze, H. Integrating Membrane Transport with Male Gametophyte Development and Function through Transcriptomics. Plant Physiol. 2006, 140, 1151–1168. [Google Scholar] [CrossRef]
- Li, D.-D.; Guan, H.; Li, F.; Liu, C.-Z.; Dong, Y.-X.; Zhang, X.-S.; Gao, X.-Q. Arabidopsis Shaker Pollen Inward K+ Channel SPIK Functions in SnRK1 Complex-Regulated Pollen Hydration on the Stigma. J. Integr. Plant Biol. 2017, 59, 604–611. [Google Scholar] [CrossRef]
- Dreyer, I.; Blatt, M.R. What Makes a Gate? The Ins and Outs of Kv-like K+ Channels in Plants. Trends Plant Sci. 2009, 14, 383–390. [Google Scholar] [CrossRef]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F.; Porée, F.; Boucherez, J.; Lebaudy, A.; Bouchez, D.; Very, A.-A.; et al. The Arabidopsis Outward K + Channel GORK Is Involved in Regulation of Stomatal Movements and Plant Transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Lebaudy, A.; Vavasseur, A.; Hosy, E.; Dreyer, I.; Leonhardt, N.; Thibaud, J.-B.; Véry, A.-A.; Simonneau, T.; Sentenac, H. Plant Adaptation to Fluctuating Environment and Biomass Production Are Strongly Dependent on Guard Cell Potassium Channels. Proc. Natl. Acad. Sci. USA 2008, 105, 5271–5276. [Google Scholar] [CrossRef]
- Ahmad, I.; Mian, A.; Maathuis, F.J.M. Overexpression of the Rice AKT1 Potassium Channel Affects Potassium Nutrition and Rice Drought Tolerance. J. Exp. Bot. 2016, 67, 2689–2698. [Google Scholar] [CrossRef]
- Feng, C.; He, C.; Wang, Y.; Xu, H.; Xu, K.; Zhao, Y.; Yao, B.; Zhang, Y.; Zhao, Y.; Idrice Carther, K.F.; et al. Genome-Wide Identification of Soybean Shaker K+ Channel Gene Family and Functional Characterization of GmAKT1 in Transgenic Arabidopsis thaliana under Salt and Drought Stress. J. Plant Physiol. 2021, 266, 153529. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Liu, Z.; Wang, Y.; Liu, W. Comparative Transcriptomic Analysis Provides Insights into the Coordinated Mechanisms of Leaves and Roots Response to Cold Stress in Common Vetch. Ind. Crops Prod. 2020, 158, 112949. [Google Scholar] [CrossRef]
- Noronha, H.; Silva, A.; Silva, T.; Frusciante, S.; Diretto, G.; Gerós, H. VviRafS5 Is a Raffinose Synthase Involved in Cold Acclimation in Grapevine Woody Tissues. Front. Plant Sci. 2022, 12, 754537. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, J. Molecular Cloning and Functional Characterization of Jatropha curcas NCED3 Involved in Cold Resistance. Plant Biotechnol. Rep. 2023, 17, 89–99. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Yao, R.; Ouyang, L.; Yu, T.; Yan, L.; Chen, Y.; Huai, D.; Wang, Z.; Kang, Y.; et al. A Systematic Identification of Cold Tolerance Genes in Peanut Using Yeast Functional Screening System. Oil Crop Sci. 2023, 8, 184–190. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, C.; Yang, J.; An, Z.; Liang, M. Genome-Wide Investigation of NAC Transcription Factors and Their Response to Cold Stress in Rubber Tree (Hevea brasiliensis). J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, C.-G.; Wu, X.; Hu, R.-X.; Yu, G.; Zhang, X.-H.; Liu, J.-L.; Pan, H.-Y. ZmDBF3, a Novel Transcription Factor from Maize (Zea mays L.), Is Involved in Multiple Abiotic Stress Tolerance. Plant Mol. Biol. Rep. 2016, 34, 353–364. [Google Scholar] [CrossRef]
- Niu, M.; Bao, C.; Zhan, J.; Yue, X.; Zou, J.; Su, N.; Cui, J. Plasma Membrane-Localized Protein BcHIPP16 Promotes the Uptake of Copper and Cadmium in Planta. Ecotoxicol. Environ. Saf. 2021, 227, 112920. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.-R.; Ma, Q.; Zhang, J.-L.; Hu, J.; Bao, A.-K.; Wei, L.; Wang, Q.; Luan, S.; Wang, S.-M. The Inward-Rectifying K+ Channel SsAKT1 Is a Candidate Involved in K+ Uptake in the Halophyte Suaeda Salsa under Saline Condition. Plant Soil. 2015, 395, 173–187. [Google Scholar] [CrossRef]
- Muguerza, M.B.; Gondo, T.; Ishigaki, G.; Shimamoto, Y.; Umami, N.; Nitthaisong, P.; Rahman, M.M.; Akashi, R. Tissue Culture and Somatic Embryogenesis in Warm-Season Grasses—Current Status and Its Applications: A Review. Plants 2022, 11, 1263. [Google Scholar] [CrossRef]
- Kimball, J.A.; Zuleta, M.C.; Martin, M.C.; Kenworthy, K.E.; Chandra, A.; Milla-Lewis, S.R. Assessment of Molecular Variation within ‘Raleigh’ St. Augustinegrass Using Amplified Fragment Length Polymorphism Markers. HortScience 2012, 47, 839–844. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Azeem, F.; Long, Y.; Boeglin, M.; Duby, G.; Mouline, K.; Hosy, E.; Vavasseur, A.; Chérel, I.; Simonneau, T.; et al. Non-Autonomous Stomatal Control by Pavement Cell Turgor via the K+ Channel Subunit AtKC1. Plant Cell 2022, 34, 2019–2037. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, M.; Jia, Y.; Yang, F.; Zhang, Y.; Xu, X.; Li, X.; Yang, F.; Lei, J.; Wang, Y.; et al. Structural Basis for the Activity Regulation of a Potassium Channel AKT1 from Arabidopsis. Nat. Commun. 2022, 13, 5682. [Google Scholar] [CrossRef]
- Pilot, G.; Gaymard, F.; Mouline, K.; Chérel, I.; Sentenac, H. Regulated Expression of Arabidopsis Shaker K+ Channel Genes Involved in K+ Uptake and Distribution in the Plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef]
- Jeanguenin, L.; Alcon, C.; Duby, G.; Boeglin, M.; Chérel, I.; Gaillard, I.; Zimmermann, S.; Sentenac, H.; Véry, A.-A. AtKC1 Is a General Modulator of Arabidopsis Inward Shaker Channel Activity. Plant J. 2011, 67, 570–582. [Google Scholar] [CrossRef]
- Musavizadeh, Z.; Najafi-Zarrini, H.; Kazemitabar, S.K.; Hashemi, S.H.; Faraji, S.; Barcaccia, G.; Heidari, P. Genome-Wide Analysis of Potassium Channel Genes in Rice: Expression of the OsAKT and OsKAT Genes under Salt Stress. Genes 2021, 12, 784. [Google Scholar] [CrossRef]
- Azeem, F.; Hussain, M.; Hussain, S.; Zubair, M.; Ali, M.A.; Afzal, M.; Siddique, M.H. Genome-Wide Analysis and Expression Profiling of Potassium. Pak. J. Agric. Sci. 2021, 58, 81–94. [Google Scholar]
- Zhang, H.; Xiao, W.; Yu, W.; Yao, L.; Li, L.; Wei, J.; Li, R. Foxtail Millet SiHAK1 Excites Extreme High-Affinity K+ Uptake to Maintain K+ Homeostasis under Low K+ or Salt Stress. Plant Cell Rep. 2018, 37, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Choi, E.-H.; Min, M.K.; Hwang, H.; Moon, S.-J.; Yoon, I.; Byun, M.-O.; Kim, B.-G. Differential Gene Expression of Two Outward-Rectifying Shaker-like Potassium Channels OsSKOR and OsGORK in Rice. J. Plant Biol. 2015, 58, 230–235. [Google Scholar] [CrossRef]
- Michard, E.; Lacombe, B.; Porée, F.; Mueller-Roeber, B.; Sentenac, H.; Thibaud, J.-B.; Dreyer, I. A Unique Voltage Sensor Sensitizes the Potassium Channel AKT2 to Phosphoregulation. J. Gen. Physiol. 2005, 126, 605–617. [Google Scholar] [CrossRef]
- Marten, I.; Hoth, S.; Deeken, R.; Ache, P.; Ketchum, K.A.; Hoshi, T.; Hedrich, R. AKT3, a Phloem-Localized K+ Channel, Is Blocked by Protons. Proc. Natl. Acad. Sci. USA 1999, 96, 7581–7586. [Google Scholar] [CrossRef]
- Song, T.; Shi, Y.; Shen, L.; Cao, C.; Shen, Y.; Jing, W.; Tian, Q.; Lin, F.; Li, W.; Zhang, W. An Endoplasmic Reticulum–Localized Cytochrome B5 Regulates High-Affinity K+ Transport in Response to Salt Stress in Rice. Proc. Natl. Acad. Sci. USA 2021, 118, e2114347118. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium Transport and Plant Salt Tolerance. Physiol. Plant 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Sanadhya, P.; Agarwal, P.; Khedia, J.; Agarwal, P.K. A Low-Affinity K+ Transporter AlHKT2;1 from Recretohalophyte Aeluropus Lagopoides Confers Salt Tolerance in Yeast. Mol. Biotechnol. 2015, 57, 489–498. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Zhu, L.; Cui, L.; Yang, L.; Wu, H.; Bie, Z. CsAKT1 Is a Key Gene for the CeO2 Nanoparticle’s Improved Cucumber Salt Tolerance: A Validation from CRISPR-Cas9 Lines. Environ. Sci. Nano 2022, 9, 4367–4381. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The Potassium Transporter OsHAK21 Functions in the Maintenance of Ion Homeostasis and Tolerance to Salt Stress in Rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deléage, G. SOPMA: Significant Improvements in Protein Secondary Structure Prediction by Consensus Prediction from Multiple Alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Kurusu, T.; Iida, H.; Kuchitsu, K. Roles of a Putative Mechanosensitive Plasma Membrane Ca2+-Permeable Channel OsMCA1 in Generation of Reactive Oxygen Species and Hypo-Osmotic Signaling in Rice. Plant Signal. Behav. 2012, 7, 796–798. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Chavanieu, A.; Jeanguenin, L.; Alcon, C.; Szponarski, W.; Estaran, S.; Chérel, I.; Zimmermann, S.; Sentenac, H.; Gaillard, I. Distinct Amino Acids in the C-Linker Domain of the Arabidopsis K+ Channel KAT2 Determine Its Subcellular Localization and Activity at the Plasma Membrane. Plant Physiol. 2014, 164, 1415–1429. [Google Scholar] [CrossRef]
- Fan, T.-F. Molecular Identification of Tobacco NtAMT1.3 That Mediated Ammonium Root-Influx with High Affinity and Improved Plant Growth on Ammonium When Overexpressed in Arabidopsis and Tobacco. Plant Sci. 2017, 264, 102–111. [Google Scholar] [CrossRef]
- Banerjee, A.; Wani, S.H.; Roychoudhury, A. Epigenetic Control of Plant Cold Responses. Front. Plant Sci. 2017, 8, 1643. [Google Scholar] [CrossRef]
- Min, X.; Wang, Q.; Wei, Z.; Liu, Z.; Liu, W. Full-Length Transcriptional Analysis Reveals the Complex Relationship of Leaves and Roots in Responses to Cold-Drought Combined Stress in Common Vetch. Front. Plant Sci. 2022, 13, 976094. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ouyang, L.; Yao, R.; Yu, T.; Yan, L.; Chen, Y.; Huai, D.; Zhou, X.; Wang, Z.; et al. Full-Length Transcriptome Sequencing Provides Insights into Alternative Splicing under Cold Stress in Peanut. Front. Plant Sci. 2024, 15, 1362277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, Z.; Gong, M. Molecular Cloning, Expression Analysis, and Preliminarily Functional Characterization of the Gene Encoding Protein Disulfide Isomerase from Jatropha curcas. Appl. Biochem. Biotechnol. 2015, 176, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Vicent, I.; Navarro, A.; Mulet, J.M.; Sharma, S.; Serrano, R. Uptake of Inorganic Phosphate Is a Limiting Factor for Saccharomyces Cerevisiae during Growth at Low Temperatures. FEMS Yeast Res. 2015, 15, fov008. [Google Scholar] [CrossRef] [PubMed]

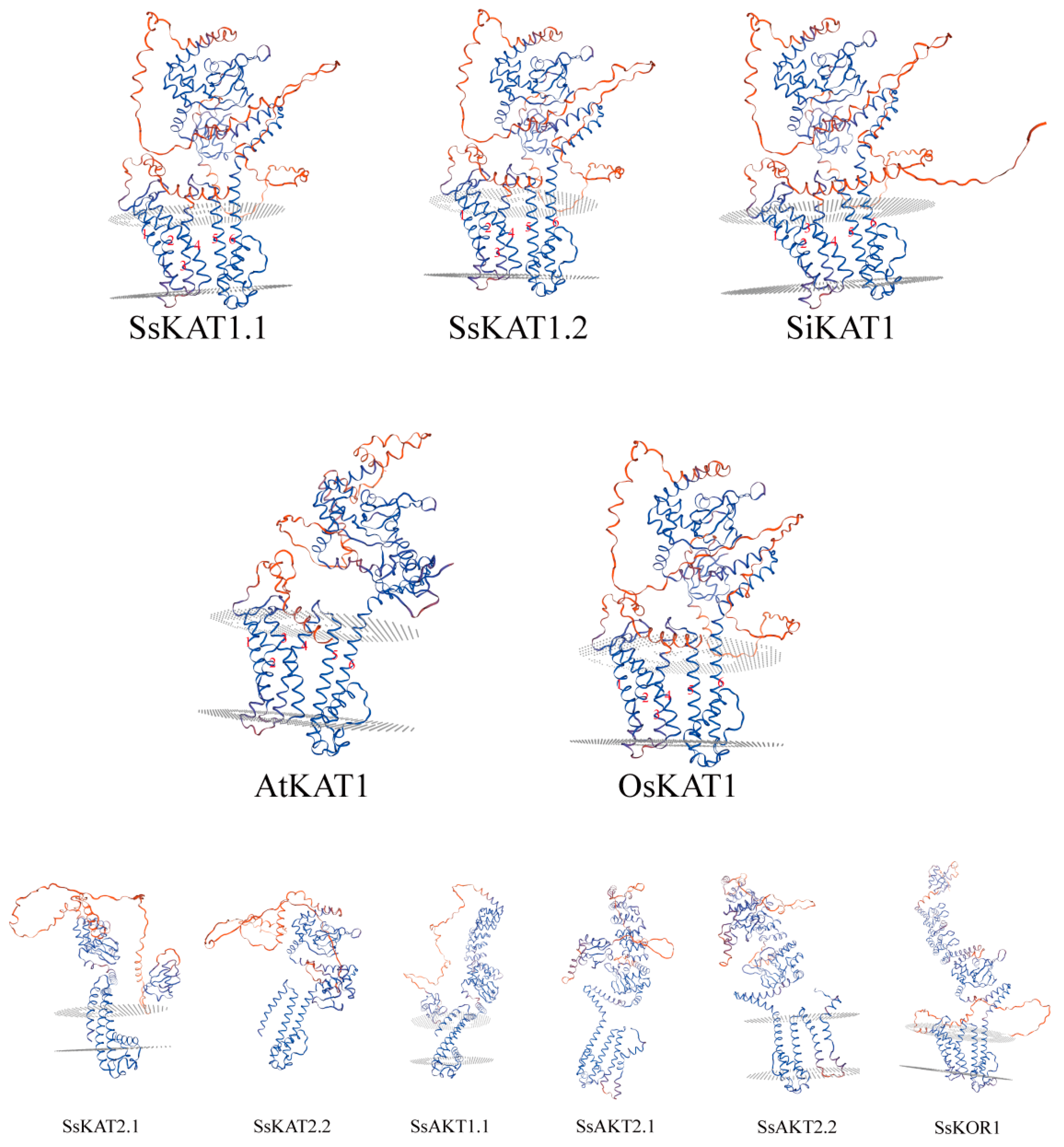

| Gene Name | CDS Length | Number of Encoded Amino Acids |
|---|---|---|
| SsKAT1.1 | 2196 | 731 |
| SsKAT1.2 | 2196 | 731 |
| SsKAT2.1 | 1761 | 586 |
| SsKAT2.2 | 1689 | 562 |
| SsAKT1.1 | 2151 | 716 |
| SsAKT1.2 | 1302 | 433 |
| SsAKT2.1 | 2184 | 727 |
| SsAKT2.2 | 2178 | 725 |
| SsKOR1 | 2559 | 852 |
| Proteins | pI | Molecular Weight (KD) | Instability Coefficient | GRAVY |
|---|---|---|---|---|
| SsKAT1.1 | 6.61 | 84.18 | 38.63 | −0.223 |
| SsKAT1.2 | 6.93 | 84.20 | 39.09 | −0.256 |
| SsKAT2.1 | 7.25 | 66.35 | 39.12 | −0.247 |
| SsKAT2.2 | 8.06 | 63.98 | 39.61 | −0.287 |
| SsAKT1.1 | 6.51 | 80.69 | 36.62 | −0.231 |
| SsAKT2.1 | 6.57 | 81.12 | 37.5 | −0.09 |
| SsAKT2.2 | 6.67 | 80.97 | 38.01 | −0.09 |
| SsKOR1 | 6.00 | 96.56 | 44.25 | −0.123 |
| Proteins | Alpha Helix (%) | Beta Turn (%) | Random Coil (%) | Extended Strand (%) |
|---|---|---|---|---|
| SsKAT1.1 | 48.43 | 3.15 | 36.11 | 12.31 |
| SsKAT1.2 | 48.02 | 3.15 | 36.53 | 12.31 |
| SsKAT2.1 | 49.49 | 2.39 | 35.49 | 12.63 |
| SsKAT2.2 | 50 | 3.38 | 33.27 | 13.35 |
| SsAKT1.1 | 47.49 | 7.82 | 37.43 | 7.26 |
| SsAKT2.1 | 55.57 | 6.05 | 27.79 | 10.59 |
| SsAKT2.2 | 52.83 | 5.66 | 29.52 | 12 |
| SsKOR1 | 53.52 | 4.81 | 31.57 | 10.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, D.-L.; Qu, J.; Wang, Z.-Y.; Sun, D.-J.; Yang, S.-N.; Liu, J.-X.; Zong, J.-Q.; Lu, H.-L. Identification of Shaker Potassium Channel Family Members and Functional Characterization of SsKAT1.1 in Stenotaphrum secundatum Suggest That SsKAT1.1 Contributes to Cold Resistance. Int. J. Mol. Sci. 2024, 25, 9480. https://doi.org/10.3390/ijms25179480
Hao D-L, Qu J, Wang Z-Y, Sun D-J, Yang S-N, Liu J-X, Zong J-Q, Lu H-L. Identification of Shaker Potassium Channel Family Members and Functional Characterization of SsKAT1.1 in Stenotaphrum secundatum Suggest That SsKAT1.1 Contributes to Cold Resistance. International Journal of Molecular Sciences. 2024; 25(17):9480. https://doi.org/10.3390/ijms25179480
Chicago/Turabian StyleHao, Dong-Li, Jia Qu, Zhi-Yong Wang, Dao-Jin Sun, Sheng-Nan Yang, Jian-Xiu Liu, Jun-Qin Zong, and Hai-Long Lu. 2024. "Identification of Shaker Potassium Channel Family Members and Functional Characterization of SsKAT1.1 in Stenotaphrum secundatum Suggest That SsKAT1.1 Contributes to Cold Resistance" International Journal of Molecular Sciences 25, no. 17: 9480. https://doi.org/10.3390/ijms25179480





