MicroRNAs in Genitourinary Malignancies: An Exciting Frontier of Cancer Diagnostics and Therapeutics
Abstract
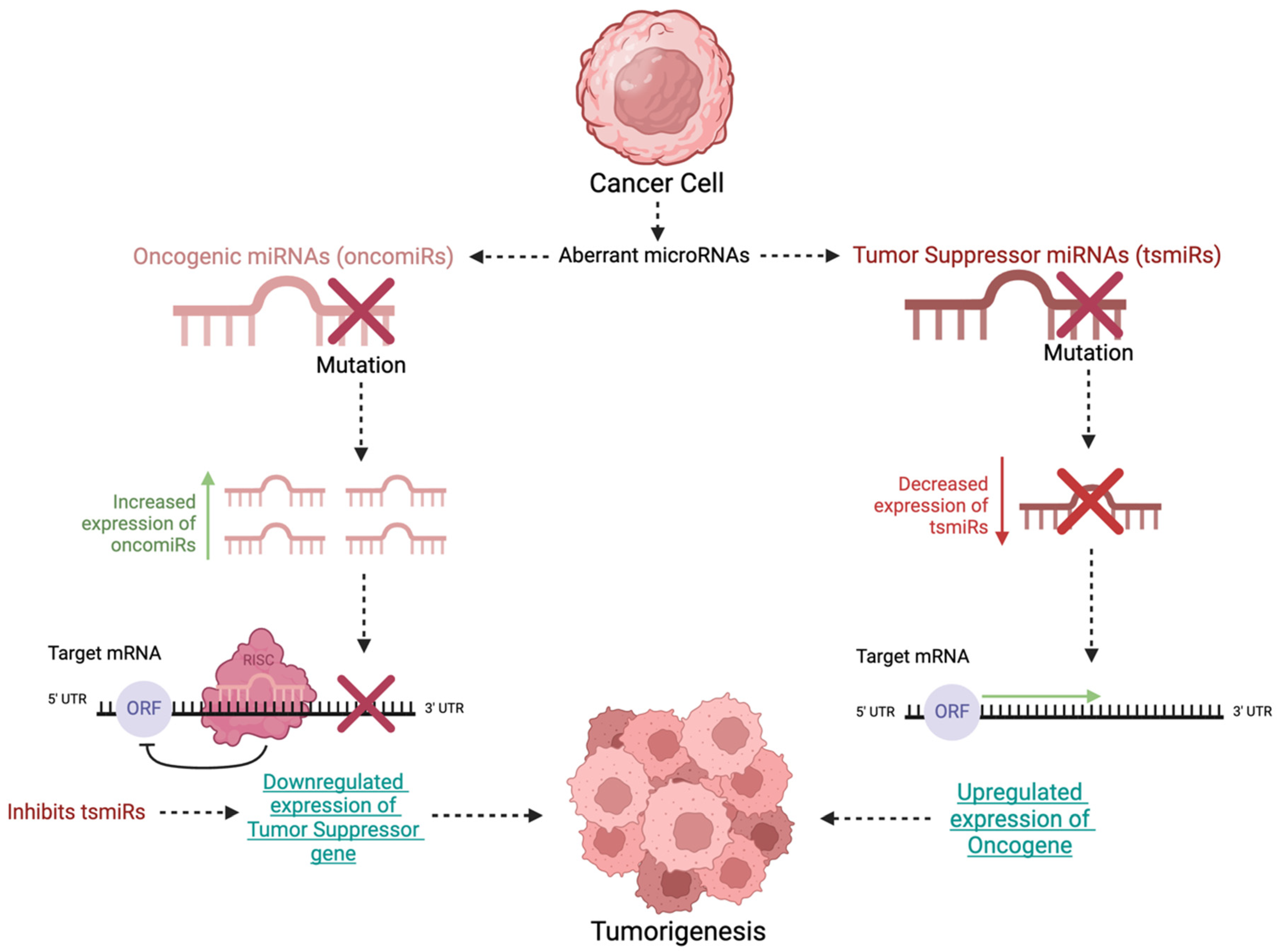
:1. Introduction
2. Prostate Cancers
3. Urothelial Cancers
4. Kidney Cancers
5. Testicular Cancers
6. Other Genitourinary Cancers
6.1. Penile Cancers
6.2. Adrenocortical Tumors
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, L.W.; Zhang, S.; Etheridge, A.; Ma, L.; Martin, D.; Galas, D.; Wang, K. Complexity of the microRNA repertoire revealed by next-generation sequencing. RNA 2010, 16, 2170–2180. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.-P.; et al. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Lotterman, C.D.; Kent, O.A.; Mendell, J.T. Functional integration of microRNAs into oncogenic and tumor suppressor pathways. Cell Cycle 2008, 7, 2493–2499. [Google Scholar] [CrossRef]
- Tay, F.C.; Lim, J.K.; Zhu, H.; Hin, L.C.; Wang, S. Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Adv. Drug Deliv. Rev. 2015, 81, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-García, J.; Osca-Verdegal, R.; Mena-Mollá, S.; García-Giménez, J.L. Epigenetic IVD Tests for Personalized Precision Medicine in Cancer. Front. Genet. 2019, 10, 621. [Google Scholar] [CrossRef]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-Q.; Yang, J.-C.; Hu, J.-J.; Ding, R.; Ye, D.-W.; Shang, J.-W. Trends and risk factors of global incidence, mortality, and disability of genitourinary cancers from 1990 to 2019: Systematic analysis for the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1119374. [Google Scholar] [CrossRef]
- Catto, J.W.F.; Miah, S.; Owen, H.C.; Bryant, H.; Myers, K.; Dudziec, E.; Larré, S.; Milo, M.; Rehman, I.; Rosario, D.J.; et al. Distinct MicroRNA Alterations Characterize High- and Low-Grade Bladder Cancer. Cancer Res. 2009, 69, 8472–8481. [Google Scholar] [CrossRef] [PubMed]
- Antolín, S.; Calvo, L.; Blanco-Calvo, M.; Santiago, M.P.; Lorenzo-Patiño, M.J.; Haz-Conde, M.; Santamarina, I.; Figueroa, A.; Antón-Aparicio, L.M.; Valladares-Ayerbes, M. Circulating miR-200c and miR-141 and outcomes in patients with breast cancer. BMC Cancer 2015, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER). Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 30 March 2021).
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. “Prostate Cancer”. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 17 May 2024).
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Filella, X.; Fernández-Galán, E.; Bonifacio, R.F.; Foj, L. Emerging biomarkers in the diagnosis of prostate cancer. Pharmacogenom. Pers. Med. 2018, 11, 83–94. [Google Scholar] [CrossRef]
- Kanwal, R.; Plaga, A.R.; Liu, X.; Shukla, G.C.; Gupta, S. MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer Lett. 2017, 407, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, X.; Cobb, G.; Anderson, T. microRNAs As oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Haldrup, C.; Kosaka, N.; Ochiya, T.; Borre, M.; Høyer, S.; Orntoft, T.F.; Sorensen, K.D. Profiling of circulating microRNAs for prostate cancer biomarker discovery. Drug Deliv. Transl. Res. 2013, 4, 19–30. [Google Scholar] [CrossRef]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sültmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2010, 128, 608–616. [Google Scholar] [CrossRef]
- Cheng, H.H.; Plets, M.; Li, H.; Higano, C.S.; Tangen, C.M.; Agarwal, N.; Vogelzang, N.J.; Hussain, M.; Thompson, I.M.; Tewari, M.; et al. Circulating microRNAs and treatment response in the Phase II SWOG S0925 study for patients with new metastatic hormone-sensitive prostate cancer. Prostate 2018, 78, 121–127. [Google Scholar] [CrossRef]
- Walter, B.A.; Valera, V.A.; Pinto, P.A.; Merino, M.J. Comprehensive microRNA Profiling of Prostate Cancer. J. Cancer 2013, 4, 350–357. [Google Scholar] [CrossRef]
- Mishra, S.; Deng, J.J.; Gowda, P.S.; Rao, M.K.; Lin, C.-L.; Chen, C.L.; Huang, T.; Sun, L.-Z. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2013, 33, 4097–4106. [Google Scholar] [CrossRef]
- E Jalava, S.; Urbanucci, A.; Latonen, L.; Waltering, K.K.; Sahu, B.; A Jänne, O.; Seppälä, J.; Lähdesmäki, H.; Tammela, T.L.J.; Visakorpi, T. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 2012, 31, 4460–4471. [Google Scholar] [CrossRef]
- Wan, X.; Pu, H.; Huang, W.; Yang, S.; Zhang, Y.; Kong, Z.; Yang, Z.; Zhao, P.; Li, A.; Li, T.; et al. Androgen-induced miR-135a acts as a tumor suppressor through downregulating RBAK and MMP11, and mediates resistance to androgen deprivation therapy. Oncotarget 2016, 7, 51284–51300. [Google Scholar] [CrossRef]
- Li, F.; Mahato, R.I. MicroRNAs and Drug Resistance in Prostate Cancers. Mol. Pharm. 2014, 11, 2539–2552. [Google Scholar] [CrossRef]
- Lima, J.F.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol. 2018, 15, 338–352. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Mercatelli, N.; Coppola, V.; Bonci, D.; Miele, F.; Costantini, A.; Guadagnoli, M.; Bonanno, E.; Muto, G.; Frajese, G.V.; De Maria, R.; et al. The Inhibition of the Highly Expressed Mir-221 and Mir-222 Impairs the Growth of Prostate Carcinoma Xenografts in Mice. PLoS ONE 2008, 3, e4029. [Google Scholar] [CrossRef]
- Mollaei, H.; Safaralizadeh, R.; Rostami, Z. MicroRNA replacement therapy in cancer. J. Cell. Physiol. 2019, 234, 12369–12384. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Wen, Y.; Song, J.H.; Parikh, N.U.; Mangala, L.S.; Blessing, A.M.; Ivan, C.; Wu, S.Y.; Varkaris, A.; Shi, Y.; et al. Chitosan nanoparticle-mediated delivery of miRNA-34a decreases prostate tumor growth in the bone and its expression induces non-canonical autophagy. Oncotarget 2015, 6, 29161–29177. [Google Scholar] [CrossRef]
- Hao, Z.; Fan, W.; Hao, J.; Wu, X.; Zeng, G.Q.; Zhang, L.J.; Nie, S.F.; Wang, X.D. Efficient delivery of micro RNA to bone-metastatic prostate tumors by using aptamer-conjugated atelocollagen in vitro and in vivo. Drug Deliv. 2014, 23, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Baker, B.F.; Pham, N.; Swayze, E.; Geary, R.S. Pharmacology of Antisense Drugs. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 81–105. [Google Scholar] [CrossRef]
- van Hoogstraten, L.M.C.; Vrieling, A.; van der Heijden, A.G.; Kogevinas, M.; Richters, A.; Kiemeney, L.A. Global trends in the epidemiology of bladder cancer: Challenges for public health and clinical practice. Nat. Rev. Clin. Oncol. 2023, 20, 287–304. [Google Scholar] [CrossRef]
- Meng, W.; Efstathiou, J.; Singh, R.; McElroy, J.; Volinia, S.; Cui, R.; Ibrahim, A.; Johnson, B.; Gupta, N.; Mehta, S.; et al. MicroRNA Biomarkers for Patients with Muscle-Invasive Bladder Cancer Undergoing Selective Bladder-Sparing Trimodality Treatment. Int. J. Radiat. Oncol. 2018, 104, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Mabjeesh, N.J. microRNA Expression profiles as decision-making biomarkers in the management of bladder cancer. Histol. Histopathol. 2017, 32, 107–119. [Google Scholar] [CrossRef]
- Browne, B.M.; Stensland, K.D.; Patel, C.K.; Sullivan, T.; Burks, E.J.; Canes, D.; Raman, J.D.; Warrick, J.; Reiger-Christ, K.M. MicroRNA Expression Profiles in Upper Tract Urothelial Carcinoma Differentiate Tumor Grade, Stage, and Survival: Implications for Clinical Decision-Making. Urology 2019, 123, 93–100. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, X.; Chen, L.; Li, H.; Gu, L.; Gao, Y.; Zhang, Y.; Li, X.; Fan, Y.; Chen, J.; et al. MicroRNAs with prognostic significance in bladder cancer: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 5619. [Google Scholar] [CrossRef]
- Rosenberg, E.; Baniel, J.; Spector, Y.; Faerman, A.; Meiri, E.; Aharonov, R.; Margel, D.; Goren, Y.; Nativ, O. Predicting progression of bladder urothelial carcinoma using microRNA expression. BJU Int. 2013, 112, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Aveta, A.; Cilio, S.; Contieri, R.; Spena, G.; Napolitano, L.; Manfredi, C.; Franco, A.; Crocerossa, F.; Cerrato, C.; Ferro, M.; et al. Urinary MicroRNAs as Biomarkers of Urological Cancers: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10846. [Google Scholar] [CrossRef] [PubMed]
- Pilala, K.-M.; Kotronopoulos, G.; Levis, P.; Giagkos, G.-C.; Stravodimos, K.; Vassilacopoulou, D.; Scorilas, A.; Avgeris, M. MIR145 Core Promoter Methylation in Pretreatment Cell-Free DNA: A Liquid Biopsy Tool for Muscle-Invasive Bladder Cancer Treatment Outcome. JCO Precis. Oncol. 2024, 8, e2300414. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, Q.; Xu, W.; Jiao, L.; Chen, Y.; Zhang, Z.; Wu, C.; Jin, T.; Pan, A.; Wei, R.; et al. miRNA-100 Inhibits Human Bladder Urothelial Carcinogenesis by Directly Targeting mTOR. Mol. Cancer Ther. 2013, 12, 207–219. [Google Scholar] [CrossRef]
- Uchino, K.; Takeshita, F.; Takahashi, R.-U.; Kosaka, N.; Fujiwara, K.; Naruoka, H.; Sonoke, S.; Yano, J.; Sasaki, H.; Nozawa, S.; et al. Therapeutic Effects of MicroRNA-582-5p and -3p on the Inhibition of Bladder Cancer Progression. Mol. Ther. 2013, 21, 610–619. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Q.-F.; Liu, X.-Q.; Guo, Z.-J.; Li, C.-Y.; Sun, G. MiR-122 targets VEGFC in bladder cancer to inhibit tumor growth and angiogenesis. Am. J. Transl. Res. 2016, 8, 3056–3066. [Google Scholar]
- Wang, X.; Wu, Q.; Xu, B.; Wang, P.; Fan, W.; Cai, Y.; Gu, X.; Meng, F. miR-124 Exerts tumor suppressive functions on the cell proliferation, motility and angiogenesis of bladder cancer by fine-tuning UHRF1. FEBS J. 2015, 282, 4376–4388. [Google Scholar] [CrossRef] [PubMed]
- Drakaki, A.; Polytarchou, C.; O’Brien, N.A.; Iliopoulos, D.; Slamon, D.J. Role and therapeutic targeting of miR-21 in bladder cancer. J. Clin. Oncol. 2015, 33, e15542. [Google Scholar] [CrossRef]
- Drakaki, A.; Koutsioumpa, M.; O’Brien, N.A.; Vorvis, C.; Iliopoulos, D.; Slamon, D.J. A chemically-modified miR-21 inhibitor (ADM-21) as a novel potential therapy in bladder cancer. J. Clin. Oncol. 2017, 35, 335. [Google Scholar] [CrossRef]
- Koutsioumpa, M.; Chen, H.-W.; O’Brien, N.; Koinis, F.; Mahurkar-Joshi, S.; Vorvis, C.; Soroosh, A.; Luo, T.; Issakhanian, S.; Pantuck, A.J.; et al. MKAD-21 Suppresses the Oncogenic Activity of the miR-21/PPP2R2A/ERK Molecular Network in Bladder Cancer. Mol. Cancer Ther. 2018, 17, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Heishima, K.; Sugito, N.; Abe, C.; Hirata, A.; Sakai, H.; Akao, Y. Targeting microRNA-145-mediated progressive phenotypes of early bladder cancer in a molecularly defined in vivo model. Mol. Ther.-Nucleic Acids 2023, 33, 960–982. [Google Scholar] [CrossRef]
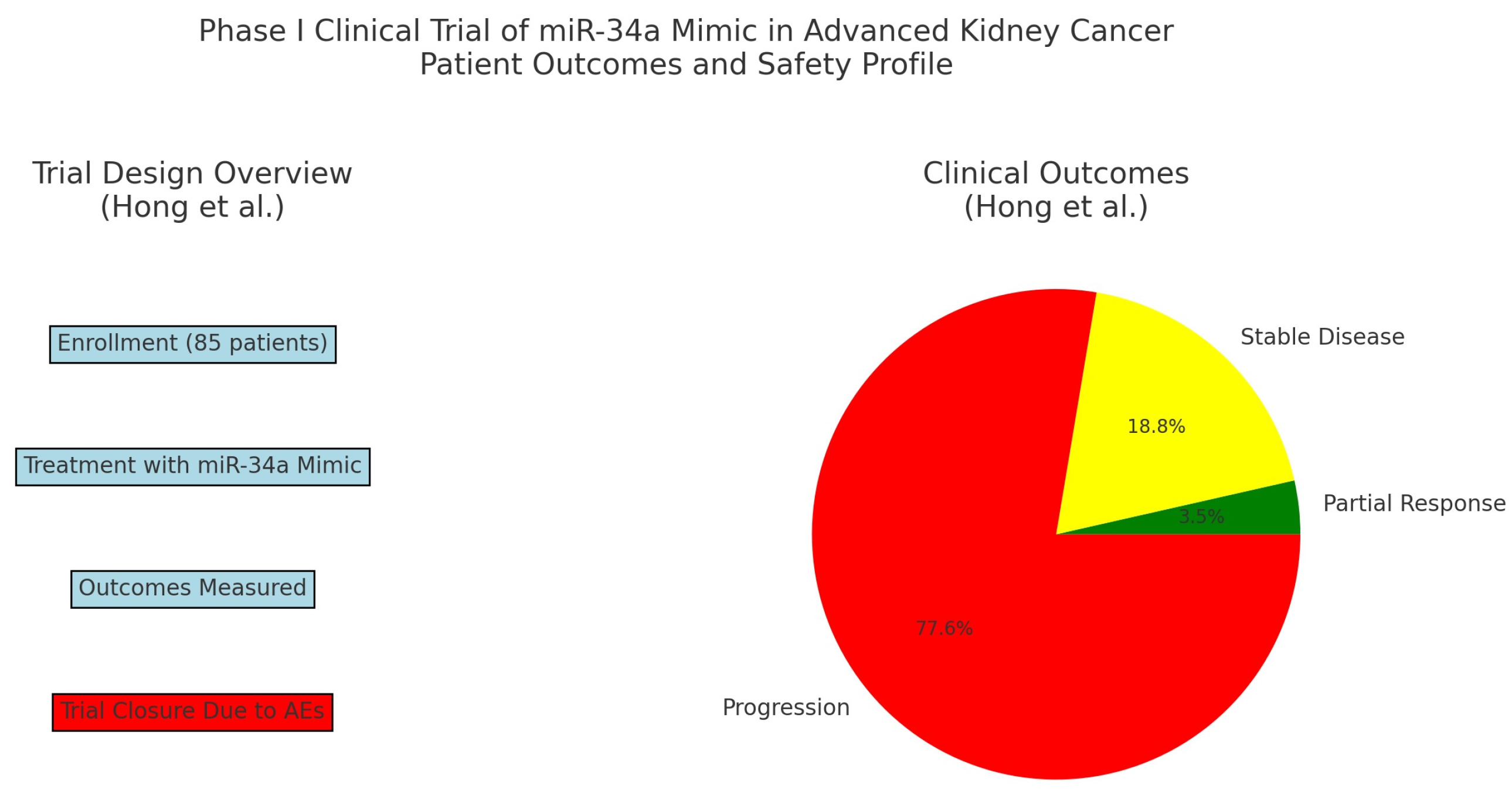
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- NCCN. Guidelines Detail. Available online: https://www.nccn.org/guidelines/guidelines-detail (accessed on 15 April 2024).
- Kathuria-Prakash, N.; Drolen, C.; Hannigan, C.A.; Drakaki, A. Immunotherapy and Metastatic Renal Cell Carcinoma: A Review of New Treatment Approaches. Life 2021, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Elballal, M.S.; Sallam, A.-A.M.; Elesawy, A.E.; Shahin, R.K.; Midan, H.M.; Elrebehy, M.A.; Elazazy, O.; El-Boghdady, R.M.; Blasy, S.H.; Amer, N.M.; et al. miRNAs As potential game-changers in renal cell carcinoma: Future clinical and medicinal uses. Pathol.-Res. Pract. 2023, 245, 154439. [Google Scholar] [CrossRef] [PubMed]
- Tusong, H.; Maolakuerban, N.; Guan, J.; Rexiati, M.; Wang, W.-G.; Azhati, B.; Nuerrula, Y.; Wang, Y.-J. Functional analysis of serum microRNAs miR-21 and miR-106a in renal cell carcinoma. Cancer Biomark. 2017, 18, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Petillo, D. MicroRNA profiling of human kidney cancer subtypes. Int. J. Oncol. 2009, 35, 109–114. [Google Scholar] [CrossRef]
- Xiao, W.; Lou, N.; Ruan, H.; Bao, L.; Xiong, Z.; Yuan, C.; Tong, J.; Xu, G.; Zhou, Y.; Qu, Y.; et al. Mir-144-3p Promotes Cell Proliferation, Metastasis, Sunitinib Resistance in Clear Cell Renal Cell Carcinoma by Downregulating ARID1A. Cell. Physiol. Biochem. 2017, 43, 2420–2433. [Google Scholar] [CrossRef]
- Berkers, J.; Govaere, O.; Wolter, P.; Beuselinck, B.; Schöffski, P.; van Kempen, L.C.; Albersen, M.; Oord, J.V.D.; Roskams, T.; Swinnen, J.; et al. A Possible Role for MicroRNA-141 Down-Regulation in Sunitinib Resistant Metastatic Clear Cell Renal Cell Carcinoma through Induction of Epithelial-to-Mesenchymal Transition and Hypoxia Resistance. J. Urol. 2012, 189, 1930–1938. [Google Scholar] [CrossRef]
- Sekino, Y.; Sakamoto, N.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. miR-130b Promotes Sunitinib Resistance through Regulation of PTEN in Renal Cell Carcinoma. Oncology 2019, 97, 164–172. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, W.; Hong, J.-Y.; Kang, D.; Park, S.; Suh, J.; You, D.; Park, Y.-Y.; Suh, N.; Hwang, J.J.; et al. miR-96-5p Targets PTEN to mediate sunitinib resistance in clear cell renal cell carcinoma. Sci. Rep. 2022, 12, 3537. [Google Scholar] [CrossRef]
- He, J.; He, J.; Min, L.; He, Y.; Guan, H.; Wang, J.; Peng, X. Extracellular vesicles transmitted miR-31-5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma. Int. J. Cancer 2019, 146, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, F.H.; Peng, L.K. MiR-200c sensitizes clear-cell renal cell carcinoma cells to sorafenib and imatinib by targeting heme oxygenase-1. Neoplasma 2014, 61, 680–689. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Yao, X.; Chen, H.; Xu, C.; Tong, L.; Shah, A.; Huang, T.; Chen, G.; Chen, J.; et al. miR-195-5p Is critical in REGγ-mediated regulation of wnt/β-catenin pathway in renal cell carcinoma. Oncotarget 2017, 8, 63986–64000. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, E.; Shen, G.; Zhu, T.; Jiang, T.; Shen, H.; Niu, L.; Wang, B.; Lu, Z.; Qian, J. Expression of microRNA-30c via lentivirus vector inhibits the proliferation and enhances the sensitivity of highly aggressive ccRCC Caki-1 cells to anticancer agents. OncoTargets Ther. 2017, 10, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lou, L.; Zhong, K.; Wan, L. MicroRNA-451 regulates chemoresistance in renal cell carcinoma by targeting ATF-2 gene. Exp. Biol. Med. 2017, 242, 1299–1305. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Qin, Z.; Lei, J.; Ye, S.; Zeng, K.; Wang, H.; Ying, M.; Gao, J.; Zeng, S.; et al. Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2. Acta Pharm. Sin. B 2019, 9, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Gaudelot, K.; Gibier, J.-B.; Pottier, N.; Hémon, B.; Van Seuningen, I.; Glowacki, F.; Leroy, X.; Cauffiez, C.; Gnemmi, V.; Aubert, S.; et al. Targeting miR-21 decreases expression of multi-drug resistant genes and promotes chemosensitivity of renal carcinoma. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Duan, L.; Yin, G.; Tan, J.; Jiang, X. miR-381, A novel intrinsic WEE1 inhibitor, sensitizes renal cancer cells to 5-FU by up-regulation of Cdc2 activities in 786-O. J. Chemother. 2013, 25, 229–238. [Google Scholar] [CrossRef]
- Ivanova, E.; Asadullina, D.; Gilyazova, G.; Rakhimov, R.; Izmailov, A.; Pavlov, V.; Khusnutdinova, E.; Gilyazova, I. Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma. Biomedicines 2023, 11, 801. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, T.; Xu, C.; Li, H.; Ma, W.; Yang, Y.; Fan, S.; Liu, Y. MicroRNA-187, down-regulated in clear cell renal cell carcinoma and associated with lower survival, inhibits cell growth and migration though targeting B7-H3. Biochem. Biophys. Res. Commun. 2013, 438, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kalantzakos, T.; Hooper, K.; Das, S.; Sullivan, T.; Canes, D.; Moinzadeh, A.; Rieger-Christ, K. MicroRNA-155-5p Targets JADE-1, Promoting Proliferation, Migration, and Invasion in Clear Cell Renal Cell Carcinoma Cells. Int. J. Mol. Sci. 2023, 24, 7825. [Google Scholar] [CrossRef]
- Liang, T.; Hu, X.-Y.; Li, Y.-H.; Tian, B.-Q.; Li, Z.-W.; Fu, Q. MicroRNA-21 Regulates the Proliferation, Differentiation, and Apoptosis of Human Renal Cell Carcinoma Cells by the mTOR-STAT3 Signaling Pathway. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 24, 371–380. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, H.; Ge, L.; Zhang, W.; Song, F.; Huang, P. A review on the role of long non-coding RNA and microRNA network in clear cell renal cell carcinoma and its tumor microenvironment. Cancer Cell Int. 2023, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Schiffgen, M.; Schmidt, D.H.; von Rücker, A.; Müller, S.C.; Ellinger, J. Epigenetic regulation of microRNA expression in renal cell carcinoma. Biochem. Biophys. Res. Commun. 2013, 436, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Wu, K.; Li, M.; Bao, L.; Shen, X.; Li, S.; Li, J.; Yang, Z. Upregulation of microRNA-492 induced by epigenetic drug treatment inhibits the malignant phenotype of clear cell renal cell carcinoma in vitro. Mol. Med. Rep. 2012, 12, 1413–1420. [Google Scholar] [CrossRef]
- Gaddam, S.; Chesnut, G. Testicular Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Smith, Z.L.; Werntz, R.P.; Eggener, S.E. Testicular Cancer. Med. Clin. N. Am. 2018, 102, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Testis Fact Sheet. International Agency for Research on Cancer, World Health Organization. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/28-testis-fact-sheet.pdf (accessed on 26 May 2024).
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2020, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Nestler, T.; Schoch, J.; Belge, G.; Dieckmann, K.-P. MicroRNA-371a-3p—The Novel Serum Biomarker in Testicular Germ Cell Tumors. Cancers 2023, 15, 3944. [Google Scholar] [CrossRef]
- Murray, M.J.; Halsall, D.J.; Hook, C.E.; Williams, D.M.; Nicholson, J.C.; Coleman, N. Identification of MicroRNAs From the miR-371∼373 and miR-302 Clusters as Potential Serum Biomarkers of Malignant Germ Cell Tumors. Am. J. Clin. Pathol. 2011, 135, 119–125. [Google Scholar] [CrossRef]
- van Agthoven, T.; Looijenga, L.H. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 2016, 8, 58037–58049. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Radtke, A.; Spiekermann, M.; Balks, T.; Matthies, C.; Becker, P.; Ruf, C.; Oing, C.; Oechsle, K.; Bokemeyer, C.; et al. Serum Levels of MicroRNA miR-371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumours. Eur. Urol. 2016, 71, 213–220. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; van den Berg, A.; Dorssers, L.C.J.; Belge, G.; Dieckmann, K.-P.; Roest, H.P.; Van Der Laan, L.J.W.; Gietema, J.; Hamilton, R.J.; et al. Identification and Validation Model for Informative Liquid Biopsy-Based microRNA Biomarkers: Insights from Germ Cell Tumor In Vitro, In Vivo and Patient-Derived Data. Cells 2019, 8, 1637. [Google Scholar] [CrossRef]
- Belge, G.; Dumlupinar, C.; Nestler, T.; Klemke, M.; Törzsök, P.; Trenti, E.; Pichler, R.; Loidl, W.; Che, Y.; Hiester, A.; et al. Detection of Recurrence through microRNA-371a-3p Serum Levels in a Follow-up of Stage I Testicular Germ Cell Tumors in the DRKS-00019223 Study. Clin. Cancer Res. 2023, 30, 404–412. [Google Scholar] [CrossRef]
- Li, H.-L.; Wei, J.-F.; Fan, L.-Y.; Wang, S.-H.; Zhu, L.; Li, T.-P.; Lin, G.; Sun, Y.; Sun, Z.-J.; Ding, J.; et al. miR-302 Regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4-dependent manner. Cell Death Dis. 2016, 7, e2078. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, X.; Dou, Y. The miR-302/367 cluster: A comprehensive update on its evolution and functions. Open Biol. 2015, 5, 150138. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Evensen, H.S.F.; Furu, K.; Haugen, T.B. miRNA-302s May act as oncogenes in human testicular germ cell tumours. Sci. Rep. 2019, 9, 9189. [Google Scholar] [CrossRef]
- Syring, I.; Bartels, J.; Holdenrieder, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Circulating Serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as Biomarkers in Patients with Testicular Germ Cell Cancer. J. Urol. 2015, 193, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Su, C.-H.; Chen, H.-M.; Wu, M.-S.; Pan, H.-A.; Chang, C.-N.; Cheng, Y.-S.; Chang, W.-T.; Chiu, C.-C.; Teng, Y.-N. MicroRNA-320a enhances LRWD1 expression through the AGO2/FXR1-dependent pathway to affect cell behaviors and the oxidative stress response in human testicular embryonic carcinoma cells. Aging 2024, 16, 3973–3988. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, L.; Liu, J.; Bian, X.; Shi, C.; Sun, C.; Zhou, X.; Wen, Y.; Hua, D.; Zhao, S.; et al. miR-320a Functions as a suppressor for gliomas by targeting SND1 and β-catenin, and predicts the prognosis of patients. Oncotarget 2017, 8, 19723–19737. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, Z.-W.; Li, W.-M.; Yang, G.; Jing, P.-Y.; Li, P.; Dang, H.-Z.; Chen, Z.; Zhou, Y.-A.; Li, X.-F. Knockdown of MALAT1 expression inhibits HUVEC proliferation by upregulation of miR-320a and downregulation of FOXM1 expression. Oncotarget 2017, 8, 61499–61509. [Google Scholar] [CrossRef]
- Elesawy, A.E.; Abulsoud, A.I.; Moustafa, H.A.M.; Elballal, M.S.; Sallam, A.-A.M.; Elazazy, O.; El-Dakroury, W.A.; Mageed, S.S.A.; Abdelmaksoud, N.M.; Midan, H.M.; et al. miRNAs Orchestration of testicular germ cell tumors—Particular emphasis on diagnosis, progression and drug resistance. Pathol.-Res. Pract. 2023, 248, 154612. [Google Scholar] [CrossRef]
- National Library of Medicine. Prospective Therapeutic De-Escalation and miRNA-M371 Biomarker Evaluation Phase II Study for Stage IIa/IIb <3 cm Seminomas. ClinicalTrials.gov identifier: NCT05529251. 25 January 2024. Available online: https://clinicaltrials.gov/study/NCT05529251 (accessed on 26 May 2024).
- National Library of Medicine. MAGESTIC Trial: MiRNA in Detecting Active Germ Cell Tumors in Early Suspected and Meta-staTIC Disease Trial. ClinicalTrials.gov identifier: NCT06060873. 3 November 2023. Available online: https://clinicaltrials.gov/study/NCT06060873 (accessed on 26 May 2024).
- National Library of Medicine. A Prospective Observational Cohort Study to Assess miRNA 371 for Outcome Prediction in Pa-tients with Newly Diagnosed Germ Cell Tumors. ClinicalTrials.gov identifier: NCT04435756. 12 December 2023. Available online: https://clinicaltrials.gov/study/NCT04435756 (accessed on 26 May 2024).
- National Library of Medicine. MicroRNA-371 as Markers for Disease Activity and as a Tool to Monitor the Effect of Chemo-therapy and Early Detection of Recurrence in Patients with Testicular Germ Cell Tumours. ClinicalTrials.gov identifier: NCT04914026. 8 August 2022. Available online: https://clinicaltrials.gov/study/NCT04914026 (accessed on 26 May 2024).
- National Library of Medicine. A Prospective, Single-Center, Clinical Trial to Evaluate the Efficacy of Sentinel Lymph Node Bi-opsy in Stage AI-IIA Germ Cell Tumors (Seminoma/Nonseminoma) (PITERLAND). ClinicalTrials.gov identifier: NCT06133699. 3 January 2024. Available online: https://clinicaltrials.gov/study/NCT06133699 (accessed on 26 May 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pow-Sang, M.R.; Ferreira, U.; Pow-Sang, J.M.; Nardi, A.C.; Destefano, V. Epidemiology and Natural History of Penile Cancer. Urology 2010, 76, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Giona, S. The Epidemiology of Penile Cancer. In Urologic Cancers; Barber, N., Ali, A., Eds.; Exon Publications: Brisbane City, Australia, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK585980/ (accessed on 25 June 2024).
- National Comprehemsive Cancer Network. Penile Cancer Guidelines. 25 October 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf (accessed on 30 May 2024).
- Zhu, Y.; Ye, D.-W. Lymph node metastases and prognosis in penile cancer. Chin. J. Cancer Res. 2012, 24, 90–96. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, P.; Shen, X.; Zhang, Y.; Xu, B.; Zhou, J.; Fan, S.; Hao, Z.; Shi, H.; Zhang, X.; et al. MicroRNA Expression Profile in Penile Cancer Revealed by Next-Generation Small RNA Sequencing. PLoS ONE 2015, 10, e0131336. [Google Scholar] [CrossRef]
- Hartz, J.M.; Engelmann, D.; Fürst, K.; Marquardt, S.; Spitschak, A.; Goody, D.; Protzel, C.; Hakenberg, O.W.; Pützer, B.M. Integrated Loss of miR-1/miR-101/miR-204 Discriminates Metastatic from Nonmetastatic Penile Carcinomas and Can Predict Patient Outcome. J. Urol. 2016, 196, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Kuasne, H.; Barros-Filho, M.C.; Busso-Lopes, A.; Marchi, F.A.; Pinheiro, M.; Muñoz, J.J.M.; Scapulatempo-Neto, C.; Faria, E.F.; Guimarães, G.C.; Lopes, A.; et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 2017, 8, 15294–15306. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.A.; Martins, D.C.; Barros-Filho, M.C.; Kuasne, H.; Lopes, A.F.B.; Brentani, H.; Filho, J.C.S.T.; Guimarães, G.C.; Faria, E.F.; Scapulatempo-Neto, C.; et al. Multidimensional integrative analysis uncovers driver candidates and biomarkers in penile carcinoma. Sci. Rep. 2017, 7, 6707. [Google Scholar] [CrossRef]
- Pinho, J.D.; Silva, G.E.B.; Júnior, A.A.L.T.; Belfort, M.R.d.C.; Mendes, J.M.; da Cunha, I.W.; Quintana, L.G.; Calixto, J.d.R.R.; Nogueira, L.R.; Coelho, R.W.P.; et al. MIR-107, MIR-223-3P and MIR-21-5P Reveals Potential Biomarkers in Penile Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Murta, C.B.; Furuya, T.K.; Carrasco, A.G.M.; Uno, M.; Sichero, L.; Villa, L.L.; Faraj, S.F.; Coelho, R.F.; Guglielmetti, G.B.; Cordeiro, M.D.; et al. miRNA And mRNA Expression Profiles Associated with Lymph Node Metastasis and Prognosis in Penile Carcinoma. Int. J. Mol. Sci. 2022, 23, 7103. [Google Scholar] [CrossRef]
- Ayoubian, H.; Heinzelmann, J.; Hölters, S.; Khalmurzaev, O.; Pryalukhin, A.; Loertzer, P.; Heinzelbecker, J.; Lohse, S.; Geppert, C.; Loertzer, H.; et al. miRNA Expression Characterizes Histological Subtypes and Metastasis in Penile Squamous Cell Carcinoma. Cancers 2021, 13, 1480. [Google Scholar] [CrossRef]
- Barzon, L.; Cappellesso, R.; Peta, E.; Militello, V.; Sinigaglia, A.; Fassan, M.; Simonato, F.; Guzzardo, V.; Ventura, L.; Blandamura, S.; et al. Profiling of Expression of Human Papillomavirus–Related Cancer miRNAs in Penile Squamous Cell Carcinomas. Am. J. Pathol. 2014, 184, 3376–3383. [Google Scholar] [CrossRef]
- Furuya, T.K.; Murta, C.B.; Carrasco, A.G.M.; Uno, M.; Sichero, L.; Villa, L.L.; Cardilli, L.; Coelho, R.F.; Guglielmetti, G.B.; Cordeiro, M.D.; et al. Disruption of miRNA-mRNA Networks Defines Novel Molecular Signatures for Penile Carcinogenesis. Cancers 2021, 13, 4745. [Google Scholar] [CrossRef]
- da Silva, J.; Nogueira, L.; Coelho, R.; Deus, A.; Khayat, A.; Marchi, R.; de Oliveira, E.; dos Santos, A.P.; Cavalli, L.; Pereira, S. HPV-associated penile cancer: Impact of copy number alterations in miRNA/mRNA interactions and potential druggable targets. Cancer Biomark. 2021, 32, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Allolio, B.; Fassnacht, M. Adrenocortical Carcinoma: Clinical Update. J. Clin. Endocrinol. Metab. 2006, 91, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Neuroendocrine and Adrenal Tumors Guidelines. 20 June 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 30 May 2024).
- Tömböl, Z.; Szabó, P.M.; Molnár, V.; Wiener, Z.; Tölgyesi, G.; Horányi, J.; Riesz, P.; Reismann, P.; Patócs, A.; Likó, I.; et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr.-Relat. Cancer 2009, 16, 895–906. [Google Scholar] [CrossRef]
- Soon, P.S.H.; Tacon, L.J.; Gill, A.J.; Bambach, C.P.; Sywak, M.S.; Campbell, P.R.; Yeh, M.W.; Wong, S.G.; Clifton-Bligh, R.J.; Robinson, B.G.; et al. miR-195 And miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin. Cancer Res. 2009, 15, 7684–7692. [Google Scholar] [CrossRef] [PubMed]
- Chabre, O.; Libé, R.; Assie, G.; Barreau, O.; Bertherat, J.; Bertagna, X.; Feige, J.-J.; Cherradi, N. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr.-Relat. Cancer 2013, 20, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.E.; Holloway, A.K.; Weng, J.; Fojo, T.; Kebebew, E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2010, 117, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.T.; Zhao, J.T.; Glover, A.R.; Gill, A.J.; Clifton-Bligh, R.; Robinson, B.G.; Ip, J.C.; Sidhu, S.B. microRNA-431 As a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma. Oncologist 2019, 24, e241–e250. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, A.S.; Parchem, R.J. KRAS Hijacks the miRNA Regulatory Pathway in Cancer. Cancer Res. 2023, 83, 1563–1572. [Google Scholar] [CrossRef]
- Igaz, P.; Igaz, I.; Nagy, Z.; Nyírő, G.; Szabó, P.M.; Falus, A.; Patócs, A.; Rácz, K. MicroRNAs in adrenal tumors: Relevance for pathogenesis, diagnosis, and therapy. Cell. Mol. Life Sci. 2014, 72, 417–428. [Google Scholar] [CrossRef]
- National Library of Medicine. Adrenal Vein Sampling as a Tool to Identify Biomarkers That Aid the Diagnosis of Adrenocortical Carcinoma (AVS for ACC) NCT05660889. 21 December 2022. Available online: https://clinicaltrials.gov/study/NCT05660889 (accessed on 30 May 2024).
- National Library of Medicine. Studying Genes in Samples From Younger Patients with Adrenocortical Tumor NCT01528956. 18 May 2016. Available online: https://clinicaltrials.gov/study/NCT01528956 (accessed on 30 May 2024).

| Clinical Trial Number | Study Type | miRNA | Malignancy | Brief Description |
|---|---|---|---|---|
| NCT05529251 | Interventional, phase II | miR-371a-3p | Seminoma, stage IIa/IIb | Investigating the correlation between miR-371 as a biomarker using positron emission tomography (PET) scanning as a tool for de-escalating treatment. |
| NCT06060873 | Observational | miR-371 | Active germ cell tumor | Investigating miR-371 as a biomarker for optimal timing of retroperitoneal lymph node dissection (RPLND). |
| NCT04435756 | Observational | miR-371 | Early-stage testicular seminoma and nonseminoma | Investigating the positive predictive value of miR-371 as a marker of disease relapse. |
| NCT04914026 | Observational | miR-371a-5p | All testicular germ cell tumors | Investigating the sensitivity and specificity of miR-371 as a biomarker at orchiectomy and during treatment and surveillance. |
| NCT06133699 | Interventional | MiRNA expression profiles | Germ cell tumors, stage IA/IIA | Investigating miRNA expression profiles as a prognostic tool throughout treatment. |
| NCT05660889 | Observational | MiRNA expression profiles | Adrenocortical carcinoma | Investigating miRNAs as diagnostic biomarkers. |
| NCT01528956 | Observational | MiRNA expression profiles | Pediatric adrenocortical tumors | Investigating miRNA expression in tumor cells compared to normal cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathuria-Prakash, N.; Dave, P.; Garcia, L.; Brown, P.; Drakaki, A. MicroRNAs in Genitourinary Malignancies: An Exciting Frontier of Cancer Diagnostics and Therapeutics. Int. J. Mol. Sci. 2024, 25, 9499. https://doi.org/10.3390/ijms25179499
Kathuria-Prakash N, Dave P, Garcia L, Brown P, Drakaki A. MicroRNAs in Genitourinary Malignancies: An Exciting Frontier of Cancer Diagnostics and Therapeutics. International Journal of Molecular Sciences. 2024; 25(17):9499. https://doi.org/10.3390/ijms25179499
Chicago/Turabian StyleKathuria-Prakash, Nikhita, Pranali Dave, Lizette Garcia, Paige Brown, and Alexandra Drakaki. 2024. "MicroRNAs in Genitourinary Malignancies: An Exciting Frontier of Cancer Diagnostics and Therapeutics" International Journal of Molecular Sciences 25, no. 17: 9499. https://doi.org/10.3390/ijms25179499






