Preparation and Molecular Dynamic Simulation of Superfine CL−20/TNT Cocrystal Based on the Opposite Spray Method
Abstract
:1. Introduction
2. Results
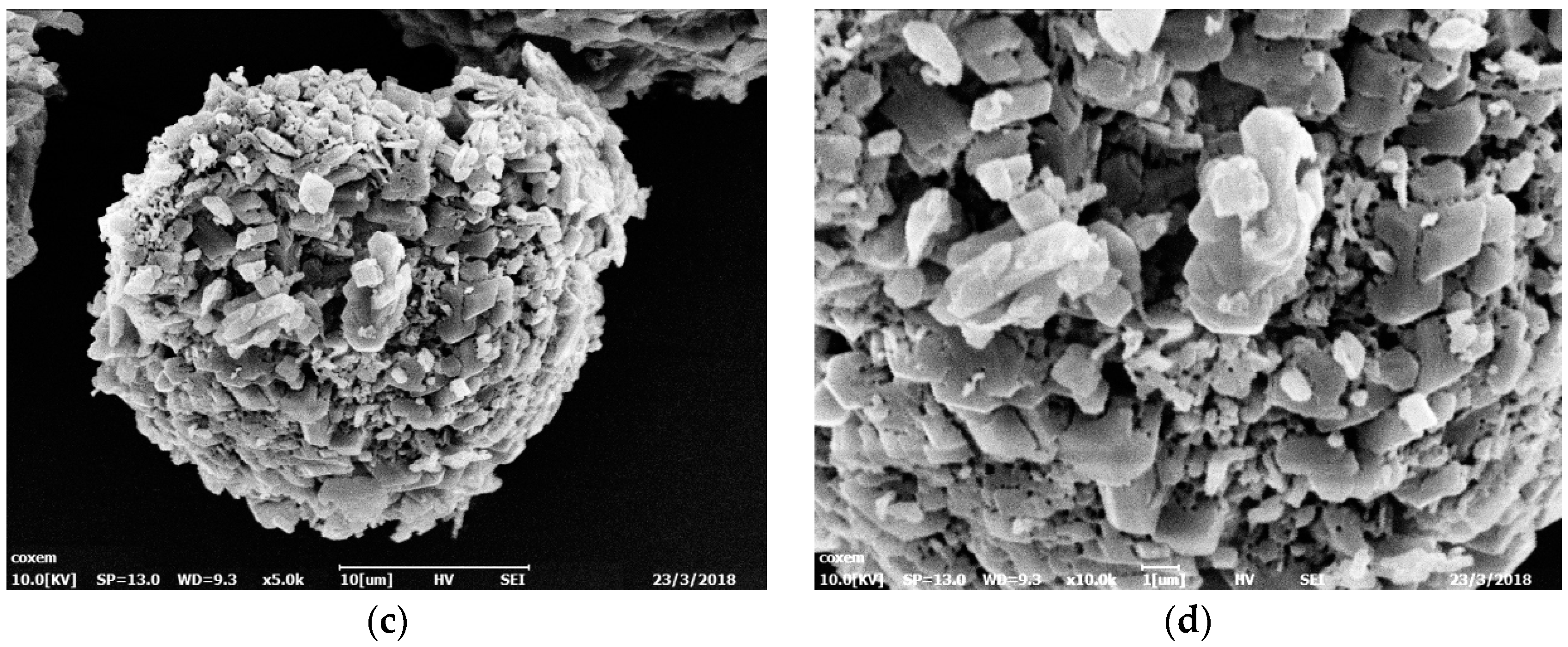
2.1. Characterization by Scanning Electron Microscopy
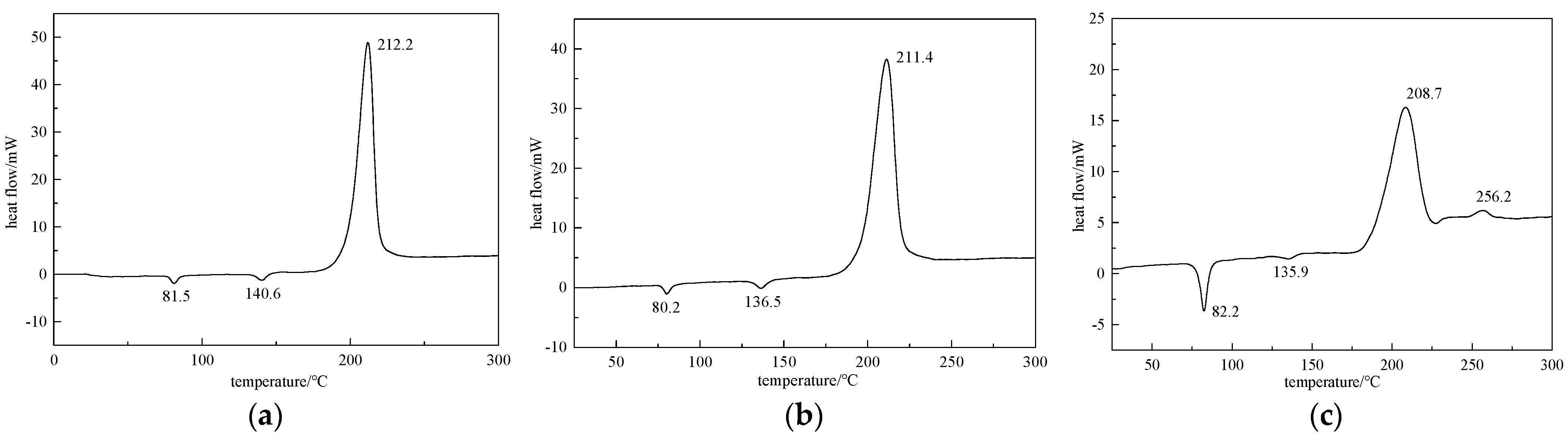
2.2. Differential Thermal Testing Results
2.3. DSC Testing Results of Explosive Cocrystal with Different Feeding Ratio
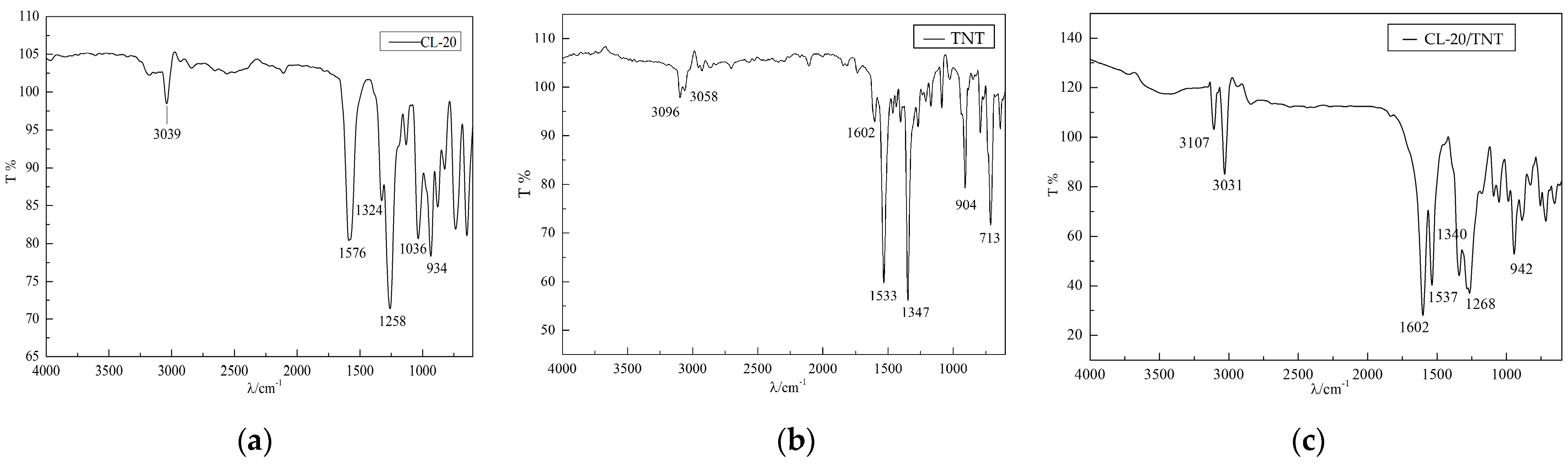
2.4. Infrared Spectrum Testing Results
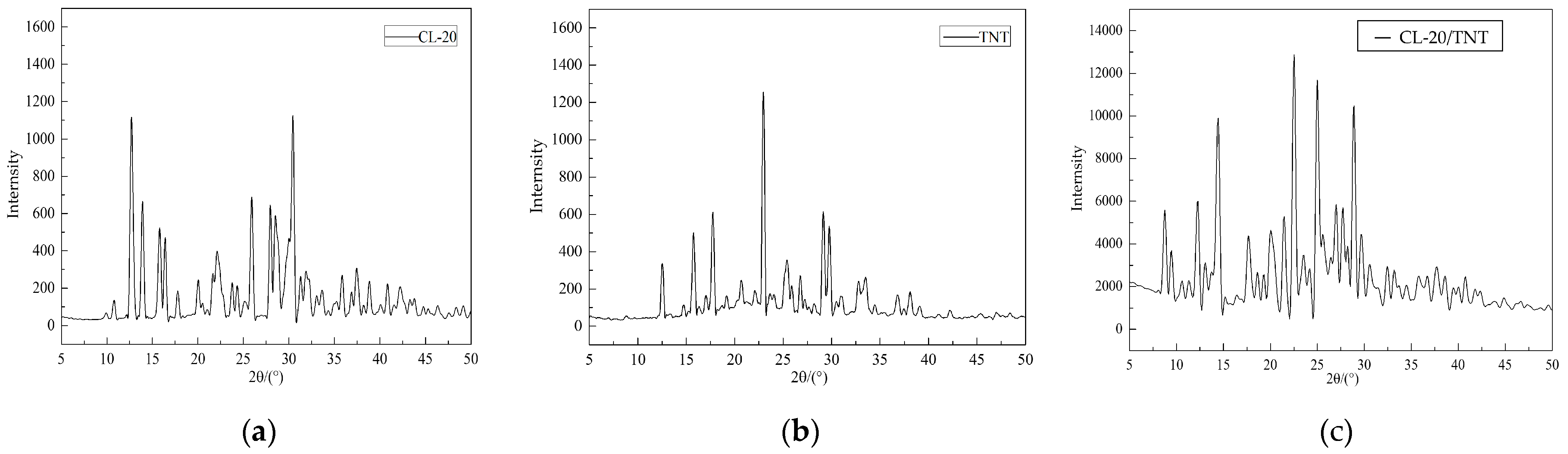
2.5. X-ray Diffraction Testing Results
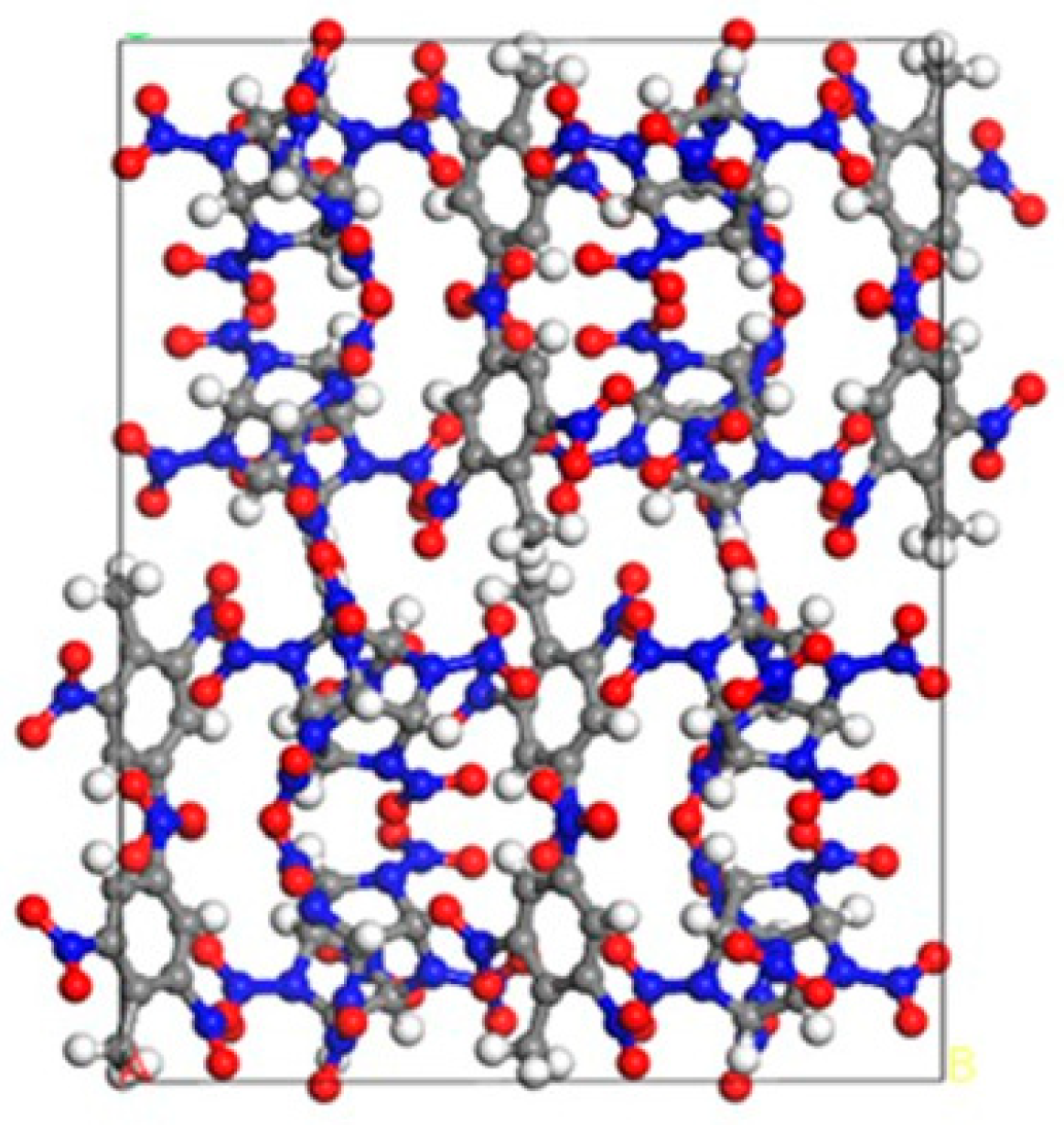
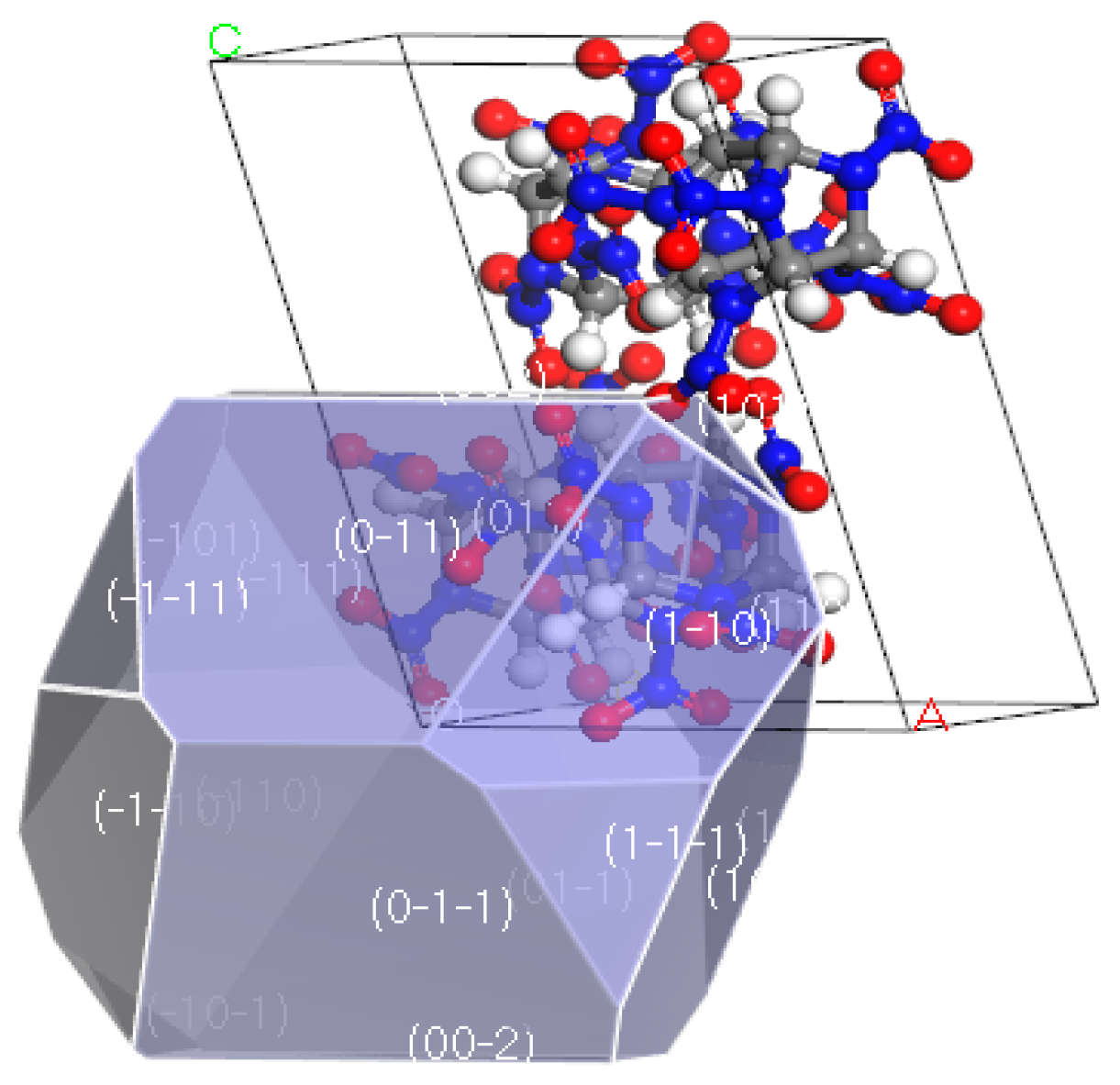
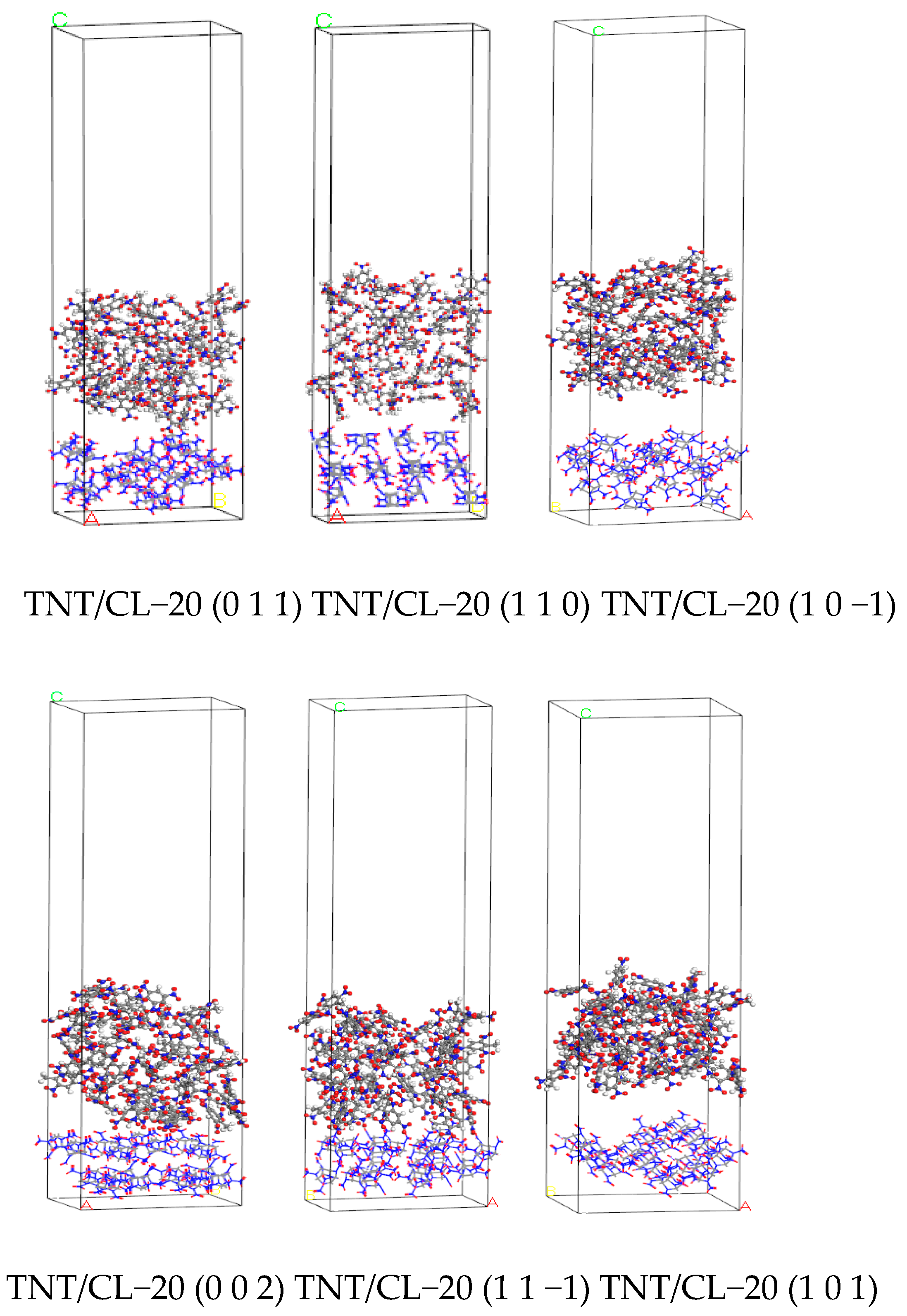
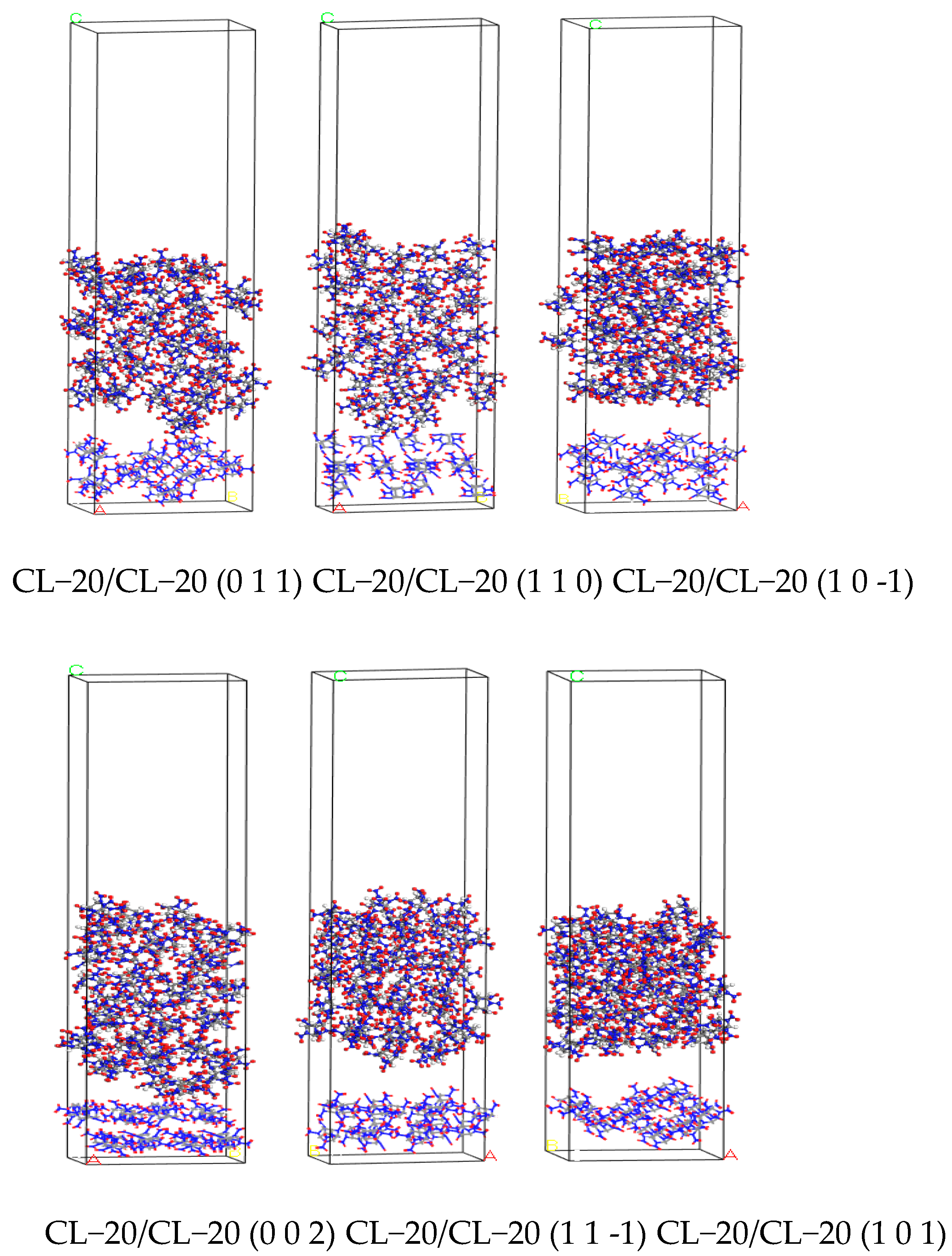
2.6. Simulated Results of Binding Energy between Molecular Crystal Planes
2.7. Calculated Results of Radial Distribution Function for CL−20/TNT Cocrystal
2.8. Testing Results of Mechanical Sensitivity
3. Discussion
3.1. Characterization Analysis of Scanning Electron Microscopy
3.2. Differential Thermal Testing Analysis
3.3. The Influence of Feeding Ratio on Explosive Cocrystal
3.4. Testing Analysis of Infrared Spectrum
3.5. Testing Analysis of X-ray Diffraction
3.6. Analysis of Binding Energy between Molecular Crystal Planes
3.7. Cocrystal Mechanism Analysis of CL−20/TNT Based on Radial Distribution Function
3.8. Testing Analysis of Mechanical Sensitivity
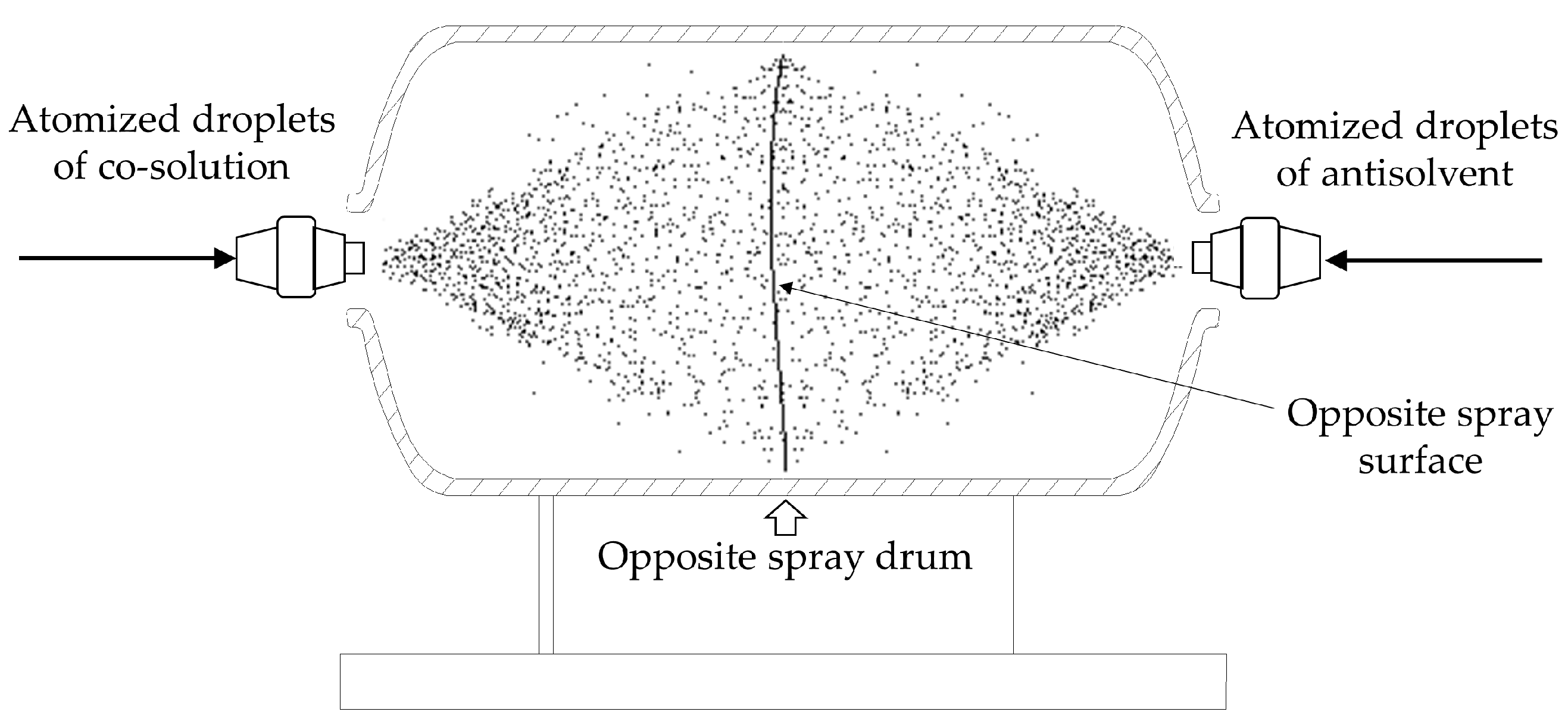
3.9. Analysis of Preparation Process Conditions for the Opposite Spray Method
4. Materials and Methods
4.1. Preparation Principle and Method of CL−20/TNT Cocrystal Explosive
4.1.1. Principle of Opposite Spray Method with Pneumatic Atomized Droplets
4.1.2. Experimental Process for Preparing CL−20/TNT Cocrystal Explosive
4.2. Experimental Chemicals and Instrumentation
4.3. Preparation of CL−20/TNT Cocrystal Explosive
4.3.1. Selection of Experimental Conditions
4.3.2. Experimental Steps for Preparing CL−20/TNT Cocrystal Explosive
4.4. Molecular Model Construction of CL−20/TNT Cocrystal Explosive
4.4.1. Molecular Crystal Plane Model of the CL−20 Explosive
4.4.2. Molecular Model of the CL−20/TNT Cocrystal Explosive
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeager, J.D.; Dattelbaum, A.M.; Orler, E.B.; Bahr, D.F.; Dattelbaum, D.M. Adhesive properties of some fluoropolymer binders with the insensitive explosive 1,3,5-triamino-2,4,6-trinitrobenzene (TATB). J. Colloid Interface Sci. 2010, 352, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Boddu, V.M.; Viswanath, D.S.; Ghosh, T.K.; Damavarapu, R. 2,4,6-triamino-1,3,5-trinitrobenzene (TATB) and TATB based formulations—A review. J. Hazard. Mater. 2010, 181, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, S.; Brousseau, P.; Ampleman, G.; Pantea, D.; Cote, S. Potential use of CL−20 in TNT/ETPE based melt cast formulations. Propellants Explos. Pyrotech. 2008, 33, 103–108. [Google Scholar] [CrossRef]
- Matyáš, R.; Šelešovský, J.; Musil, T. Sensitivity to friction for primary explosives. J. Hazard. Mater. 2012, 213, 236–241. [Google Scholar] [CrossRef]
- Wang, X.; Peng, C. Development of Hexanitrohexaazaisowurtaitane at abroad. Chin. J. Explos. Propellants 2007, 30, 45–48. [Google Scholar]
- Dunitz, J.D. Crystal and co-crystal: A second opinion. CrystEngComm 2003, 5, 506. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Wu, Y.; Jin, S.; Wang, X.; Wang, Y.; Shang, F.; Chen, K.; Du, J.; Shu, K. A novel cocrystal composed of CL−20 and an energetic ionic salt. Chem. Commun. 2018, 54, 13268–13270. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, S.; Li, X.; Liu, M.; Sun, F.; Yang, Y.; Hao, G.; Jiang, W. Preparation and characterization of nano-CL−20/TNT cocrystal explosives by mechanical ball-milling method. ACS Omega 2020, 5, 17761–17766. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Duan, X.; Li, H.; Pei, C. A novel high-energetic and good-sensitive cocrystal composed of CL−20 and TATB by a rapid solvent/non-solvent method. RSC Adv. 2015, 5, 95764–95770. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. 2011, 123, 9122–9125. [Google Scholar] [CrossRef]
- Landenberger, K.B.; Matzger, A.J. Cocrystal engineering of a prototype energetic material: Supramolecular chemistry of 2,4,6-trinitrotoluene. Cryst. Growth Des. 2010, 10, 5341–5347. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Wang, X.; Liu, X.; Liu, Y.; Sun, J. Preparation and characterization of 7 types of BTF cocrystal. Chin. J. Energ. Mater. 2012, 20, 503–504. [Google Scholar]
- Wang, Y.; Yang, Z.; Li, H.; Wang, J.; Zhou, X.; Zhang, Q. Preparation and Characterization of CL−20/DNB Cocrystal Explosive. Chin. J. Energ. Mater. 2013, 21, 554–555. [Google Scholar]
- Ma, Y.; Hao, S.; Li, H.; Liu, Y.; Yang, Z. Preparation and Performance of BTF-DNAN Cocrystal Explosive. Chin. J. Energ. Mater. 2015, 23, 1228–1230. [Google Scholar]
- Song, X.; Wang, Y.; Song, Z.; Song, D.; An, C.; Wang, J.; Zhang, J. Preparation od CL−20/DNT Cocrystal Explosive and Study on Its Performance. Chin. J. Explos. Propellants 2016, 39, 23–27. [Google Scholar]
- Feng, C.; Deng, P.; Fan, X.; Ma, D. Application of Solvent-Non-Solvent Method in Energetic Materials. Chem. Propellants Polym. Mater. 2010, 8, 38–41. [Google Scholar]
- Chen, J.; Duan, X.; Pei, C. Preparation and Characterization of HMX/AP Co-crystal. Chin. J. Energ. Mater. 2013, 21, 409–413. [Google Scholar]
- Jayasankar, A.; Somwangthanaroj, A.; Shao, Z.J.; Rodríguez-Hornedo, N. Cocrystal formation during cogrinding and storage is mediated by amorphous phase. Pharm. Res. 2006, 23, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Friscic, T.; Jones, W. Recent advances in understanding the mechanism of cocrystal formation via grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Lu, J.; Rohani, S. Preparation and characterization of theophylline−nicotinamide cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Huang, H.; Zhou, X.; Li, J.; Nie, F. Preparation and performance of a HNIW/TNT cocrystal explosive. Propellants Explos. Pyrotech. 2013, 38, 495–501. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Dong, X.; Fan, L.; Zhu, Y. Study on the Preparation and Composition of Ultrafine CL-18/HNS. J. Phys. Conf. Ser. 2022, 2239, 012023. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Velaga, S.P. Formation of cocrystals from stoichiometric solutions of incongruently saturating systems by spray drying. Cryst. Growth Des. 2010, 10, 3302–3305. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; An, C.; Guo, W. Preparation and characterization of ultrafine CL−20/TNT cocrystal explosive by spray drying method. Chin. J. Energ. Mater. 2015, 23, 1103–1106. [Google Scholar]
- Liu, J.; Yan, Z.; Chi, D.; Yang, L. Synthesis of the microspheric cocrystal CL−20/2,4-DNI with high energy and low sensitivity by a spray-drying process. New J. Chem. 2019, 43, 17390–17394. [Google Scholar] [CrossRef]
- Guo, D.; An, Q.; Zybin, S.V.; Goddard III, W.A.; Huang, F.; Tang, B. The co-crystal of TNT/CL−20 leads to decreased sensitivity toward thermal decomposition from first principles based reactive molecular dynamics. J. Mater. Chem. A 2015, 3, 5409–5419. [Google Scholar] [CrossRef]
- Ren, C.; Li, X.; Guo, L. Chemical insight on decreased sensitivity of CL−20/TNT cocrystal revealed by ReaxFF MD simulations. J. Chem. Inf. Model. 2019, 59, 2079–2092. [Google Scholar] [CrossRef]
- Pang, W.; Wang, K.; Zhang, W.; De Luca, L.T.; Fan, X.; Li, J. CL−20-based cocrystal energetic materials: Simulation, preparation and performance. Molecules 2020, 25, 4311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, W.; Huang, H. CL−20/TNT decomposition under shock: Cocrystalline versus amorphous. RSC Adv. 2022, 12, 6938–6946. [Google Scholar] [CrossRef]
- Hang, G.; Yu, W.; Wang, T.; Li, Z. Comparative studies on structures, mechanical properties, sensitivity, stabilities and detonation performance of CL−20/TNT cocrystal and composite explosives by molecular dynamics simulation. J. Mol. Model. 2017, 23, 281. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Selvaraj, G.; Ji, G.; Chen, X.; Wei, D. Towards the low-sensitive and high-energetic co-crystal explosive CL−20/TNT: From intermolecular interactions to structures and properties. Phys. Chem. Chem. Phys. 2018, 20, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Duan, X.; Zhu, L.; Liu, X.; Pei, C. Directly insight into the inter-and intramolecular interactions of CL−20/TNT energetic cocrystal through the theoretical simulations of THz spectroscopy. J. Phys. Chem. A 2016, 120, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xiao, J.; Zhang, J.; Zhao, F.; He, Z.; Xiao, H. Molecular dynamics simulation on CL−20/TNT cocrystal explosive. Chem. J. Chin. Univ. 2016, 37, 559–566. [Google Scholar]
- Zhu, S.; Ji, J.; Zhu, W. Intermolecular interactions, vibrational spectra, and detonation performance of CL−20/TNT cocrystal. J. Chin. Chem. Soc. 2020, 67, 1742–1752. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Kaliamurthi, S.; Selvaraj, G.; Ji, G.; Wei, D. Initial decomposition of the co-crystal of CL−20/TNT: Sensitivity decrease under shock loading. J. Phys. Chem. C 2018, 122, 24270–24278. [Google Scholar] [CrossRef]
- Guo, D.; An, Q.; Goddard III, W.A.; Zybin, S.V.; Huang, F. Compressive shear reactive molecular dynamics studies indicating that cocrystals of TNT/CL−20 decrease sensitivity. J. Phys. Chem. C 2014, 118, 30202–30208. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, X. Impact sensitivity and moisture adsorption on the surface of CL−20/TNT cocrystal by molecular dynamics simulation. Appl. Surf. Sci. 2019, 483, 91–97. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J. Molecular dynamics simulations for effects of fluoropolymer binder content in CL−20/TNT based polymer-bonded explosives. Molecules 2021, 26, 4876. [Google Scholar] [CrossRef]
- Li, H.; Shu, Y.; Gao, S.; Chen, L.; Ma, Q.; Ju, X. Easy methods to study the smart energetic TNT/CL−20 co-crystal. J. Mol. Model. 2013, 19, 4909–4917. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, X.; Cao, Y.; Zhou, J.; Zhang, A.; Li, H.; Zhou, Y.; Xu, R.; Gao, T. Toward low-sensitive and high-energetic co-crystal II: Structural, electronic and energetic features of CL−20 polymorphs and the observed CL−20-based energetic–energetic co-crystals. CrystEngComm 2014, 16, 5905–5916. [Google Scholar] [CrossRef]
- Hang, G.; Yu, W.; Wang, T.; Wang, J. Theoretical investigations into effects of adulteration crystal defect on properties of CL−20/TNT cocrystal explosive. Comput. Mater. Sci. 2019, 156, 77–83. [Google Scholar] [CrossRef]
- Hang, G.; Yu, W.; Wang, T.; Wang, J. Theoretical investigations on structures, stability, energetic performance, sensitivity, and mechanical properties of CL−20/TNT/HMX cocrystal explosives by molecular dynamics simulation. J. Mol. Model. 2019, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- GJB772A-1997; Test Methods for Explosives. National Military Standard of China: Beijing, China, 1997.
- Yang, Z.; Zhang, Y.; Li, H.; Zhou, X.; Nie, F.; Li, J.; Huang, H. Preparation, Structure and Properties of CL−20/TNT Cocrystal. Chin. J. Energ. Mater. 2012, 20, 674–679. [Google Scholar]
- Niu, S.; Gao, H.; Qu, W.; Li, N.; Zhao, F. Research Progress on Behavior and Mechanism of Crystal Transformation of CL−20. Chin. J. Explos. Propellants 2017, 40, 1–7. [Google Scholar]



| Sample | Etotal /(kcal/mol) | Elayer (1) /(kcal/mol) | Elayer (2) /(kcal/mol) | Ebind /(kcal/mol) |
|---|---|---|---|---|
| CL−20/TNT | −7424.058 | 1925.070 | −9248.818 | 100.310 |
| CL−20/CL−20 | −18,489.851 | −9266.570 | −9215.049 | 8.232 |
| TNT/TNT | 2485.858 | 1220.582 | 1269.425 | 4.149 |
| Sample | Etotal /(kcal/mol) | Elayer (1) /(kcal/mol) | Elayer (2) /(kcal/mol) | Ebind /(kcal/mol) |
|---|---|---|---|---|
| CL−20/TNT | −7454.284 | 1893.402 | −9162.901 | 184.785 |
| CL−20/CL−20 | −1,8434.556 | −9196.321 | −9223.500 | 14.735 |
| TNT/TNT | 2464.076 | 1277.202 | 1308.020 | 121.146 |
| Sample | Etotal /(kcal/mol) | Elayer (1) /(kcal/mol) | Elayer (2) /(kcal/mol) | Ebind /(kcal/mol) |
|---|---|---|---|---|
| CL−20/TNT | −7411.435 | 1950.566 | −9202.705 | 159.296 |
| CL−20/CL−20 | −18,492.884 | −9219.501 | −9263.576 | 9.807 |
| TNT/TNT | 2475.853 | 1272.912 | 1297.089 | 94.148 |
| Crystal face | Etotal /(kcal/mol) | Elayer (1) /(kcal/mol) | Elayer (2) /(kcal/mol) | Ebind /(kcal/mol) |
|---|---|---|---|---|
| 0 1 1 | −2819.4 | −4620.6 | 1820.8 | 19.6 |
| 1 1 0 | −2681.4 | −4495.6 | 1837.1 | 22.9 |
| 1 0 −1 | −2930.2 | −4786.3 | 1860.9 | 4.8 |
| 0 0 2 | −3104.7 | −4858.1 | 1842.2 | 88.8 |
| 1 1 −1 | −2523.3 | −4303.4 | 1877.4 | 97.3 |
| 1 0 1 | −2861.9 | −4741.3 | 1884.6 | 5.2 |
| Crystal face | Etotal /(kcal/mol) | Elayer (1) /(kcal/mol) | Elayer (2) /(kcal/mol) | Ebind /(kcal/mol) |
|---|---|---|---|---|
| 0 1 1 | −18,541.5 | −4620.5 | −13,895.1 | 25.9 |
| 1 1 0 | −18,521.4 | −4495.4 | −13,996.9 | 29.1 |
| 1 0 −1 | −18,726.8 | −4786.1 | −13,933.4 | 7.3 |
| 0 0 2 | −18,824.9 | −4857.9 | −13,916.8 | 50.2 |
| 1 1 −1 | −18,230.4 | −4303.3 | −13,920.1 | 7.0 |
| 1 0 1 | −18,652.8 | −4741.2 | −13,905.5 | 6.1 |
| Sample | Impact Sensitivity H50/cm | Friction Sensitivity P/% |
|---|---|---|
| Raw CL−20 | 15 | 100 |
| Raw TNT | 102 | 6 |
| CL−20/TNT | 36 | 48 |
| Name | Specifications | Properties |
|---|---|---|
| CL−20 | - | White powder, easily soluble in acetone, methanol, acetonitrile, and ethyl acetate, slightly soluble in ethanol, insoluble in chlorinated hydrocarbons and water. |
| TNT | - | Yellow flake or powder, highly soluble in acetone, toluene, benzene, and chloroform, slightly soluble in ethanol and carbon tetrachloride, insoluble in water. |
| Acetone | analytical pure | Colorless transparent liquid, flammable and volatile. |
| Name | Model | Manufacturer |
|---|---|---|
| Electronic balance | AL204-IC | Mettler Toledo Instruments Co., Ltd., Shanghai, China. |
| Ultrasonic cleaner | KQ-250DE | Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, China. |
| Air compressor | 600W-30L | Shanghai Shengxi Hardware and Electromechanical Co., Ltd., Shanghai, China. |
| Opposite spray drum | 5 L | Anqing Shengxing Plastic Industry, Anqing, China. |
| Circulating water multi-purpose vacuum pump | SHB-IIIS | Zhengzhou Changcheng Science and Technology Industry and Trade Co., Ltd., Zhengzhou, China. |
| Thermostatic incubator | SPX-150B-D | Shanghai Boxun Industrial Medical Equipment Factory, Shanghai, China |
| Scanning electron | EM-30 | COXEM Company, Datian city, Korea |
| DSC | HCT-1 | Beijing Hengjiu Technology Co., Ltd., Beijing, China. |
| Infrared spectrometer | Perkin Elmer Spectrum 100 | Platinum Elmer Inc., MA, USA |
| X-ray diffractometer | DX-2700 | Dandong Haoyuan Instrument Co., Ltd., Dandong, China. |
| Impact sensitivity device | WL-1 | Shanxi Institute of Applied Physics and Chemistry, Xi’an, China |
| Friction sensitivity device | MGY-I | Shanxi Institute of Applied Physics and Chemistry, Xi’an, China |
| hkl | Multiplicity | dhkl | Surface Area | Eatt(Total) | Distance | Area Ratio |
|---|---|---|---|---|---|---|
| (0 1 1) | 4 | 8.909 | 156.626 | −83.975 | 83.975 | 38.914 |
| (1 0 −1) | 2 | 8.180 | 170.570 | −85.080 | 85.080 | 13.749 |
| (1 1 0) | 4 | 6.981 | 199.874 | −89.337 | 89.337 | 26.114 |
| (1 1 −1) | 4 | 6.841 | 203.976 | −94.024 | 94.024 | 8.421 |
| (0 0 2) | 2 | 6.364 | 109.634 | −90.810 | 90.810 | 10.713 |
| (1 0 1) | 2 | 6.251 | 223.204 | −109.305 | 109.305 | 2.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Liu, Z.; Han, T.; Li, J.; Han, P.; Wang, J. Preparation and Molecular Dynamic Simulation of Superfine CL−20/TNT Cocrystal Based on the Opposite Spray Method. Int. J. Mol. Sci. 2024, 25, 9501. https://doi.org/10.3390/ijms25179501
Yuan J, Liu Z, Han T, Li J, Han P, Wang J. Preparation and Molecular Dynamic Simulation of Superfine CL−20/TNT Cocrystal Based on the Opposite Spray Method. International Journal of Molecular Sciences. 2024; 25(17):9501. https://doi.org/10.3390/ijms25179501
Chicago/Turabian StyleYuan, Junming, Zhenyang Liu, Tao Han, Junyi Li, Peijiang Han, and Jing Wang. 2024. "Preparation and Molecular Dynamic Simulation of Superfine CL−20/TNT Cocrystal Based on the Opposite Spray Method" International Journal of Molecular Sciences 25, no. 17: 9501. https://doi.org/10.3390/ijms25179501




