The Pathological Mechanisms and Therapeutic Molecular Targets in Arteriovenous Fistula Dysfunction
Abstract
1. Introduction
2. The Clinical Characteristics of AVF Dysfunction
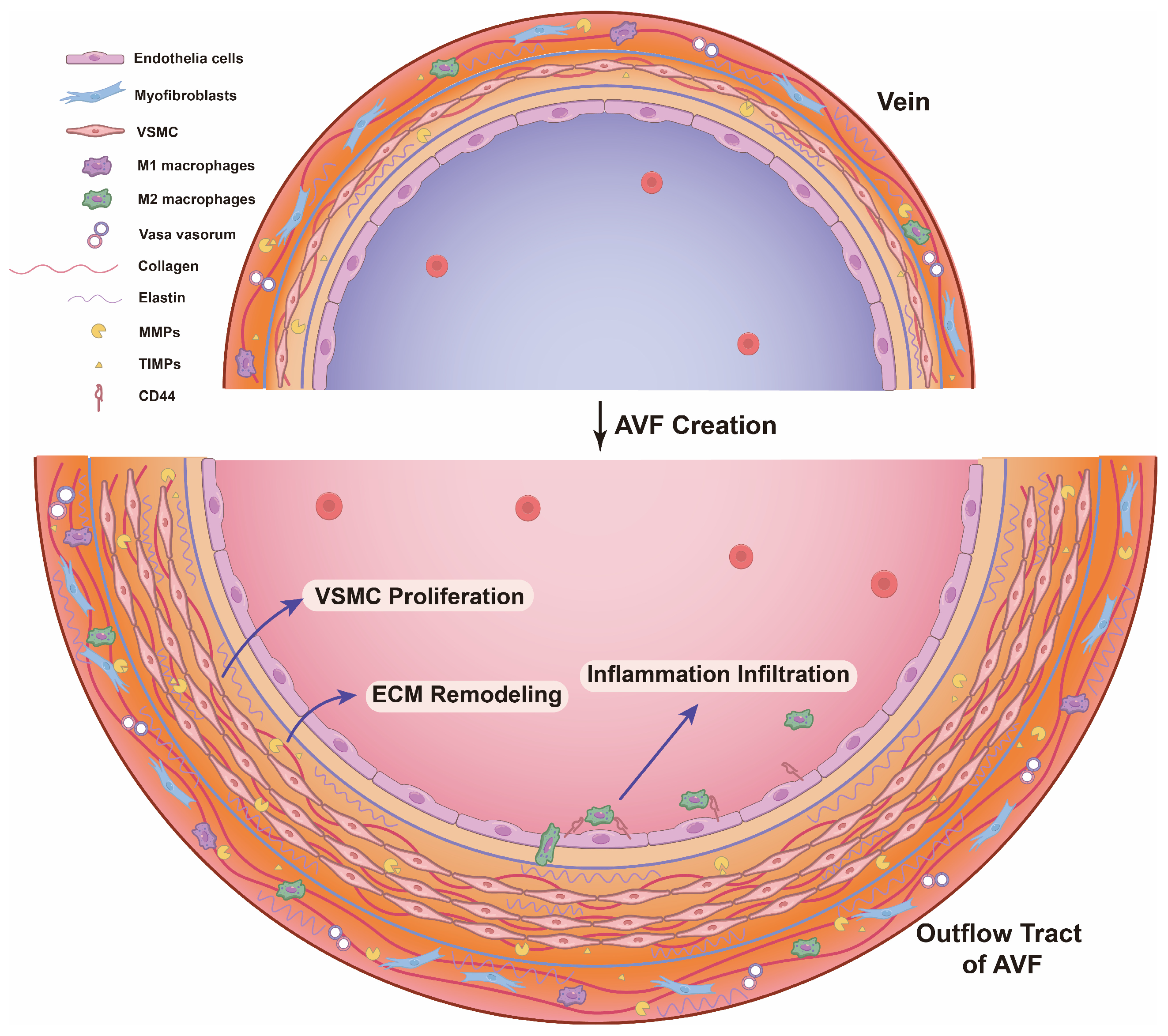
3. The Pathophysiological Features of AVF Dysfunction
4. The Pathological Mechanisms Underlying Outward Remodeling Failure
4.1. Differentiated VSMC Proliferation
4.2. ECM Degradation, Deposit, and Rearrangement
4.3. Inflammation Infiltration
5. The Potential Mechanisms Underlying Excessive Inward Remodeling
5.1. Endothelia Cells
5.2. Vascular Smooth Muscle Cells
5.3. Myofibroblasts and Fibroblasts
5.4. Inflammatory Cell Infiltration
5.5. Bone-Marrow-Derived Cells
6. Outward Remodeling and Inward Remodeling: The Friends or the Foes?
7. The Potential Therapeutic Molecular Targets in AVF Dysfunction
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bello, A.K.; Okpechi, I.G.; Levin, A.; Ye, F.; Damster, S.; Arruebo, S.; Donner, J.A.; Caskey, F.J.; Cho, Y.; Davids, M.R.; et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob. Health 2024, 12, e382–e395. [Google Scholar] [CrossRef]
- Johansen, K.L.; Gilbertson, D.T.; Li, S.; Li, S.; Liu, J.; Roetker, N.S.; Ku, E.; Schulman, I.H.; Greer, R.C.; Chan, K.; et al. US Renal Data System 2023 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2024, 83 (Suppl. S1), A8–A13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Zhang, M.; Hu, C.; Zhang, X.; Li, C.; Nie, S.; Huang, Z.; Zhao, Z.; Hou, F.F.; et al. Prevalence of Chronic Kidney Disease in China: Results From the Sixth China Chronic Disease and Risk Factor Surveillance. JAMA Intern. Med. 2023, 183, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Eustace, J.A.; Powe, N.R.; Klag, M.J.; Fink, N.E.; Coresh, J. Type of vascular access and survival among incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J. Am. Soc. Nephrol. 2005, 16, 1449–1455. [Google Scholar] [CrossRef]
- Banerjee, T.; Kim, S.J.; Astor, B.; Shafi, T.; Coresh, J.; Powe, N.R. Vascular access type, inflammatory markers, and mortality in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am. J. Kidney Dis. 2014, 64, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Duque, J.C.; Tabbara, M.; Paez, A.; Selman, G.; Hernandez, D.R.; Sundberg, C.A.; Tey, J.C.S.; Shiu, Y.T.; Cheung, A.K.; et al. Fibrotic Venous Remodeling and Nonmaturation of Arteriovenous Fistulas. J. Am. Soc. Nephrol. 2018, 29, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Brescia, M.J.; Cimino, J.E.; Appel, K.; Hurwich, B.J. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N. Engl. J. Med. 1966, 275, 1089–1092. [Google Scholar] [CrossRef]
- Schmidli, J.; Widmer, M.K.; Basile, C.; de Donato, G.; Gallieni, M.; Gibbons, C.P.; Haage, P.; Hamilton, G.; Hedin, U.; Kamper, L.; et al. Editor’s Choice–Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 757–818. [Google Scholar] [CrossRef]
- Al-Jaishi, A.A.; Oliver, M.J.; Thomas, S.M.; Lok, C.E.; Zhang, J.C.; Garg, A.X.; Kosa, S.D.; Quinn, R.R.; Moist, L.M. Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 63, 464–478. [Google Scholar] [CrossRef]
- Manns, B.; Tonelli, M.; Yilmaz, S.; Lee, H.; Laupland, K.; Klarenbach, S.; Radkevich, V.; Murphy, B. Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J. Am. Soc. Nephrol. 2005, 16, 201–209. [Google Scholar] [CrossRef]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef] [PubMed]
- Robbin, M.L.; Oser, R.F.; Lee, J.Y.; Heudebert, G.R.; Mennemeyer, S.T.; Allon, M. Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int. 2006, 69, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Bacchini, G.; Cappello, A.; La Milia, V.; Andrulli, S.; Locatelli, F. Color doppler ultrasonography imaging to guide transluminal angioplasty of venous stenosis. Kidney Int. 2000, 58, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Rabin, I.; Shani, M.; Mursi, J.; Peer, A.; Beberashvili, I.; Bass, A.; Feldman, L. Effect of timing of thrombectomy on survival of thrombosed arteriovenous hemodialysis grafts. Vasc. Endovasc. Surg. 2013, 47, 342–345. [Google Scholar] [CrossRef]
- Laboyrie, S.L.; de Vries, M.R.; Bijkerk, R.; Rotmans, J.I. Building a Scaffold for Arteriovenous Fistula Maturation: Unravelling the Role of the Extracellular Matrix. Int. J. Mol. Sci. 2023, 24, 10825. [Google Scholar] [CrossRef]
- Giudici, A.; Wilkinson, I.B.; Khir, A.W. Review of the Techniques Used for Investigating the Role Elastin and Collagen Play in Arterial Wall Mechanics. IEEE Rev. Biomed. Eng. 2021, 14, 256–269. [Google Scholar] [CrossRef]
- Mozaffar, M.; Fallah, M.; Lotfollahzadeh, S.; Sobhiyeh, M.R.; Gholizadeh, B.; Jabbehdari, S.; Mahdi, Z. Comparison of efficacy of side to side versus end to side arteriovenous fistulae formation in chronic renal failure as a permanent hemodialysis access. Nephrourol. Mon. 2013, 5, 827–830. [Google Scholar] [CrossRef]
- Röhl, L.; Franz, H.E.; Möhring, K.; Ritz, E.; Schüler, H.W.; Uhse, H.G.; Ziegler, M. Direct arteriovenous fistula for hemodialysis. Scand. J. Urol. Nephrol. 1968, 2, 191–195. [Google Scholar] [CrossRef]
- Clinical practice guidelines for vascular access. Am. J. Kidney Dis. 2006, 48 (Suppl. S1), S176–S247. [CrossRef]
- Sidawy, A.N.; Spergel, L.M.; Besarab, A.; Allon, M.; Jennings, W.C.; Padberg, F.T., Jr.; Murad, M.H.; Montori, V.M.; O’Hare, A.M.; Calligaro, K.D.; et al. The Society for Vascular Surgery: Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J. Vasc. Surg. 2008, 48, 2s–25s. [Google Scholar] [CrossRef]
- Gorecka, J.; Fereydooni, A.; Gonzalez, L.; Lee, S.R.; Liu, S.; Ono, S.; Xu, J.; Liu, J.; Taniguchi, R.; Matsubara, Y.; et al. Molecular Targets for Improving Arteriovenous Fistula Maturation and Patency. Vasc. Investig. Ther. 2019, 2, 33–41. [Google Scholar] [PubMed]
- Lawson, J.H.; Niklason, L.E.; Roy-Chaudhury, P. Challenges and novel therapies for vascular access in haemodialysis. Nat. Rev. Nephrol. 2020, 16, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Patel, S.; Hanisch, J.J.; Santana, J.M.; Hashimoto, T.; Bai, H.; Kudze, T.; Foster, T.R.; Guo, J.; Yatsula, B.; et al. Future research directions to improve fistula maturation and reduce access failure. Semin. Vasc. Surg. 2016, 29, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Robbin, M.L.; Young, C.J.; Deierhoi, M.H.; Goodman, J.; Hanaway, M.; Lockhart, M.E.; Litovsky, S. Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin. J. Am. Soc. Nephrol. 2013, 8, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Duband, J.L.; Gimona, M.; Scatena, M.; Sartore, S.; Small, J.V. Calponin and SM 22 as differentiation markers of smooth muscle: Spatiotemporal distribution during avian embryonic development. Differentiation 1993, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ip, H.S.; Lu, M.M.; Clendenin, C.; Parmacek, M.S. A serum response factor-dependent transcriptional regulatory program identifies distinct smooth muscle cell sublineages. Mol. Cell. Biol. 1997, 17, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miano, J.M.; Cserjesi, P.; Olson, E.N. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circul. Res. 1996, 78, 188–195. [Google Scholar] [CrossRef]
- Gabbiani, G.; Schmid, E.; Winter, S.; Chaponnier, C.; de Ckhastonay, C.; Vandekerckhove, J.; Weber, K.; Franke, W.W. Vascular smooth muscle cells differ from other smooth muscle cells: Predominance of vimentin filaments and a specific alpha-type actin. Proc. Natl. Acad. Sci. USA 1981, 78, 298–302. [Google Scholar] [CrossRef]
- Mack, C.P.; Owens, G.K. Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5’ and first intron promoter regions. Circ. Res. 1999, 84, 852–861. [Google Scholar] [CrossRef]
- Arimura, C.; Suzuki, T.; Yanagisawa, M.; Imamura, M.; Hamada, Y.; Masaki, T. Primary structure of chicken skeletal muscle and fibroblast alpha-actinins deduced from cDNA sequences. Eur. J. Biochem. 1988, 177, 649–655. [Google Scholar] [CrossRef]
- Babij, P.; Kelly, C.; Periasamy, M. Characterization of a mammalian smooth muscle myosin heavy-chain gene: Complete nucleotide and protein coding sequence and analysis of the 5’ end of the gene. Proc. Natl. Acad. Sci. USA 1991, 88, 10676–10680. [Google Scholar] [CrossRef] [PubMed]
- Frid, M.G.; Printesva, O.Y.; Chiavegato, A.; Faggin, E.; Scatena, M.; Koteliansky, V.E.; Pauletto, P.; Glukhova, M.A.; Sartore, S. Myosin heavy-chain isoform composition and distribution in developing and adult human aortic smooth muscle. J. Vasc. Res. 1993, 30, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Miano, J.M.; Cserjesi, P.; Ligon, K.L.; Periasamy, M.; Olson, E.N. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ. Res. 1994, 75, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Miano, J.M.; Carlson, M.J.; Spencer, J.A.; Misra, R.P. Serum response factor-dependent regulation of the smooth muscle calponin gene. J. Biol. Chem. 2000, 275, 9814–9822. [Google Scholar] [CrossRef]
- Van der Loop, F.T.; Schaart, G.; Timmer, E.D.; Ramaekers, F.C.; van Eys, G.J. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J. Cell Biol. 1996, 134, 401–411. [Google Scholar] [CrossRef]
- Jahn, L.; Kreuzer, J.; von Hodenberg, E.; Kübler, W.; Franke, W.W.; Allenberg, J.; Izumo, S. Cytokeratins 8 and 18 in smooth muscle cells. Detection in human coronary artery, peripheral vascular, and vein graft disease and in transplantation-associated arteriosclerosis. Arterioscler. Thromb. 1993, 13, 1631–1639. [Google Scholar] [CrossRef]
- Ehler, E.; Jat, P.S.; Noble, M.D.; Citi, S.; Draeger, A. Vascular smooth muscle cells of H-2Kb-tsA58 transgenic mice. Characterization of cell lines with distinct properties. Circulation 1995, 92, 3289–3296. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Hastings, N.E.; Blackman, B.R.; Wamhoff, B.R. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J. Vasc. Res. 2010, 47, 168–180. [Google Scholar] [CrossRef]
- Zhao, J.; Jourd’heuil, F.L.; Xue, M.; Conti, D.; Lopez-Soler, R.I.; Ginnan, R.; Asif, A.; Singer, H.A.; Jourd’heuil, D.; Long, X. Dual Function for Mature Vascular Smooth Muscle Cells During Arteriovenous Fistula Remodeling. J. Am. Heart Assoc. 2017, 6, e004891. [Google Scholar] [CrossRef]
- Hu, H.; Lee, S.R.; Bai, H.; Guo, J.; Hashimoto, T.; Isaji, T.; Guo, X.; Wang, T.; Wolf, K.; Liu, S.; et al. TGFβ (Transforming Growth Factor-Beta)-Activated Kinase 1 Regulates Arteriovenous Fistula Maturation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e203–e213. [Google Scholar] [CrossRef]
- Matsubara, Y.; Kiwan, G.; Liu, J.; Gonzalez, L.; Langford, J.; Gao, M.; Gao, X.; Taniguchi, R.; Yatsula, B.; Furuyama, T.; et al. Inhibition of T-Cells by Cyclosporine A Reduces Macrophage Accumulation to Regulate Venous Adaptive Remodeling and Increase Arteriovenous Fistula Maturation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e160–e174. [Google Scholar] [CrossRef] [PubMed]
- Bezhaeva, T.; Wong, C.; de Vries, M.R.; van der Veer, E.P.; van Alem, C.M.A.; Que, I.; Lalai, R.A.; van Zonneveld, A.J.; Rotmans, J.I.; Quax, P.H.A. Deficiency of TLR4 homologue RP105 aggravates outward remodeling in a murine model of arteriovenous fistula failure. Sci. Rep. 2017, 7, 10269. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.; Fernandez-Catalan, C.; Grams, F.; Gomis-Rüth, F.X.; Nagase, H.; Tschesche, H.; Maskos, K. Insights into MMP-TIMP interactions. Ann. N. Y. Acad. Sci. 1999, 878, 73–91. [Google Scholar] [CrossRef]
- Lee, E.S.; Shen, Q.; Pitts, R.L.; Guo, M.; Wu, M.H.; Sun, S.C.; Yuan, S.Y. Serum metalloproteinases MMP-2, MMP-9, and metalloproteinase tissue inhibitors in patients are associated with arteriovenous fistula maturation. J. Vasc. Surg. 2011, 54, 454–459; discussion 459–460. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Chen, Y.S.; Ma, M.C.; Chen, C.F. Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats. J. Vasc. Surg. 2007, 45, 804–811. [Google Scholar] [CrossRef]
- Harris, L.K.; Aplin, J.D. Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reprod. Sci. 2007, 14, 28–34. [Google Scholar] [CrossRef]
- Harris, L.K.; Smith, S.D.; Keogh, R.J.; Jones, R.L.; Baker, P.N.; Knöfler, M.; Cartwright, J.E.; Whitley, G.S.; Aplin, J.D. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am. J. Pathol. 2010, 177, 2103–2115. [Google Scholar] [CrossRef]
- Saracini, C.; Bolli, P.; Sticchi, E.; Pratesi, G.; Pulli, R.; Sofi, F.; Pratesi, C.; Gensini, G.F.; Abbate, R.; Giusti, B. Polymorphisms of genes involved in extracellular matrix remodeling and abdominal aortic aneurysm. J. Vasc. Surg. 2012, 55, 171–179.e2. [Google Scholar] [CrossRef]
- Wong, C.Y.; Rothuizen, T.C.; de Vries, M.R.; Rabelink, T.J.; Hamming, J.F.; van Zonneveld, A.J.; Quax, P.H.; Rotmans, J.I. Elastin is a key regulator of outward remodeling in arteriovenous fistulas. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 480–486. [Google Scholar] [CrossRef]
- Bezhaeva, T.; de Vries, M.R.; Geelhoed, W.J.; van der Veer, E.P.; Versteeg, S.; van Alem, C.M.A.; Voorzaat, B.M.; Eijkelkamp, N.; van der Bogt, K.E.; Agoulnik, A.I.; et al. Relaxin receptor deficiency promotes vascular inflammation and impairs outward remodeling in arteriovenous fistulas. FASEB J. 2018, 32, fj201800437R. [Google Scholar] [CrossRef]
- Peden, E.K.; O’Connor, T.P.; Browne, B.J.; Dixon, B.S.; Schanzer, A.S.; Jensik, S.C.; Sam, A.D., 2nd; Burke, S.K. Arteriovenous fistula patency in the 3 years following vonapanitase and placebo treatment. J. Vasc. Surg. 2017, 65, 1113–1120. [Google Scholar] [CrossRef][Green Version]
- Hall, M.R.; Yamamoto, K.; Protack, C.D.; Tsuneki, M.; Kuwahara, G.; Assi, R.; Brownson, K.E.; Bai, H.; Madri, J.A.; Dardik, A. Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. J. Vasc. Access 2015, 16, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Shiu, Y.T.; He, Y.; Tey, J.C.S.; Knysheva, M.; Anderson, B.; Kauser, K. Natural Vascular Scaffolding Treatment Promotes Outward Remodeling During Arteriovenous Fistula Development in Rats. Front. Bioeng. Biotechnol. 2021, 9, 622617. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Rojas, M.G.; Tabbara, M.; Pereira-Simon, S.; Santos Falcon, N.; Rauf, M.A.; Challa, A.; Zigmond, Z.M.; Griswold, A.J.; Duque, J.C.; et al. The Transcriptomics of the Human Vein Transformation After Arteriovenous Fistula Anastomosis Uncovers Layer-Specific Remodeling and Hallmarks of Maturation Failure. Kidney Int. Rep. 2023, 8, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Bezhaeva, T.; Rothuizen, T.C.; Metselaar, J.M.; de Vries, M.R.; Verbeek, F.P.; Vahrmeijer, A.L.; Wezel, A.; van Zonneveld, A.J.; Rabelink, T.J.; et al. Liposomal prednisolone inhibits vascular inflammation and enhances venous outward remodeling in a murine arteriovenous fistula model. Sci. Rep. 2016, 6, 30439. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Kiwan, G.; Fereydooni, A.; Langford, J.; Dardik, A. Distinct subsets of T cells and macrophages impact venous remodeling during arteriovenous fistula maturation. JVS Vasc. Sci. 2020, 1, 207–218. [Google Scholar] [CrossRef]
- Nguyen, M.; Thankam, F.G.; Agrawal, D.K. Sterile inflammation in the pathogenesis of maturation failure of arteriovenous fistula. J. Mol. Med. 2021, 99, 729–741. [Google Scholar] [CrossRef]
- Shiu, Y.T.; Rotmans, J.I.; Geelhoed, W.J.; Pike, D.B.; Lee, T. Arteriovenous conduits for hemodialysis: How to better modulate the pathophysiological vascular response to optimize vascular access durability. Am. J. Physiol. Renal Physiol. 2019, 316, F794–F806. [Google Scholar] [CrossRef]
- Stolic, R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med. Princ. Pract. 2013, 22, 220–228. [Google Scholar] [CrossRef] [PubMed]
- De Donatis, A.; Comito, G.; Buricchi, F.; Vinci, M.C.; Parenti, A.; Caselli, A.; Camici, G.; Manao, G.; Ramponi, G.; Cirri, P. Proliferation versus migration in platelet-derived growth factor signaling: The key role of endocytosis. J. Biol. Chem. 2008, 283, 19948–19956. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fereydooni, A.; Isaji, T.; Gorecka, J.; Liu, S.; Hu, H.; Ono, S.; Alozie, M.; Lee, S.R.; Taniguchi, R.; et al. Inhibition of the Akt1-mTORC1 Axis Alters Venous Remodeling to Improve Arteriovenous Fistula Patency. Sci. Rep. 2019, 9, 11046. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, G.; Hashimoto, T.; Tsuneki, M.; Yamamoto, K.; Assi, R.; Foster, T.R.; Hanisch, J.J.; Bai, H.; Hu, H.; Protack, C.D.; et al. CD44 Promotes Inflammation and Extracellular Matrix Production During Arteriovenous Fistula Maturation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117. [Google Scholar]
- Matsubara, Y.; Gonzalez, L.; Kiwan, G.; Liu, J.; Langford, J.; Gao, M.; Gao, X.; Taniguchi, R.; Yatsula, B.; Furuyama, T.; et al. PD-L1 (Programmed Death Ligand 1) Regulates T-Cell Differentiation to Control Adaptive Venous Remodeling. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2909–2922. [Google Scholar]
- Duque, J.C.; Martinez, L.; Mesa, A.; Wei, Y.; Tabbara, M.; Salman, L.H.; Vazquez-Padron, R.I. CD4(+) lymphocytes improve venous blood flow in experimental arteriovenous fistulae. Surgery 2015, 158, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Khan, R.; Campos, B.; Wang, Y.; Kurian, M.; Lee, T.; Arend, L.; Munda, R. Pathogenetic role for early focal macrophage infiltration in a pig model of arteriovenous fistula (AVF) stenosis. J. Vasc. Access 2014, 15, 25–28. [Google Scholar] [CrossRef]
- Huynh, N.N.; Chin-Dusting, J. Amino acids, arginase and nitric oxide in vascular health. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1–8. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Higman, D.J.; Strachan, A.M.; Buttery, L.; Hicks, R.C.; Springall, D.R.; Greenhalgh, R.M.; Powell, J.T. Smoking impairs the activity of endothelial nitric oxide synthase in saphenous vein. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Hingorani, A. Endothelial nitric oxide in humans in health and disease. Int. J. Exp. Pathol. 1999, 80, 291–303. [Google Scholar] [CrossRef]
- Kang, H.; Fan, Y.; Deng, X. Vascular smooth muscle cell glycocalyx modulates shear-induced proliferation, migration, and NO production responses. Am. J. Physiol. Heart Circul. Physiol. 2011, 300, H76–H83. [Google Scholar] [CrossRef] [PubMed]
- Pike, D.; Shiu, Y.T.; Cho, Y.F.; Le, H.; Somarathna, M.; Isayeva, T.; Guo, L.; Symons, J.D.; Kevil, C.G.; Totenhagen, J.; et al. The effect of endothelial nitric oxide synthase on the hemodynamics and wall mechanics in murine arteriovenous fistulas. Sci. Rep. 2019, 9, 4299. [Google Scholar]
- Hong, S.; Cho, Y.W.; Yu, L.R.; Yu, H.; Veenstra, T.D.; Ge, K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl. Acad. Sci. USA 2007, 104, 18439–18444. [Google Scholar] [CrossRef]
- Feng, S.; Peden, E.K.; Guo, Q.; Lee, T.H.; Li, Q.; Yuan, Y.; Chen, C.; Huang, F.; Cheng, J. Downregulation of the endothelial histone demethylase JMJD3 is associated with neointimal hyperplasia of arteriovenous fistulas in kidney failure. J. Biol. Chem. 2022, 298, 101816. [Google Scholar] [CrossRef] [PubMed]
- Pintavorn, P.; Ballermann, B.J. TGF-beta and the endothelium during immune injury. Kidney Int. 1997, 51, 1401–1412. [Google Scholar] [CrossRef]
- Liang, A.; Wang, Y.; Han, G.; Truong, L.; Cheng, J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am. J. Physiol. Renal Physiol. 2013, 304, F1413–F1420. [Google Scholar] [CrossRef] [PubMed]
- Dardik, A.; Yamashita, A.; Aziz, F.; Asada, H.; Sumpio, B.E. Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1alpha. J. Vasc. Surg. 2005, 41, 321–331. [Google Scholar] [CrossRef]
- Jia, L.; Wang, L.; Wei, F.; Li, C.; Wang, Z.; Yu, H.; Chen, H.; Wang, B.; Jiang, A. Effects of Caveolin-1-ERK1/2 pathway on endothelial cells and smooth muscle cells under shear stress. Exp. Biol. Med. 2020, 245, 21–33. [Google Scholar] [CrossRef]
- Huang, X.; Guan, J.; Sheng, Z.; Wang, M.; Xu, T.; Guo, G.; Wan, P.; Tian, B.; Zhou, J.; Huang, A.; et al. Effect of local anti-vascular endothelial growth factor therapy to prevent the formation of stenosis in outflow vein in arteriovenous fistula. J. Transl. Int. Med. 2021, 9, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Cooley, B.C.; Nevado, J.; Mellad, J.; Yang, D.; St Hilaire, C.; Negro, A.; Fang, F.; Chen, G.; San, H.; Walts, A.D.; et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 2014, 6, 227ra34. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lai, Y.J.; Tung, Y.C.; Wu, L.S.; Hsu, L.A.; Tseng, C.N.; Chang, G.J.; Yang, K.C.; Yeh, Y.H. Osteopontin mediation of disturbed flow-induced endothelial mesenchymal transition through CD44 is a novel mechanism of neointimal hyperplasia in arteriovenous fistulae for hemodialysis access. Kidney Int. 2023, 103, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Hsu, S.C.; Hsieh, H.L.; Chen, C.H.; Chen, C.Y.; Sue, Y.M.; Chen, T.H.; Hsu, Y.H.; Lin, F.Y.; Shih, C.M.; et al. Inhibition of β-catenin signaling attenuates arteriovenous fistula thickening in mice by suppressing myofibroblasts. Mol. Med. 2022, 28, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, A.; Luo, J.; Liang, M.; Han, G.; Mitch, W.E.; Cheng, J. Blocking Notch in endothelial cells prevents arteriovenous fistula failure despite CKD. J. Am. Soc. Nephrol. 2014, 25, 773–783. [Google Scholar] [CrossRef]
- Tian, D.; Zeng, X.; Wang, W.; Wang, Z.; Zhang, Y.; Wang, Y. Protective effect of rapamycin on endothelial-to-mesenchymal transition in HUVECs through the Notch signaling pathway. Vasc. Pharmacol. 2019, 113, 20–26. [Google Scholar] [CrossRef]
- Liang, M.; Guo, Q.; Huang, F.; Han, G.; Song, K.; Luo, J.; Cheng, H.; Hu, H.; Peden, E.K.; Chen, C.; et al. Notch signaling in bone marrow-derived FSP-1 cells initiates neointima formation in arteriovenous fistulas. Kidney Int. 2019, 95, 1347–1358. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Y.; Liang, A.; Mitch, W.E.; Roy-Chaudhury, P.; Han, G.; Cheng, J. Migration of smooth muscle cells from the arterial anastomosis of arteriovenous fistulas requires Notch activation to form neointima. Kidney Int. 2015, 88, 490–502. [Google Scholar] [CrossRef]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef]
- Wolf, K.; Hu, H.; Isaji, T.; Dardik, A. Molecular identity of arteries, veins, and lymphatics. J. Vasc. Surg. 2019, 69, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Protack, C.D.; Foster, T.R.; Hashimoto, T.; Yamamoto, K.; Lee, M.Y.; Kraehling, J.R.; Bai, H.; Hu, H.; Isaji, T.; Santana, J.M.; et al. Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency. Sci. Rep. 2017, 7, 15386. [Google Scholar] [CrossRef] [PubMed]
- Kudo, F.A.; Muto, A.; Maloney, S.P.; Pimiento, J.M.; Bergaya, S.; Fitzgerald, T.N.; Westvik, T.S.; Frattini, J.C.; Breuer, C.K.; Cha, C.H.; et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Yi, T.; Harrison, K.D.; Dávalos, A.; Fancher, T.T.; Ziegler, K.R.; Feigel, A.; Kondo, Y.; Nishibe, T.; Sessa, W.C.; et al. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J. Exp. Med. 2011, 208, 561–575. [Google Scholar] [CrossRef]
- Liang, M.; Liang, A.; Wang, Y.; Jiang, J.; Cheng, J. Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res. Cardiol. 2014, 109, 431. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Herring, B.P.; Hoggatt, A.M.; Burlak, C.; Offermanns, S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc. Cell. 2014, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Song, A.N.; Yin, X.J.; Gao, P.; Tang, H.; Meng, X.F.; Zhang, C. Inhibition of MAD2B alleviates venous neointimal formation by suppressing VSMCs proliferation and migration. FASEB J. 2021, 35, e21959. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, G.; Cheng, H.; Qing, Y.; Truong, L.; Ma, Q.; Wang, Y.; Cheng, J. Temporal regulation of notch activation improves arteriovenous fistula maturation. J. Transl. Med. 2022, 20, 543. [Google Scholar] [CrossRef]
- Misra, S.; Doherty, M.G.; Woodrum, D.; Homburger, J.; Mandrekar, J.N.; Elkouri, S.; Sabater, E.A.; Bjarnason, H.; Fu, A.A.; Glockner, J.F.; et al. Adventitial remodeling with increased matrix metalloproteinase-2 activity in a porcine arteriovenous polytetrafluoroethylene grafts. Kidney Int. 2005, 68, 2890–2900. [Google Scholar] [CrossRef][Green Version]
- Misra, S.; Fu, A.A.; Rajan, D.K.; Juncos, L.A.; McKusick, M.A.; Bjarnason, H.; Mukhopadhyay, D. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J. Vasc. Interv. Radiol. 2008, 19, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Shergill, U.; Yang, B.; Janardhanan, R.; Misra, K.D. Increased expression of HIF-1alpha, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J. Vasc. Interv. Radiol. 2010, 21, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Fu, A.A.; Puggioni, A.; Karimi, K.M.; Mandrekar, J.N.; Glockner, J.F.; Juncos, L.A.; Anwer, B.; McGuire, A.M.; Mukhopadhyay, D. Increased shear stress with upregulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am. J. Physiol. Heart Circul. Physiol. 2008, 294, H2219–H2230. [Google Scholar] [CrossRef]
- Yang, B.; Janardhanan, R.; Vohra, P.; Greene, E.L.; Bhattacharya, S.; Withers, S.; Roy, B.; Nieves Torres, E.C.; Mandrekar, J.; Leof, E.B.; et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int. 2014, 85, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Qing, Y.; Guo, Q.; Peden, E.K.; Chen, C.; Mitch, W.E.; Truong, L.; Cheng, J. PDGFRA in vascular adventitial MSCs promotes neointima formation in arteriovenous fistula in chronic kidney disease. JCI Insight 2020, 5, e137298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Martinez, L.; Zigmond, Z.; Woltmann, D.; Singer, D.V.; Singer, H.A.; Vazquez-Padron, R.I.; Salman, L.H. Functions for platelet factor 4 (PF4/CXCL4) and its receptors in fibroblast-myofibroblast transition and fibrotic failure of arteriovenous fistulas (AVFs). J. Vasc. Access 2023, 11297298231192386. [Google Scholar] [CrossRef]
- Bojakowski, K.; Dzabic, M.; Kurzejamska, E.; Styczynski, G.; Andziak, P.; Gaciong, Z.; Söderberg-Nauclér, C.; Religa, P. A high red blood cell distribution width predicts failure of arteriovenous fistula. PLoS ONE 2012, 7, e36482. [Google Scholar] [CrossRef]
- Misra, S.; Kilari, S.; Yang, B.; Sharma, A.; Wu, C.C.; Vazquez-Padron, R.I.; Broadwater, J. Anti Human CX3CR1 VHH Molecule Attenuates Venous Neointimal Hyperplasia of Arteriovenous Fistula in Mouse Model. J. Am. Soc. Nephrol. 2021, 32, 1630–1648. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Wang, Y.; Cornea, V.; Roy-Chaudhury, P.; Campos, B. Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis: Opportunities for Intervention. Int. J. Mol. Sci. 2021, 22, 12285. [Google Scholar] [CrossRef]
- Chen, B.; Ding, X.; Yang, Y. Hirudin Regulates Vascular Function in Chronic Renal Failure through Modulating Macrophage Polarization. Biomed. Res. Int. 2022, 2022, 6043698. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Oh, J.; Bong, S.K.; Kim, J.S.; Park, S.; Kim, S.; Park, S.; Lee, S.H.; Jang, Y. Macrophage polarization and acceleration of atherosclerotic plaques in a swine model. PLoS ONE 2018, 13, e0193005. [Google Scholar] [CrossRef]
- Pourcet, B.; Staels, B. Alternative macrophages in atherosclerosis: Not always protective! J. Clin. Investig. 2018, 128, 910–912. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Wang, J.; Zhang, C.; Luo, T. Factors associated with dysfunction of autogenous arteriovenous fistula in patients with maintenance hemodialysis: A retrospective study. Ann. Pallliat. Med. 2021, 10, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Boscá, L.; Zandbergen, H.R.; Kovacic, J.C.; Narula, N.; González-Ramos, S.; Fernandez-Velasco, M.; Agrawal, S.; Paz-García, M.; Gupta, S.; et al. Transition of Macrophages to Fibroblast-Like Cells in Healing Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 74, 3124–3135. [Google Scholar] [CrossRef]
- Sata, M.; Saiura, A.; Kunisato, A.; Tojo, A.; Okada, S.; Tokuhisa, T.; Hirai, H.; Makuuchi, M.; Hirata, Y.; Nagai, R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat. Med. 2002, 8, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Castier, Y.; Lehoux, S.; Hu, Y.; Foteinos, G.; Tedgui, A.; Xu, Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int. 2006, 70, 315–320. [Google Scholar] [CrossRef]
- Kokubo, T.; Ishikawa, N.; Uchida, H.; Chasnoff, S.E.; Xie, X.; Mathew, S.; Hruska, K.A.; Choi, E.T. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J. Am. Soc. Nephrol. 2009, 20, 1236–1245. [Google Scholar] [CrossRef]
- Alexander, M.R.; Moehle, C.W.; Johnson, J.L.; Yang, Z.; Lee, J.K.; Jackson, C.L.; Owens, G.K. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Investig. 2012, 122, 70–79. [Google Scholar] [CrossRef]
- Rai, V.; Singh, H.; Agrawal, D.K. Targeting the Crosstalk of Immune Response and Vascular Smooth Muscle Cells Phenotype Switch for Arteriovenous Fistula Maturation. Int. J. Mol. Sci. 2022, 23, 12012. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, X.; Tan, F.; Cao, X.; Guo, S.; Li, X.; Huang, Z.; Diabakte, K.; Wang, L.; Liu, M.; et al. Early modulation of macrophage ROS-PPARγ-NF-κB signalling by sonodynamic therapy attenuates neointimal hyperplasia in rabbits. Sci. Rep. 2020, 10, 11638. [Google Scholar] [CrossRef] [PubMed]
- Stabile, E.; Zhou, Y.F.; Saji, M.; Castagna, M.; Shou, M.; Kinnaird, T.D.; Baffour, R.; Ringel, M.D.; Epstein, S.E.; Fuchs, S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ. Res. 2003, 93, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Jadlowiec, C.C.; Feigel, A.; Yang, C.; Feinstein, A.J.; Kim, S.T.; Collins, M.J.; Kondo, Y.; Muto, A.; Dardik, A. Reduced adult endothelial cell EphB4 function promotes venous remodeling. Am. J. Physiol. Cell Physiol. 2013, 304, C627–C635. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, R.; Ohashi, Y.; Lee, J.S.; Hu, H.; Gonzalez, L.; Zhang, W.; Langford, J.; Matsubara, Y.; Yatsula, B.; Tellides, G.; et al. Endothelial Cell TGF-β (Transforming Growth Factor-Beta) Signaling Regulates Venous Adaptive Remodeling to Improve Arteriovenous Fistula Patency. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 868–883. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Insulin-PI3K signalling: An evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 2020, 16, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Benchoula, K.; Parhar, I.S.; Wong, E.H. The crosstalk of hedgehog, PI3K and Wnt pathways in diabetes. Arch. Biochem. Biophys. 2021, 698, 108743. [Google Scholar] [CrossRef]
- Maffei, A.; Lembo, G.; Carnevale, D. PI3Kinases in Diabetes Mellitus and Its Related Complications. Int. J. Mol. Sci. 2018, 19, 4098. [Google Scholar] [CrossRef] [PubMed]
- Preite, S.; Gomez-Rodriguez, J.; Cannons, J.L.; Schwartzberg, P.L. T and B-cell signaling in activated PI3K delta syndrome: From immunodeficiency to autoimmunity. Immunol. Rev. 2019, 291, 154–173. [Google Scholar] [CrossRef]
- Patel, R.K.; Mohan, C. PI3K/AKT signaling and systemic autoimmunity. Immunol. Rev. 2005, 31, 47–55. [Google Scholar] [CrossRef]
- Chen, Z.; Lee, F.Y.; Bhalla, K.N.; Wu, J. Potent inhibition of platelet-derived growth factor-induced responses in vascular smooth muscle cells by BMS-354825 (dasatinib). Mol. Pharmacol. 2006, 69, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Calvieri, C.; Ferro, D.; Pignatelli, P. Statins as antithrombotic drugs. Circulation 2013, 127, 251–257. [Google Scholar] [CrossRef]
- Bourcier, T.; Libby, P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 556–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, J.; Kessinger, C.W.; Jhajj, H.S.; Grau, M.S.; Misra, S.; Libby, P.; McCarthy, J.R.; Jaffer, F.A. Atorvastatin Reduces In Vivo Fibrin Deposition and Macrophage Accumulation, and Improves Primary Patency Duration and Maturation of Murine Arteriovenous Fistula. J. Am. Soc. Nephrol. 2020, 31, 931–945. [Google Scholar] [CrossRef]
- Janardhanan, R.; Yang, B.; Vohra, P.; Roy, B.; Withers, S.; Bhattacharya, S.; Mandrekar, J.; Kong, H.; Leof, E.B.; Mukhopadhyay, D.; et al. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int. 2013, 84, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Elder, S.J.; Bragg-Gresham, J.L.; Pisoni, R.L.; Yamazaki, S.; Akizawa, T.; Jadoul, M.; Hugh, R.C.; Port, F.K.; Fukuhara, S. Consistent aspirin use associated with improved arteriovenous fistula survival among incident hemodialysis patients in the dialysis outcomes and practice patterns study. Clin. J. Am. Soc. Nephrol. 2008, 3, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.; Kamarizan, M.F.A.; Silva, A.D. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst. Rev. 2021, 23. [Google Scholar]
- Zhang, C.; Baker, D.L.; Yasuda, S.; Makarova, N.; Balazs, L.; Johnson, L.R.; Marathe, G.K.; McIntyre, T.M.; Xu, Y.; Prestwich, G.D.; et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 2004, 199, 763–774. [Google Scholar] [CrossRef]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. Lysophospholipid-Related Diseases and PPARγ Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 2730. [Google Scholar] [CrossRef]
- Tanaka, H.; Zaima, N.; Yamamoto, N.; Suzuki, M.; Mano, Y.; Konno, H.; Unno, N.; Setou, M. Distribution of phospholipid molecular species in autogenous access grafts for hemodialysis analyzed using imaging mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 1873–1880. [Google Scholar] [CrossRef]
- Schneider, G.; Sellers, Z.P.; Abdel-Latif, A.; Morris, A.J.; Ratajczak, M.Z. Bioactive lipids, LPC and LPA, are novel prometastatic factors and their tissue levels increase in response to radio/chemotherapy. Mol. Cancer Res. 2014, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; St Hilaire, A.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, C.; Motta, I.; Vasuri, F.; Palumbo, T.; Lisi, A.P.; Costa, A.; Astolfi, A.; Valente, S.; Versura, P.; Fornasiero, E.F.; et al. The PPAR-γ Agonist Pioglitazone Modulates Proliferation and Migration in HUVEC, HAOSMC and Human Arteriovenous Fistula-Derived Cells. Int. J. Mol. Sci. 2023, 24, 4424. [Google Scholar] [CrossRef]
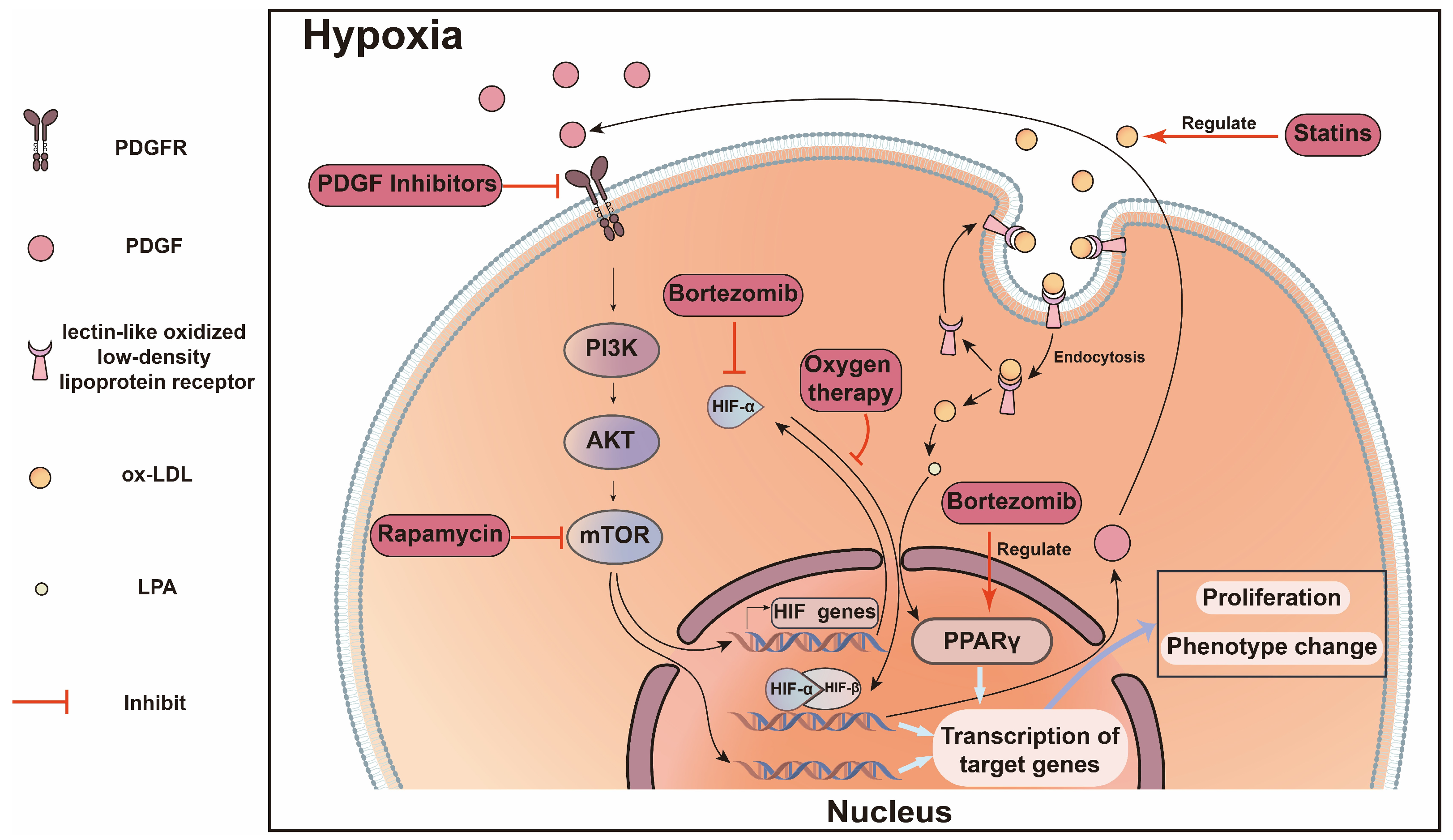
- Sadaghianloo, N.; Contenti, J.; Dardik, A.; Mazure, N.M. Role of Hypoxia and Metabolism in the Development of Neointimal Hyperplasia in Arteriovenous Fistulas. Int. J. Mol. Sci. 2019, 20, 5387. [Google Scholar] [CrossRef] [PubMed]
- Sadaghianloo, N.; Contenti, J.; Declemy, S.; Ambrosetti, D.; Zdralevic, M.; Tannour-Louet, M.; Fabbri, L.; Pagès, G.; Bost, F.; Hassen-Khodja, R.; et al. Hypoxia and hypoxia-inducible factors promote the development of neointimal hyperplasia in arteriovenous fistula. J. Physiol. 2021, 599, 2299–2321. [Google Scholar] [CrossRef]
- Wan, J.; Lata, C.; Santilli, A.; Green, D.; Roy, S.; Santilli, S. Supplemental oxygen reverses hypoxia-induced smooth muscle cell proliferation by modulating HIF-alpha and VEGF levels in a rabbit arteriovenous fistula model. Ann. Vasc. Surg. 2014, 28, 725–736. [Google Scholar] [CrossRef]
- Vachharajani, T.J.; Taliercio, J.J.; Anvari, E. New Devices and Technologies for Hemodialysis Vascular Access A Review. Am. J. Kidney Dis. 2021, 78, 116–124. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Wang, P.; Wang, Y.Z.; Zhang, L.H.; Zhao, Y.; Li, H.; He, Q.; Liu, H.; Luo, J.F.; Jia, X.; et al. Drug-Coated Balloon Angioplasty for Dysfunctional Arteriovenous Hemodialysis Fistulae: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 336–344. [Google Scholar] [CrossRef]
- Tang, T.Y.; Soon, S.X.Y.; Yap, C.J.Q.; Chan, S.L.; Tan, R.Y.; Pang, S.C.; Lee, S.Q.W.; Yap, H.Y.; Choke, E.T.C.; Tan, C.S.; et al. Early (6 months) results of a pilot prospective study to investigate the efficacy and safety of sirolimus coated balloon angioplasty for dysfunctional arterio-venous fistulas: MAgicTouch Intervention Leap for Dialysis Access (MATILDA) Trial. PLoS ONE 2020, 15, e0241321. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, R.; Song, A.; Zhang, C. The Pathological Mechanisms and Therapeutic Molecular Targets in Arteriovenous Fistula Dysfunction. Int. J. Mol. Sci. 2024, 25, 9519. https://doi.org/10.3390/ijms25179519
Yan R, Song A, Zhang C. The Pathological Mechanisms and Therapeutic Molecular Targets in Arteriovenous Fistula Dysfunction. International Journal of Molecular Sciences. 2024; 25(17):9519. https://doi.org/10.3390/ijms25179519
Chicago/Turabian StyleYan, Ruiwei, Anni Song, and Chun Zhang. 2024. "The Pathological Mechanisms and Therapeutic Molecular Targets in Arteriovenous Fistula Dysfunction" International Journal of Molecular Sciences 25, no. 17: 9519. https://doi.org/10.3390/ijms25179519
APA StyleYan, R., Song, A., & Zhang, C. (2024). The Pathological Mechanisms and Therapeutic Molecular Targets in Arteriovenous Fistula Dysfunction. International Journal of Molecular Sciences, 25(17), 9519. https://doi.org/10.3390/ijms25179519





