Systematic Review and Meta-Analysis of Clinical Efficacy and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Search
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
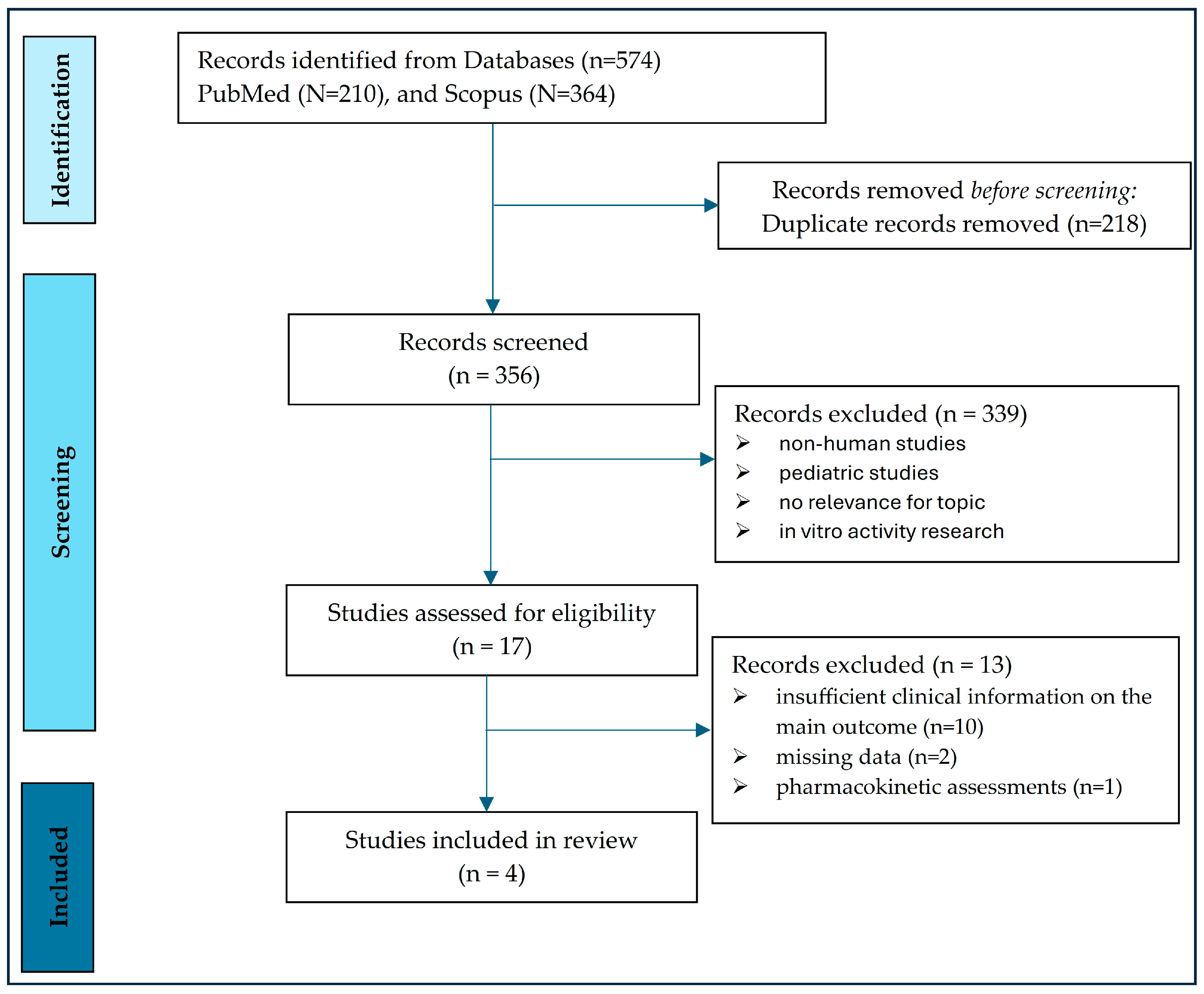
2.4. The Articles Selection Scheme
2.5. Statistical Analysis
3. Results
3.1. Microbiological Characteristics of the Studies
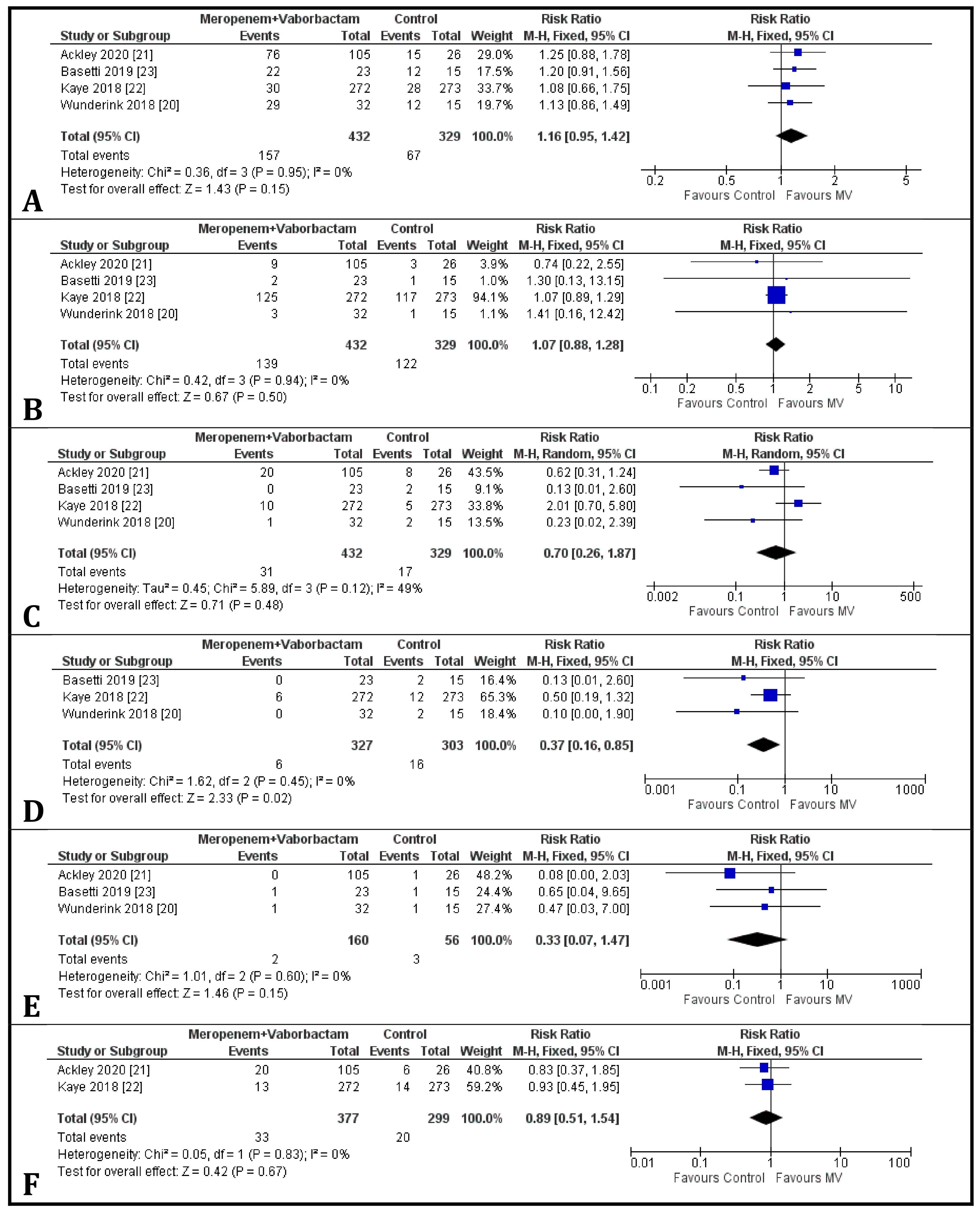
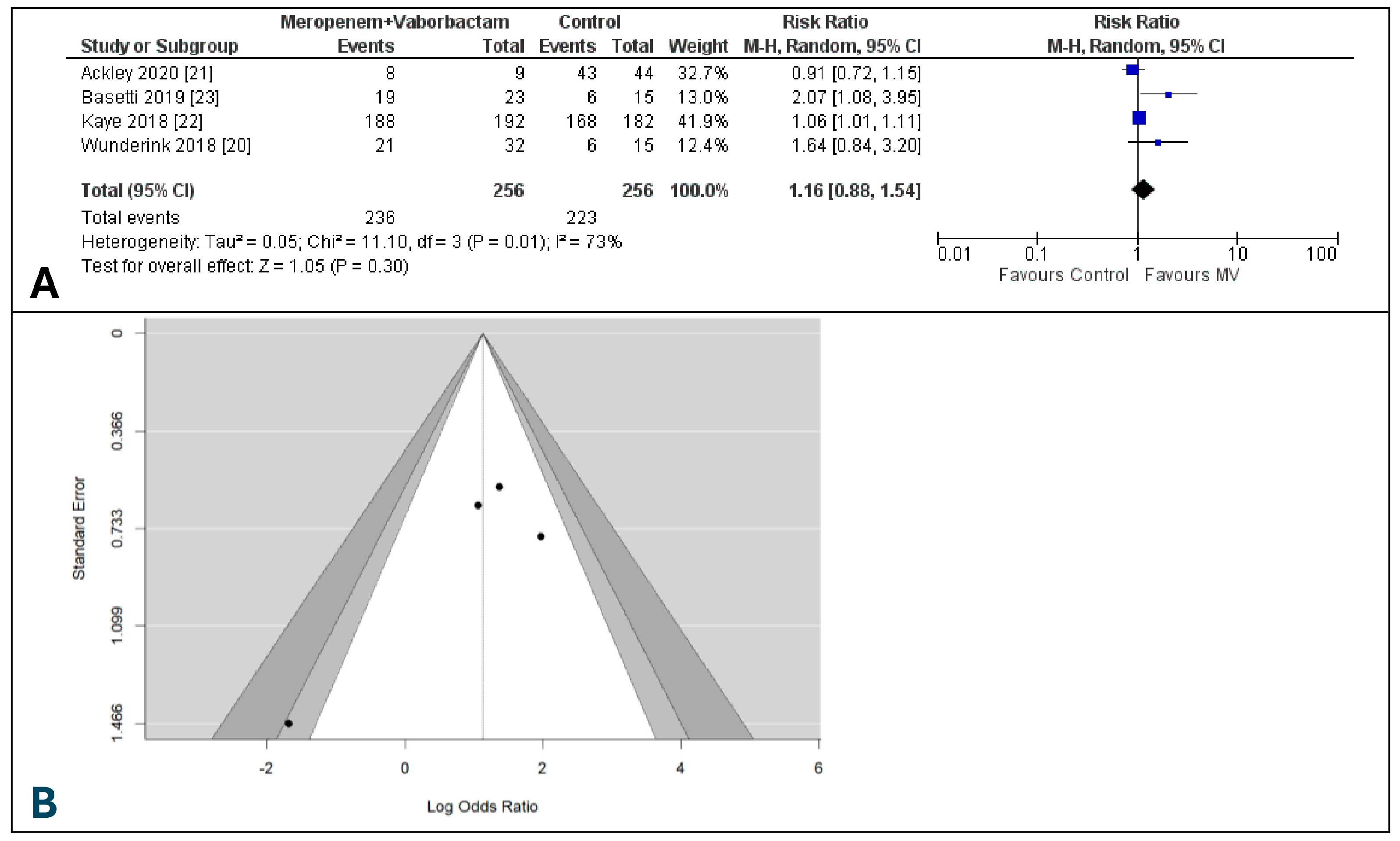
3.2. Clinical Cure at the End of Treatment
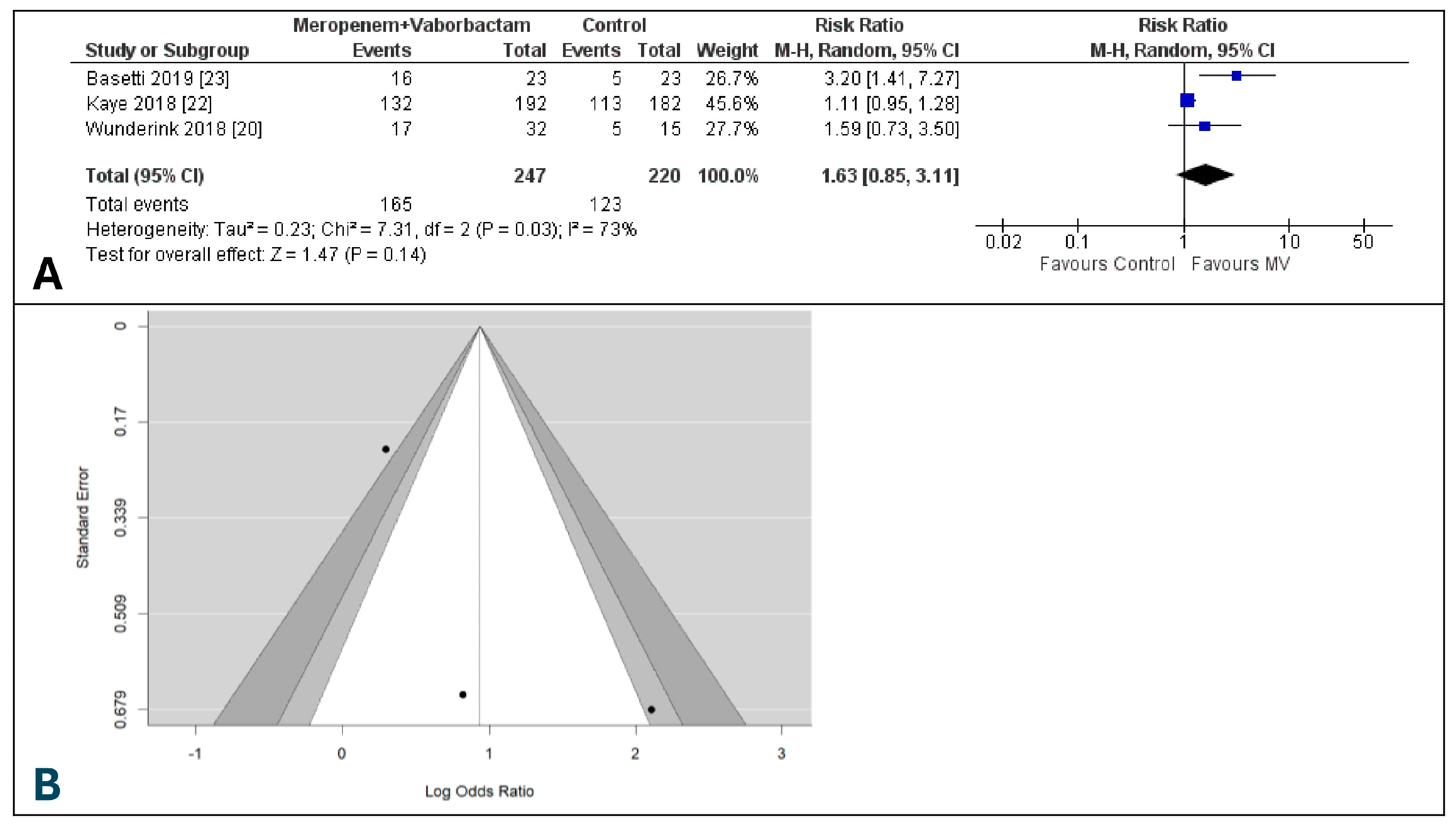
3.3. Clinical Cure at the Test of Cure
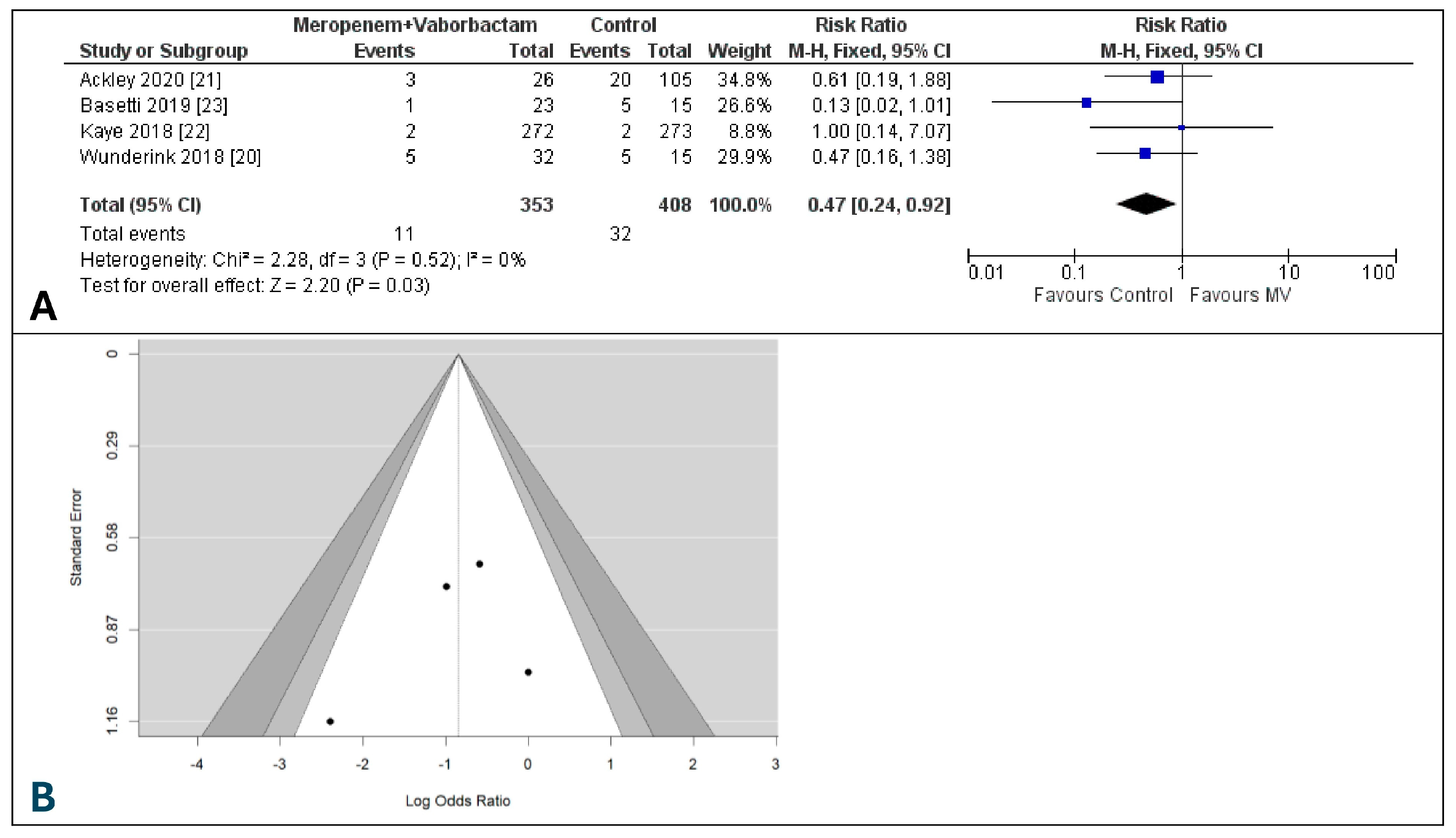
3.4. Microbiological Eradication Rate at the End of Treatment
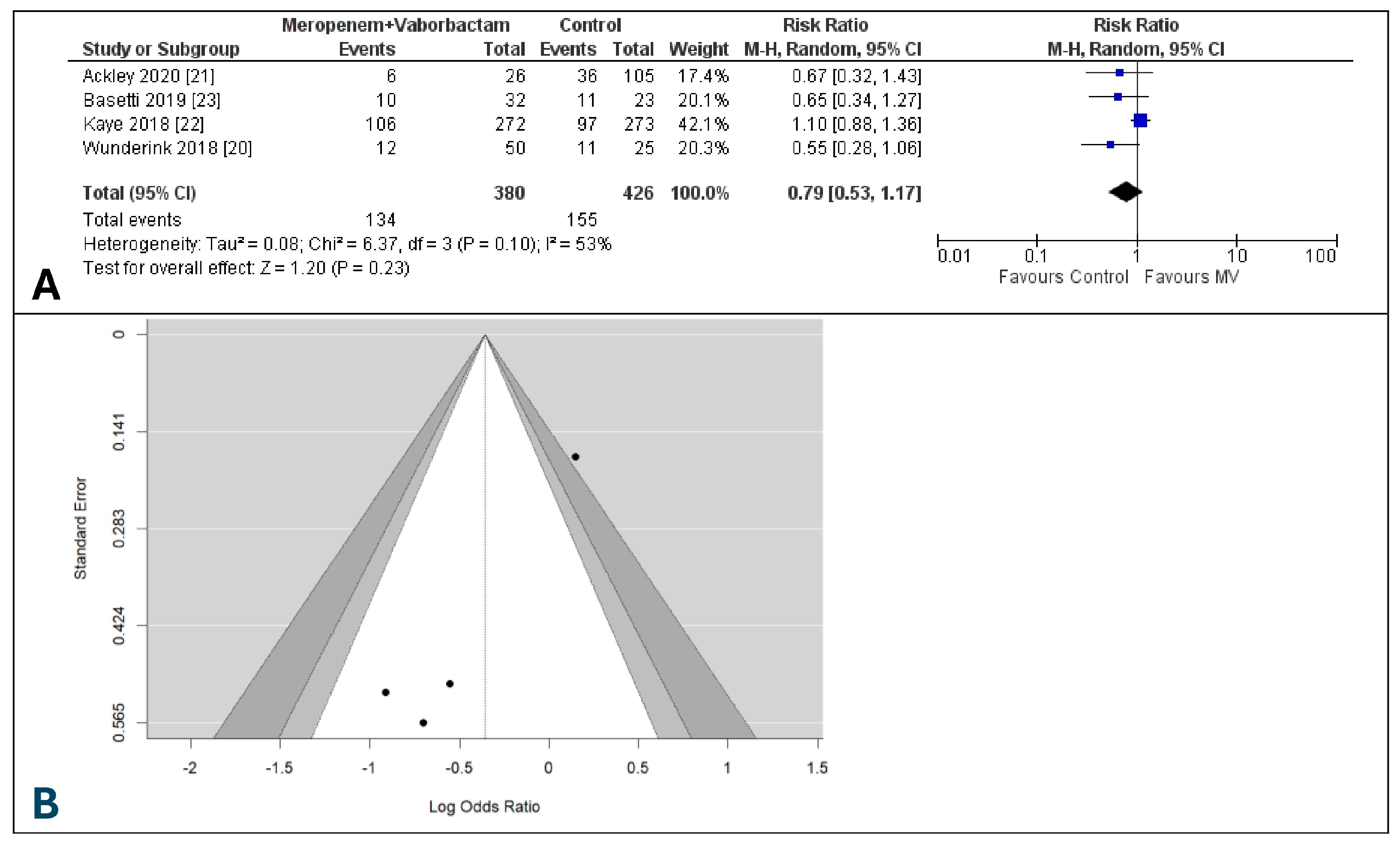
3.5. Microbiological Eradication Rate at Test of Cure
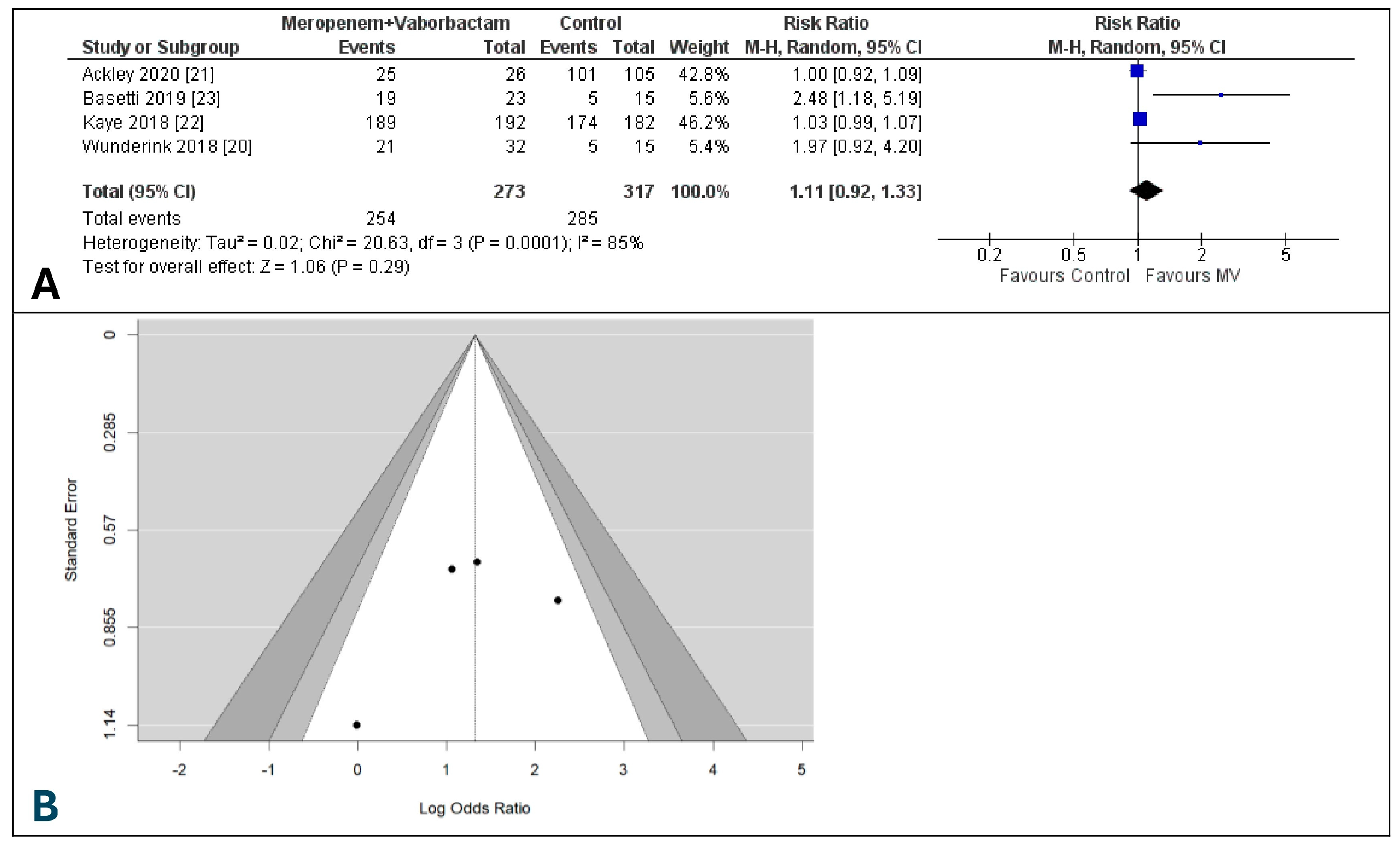
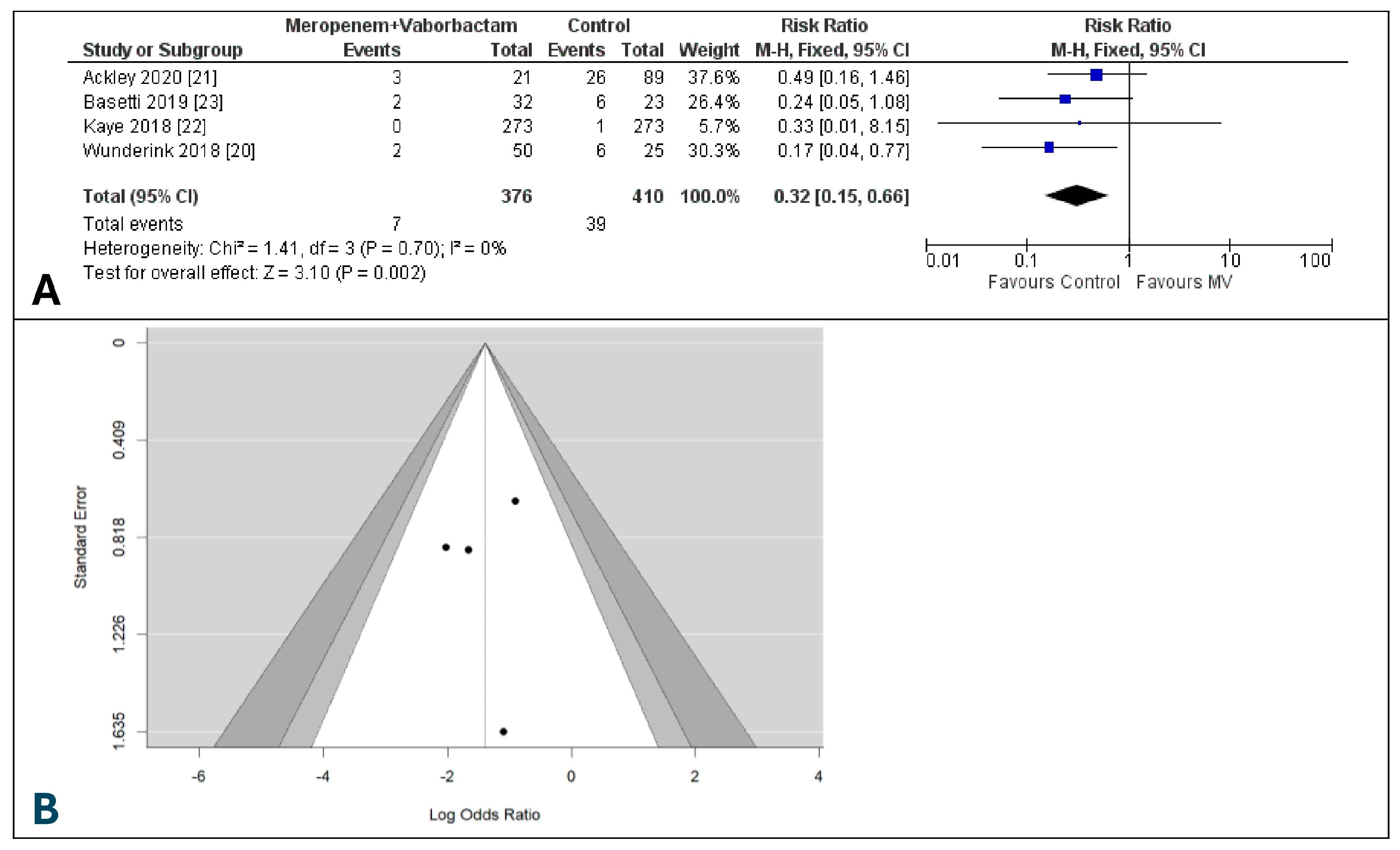
3.6. Day-28 All-Cause Mortality
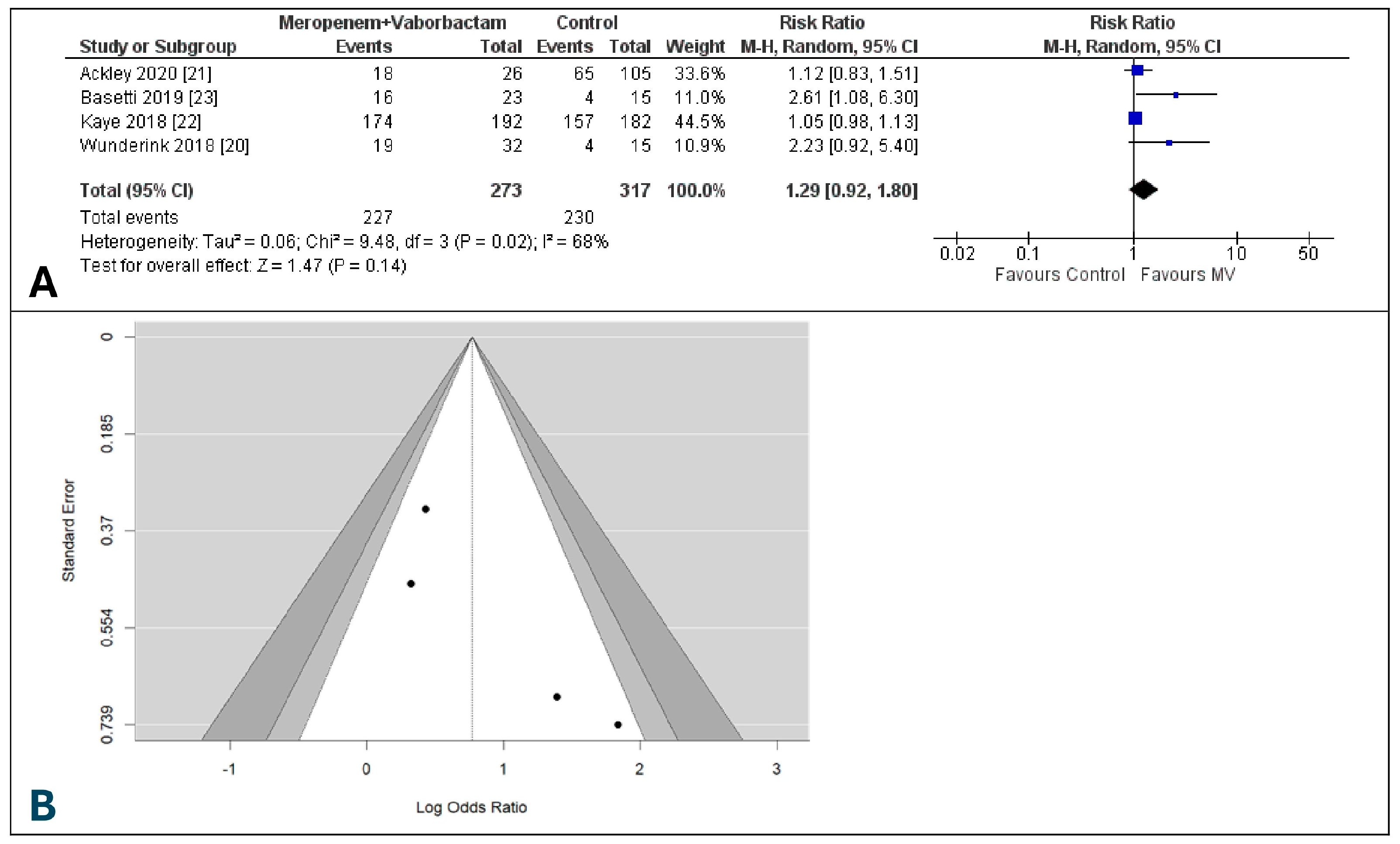
3.7. Risk of Adverse Events of Meropenem-Vaborbactam and Comparators
3.8. Risk of Renal-Related Adverse Events between Meropenem-Vaborbactam vs. Comparators
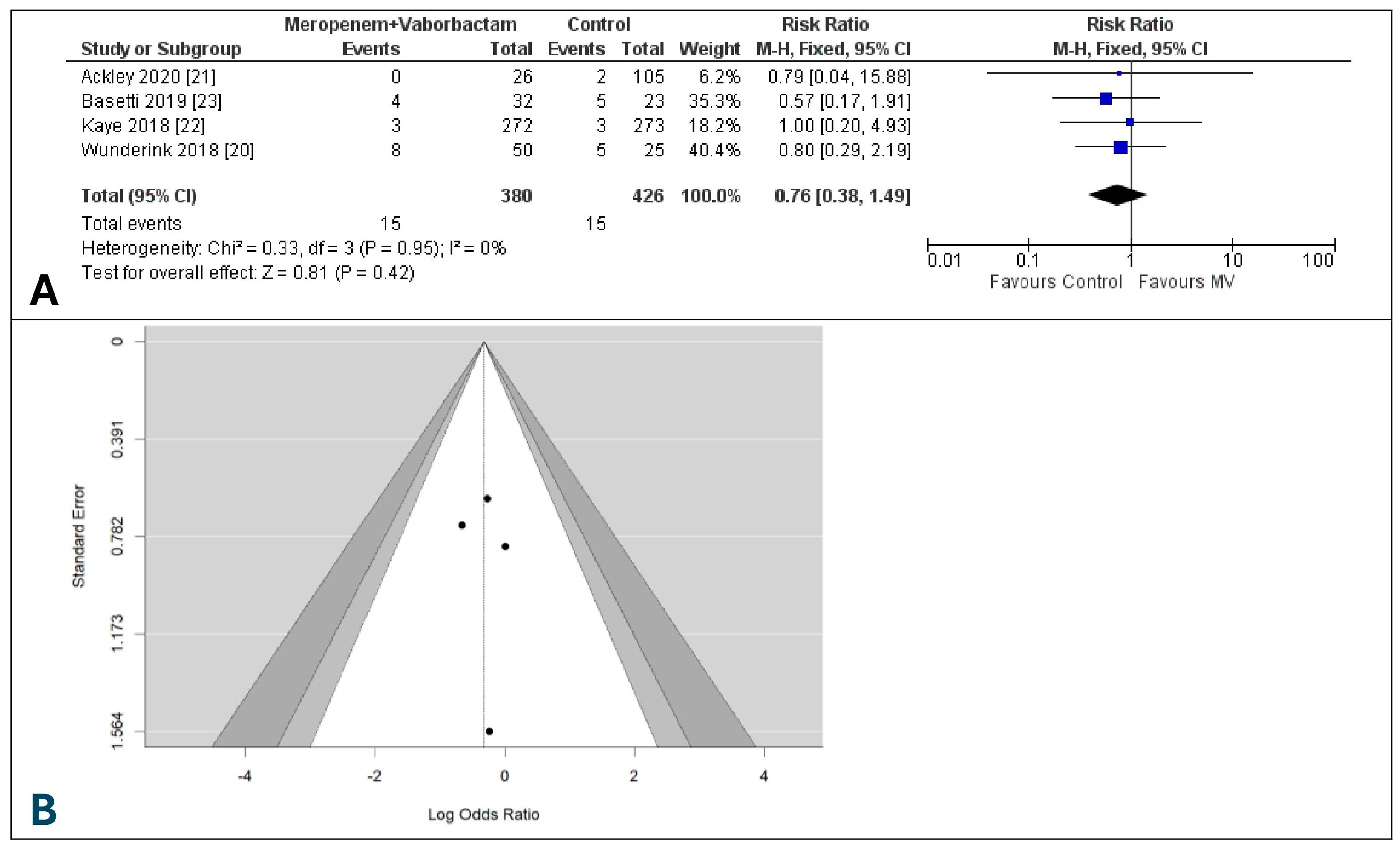
3.9. Discontinuation of Study Due to Drug-Related Adverse Events
3.10. Discontinuation of Study Drug Due to Drug-Related AEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 27 July 2024).
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats Report. Antimicrobial Resistance. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 27 July 2024).
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- De Angelis, G.; Del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular Mechanisms, Epidemiology, and Clinical Importance of β-Lactam Resistance in Enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef]
- Potter, R.F.; D’Souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2016, 29, 30–46. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215 (Suppl. S1), S28–S36. [Google Scholar] [CrossRef]
- Smith, H.Z.; Hollingshead, C.M.; Kendall, B. Carbapenem-Resistant Enterobacterales. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bush, K. Beta-Lactamase Inhibitors from Laboratory to Clinic. Clin. Microbiol. Rev. 1988, 1, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Hraiech, S.; Kernéis, S.; Veluppillai, N.; Pajot, O.; Poissy, J.; Roux, D.; Zahar, J.-R.; French Intensive Care Society. Rationale and Evidence for the Use of New Beta-Lactam/Beta-Lactamase Inhibitor Combinations and Cefiderocol in Critically Ill Patients. Ann. Intensive Care 2023, 13, 65. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America Antimicrobial-Resistant Treatment Guidance: Gram-Negative Bacterial Infections. Infect. Dis. Soc. Am. 2024, 6. Available online: https://www.idsociety.org/practice-guideline/amr-guidance/ (accessed on 30 July 2024).
- Durante-Mangoni, E.; Andini, R.; Zampino, R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin. Microbiol. Infect. 2019, 25, 943–950. [Google Scholar] [CrossRef]
- Dhillon, S. Meropenem/Vaborbactam: A Review in Complicated Urinary Tract Infections. Drugs 2018, 78, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of Beta-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e01443-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liao, X.; Ding, T.; Ahn, J. Role of β-Lactamase Inhibitors as Potentiators in Antimicrobial Chemotherapy Targeting Gram-Negative Bacteria. Antibiotics 2024, 13, 260. [Google Scholar] [CrossRef]
- Wilson, W.R.; Kline, E.G.; Jones, C.E.; Morder, K.T.; Mettus, R.T.; Doi, Y.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K. Effects of KPC Variant and Porin Genotype on the In Vitro Activity of Meropenem-Vaborbactam against Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e02048-18. [Google Scholar] [CrossRef] [PubMed]
- Hackel, M.A.; Lomovskaya, O.; Dudley, M.N.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of Meropenem-Vaborbactam against Clinical Isolates of KPC-Positive Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e01904-17. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Huband, M.D.; Mendes, R.E.; Flamm, R.K.; Castanheira, M. In Vitro Activity of Meropenem/Vaborbactam and Characterisation of Carbapenem Resistance Mechanisms among Carbapenem-Resistant Enterobacteriaceae from the 2015 Meropenem/Vaborbactam Surveillance Programme. Int. J. Antimicrob. Agents 2018, 52, 144–150. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef]
- Ackley, R.; Roshdy, D.; Meredith, J.; Minor, S.; Anderson, W.E.; Capraro, G.A.; Polk, C. Meropenem-Vaborbactam versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2020, 64, e02313-19. [Google Scholar] [CrossRef]
- Kaye, K.S.; Bhowmick, T.; Metallidis, S.; Bleasdale, S.C.; Sagan, O.S.; Stus, V.; Vazquez, J.; Zaitsev, V.; Bidair, M.; Chorvat, E.; et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. JAMA 2018, 319, 788. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Patel, N.; Tillotson, G.; Massey, J. Efficacy and Safety of Meropenem-Vaborbactam Versus Best Available Therapy for the Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in Patients Without Prior Antimicrobial Failure: A Post Hoc Analysis. Adv. Ther. 2019, 36, 1771–1777. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cressman, L.; Lautenbach, E. Real-World Clinical Outcomes of Meropenem/Vaborbactam for Treatment of Carbapenem-Resistant Enterobacterales Infections. J. Glob. Antimicrob. Resist. 2021, 27, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; McCreary, E.K.; Marini, R.V.; Kline, E.G.; Jones, C.E.; Hao, B.; Chen, L.; Kreiswirth, B.N.; Doi, Y.; Clancy, C.J.; et al. Early Experience with Meropenem-Vaborbactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect Dis. 2020, 71, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, C.G.; Shortridge, D.; Sader, H.S.; Castanheira, M. Activity of Meropenem-Vaborbactam against Bacterial Isolates Causing Pneumonia in Patients in U.S. Hospitals during 2014 to 2018. Antimicrob. Agents Chemother. 2020, 64, e02177-19. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Carvalhaes, C.; Deshpande, L.; Castanheira, M. Activity of Meropenem/Vaborbactam and Comparators against Gram-Negative Isolates from Eastern and Western European Patients Hospitalized with Pneumonia Including Ventilator-Associated Pneumonia (2014-19). J. Antimicrob. Chemother. 2021, 76, 2600–2605. [Google Scholar] [CrossRef]
- Hsueh, S.C.; Chao, C.M.; Wang, C.Y.; Lai, C.C.; Chen, C.H. Clinical efficacy and safety of cefiderocol in the treatment of acute bacterial infections: A systematic review and meta-analysis of randomised controlled trials. J. Glob. Antimicrob. Resist. 2021, 24, 376–382. [Google Scholar] [CrossRef]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; De Pascale, G.; Cascio, A.; De Rosa, F.G.; Cattelan, A.M.; Oliva, A.; Saracino, A.; Bassetti, M.; et al. Outcomes and Predictors of Mortality in Patients With KPC-Kp Infections Treated with Meropenem Vaborbactam: An Observational Multicenter Study. Open Forum Infect. Dis. 2024, 11, ofae273. [Google Scholar] [CrossRef]
- Alosaimy, S.; Jorgensen, S.C.J.; Lagnf, A.M.; Melvin, S.; Mynatt, R.P.; Carlson, T.J.; Garey, K.W.; Allen, D.; Venugopalan, V.; Veve, M.; et al. Real-World Multicenter Analysis of Clinical Outcomes and Safety of Meropenem-Vaborbactam in Patients Treated for Serious Gram-Negative Bacterial Infections. Open Forum Infect. Dis. 2020, 7, ofaa051. [Google Scholar] [CrossRef]
- Rubino, C.M.; Bhavnani, S.M.; Loutit, J.S.; Morgan, E.E.; White, D.; Dudley, M.N.; Griffith, D.C. Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of Vaborbactam and Meropenem Alone and in Combination Following Single and Multiple Doses in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2018, 62, e02228-17. [Google Scholar] [CrossRef]
- Tiseo, G.; Galfo, V.; Riccardi, N.; Suardi, L.R.; Pogliaghi, M.; Giordano, C.; Leonildi, A.; Barnini, S.; Falcone, M. Real-world experience with meropenem/vaborbactam for the treatment of infections caused by ESBL-producing Enterobacterales and carbapenem-resistant Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucataru, A.; Turcu-Stiolica, A.; Calina, D.; Balasoiu, A.T.; Zlatian, O.M.; Osman, A.; Balasoiu, M.; Ghenea, A.E. Systematic Review and Meta-Analysis of Clinical Efficacy and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Int. J. Mol. Sci. 2024, 25, 9574. https://doi.org/10.3390/ijms25179574
Bucataru A, Turcu-Stiolica A, Calina D, Balasoiu AT, Zlatian OM, Osman A, Balasoiu M, Ghenea AE. Systematic Review and Meta-Analysis of Clinical Efficacy and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. International Journal of Molecular Sciences. 2024; 25(17):9574. https://doi.org/10.3390/ijms25179574
Chicago/Turabian StyleBucataru, Alexandra, Adina Turcu-Stiolica, Daniela Calina, Andrei Theodor Balasoiu, Ovidiu Mircea Zlatian, Andrei Osman, Maria Balasoiu, and Alice Elena Ghenea. 2024. "Systematic Review and Meta-Analysis of Clinical Efficacy and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections" International Journal of Molecular Sciences 25, no. 17: 9574. https://doi.org/10.3390/ijms25179574
APA StyleBucataru, A., Turcu-Stiolica, A., Calina, D., Balasoiu, A. T., Zlatian, O. M., Osman, A., Balasoiu, M., & Ghenea, A. E. (2024). Systematic Review and Meta-Analysis of Clinical Efficacy and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. International Journal of Molecular Sciences, 25(17), 9574. https://doi.org/10.3390/ijms25179574










