Mesenchymal Stem/Stromal Cells Derived from Dental Tissues Mediate the Immunoregulation of T Cells through the Purinergic Pathway
Abstract
:1. Introduction
2. Results
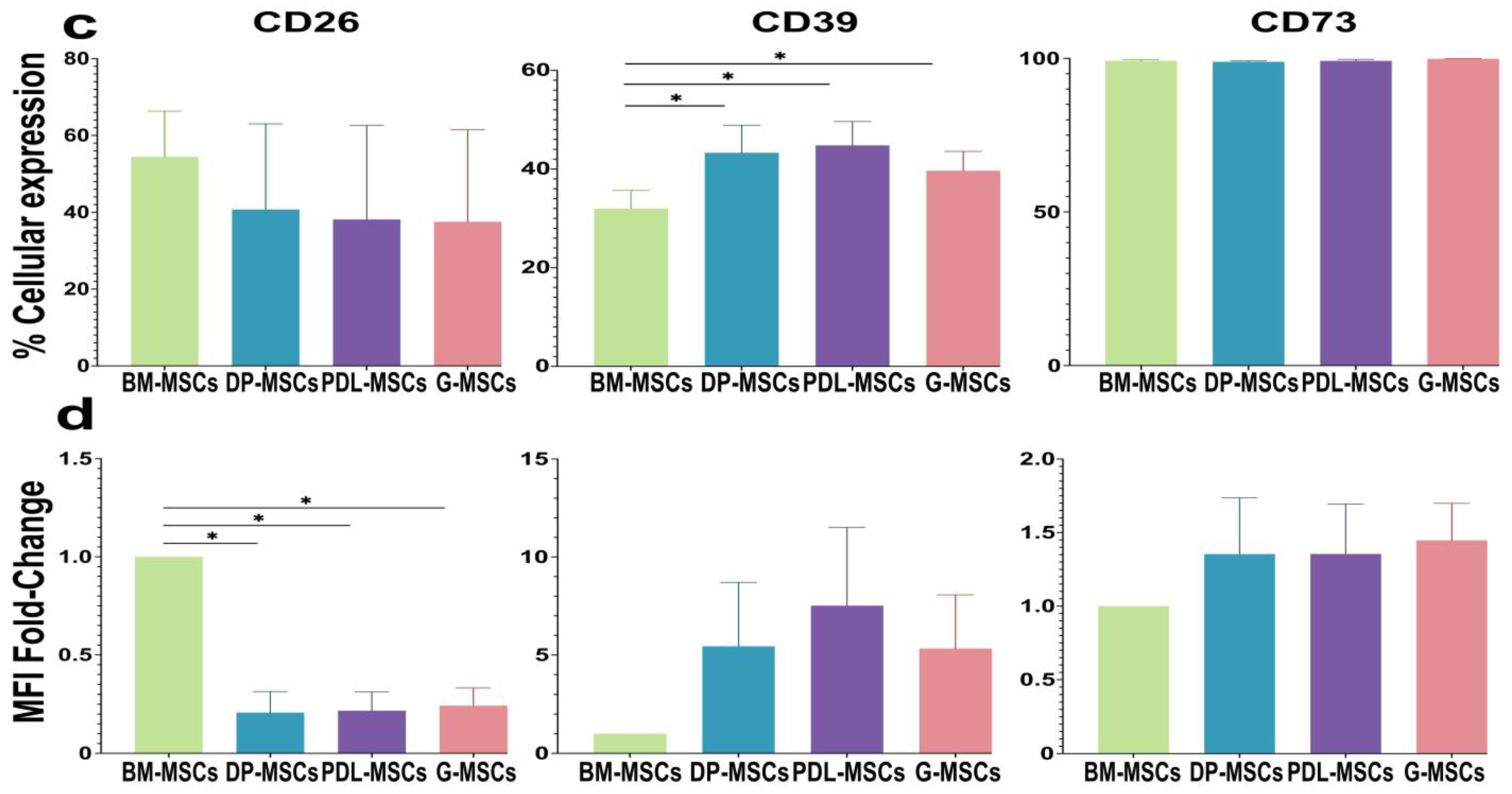
2.1. DP-MSCs, PDL-MSCs, and G-MSCs Express CD26, CD39, and CD73
2.2. DT-MSCs Produce ADO from ATP or AMP via CD39 and CD73
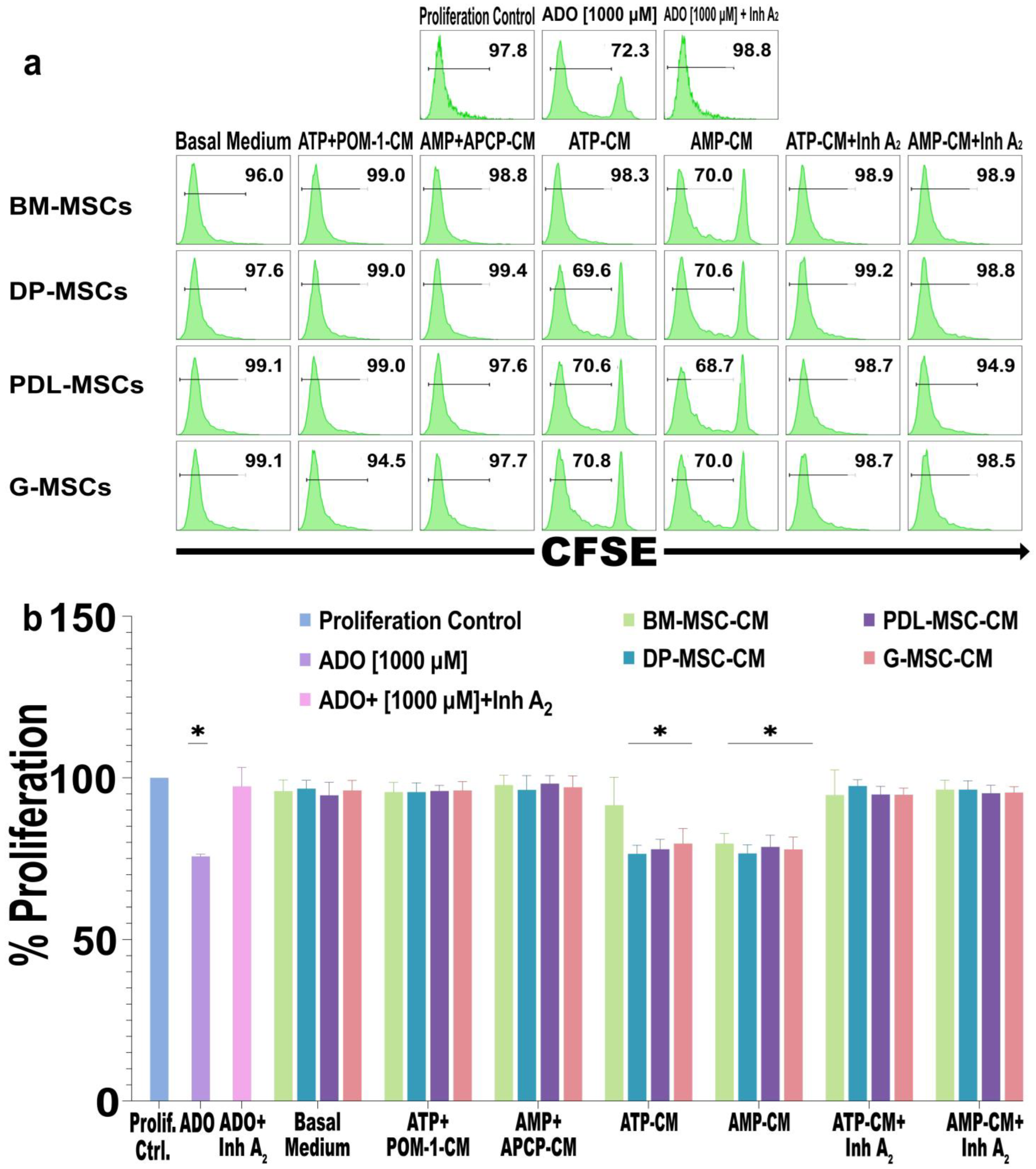
2.3. The Concentration of ADO Generated by DT-MSCs Decreases the Proliferation of CD3+ Lymphocytes
2.4. ADO Produced by DT-MSCs Induces the Generation of CD4+CD25+FoxP3+ Tregs and Induces the Coexpression of CD39+CD73+
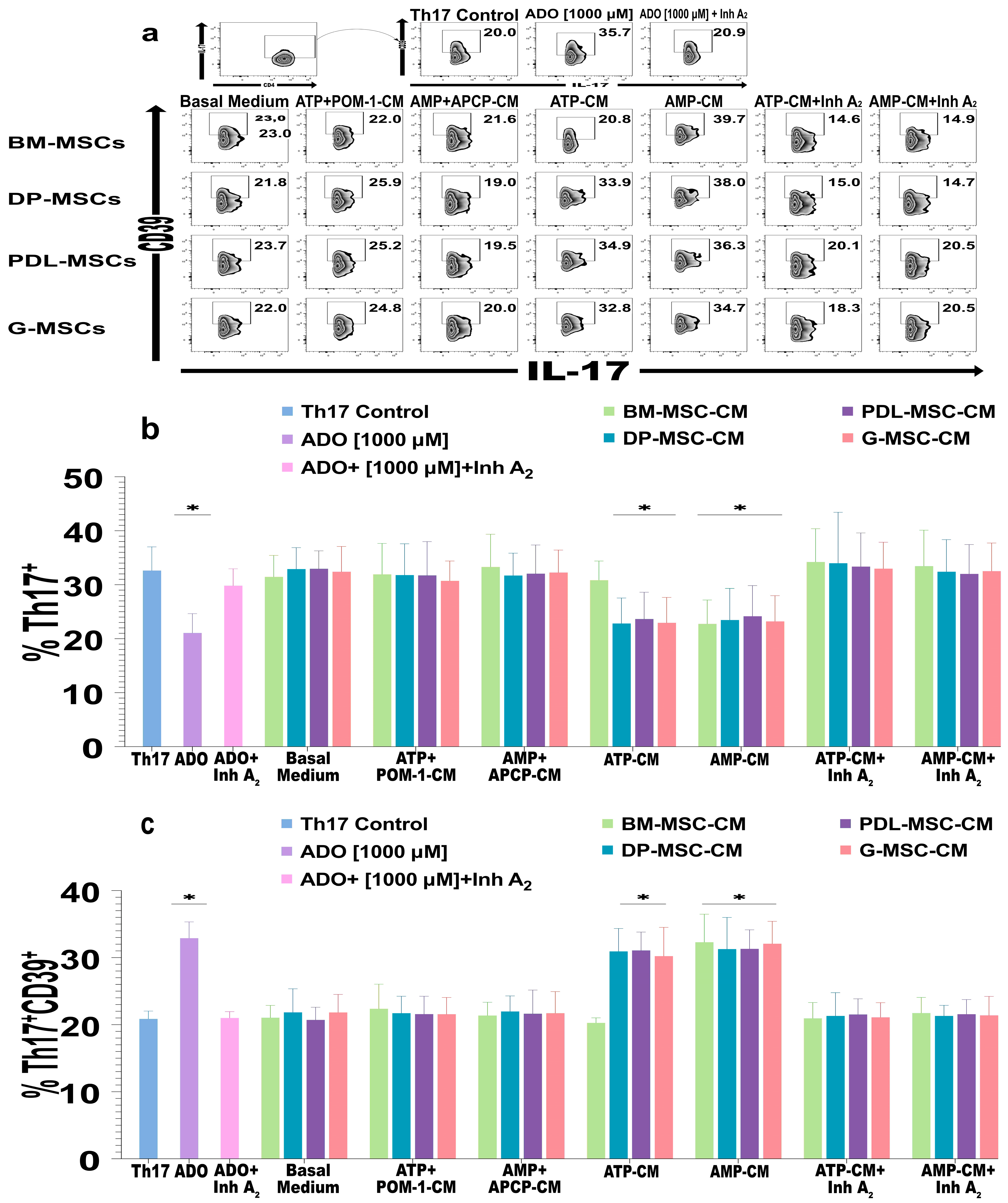
2.5. The ADO Produced by DT-MSCs Induces the Expression of CD39 in Th17 Lymphocytes
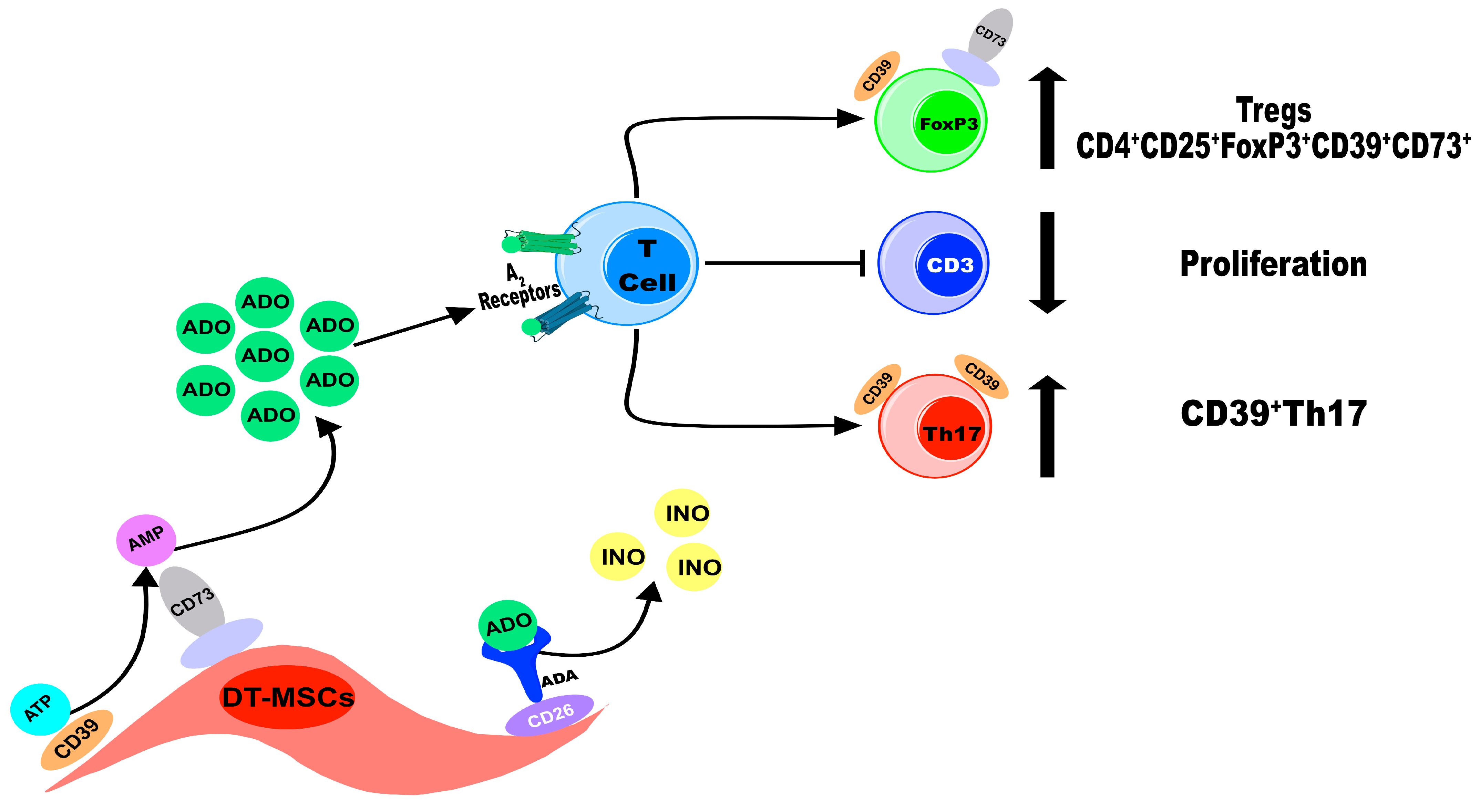
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Mesenchymal Stromal Cells (MSCs)
4.1.1. Isolation of BM-MSCs
4.1.2. Isolation of DT-MSCs
4.2. Cytometry and Immunophenotyping of DT-MSCs and BM-MSCs
4.3. Morphology and Induction of Differentiation of DT-MSCs and BM-MSCs
4.4. Expression of CD26, CD39, and CD73 in DT-MSCs and BM-MSCs
4.5. Phosphohydrolytic Activity of CD39/CD73 by Silica Gel Thin Layer Chromatography (TLC)
4.6. Ultraperformance Liquid Chromatography (UPLC)
4.7. Obtaining Mononuclear Cells from Peripheral Blood and Enrichment of CD3+ and CD4+ T Lymphocytes
4.8. Effect of ADO Produced by DT-MSCs and BM-MSCs on T Lymphocytes
4.8.1. Effect of DT-MSC-Induced ADO on T Lymphocyte Proliferation
4.8.2. Generation of FoxP3+CD39+CD73+ Treg Lymphocytes with ADO Produced by DT-MSCs and BM-MSCs
4.8.3. Effect of ADO Produced by DT-MSCs and BM-MSCs on Th17 Lymphocytes
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyndall, A. Successes and Failures of Stem Cell Transplantation in Autoimmune Diseases. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 280–284. [Google Scholar] [CrossRef]
- Gao, G.; Fan, C.; Li, W.; Liang, R.; Wei, C.; Chen, X.; Yang, Y.; Zhong, Y.; Shao, Y.; Kong, Y.; et al. Mesenchymal Stem Cells: Ideal Seeds for Treating Diseases. Hum. Cell 2021, 34, 1585–1600. [Google Scholar] [CrossRef]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of Mesenchymal Stem Cell In Vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef]
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-Cell Immunosuppression by Mesenchymal Stromal Cells: What Do We Know So Far? Biomed. Res. Int. 2014, 2014, 216806. [Google Scholar] [CrossRef]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging In Vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45(low) CD271(+) Phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.R.; Fernandez-Gutierrez, B.; Mucientes, A.; Marco, F.; Lopiz, Y.; Jover, J.A.; Abasolo, L.; Rodriguez-Rodriguez, L. RNA Sequencing of Mesenchymal Stem Cells Reveals a Blocking of Differentiation and Immunomodulatory Activities under Inflammatory Conditions in Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2019, 21, 112. [Google Scholar] [CrossRef]
- Sibov, T.T.; Severino, P.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; F Gamarra, L.; et al. Mesenchymal Stem Cells from Umbilical Cord Blood: Parameters for Isolation, Characterization and Adipogenic Differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.B.; Furlan, J.M.; Salton, G.D.; Schmalfuss, T.; Röhsig, L.M.; Silla, L.M.R.; Passos, E.P.; Paz, A.H. Isolation of Human Mesenchymal Stem Cells from Amnion, Chorion, Placental Decidua and Umbilical Cord: Comparison of Four Enzymatic Protocols. Biotechnol. Lett. 2018, 40, 989–998. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Bonifaz, L.; Castro-Escamilla, O.; Monroy-Garcia, A.; Cortes-Morales, A.; Hernandez-Estevez, E.; Hernandez-Cristino, J.; Mayani, H.; Montesinos, J.J. Mesenchymal Stromal Cells from the Epidermis and Dermis of Psoriasis Patients: Morphology, Immunophenotype, Differentiation Patterns, and Regulation of T Cell Proliferation. Stem Cells Int. 2019, 2019, 4541797. [Google Scholar] [CrossRef]
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef]
- Poblano-Pérez, L.I.; Castro-Manrreza, M.E.; González-Alva, P.; Fajardo-Orduña, G.R.; Montesinos, J.J. Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential. Int. J. Mol. Sci. 2024, 25, 1986. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory Properties of Dental Tissue-Derived Mesenchymal Stem Cells: Implication in Disease and Tissue Regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Trubiani, O.; Diomede, F.; Pisciotta, A.; Paganelli, R. Immunomodulating Profile of Dental Mesenchymal Stromal Cells: A Comprehensive Overview. Front. Oral Health 2021, 2, 635055. [Google Scholar] [CrossRef]
- Saldanha-Araujo, F.; Ferreira, F.I.; Palma, P.V.; Araujo, A.G.; Queiroz, R.H.; Covas, D.T.; Zago, M.A.; Panepucci, R.A. Mesenchymal Stromal Cells Up-Regulate CD39 and Increase Adenosine Production to Suppress Activated T-Lymphocytes. Stem Cell Res. 2011, 7, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jeong, H.J.; Kim, M.K.; Wee, W.R.; Lee, W.W.; Kim, S.U.; Sung, C.; Yang, Y.H. CD39-Mediated Effect of Human Bone Marrow-Derived Mesenchymal Stem Cells on the Human Th17 Cell Function. Purinergic Signal. 2014, 10, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Laitinen, A.; Rabina, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T Cells. Stem Cells 2016, 34, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; Sáez, T.; de Vos, P. Extracellular ATP and Adenosine: The Yin and Yang in Immune Responses? Mol. Asp. Med. 2017, 55, 9–19. [Google Scholar] [CrossRef]
- Feng, L.L.; Cai, Y.Q.; Zhu, M.C.; Xing, L.J.; Wang, X. The Yin and Yang Functions of Extracellular ATP and Adenosine in Tumor Immunity. Cancer Cell Int. 2020, 20, 110. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Haskó, G. Adenosine Regulation of the Immune System. In The Adenosine Receptors; Borea, P.A., Varani, K., Gessi, S., Merighi, S., Vincenzi, F., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 499–514. [Google Scholar]
- Csóka, B.; Selmeczy, Z.; Koscsó, B.; Németh, Z.H.; Pacher, P.; Murray, P.J.; Kepka-Lenhart, D.; Morris, S.M., Jr.; Gause, W.C.; Leibovich, S.J.; et al. Adenosine Promotes Alternative Macrophage Activation via A2A and A2B Receptors. FASEB J. 2012, 26, 376–386. [Google Scholar] [CrossRef]
- Koscso, B.; Csoka, B.; Kokai, E.; Nemeth, Z.H.; Pacher, P.; Virag, L.; Leibovich, S.J.; Hasko, G. Adenosine Augments IL-10-Induced STAT3 Signaling in M2c Macrophages. J. Leukoc. Biol. 2013, 94, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Mou, K.J.; Shen, K.F.; Li, Y.L.; Wu, Z.F.; Duan, W. Adenosine A(2A) Receptor in Bone Marrow-Derived Cells Mediated Macrophages M2 Polarization via PPARgamma-P65 Pathway in Chronic Hypoperfusion Situation. Front. Aging Neurosci. 2021, 13, 792733. [Google Scholar] [CrossRef]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The Development and Immunosuppressive Functions of CD4(+) CD25(+) Foxp3(+) Regulatory T Cells are under Influence of the Adenosine-A2A Adenosine Receptor Pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, H.; Tsukimoto, M.; Harada, H.; Kojima, S. Adenosine A2B Receptor Antagonist Suppresses Differentiation to Regulatory T Cells without Suppressing Activation of T Cells. Biochem. Biophys. Res. Commun. 2011, 409, 114–119. [Google Scholar] [CrossRef]
- Zarek, P.E.; Huang, C.T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A Receptor Signaling Promotes Peripheral Tolerance by Inducing T-Cell Anergy and the Generation of Adaptive Regulatory T Cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ehrentraut, H.; Westrich, J.A.; Eltzschig, H.K.; Clambey, E.T. AdorA2B Adenosine Receptor Engagement Enhances Regulatory T Cell Abundance during Endotoxin-Induced Pulmonary Inflammation. PLoS ONE 2012, 7, e32416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, J.; Fu, J.; Chen, G.; Ma, T. Improvement of the Sepsis Survival Rate by Adenosine 2A Receptor Antagonists Depends on Immune Regulatory Functions of Regulatory T-cells. Front. Immunol. 2022, 13, 996446. [Google Scholar] [CrossRef]
- Leone, R.D.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Wen, J.; Englert, J.; Powell, J.D. Inhibition of the Adenosine A2a Receptor Modulates Expression of T Cell Coinhibitory Receptors and Improves Effector Function for Enhanced Checkpoint Blockade and ACT in Murine Cancer Models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef]
- Leikeim, L.; Li, H.; An, L.; Sticht, C.; Kramer, B.K.; Yard, B.; Leipe, J.; Kalsch, A.I. Adenosine Signalling in T-Cell Activation Favours Development of IL-17 Positive Cells with Suppressive Properties. Immunology 2023, 169, 42–56. [Google Scholar] [CrossRef]
- Whitmore, K.V.; Gaspar, H.B. Adenosine Deaminase Deficiency–More Than Just an Immunodeficiency. Front. Immunol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Kameoka, J.; Tanaka, T.; Nojima, Y.; Schlossman, S.F.; Morimoto, C. Direct Association of Adenosine Deaminase with a T Cell Activation Antigen, CD26. Science 1993, 261, 466–469. [Google Scholar] [CrossRef]
- De la Rosa-Ruiz, M.d.P.; Álvarez-Pérez, M.A.; Cortés-Morales, V.A.; Monroy-García, A.; Mayani, H.; Fragoso-González, G.; Caballero-Chacón, S.; Diaz, D.; Candanedo-González, F.; Montesinos, J.J. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues: A Comparative In Vitro Evaluation of Their Immunoregulatory Properties Against T cells. Cells 2019, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Antonioli, L.; Colucci, R.; La Motta, C.; Tuccori, M.; Awwad, O.; Da Settimo, F.; Blandizzi, C.; Fornai, M. Adenosine Deaminase in the Modulation of Immune System and its Potential as a Novel Target for Treatment of Inflammatory Disorders. Curr. Drug Targets 2012, 13, 842–862. [Google Scholar] [CrossRef]
- Netsch, P.; Elvers-Hornung, S.; Uhlig, S.; Kluter, H.; Huck, V.; Kirschhofer, F.; Brenner-Weiss, G.; Janetzko, K.; Solz, H.; Wuchter, P.; et al. Human Mesenchymal Stromal Cells Inhibit Platelet Activation and Aggregation Involving CD73-Converted Adenosine. Stem Cell Res. Ther. 2018, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Burr, A.; Parekkadan, B. Kinetics of MSC-Based Enzyme Therapy for Immunoregulation. J. Transl. Med. 2019, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- Beckenkamp, L.R.; da Fontoura, D.M.S.; Korb, V.G.; de Campos, R.P.; Onzi, G.R.; Iser, I.C.; Bertoni, A.P.S.; Sevigny, J.; Lenz, G.; Wink, M.R. Immortalization of Mesenchymal Stromal Cells by TERT Affects Adenosine Metabolism and Impairs their Immunosuppressive Capacity. Stem Cell Rev. Rep. 2020, 16, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Niehage, C.; Karbanova, J.; Steenblock, C.; Corbeil, D.; Hoflack, B. Cell Surface Proteome of Dental Pulp Stem Cells Identified by Label-Free Mass Spectrometry. PLoS ONE 2016, 11, e0159824. [Google Scholar] [CrossRef]
- Ahmadi, P.; Yan, M.; Bauche, A.; Smeets, R.; Muller, C.E.; Koch-Nolte, F.; Haag, F.; Fliegert, R.; Kluwe, L.; Schulze Zur Wiesch, J.; et al. Human Dental Pulp Cells Modulate CD8(+) T Cell Proliferation and Efficiently Degrade Extracellular ATP to Adenosine In Vitro. Cell Immunol. 2022, 380, 104589. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Dang, J.; Zhang, X.; Wang, J.; Chen, Y.; Liang, J.; Li, D.; Ma, J.; Yuan, J.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Ameliorate Streptozoticin-induced T1DM in Mice via Suppression of T effector cells and Up-regulating Treg Subsets. Sci. Rep. 2017, 7, 15249. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Huang, F.; Wang, J.; Su, W.; Zhou, J.; Qi, Q.; Cao, F.; Sun, B.; Liu, Z.; et al. Human Gingiva Tissue-Derived MSC Ameliorates Immune-Mediated Bone Marrow Failure of Aplastic Anemia via Suppression of Th1 and Th17 Cells and Enhancement of CD4+Foxp3+ Regulatory T Cells Differentiation. Am. J. Transl. Res. 2019, 11, 7627–7643. [Google Scholar] [PubMed]
- Chen, W.; Yu, Y.; Zheng, S.G.; Lin, J. Human Gingival Tissue-Derived Mesenchymal Stem Cells Inhibit Proliferation and Invasion of Rheumatoid Fibroblast-Like Synoviocytes via the CD39/CD73 Signaling Pathway. Rheumatol. Autoimmun. 2023, 3, 90–99. [Google Scholar] [CrossRef]
- Mora-García, M.; García-Rocha, R.; Morales-Ramírez, O.; Montesinos, J.J.; Weiss-Steider, B.; Hernández-Montes, J.; Ávila-Ibarra, L.R.; Don-López, C.A.; Velasco-Velázquez, M.A.; Gutiérrez-Serrano, V.; et al. Mesenchymal Stromal Cells Derived from Cervical Cancer Produce High Amounts of Adenosine to Suppress Cytotoxic T Lymphocyte Functions. J. Transl. Med. 2016, 14, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Terraza-Aguirre, C.; Campos-Mora, M.; Elizondo-Vega, R.; Contreras-Lopez, R.A.; Luz-Crawford, P.; Jorgensen, C.; Djouad, F. Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells 2020, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, W.; Lin, X.; Guo, Z.; Wang, J.; Zhang, Q.; Brand, D.; Ryffel, B.; Huang, J.; Liu, Z.; et al. Adoptive Transfer of Human Gingiva-Derived Mesenchymal Stem Cells Ameliorates Collagen-Induced Arthritis via Suppression of Th1 and Th17 Cells and Enhancement of Regulatory T Cell Differentiation. Arthritis Rheum. 2013, 65, 1181–1193. [Google Scholar] [CrossRef]
- Tokano, M.; Matsushita, S.; Takagi, R.; Yamamoto, T.; Kawano, M. Extracellular Adenosine Induces Hypersecretion of IL-17A by T-helper 17 Cells through the Adenosine A2a Receptor. Brain Behav. Immun. Health 2022, 26, 100544. [Google Scholar] [CrossRef]
- Hassan, M.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 9529465. [Google Scholar] [CrossRef]
- Jankovic, M.G.; Stojkovic, M.; Bojic, S.; Jovicic, N.; Kovacevic, M.M.; Ivosevic, Z.; Juskovic, A.; Kovacevic, V.; Ljujic, B. Scaling Up Human Mesenchymal Stem Cell Manufacturing Using Bioreactors for Clinical Uses. Curr. Res. Transl. Med. 2023, 71, 103393. [Google Scholar] [CrossRef]
- Srinivasan, A.; Sathiyanathan, P.; Yin, L.; Liu, T.M.; Lam, A.; Ravikumar, M.; Smith, R.A.A.; Loh, H.P.; Zhang, Y.; Ling, L.; et al. Strategies to Enhance Immunomodulatory Properties and Reduce Heterogeneity in Mesenchymal Stromal Cells during Ex Vivo Expansion. Cytotherapy 2022, 24, 456–472. [Google Scholar] [CrossRef]
- Miclau, K.; Hambright, W.S.; Huard, J.; Stoddart, M.J.; Bahney, C.S. Cellular Expansion of MSCs: Shifting the Regenerative Potential. Aging Cell 2023, 22, e13759. [Google Scholar] [CrossRef]
- Govindasamy, V.; Ronald, V.S.; Abdullah, A.N.; Ganesan Nathan, K.R.; Aziz, Z.A.; Abdullah, M.; Zain, R.B.; Kasim, N.H.; Musa, S.; Bhonde, R.R. Human Platelet Lysate Permits Scale-Up of Dental Pulp Stromal Cells for Clinical Applications. Cytotherapy 2011, 13, 1221–1233. [Google Scholar] [CrossRef]
- Eubanks, E.J.; Tarle, S.A.; Kaigler, D. Tooth Storage, Dental Pulp Stem Cell Isolation, and Clinical Scale Expansion without Animal Serum. J. Endod. 2014, 40, 652–657. [Google Scholar] [CrossRef]
- Wu, R.X.; Yu, Y.; Yin, Y.; Zhang, X.Y.; Gao, L.N.; Chen, F.M. Platelet Lysate Supports the In Vitro Expansion of Human Periodontal Ligament Stem Cells for Cytotherapeutic Use. J. Tissue Eng. Regen. Med. 2017, 11, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Földes, A.; Reider, H.; Varga, A.; Nagy, K.S.; Perczel-Kovach, K.; Kis-Petik, K.; DenBesten, P.; Ballagi, A.; Varga, G. Culturing and Scaling up Stem Cells of Dental Pulp Origin Using Microcarriers. Polymers 2021, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Roemeling-van Rhijn, M.; Khairoun, M.; Korevaar, S.S.; Lievers, E.; Leuning, D.G.; Ijzermans, J.N.; Betjes, M.G.; Genever, P.G.; van Kooten, C.; de Fijter, H.J.; et al. Human Bone Marrow- and Adipose Tissue-derived Mesenchymal Stromal Cells are Immunosuppressive In Vitro and in a Humanized Allograft Rejection Model. J. Stem Cell Res. Ther. 2013, 1 (Suppl. S6), 20780. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Mayani, H.; Monroy-Garcia, A.; Flores-Figueroa, E.; Chavez-Rueda, K.; Legorreta-Haquet, V.; Santiago-Osorio, E.; Montesinos, J.J. Human Mesenchymal Stromal Cells from Adult and Neonatal Sources: A Comparative In Vitro Analysis of Their Immunosuppressive Properties Against T Cells. Stem Cells Deveploment 2014, 23, 1217–1232. [Google Scholar] [CrossRef]
- Sattler, C.; Steinsdoerfer, M.; Offers, M.; Fischer, E.; Schierl, R.; Heseler, K.; Daubener, W.; Seissler, J. Inhibition of T-Cell Proliferation by Murine Multipotent Mesenchymal Stromal Cells is Mediated by CD39 Expression and Adenosine Generation. Cell Transplant. 2011, 20, 1221–1230. [Google Scholar] [CrossRef]
- Naasani, L.I.S.; Rodrigues, C.; de Campos, R.P.; Beckenkamp, L.R.; Iser, I.C.; Bertoni, A.P.S.; Wink, M.R. Extracellular Nucleotide Hydrolysis in Dermal and Limbal Mesenchymal Stem Cells: A Source of Adenosine Production. J. Cell Biochem. 2017, 118, 2430–2442. [Google Scholar] [CrossRef]
- Schuler, P.J.; Westerkamp, A.-M.; Kansy, B.A.; Bruderek, K.; Dissmann, P.A.; Dumitru, C.A.; Lang, S.; Jackson, E.K.; Brandau, S. Adenosine Metabolism of Human Mesenchymal Stromal Cells Isolated from Patients with Head and Neck Squamous Cell Carcinoma. Immunobiology 2017, 222, 66–74. [Google Scholar] [CrossRef]
- Baghbani, E.; Noorolyai, S.; Shanehbandi, D.; Mokhtarzadeh, A.; Aghebati-Maleki, L.; Shahgoli, V.K.; Brunetti, O.; Rahmani, S.; Shadbad, M.A.; Baghbanzadeh, A.; et al. Regulation of Immune Responses through CD39 and CD73 in Cancer: Novel Checkpoints. Life Sci. 2021, 282, 119826. [Google Scholar] [CrossRef]
- Sundin, M.; D’Arcy, P.; Johansson, C.C.; Barrett, A.J.; Lönnies, H.; Sundberg, B.; Nava, S.; Kiessling, R.; Mougiakakos, D.; Le Blanc, K. Multipotent Mesenchymal Stromal Cells Express FoxP3: A Marker for the Immunosuppressive Capacity? J. Immunother. 2011, 34, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Okuda, K.; Yoshie, H. Extracellular ATP and ATPgammaS Suppress the Proliferation of Human Periodontal Ligament Cells by Different Mechanisms. J. Periodontol. 2007, 78, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Ontyd, J.; Schrader, J. Measurement of Adenosine, Inosine, and Hypoxanthine in Human Plasma. J. Chromatogr. 1984, 307, 404–409. [Google Scholar] [CrossRef]
- Moser, G.H.; Schrader, J.; Deussen, A. Turnover of Adenosine in Plasma of Human and Dog Blood. Am. J. Physiol. 1989, 256, C799–C806. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Sitkovsky, M.V.; Szabó, C. Immunomodulatory and Neuroprotective Effects of Inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef]
- Zhulai, G.; Oleinik, E.; Shibaev, M.; Ignatev, K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules 2022, 12, 418. [Google Scholar] [CrossRef]
- Nakajima, M.; Nito, C.; Sowa, K.; Suda, S.; Nishiyama, Y.; Nakamura-Takahashi, A.; Nitahara-Kasahara, Y.; Imagawa, K.; Hirato, T.; Ueda, M.; et al. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol. Ther. Methods Clin. Dev. 2017, 6, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, H.; Yuan, X.; Ma, H.; Shi, X.; Ding, Y. Interleukin-10 Secreted by Mesenchymal Stem Cells Attenuates Acute Liver Failure through Inhibiting Pyroptosis. Hepatol. Res. 2018, 48, E194–E202. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, G.; Wei, Z.; Nie, K.; Liu, Z.; Deng, C.; Wang, D. IL-10 Gene-Modified Human Amniotic Mesenchymal Stem Cells Augment Regenerative Wound Healing by Multiple Synergistic Effects. Stem Cells Int. 2019, 2019, 9158016. [Google Scholar] [CrossRef]
- Zhang, C.; Delawary, M.; Huang, P.; Korchak, J.A.; Suda, K.; Zubair, A.C. IL-10 mRNA Engineered MSCs Demonstrate Enhanced Anti-Inflammation in an Acute GvHD Model. Cells 2021, 10, 3101. [Google Scholar] [CrossRef]
- Niu, J.; Yue, W.; Le-Le, Z.; Bin, L.; Hu, X. Mesenchymal Stem Cells Inhibit T Cell Activation by Releasing TGF-β1 from TGF-β1/GARP Complex. Oncotarget 2017, 8, 99784–99800. [Google Scholar] [CrossRef]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-Secreted TGF-beta Regulates Lipopolysaccharide-Stimulated Macrophage M2-Like Polarization via the Akt/FoxO1 Pathway. Stem Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.; Treacy, O.; Chen, X.; Murphy, N.; Lohan, P.; Islam, M.N.; Donohoe, E.; Griffin, M.D.; Watson, L.; McLoughlin, S.; et al. TGF-beta1-Licensed Murine MSCs Show Superior Therapeutic Efficacy in Modulating Corneal Allograft Immune Rejection In Vivo. Mol. Ther. 2020, 28, 2023–2043. [Google Scholar] [CrossRef]
- Yanez, R.; Oviedo, A.; Aldea, M.; Bueren, J.A.; Lamana, M.L. Prostaglandin E2 Plays a Key Role in the Immunosuppressive Properties of Adipose and Bone Marrow Tissue-Derived Mesenchymal Stromal Cells. Exp. Cell Res. 2010, 316, 3109–3123. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tang, X.; Li, W.; Chen, W.; Yao, G.; Sun, L. Mesenchymal Stem Cells Inhibited the Differentiation of MDSCs via COX2/PGE2 in Experimental Sialadenitis. Stem Cell Res. Ther. 2020, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Su, Y.; Liu, X.; Liu, F.; Zhang, G.; Chen, Q.; Wang, C.; Fu, H.; Zhu, X.; Liu, K.; et al. PGE2 Dependent Inhibition of Macrophage Pyroptosis By MSCs Contributes to Alleviating aGVHD. Blood 2020, 136 (Suppl. S1), 15. [Google Scholar] [CrossRef]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal Stem Cells: A Double-Edged Sword in Regulating Immune Responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef]
- Maria, A.T.J.; Rozier, P.; Fonteneau, G.; Sutra, T.; Maumus, M.; Toupet, K.; Cristol, J.P.; Jorgensen, C.; Guilpain, P.; Noel, D. iNOS Activity Is Required for the Therapeutic Effect of Mesenchymal Stem Cells in Experimental Systemic Sclerosis. Front. Immunol. 2018, 9, 3056. [Google Scholar] [CrossRef]
- Meesuk, L.; Tantrawatpan, C.; Kheolamai, P.; Manochantr, S. The Immunosuppressive Capacity of Human Mesenchymal Stromal Cells Derived from Amnion and Bone Marrow. Biochem. Biophys. Rep. 2016, 8, 34–40. [Google Scholar] [CrossRef]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Uhlig, S.; Elvers-Hornung, S.; Kluter, H.; Bieback, K. Human Adipose Tissue-Derived Stromal Cells Suppress Human, but Not Murine Lymphocyte Proliferation, via Indoleamine 2,3-Dioxygenase Activity. Cells 2020, 9, 2419. [Google Scholar] [CrossRef]
- Lopez-Garcia, L.; Castro-Manrreza, M.E. TNF-alpha and IFN-gamma Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 9531. [Google Scholar] [CrossRef]
- Linden, J.; Cekic, C. Regulation of Lymphocyte Function by Adenosine. Arter. Thromb. Vasc. Biol. 2012, 32, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.R.; Fernández, D.; Flores-Santibáñez, F.; Rosemblatt, M.; Sauma, D. CD73 and CD39 Ectonucleotidases in T Cell Differentiation: Beyond Immunosuppression. FEBS Lett. 2015, 589, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-Cells in Autoimmune Diseases: Challenges, Controversies and—Yet—Unanswered Questions. Autoimmun. Rev. 2015, 14, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ni, X.; Pan, X.; Lu, H.; Lu, Y.; Zhao, J.; Guo Zheng, S.; Hippen, K.L.; Wang, X.; Lu, L. Human CD39(hi) Regulatory T Cells Present Stronger Stability and Function under Inflammatory Conditions. Cell Mol. Immunol. 2017, 14, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Himer, L.; Selmeczy, Z.; Vizi, E.S.; Pacher, P.; Ledent, C.; Deitch, E.A.; Spolarics, Z.; Németh, Z.H.; Haskü, G. Adenosine A2A Receptor Activation Inhibits T Helper 1 And T Helper 2 Cell Development and Effector Function. FASEB J. 2008, 22, 3491–3499. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Shao, H.; Chen, M.; Kaplan, H.J.; Sun, D. Anti-Inflammatory or Proinflammatory Effect of an Adenosine Receptor Agonist on the Th17 Autoimmune Response is Inflammatory Environment-Dependent. J. Immunol. 2014, 193, 5498–5505. [Google Scholar] [CrossRef]
- Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Raish, M.; Ahmad, S.F. Adenosine A2A Receptor Modulates Neuroimmune Function through Th17/Retinoid-Related Orphan Receptor Gamma T (Rorγt) Signaling in a BTBR T+ Itpr3tf/J Mouse Model of Autism. Cell Signal. 2017, 36, 14–24. [Google Scholar] [CrossRef]
- Wei, W.; Du, C.; Lv, J.; Zhao, G.; Li, Z.; Wu, Z.; Hasko, G.; Xie, X. Blocking A2B Adenosine Receptor Alleviates Pathogenesis of Experimental Autoimmune Encephalomyelitis via Inhibition of IL-6 Production and Th17 Differentiation. J. Immunol. 2013, 190, 138–146. [Google Scholar] [CrossRef]
- Dong, L.W.; Ma, Z.C.; Fu, J.; Huang, B.L.; Liu, F.J.; Sun, D.; Lan, C. Upregulated Adenosine 2A Receptor Accelerates Post-Infectious Irritable Bowel Syndrome by Promoting CD4+ T Cells’ T Helper 17 Polarization. World J. Gastroenterol. 2022, 28, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Tokano, M.; Kawano, M.; Takagi, R.; Matsushita, S. Istradefylline, an Adenosine A2A Receptor Antagonist, Ameliorates Neutrophilic Airway Inflammation and Psoriasis in Mice. Clin. Exp. Neuroimmunol. 2021, 12, 268–275. [Google Scholar] [CrossRef]
- Longhi, M.S.; Moss, A.; Bai, A.; Wu, Y.; Huang, H.; Cheifetz, A.; Quintana, F.J.; Robson, S.C. Characterization of Human CD39+ Th17 Cells with Suppressor Activity and Modulation in Inflammatory Bowel Disease. PLoS ONE 2014, 9, e87956. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.J.; Flores-Figueroa, E.; Castillo-Medina, S.; Flores-Guzman, P.; Hernandez-Estevez, E.; Fajardo-Orduna, G.; Orozco, S.; Mayani, H. Human Mesenchymal Stromal Cells from Adult and neonatal Sources: Comparative Analysis of their Morphology, Immunophenotype, Differentiation Patterns and Neural Protein Expression. Cytotherapy 2009, 11, 163–176. [Google Scholar] [CrossRef]
- Freudenberg, K.; Lindner, N.; Dohnke, S.; Garbe, A.I.; Schallenberg, S.; Kretschmer, K. Critical Role of TGF-beta and IL-2 Receptor Signaling in Foxp3 Induction by an Inhibitor of DNA Methylation. Front. Immunol. 2018, 9, 125. [Google Scholar] [CrossRef]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poblano-Pérez, L.I.; Monroy-García, A.; Fragoso-González, G.; Mora-García, M.d.L.; Castell-Rodríguez, A.; Mayani, H.; Álvarez-Pérez, M.A.; Pérez-Tapia, S.M.; Macías-Palacios, Z.; Vallejo-Castillo, L.; et al. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues Mediate the Immunoregulation of T Cells through the Purinergic Pathway. Int. J. Mol. Sci. 2024, 25, 9578. https://doi.org/10.3390/ijms25179578
Poblano-Pérez LI, Monroy-García A, Fragoso-González G, Mora-García MdL, Castell-Rodríguez A, Mayani H, Álvarez-Pérez MA, Pérez-Tapia SM, Macías-Palacios Z, Vallejo-Castillo L, et al. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues Mediate the Immunoregulation of T Cells through the Purinergic Pathway. International Journal of Molecular Sciences. 2024; 25(17):9578. https://doi.org/10.3390/ijms25179578
Chicago/Turabian StylePoblano-Pérez, Luis Ignacio, Alberto Monroy-García, Gladis Fragoso-González, María de Lourdes Mora-García, Andrés Castell-Rodríguez, Héctor Mayani, Marco Antonio Álvarez-Pérez, Sonia Mayra Pérez-Tapia, Zaira Macías-Palacios, Luis Vallejo-Castillo, and et al. 2024. "Mesenchymal Stem/Stromal Cells Derived from Dental Tissues Mediate the Immunoregulation of T Cells through the Purinergic Pathway" International Journal of Molecular Sciences 25, no. 17: 9578. https://doi.org/10.3390/ijms25179578





