Repurposing Niclosamide to Modulate Renal RNA-Binding Protein HuR for the Treatment of Diabetic Nephropathy in db/db Mice
Abstract
1. Introduction
2. Results
2.1. HuR Is Increased in Diabetic Kidneys in db/db Mice
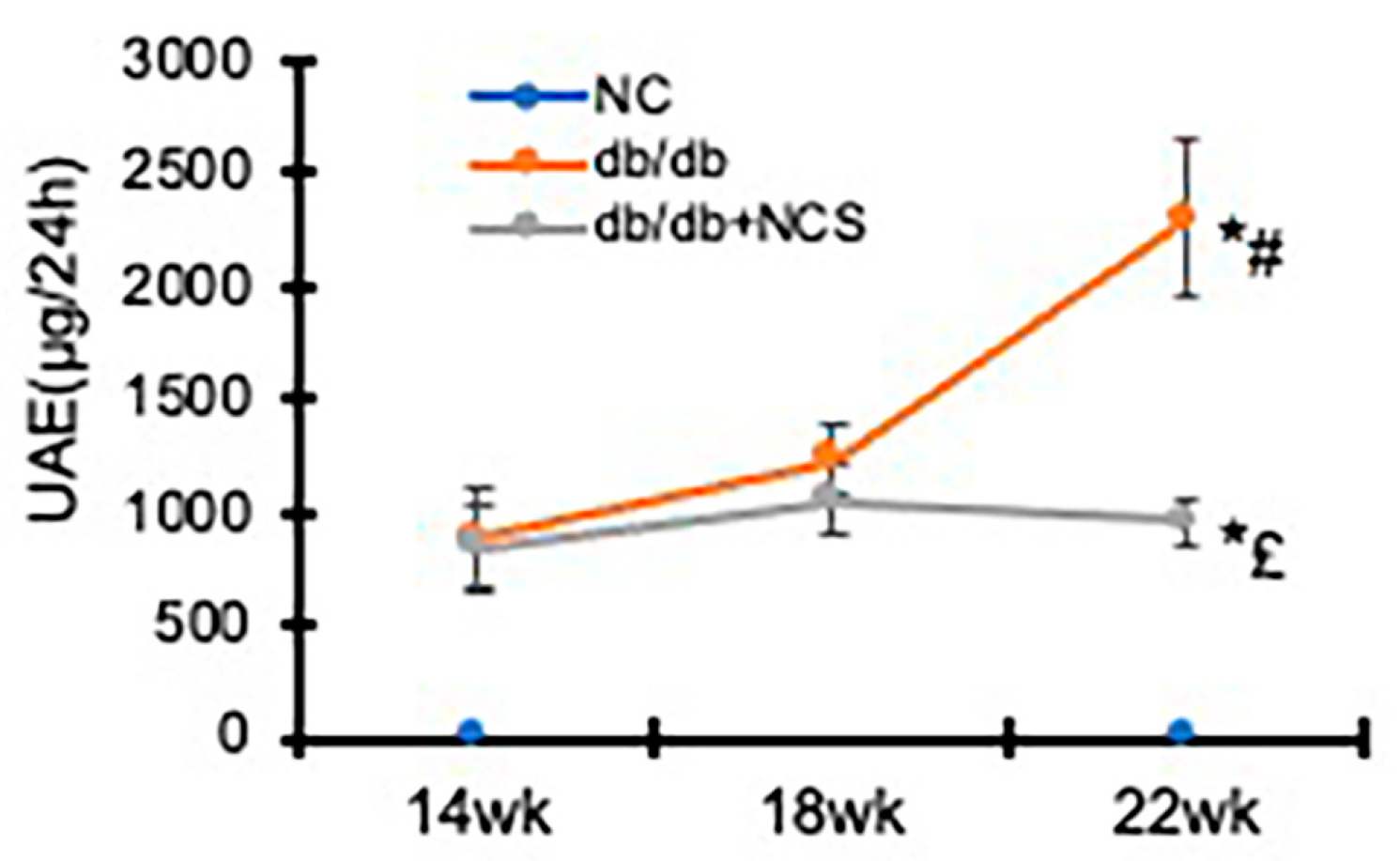
2.2. Treatment with NCS Reduces Hyperglycemia and Albuminuria
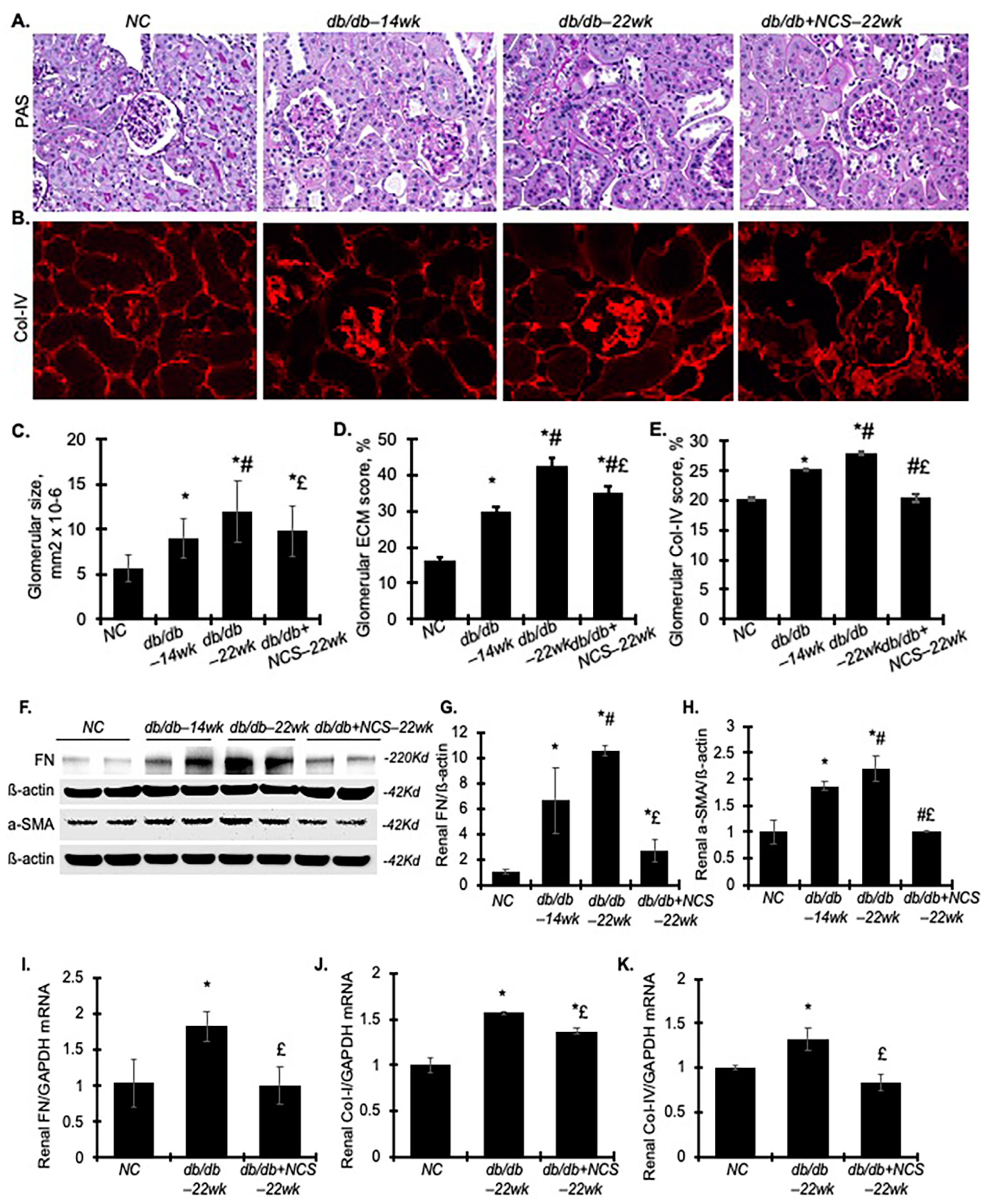
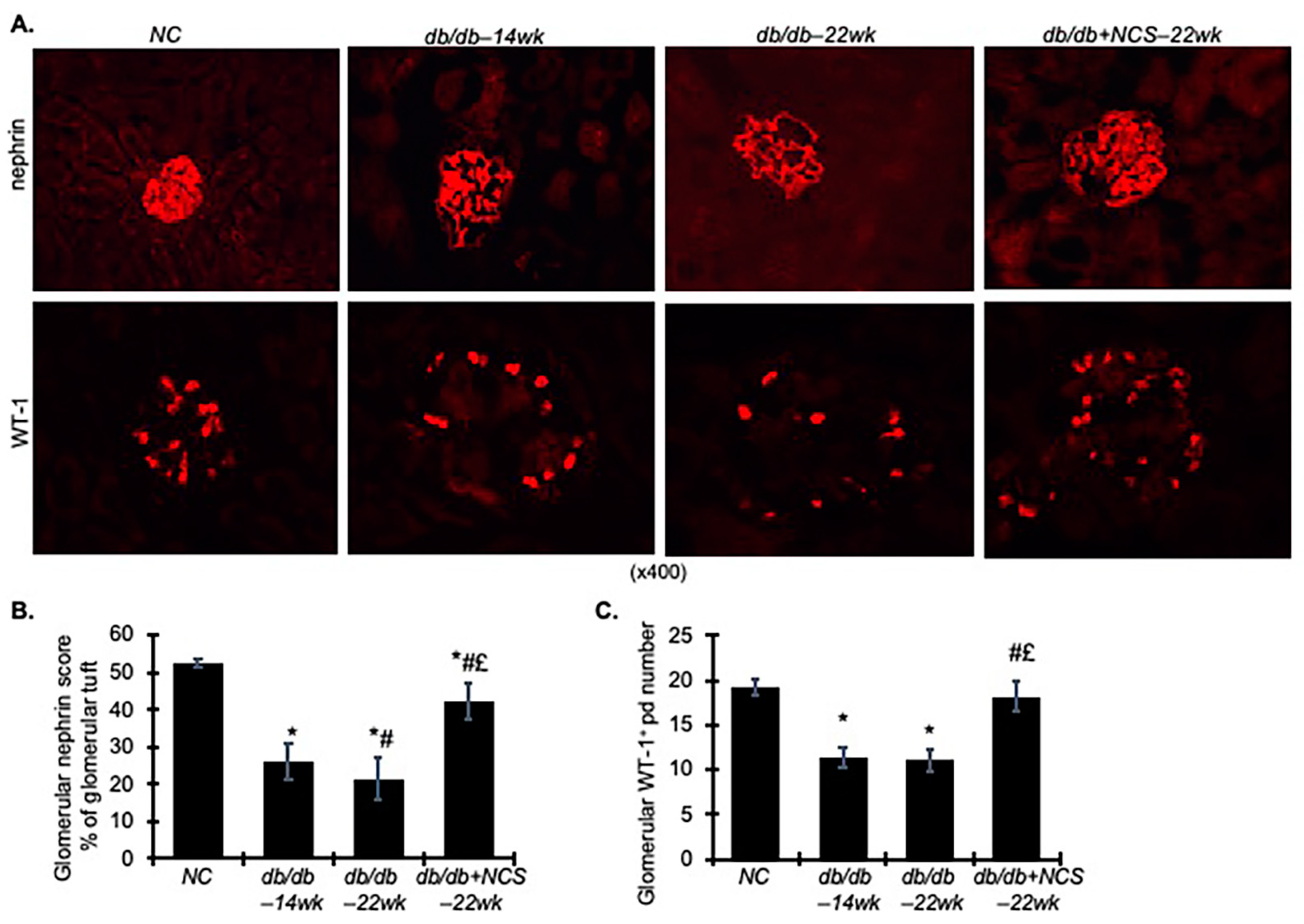
2.3. Treatment with NCS Reduces Kidney Hypertrophy, Fibrosis and Podocyte Injury
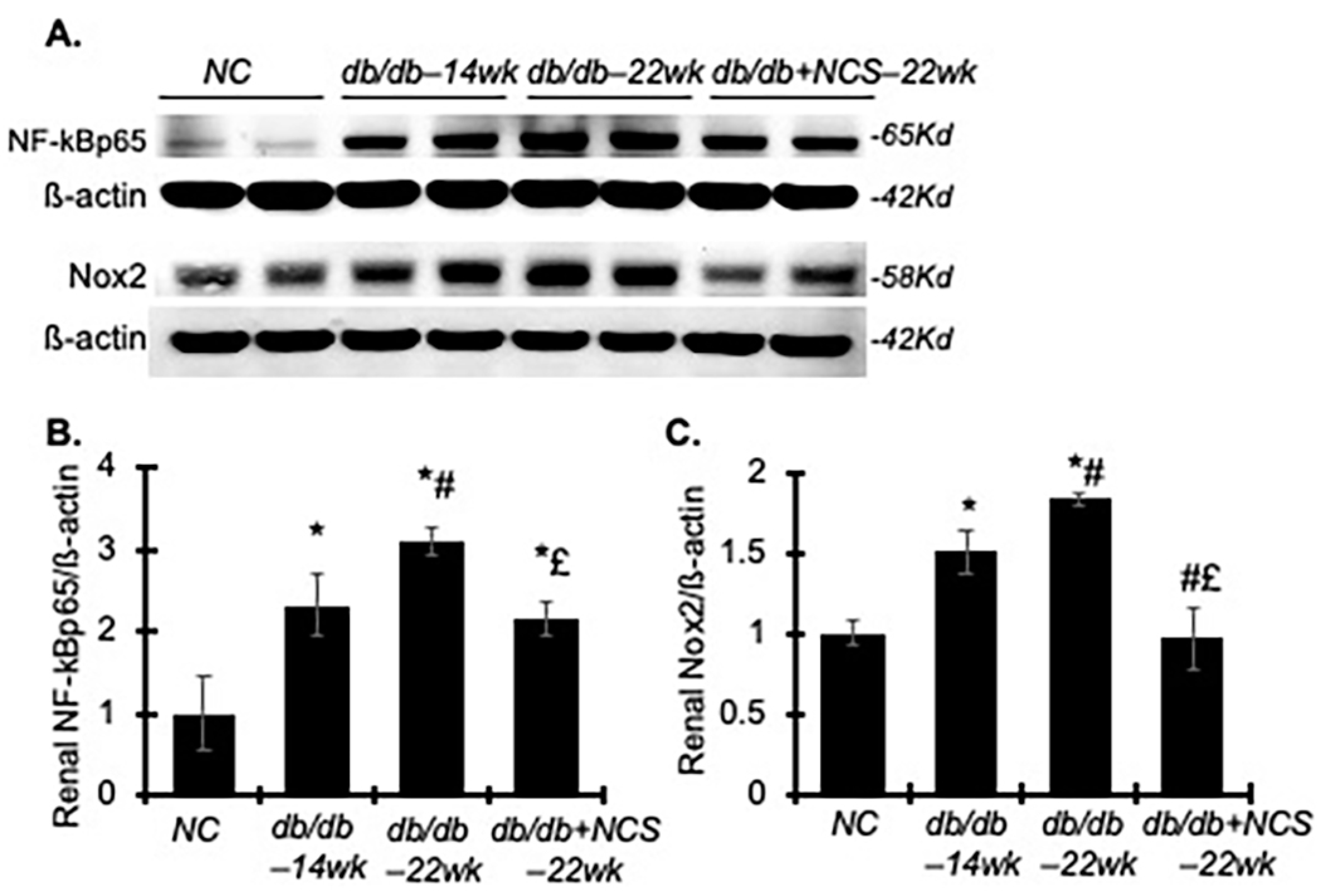
2.4. Treatment with NCS Reduces Inflammation and Oxidative Stress Involved in the Progression of Diabetic Nephropathy
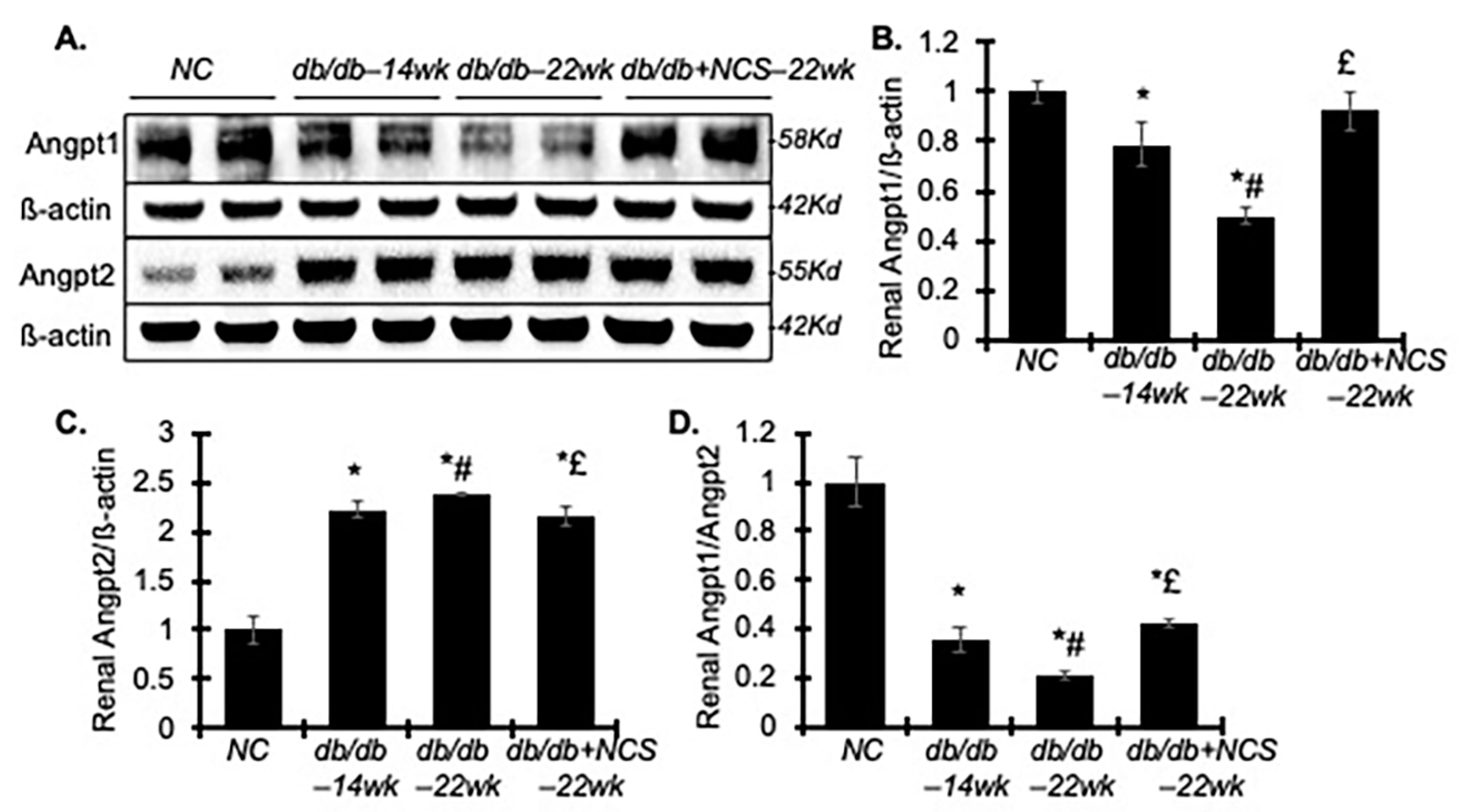
2.5. Treatment with NCS Improves the Renal Imbalance of Angiopoietin (Angpt) 1 and 2 Involved in Diabetes-Induced Endothelial Dysfunction
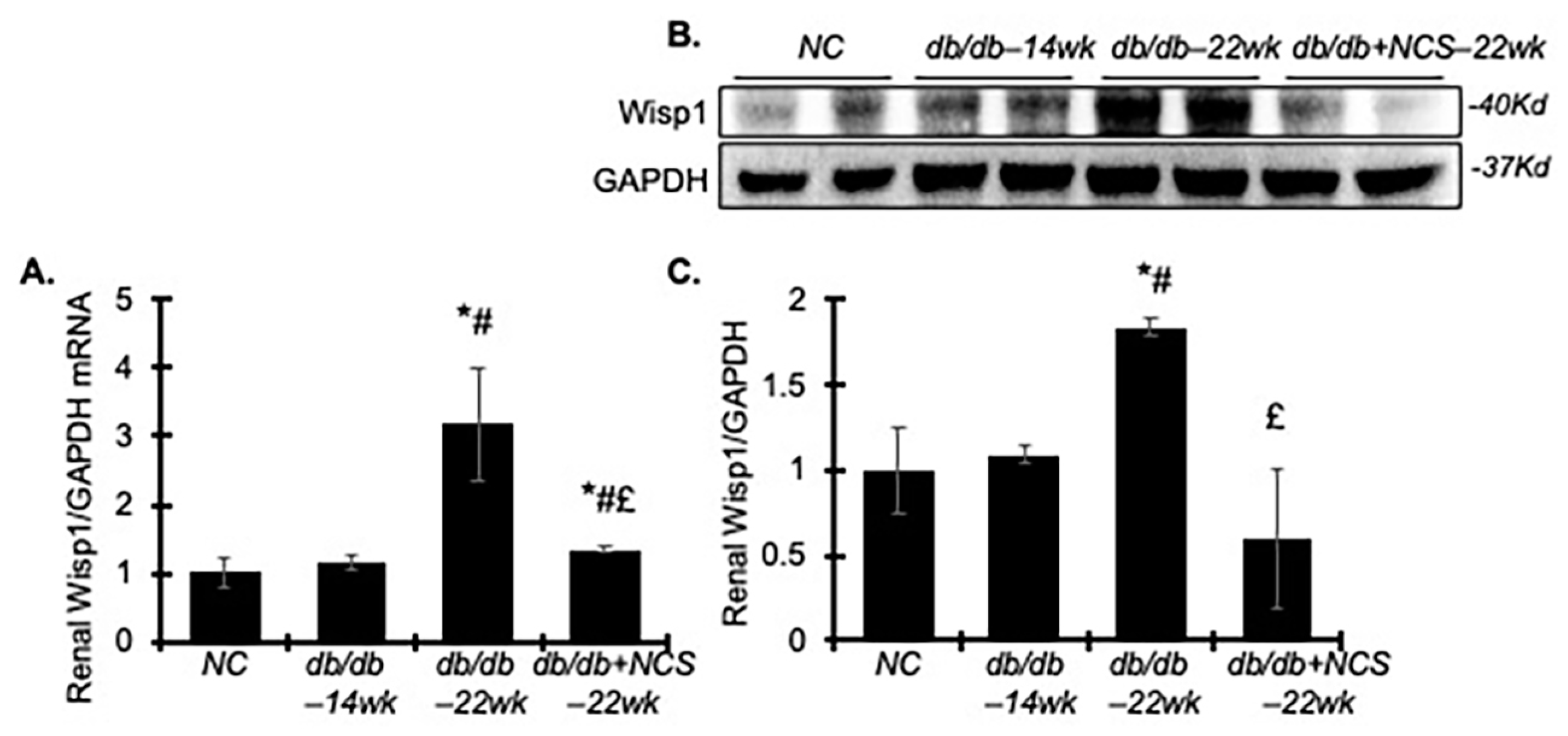
2.6. Treatment with NCS Downregulates Renal WNT1-Inducible-Signaling Pathway Protein 1 (Wisp1) in db/db Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Experimental Designs
4.3. Euthanasia
4.4. Measurement of Clinical Parameters
4.5. Histological Analysis of Kidney Tissue
4.6. Western Blot Analysis of Kidney Tissue
4.7. RNA Isolation and Real-Time RT-PCR Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, W.J.; Cheng, S.; Campbell, C.; Wright, A.; Furneaux, H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 1996, 271, 8144–8151. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Corcoran, D.L.; Nusbaum, J.D.; Reid, D.W.; Georgiev, S.; Hafner, M.; Ascano, M.; Tuschl, T.; Ohler, U.; Keene, J.D. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell 2011, 43, 327–339. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, Z. Emerging role of HuR in inflammatory response in kidney diseases. Acta Biochim. Biophys. Sin. 2017, 49, 753–763. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Q.; Wang, X.; Duan, Y.; Wang, Z.; Wei, X.; Zhang, Y.; Wang, H.; Wang, R.; Yi, F. Identification of NOD2 as a novel target of RNA-binding protein HuR: Evidence from NADPH oxidase-mediated HuR signaling in diabetic nephropathy. Free Radic. Biol. Med. 2015, 79, 217–227. [Google Scholar] [CrossRef]
- Yu, C.; Xin, W.; Zhen, J.; Liu, Y.; Javed, A.; Wang, R.; Wan, Q. Human antigen R mediated post-transcriptional regulation of epithelial-mesenchymal transition related genes in diabetic nephropathy. J. Diabetes 2015, 7, 562–572. [Google Scholar] [CrossRef]
- Doller, A.; Gauer, S.; Sobkowiak, E.; Geiger, H.; Pfeilschifter, J.; Eberhardt, W. Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am. J. Pathol. 2009, 174, 1252–1263. [Google Scholar] [CrossRef]
- Ceolotto, G.; De Kreutzenberg, S.V.; Cattelan, A.; Fabricio, A.S.; Squarcina, E.; Gion, M.; Semplicini, A.; Fadini, G.P.; Avogaro, A. Sirtuin 1 stabilization by HuR represses TNF-alpha- and glucose-induced E-selectin release and endothelial cell adhesiveness in vitro: Relevance to human metabolic syndrome. Clin. Sci. 2014, 127, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Danilin, S.; Sourbier, C.; Thomas, L.; Lindner, V.; Rothhut, S.; Dormoy, V.; Helwig, J.-J.; Jacqmin, D.; Lang, H.; Massfelder, T. Role of the RNA-binding protein HuR in human renal cell carcinoma. Carcinogenesis 2010, 31, 1018–1026. [Google Scholar] [CrossRef]
- Feigerlova, E.; Battaglia-Hsu, S.F. Role of post-transcriptional regulation of mRNA stability in renal pathophysiology: Focus on chronic kidney disease. FASEB J. 2017, 31, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, Z.; Tang, A.; Wu, X.; Aube, J.; Xu, L.; Xing, C.; Huang, Y. Inhibition of RNA-binding protein HuR reduces glomerulosclerosis in experimental nephritis. Clin. Sci. 2020, 134, 1433–1448. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Tang, A.; Wu, X.; Aube, J.; Xu, L.; Huang, Y. Targeting RNA-binding protein HuR to inhibit the progression of renal tubular fibrosis. J. Transl. Med. 2023, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, S.; Li, Q.; Wu, L.; Zhang, C.; Duan, S.; Zhang, B.; Yuan, Y.; Huang, Z.; Xing, C.; et al. The association of RNA-binding protein Human antigen R with kidney clinicopathologic features and renal outcomes in patients with diabetic nephropathy. Diabetes Res. Clin. Pract. 2022, 193, 110142. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Anthony, S.R.; Slone, S.; Lanzillotta, L.; Nieman, M.L.; Wu, X.; Robbins, N.; Jones, S.M.; Roy, S.; Owens, A.P.; et al. Human antigen R as a therapeutic target in pathological cardiac hypertrophy. JCI Insight 2019, 4, e121541. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lv, J.; Chang, S.; Chen, Z.; Lu, W.; Xu, C.; Liu, M.; Pang, X. Inhibiting cytoplasmic accumulation of HuR synergizes genotoxic agents in urothelial carcinoma of the bladder. Oncotarget 2016, 7, 45249–45262. [Google Scholar] [CrossRef]
- Andrews, P.; Thyssen, J.; Lorke, D. The biology and toxicology of molluscicides, Bayluscide. Pharmacol. Ther. 1982, 19, 245–295. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Wu, X.; Hao, S.; Hao, X.; Jones, E.; Zhang, Y.; Qiu, J.; Xu, L. Repurposing Niclosamide as a Novel Anti-SARS-CoV-2 Drug by Restricting Entry Protein CD147. Biomedicines 2023, 11, 2019. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Hao, X.; Dandreo, L.J.; He, L.; Zhang, Y.; Wang, F.; Wu, X.; Xu, L. Niclosamide improves cancer immunotherapy by modulating RNA-binding protein HuR-mediated PD-L1 signaling. Cell Biosci. 2023, 13, 192. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.K.; Roberts, M.J.; Arend, R.C.; Samant, R.S.; Buchsbaum, D.J. Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Lett. 2014, 349, 8–14. [Google Scholar] [CrossRef]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; A Koetzner, C.; Allen, C.; A Jones, S.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A.; Jr Premont, R.T.; Wang, J. Niclosamide: Beyond an antihelminthic drug. Cell Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Chang, X.; Zhen, X.; Liu, J.; Ren, X.; Hu, Z.; Zhou, Z.; Zhu, F.; Ding, K.; Nie, J. The antihelmenthic phosphate niclosamide impedes renal fibrosis by inhibiting homeodomain-interacting protein kinase 2 expression. Kidney Int. 2017, 92, 612–624. [Google Scholar] [CrossRef]
- El-Fatatry, B.M.; El-Haggar, S.M.; Ibrahim, O.M.; Shalaby, K.H. Niclosamide from an anthelmintic drug to a promising adjuvant therapy for diabetic kidney disease: Randomized clinical trial. Diabetol. Metab. Syndr. 2023, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Border, W.A.; Yu, L.; Zhang, J.; Lawrence, D.A.; Noble, N.A. A PAI-1 Mutant, PAI-1R, Slows Progression of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, J.; Noble, N.A.; Peng, X.R.; Huang, Y. An additive effect of anti-PAI-1 antibody to ACE inhibitor on slowing the progression of diabetic kidney disease. Am. J. Physiol. Renal. Physiol. 2016, 311, F852–F863. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, Y.; Tian, M.; Sjöström, C.D.; Johansson, U.; Peng, X.-R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef]
- Liu, G.; Kaw, B.; Kurfis, J.; Rahmanuddin, S.; Kanwar, Y.S.; Chugh, S.S. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J. Clin. Investig. 2003, 112, 209–221. [Google Scholar] [CrossRef]
- Mundlos, S.; Pelletier, J.; Darveau, A.; Bachmann, M.; Winterpacht, A.; Zabel, B. Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development 1993, 119, 1329–1341. [Google Scholar] [CrossRef]
- Guo, J.K.; Menke, A.L.; Gubler, M.C.; Clarke, A.R.; Harrison, D.; Hammes, A.; Hastie, N.D.; Schedl, A. WT1 is a key regulator of podocyte function: Reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum. Mol. Genet. 2002, 11, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Vithian, K.; Hurel, S. Microvascular complications: Pathophysiology and management. Clin. Med. 2010, 10, 505–509. [Google Scholar] [CrossRef]
- Gnudi, L. Angiopoietins and diabetic nephropathy. Diabetologia 2016, 59, 1616–1620. [Google Scholar] [CrossRef][Green Version]
- Tian, M.; Carroll, L.S.; Tang, L.; Uehara, H.; Westenfelder, C.; Ambati, B.K.; Huang, Y. Systemic AAV10.COMP-Ang1 rescues renal glomeruli and pancreatic islets in type 2 diabetic mice. BMJ Open Diabetes Res. Care 2020, 8, e000882. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Slone, S.; Anthony, S.R.; Guarnieri, A.R.; Parkins, S.; Shearer, S.M.; Nieman, M.L.; Roy, S.; Aube, J.; Wu, X.; et al. HuR-dependent expression of Wisp1 is necessary for TGFbeta-induced cardiac myofibroblast activity. J. Mol. Cell. Cardiol. 2023, 174, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhan, H.; Shao, M.; Wang, W.; Song, G.; Yu, X.; Zhang, C.; Ge, N.; Yi, T.; Li, S.; et al. Niclosamide ethanolamine improves kidney injury in db/db mice. Diabetes Res. Clin. Pract. 2018, 144, 25–33. [Google Scholar] [CrossRef]
- Guo, J.; Lei, M.; Cheng, F.; Liu, Y.; Zhou, M.; Zheng, W.; Zhou, Y.; Gong, R.; Liu, Z. RNA-binding proteins tristetraprolin and human antigen R are novel modulators of podocyte injury in diabetic kidney disease. Cell Death Dis. 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Lee, D.Y.; Feliers, D.; Abboud, H.E.; Bhat, M.A.; Gorin, Y. Interplay between RNA-binding protein HuR and Nox4 as a novel therapeutic target in diabetic kidney disease. Mol. Metab. 2020, 36, 100968. [Google Scholar] [CrossRef]
- Supe, S.; Dighe, V.; Upadhya, A.; Singh, K. Analysis of RNA Interference Targeted Against Human Antigen R (HuR) to Reduce Vascular Endothelial Growth Factor (VEGF) Protein Expression in Human Retinal Pigment Epithelial Cells. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Amadio, M.; Pascale, A.; Cupri, S.; Pignatello, R.; Osera, C.; D’Agata, V.; Amico, A.G.D.; Leggio, G.M.; Ruozi, B.; Govoni, S.; et al. Nanosystems based on siRNA silencing HuR expression counteract diabetic retinopathy in rat. Pharmacol. Res. 2016, 111, 713–720. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Luis-Rodríguez, D.; Martín-Núñez, E.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Rodríguez-Rodríguez, A.E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory Targets in Diabetic Nephropathy. J. Clin. Med. 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Leehey, D.J. Targeting Inflammation in Diabetic Kidney Disease: Is There a Role for Pentoxifylline? Kidney360 2020, 1, 292–299. [Google Scholar] [CrossRef]
- Maiese, K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr. Neurovasc. Res. 2014, 11, 378–389. [Google Scholar] [CrossRef]
- Gurbuz, I.; Chiquet-Ehrismann, R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): A focus on its role in cancer. Int. J. Biochem. Cell Biol. 2015, 62, 142–146. [Google Scholar] [CrossRef]
- Maeda, A.; Ono, M.; Holmbeck, K.; Li, L.; Kilts, T.M.; Kram, V.; Noonan, M.L.; Yoshioka, Y.; McNerny, E.M.; Tantillo, M.A.; et al. WNT1-induced Secreted Protein-1 (WISP1), a Novel Regulator of Bone Turnover and Wnt Signaling. J. Biol. Chem. 2015, 290, 14004–14018. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.; Jing, H.; Tang, S.; Huang, Z.; Liao, M.; Lin, S.; Zhong, J.; Zhou, J. LncRNA Gm12840 mediates WISP1 to regulate ischemia-reperfusion-induced renal fibrosis by sponging miR-677-5p. Epigenomics 2020, 12, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ding, X.; Ding, C.; Tesch, G.; Zheng, J.; Tian, P.; Ricardo, S.; Shen, H.H.; Xue, W. WNT1-inducible-signaling pathway protein 1 regulates the development of kidney fibrosis through the TGF-beta1 pathway. FASEB J. 2020, 34, 14507–14520. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Shao, M.; Guo, L.; Wang, W.; Song, G.; Yu, X.; Zhang, C.; Ge, N.; Yi, T.; Li, S.; et al. Niclosamide ethanolamine improves diabetes and diabetic kidney disease in mice. Am. J. Transl. Res. 2018, 10, 1071–1084. [Google Scholar]
- Weng, W.; Liu, H.; Sun, Z.; Zhou, P.; Yu, X.; Shao, M.; Han, P.; Sun, H. Combined treatment with niclosamide ethanolamine and artemether combination improves type 1 diabetes via the targeting of liver mitochondria. Exp. Ther. Med. 2022, 23, 239. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Zeng, X.; Shulman, G.I.; Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014, 20, 1263–1269. [Google Scholar] [CrossRef]
- Bhagat, H.A.; Compton, S.A.; Musso, D.L.; Laudeman, C.P.; Jackson, K.M.P.; Yi, N.Y.; Nierobisz, L.S.; Forsberg, L.; Brenman, J.E.; Sexton, J.Z. N-substituted phenylbenzamides of the niclosamide chemotype attenuate obesity related changes in high fat diet fed mice. PLoS ONE 2018, 13, e0204605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Cheung, A.K.; Liu, X.; Huang, Y. Valsartan slows the progression of diabetic nephropathy in db/db mice via a reduction in podocyte injury, and renal oxidative stress and inflammation. Clin. Sci. 2014, 126, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Tang, L.; Wu, Y.; Beddhu, S.; Huang, Y. Adiponectin attenuates kidney injury and fibrosis in deoxycorticosterone acetate-salt and angiotensin II-induced CKD mice. Am. J. Physiol. Renal. Physiol. 2018, 315, F558–F571. [Google Scholar] [CrossRef] [PubMed]

| NC–22wk (n = 8) | db/db–14wk (n = 9) | db/db–22wk (n = 9) | db/db + NCS–22wk (n = 9) | |
|---|---|---|---|---|
| BW, initial, g | 26.3 ± 1.08 | 47.3 ± 2.49 * | 48.2 ± 2.19 * | 48.5 ± 3.55 * |
| BW, 18wk, g | 27.4 ± 1.69 | 51.1 ± 4.35 * | 54.3 ± 3.53 * | |
| BW, final, g | 28.3 ± 1.79 | 47.3 ± 6.75 * | 47.4 ± 6.95 * | |
| plasma glucose, initial, mg/dL | 128.3 ± 34.7 | 500.3 ± 109.6 * | 504.7 ± 67.0 * | 504 ± 77.7 * |
| plasma glucose, final, mg/dL | 161.8 ± 52.8 | 593.5 ± 18.3 *#$ | 557.7 ± 45.1 *#£$ | |
| HbA1c, initial, % | 4.13 ± 0.15 | 7.77 ± 1.17 * | 7.57 ± 1.14 * | 7.64 ± 0.87 * |
| HbA1c, final, % | 4.52 ± 0.36 | 13.35 ± 0.97 *#$ | 10.87 ± 1.09 *#£$ | |
| urine volume, initial, mL/d | 0.8 ± 0.67 | 13.0 ± 5.96 * | 14.75 ± 5.72 * | 11.06 ± 4.93 * |
| urine volume, 18wk, mL/d | 26.4 ± 5.98 $ | 24.0 ± 6.05 $ | ||
| urine volume, final, mL/d | 0.67 ± 0.36 | 35.7 ± 4.65 *& | 13.0 ± 2.51 *£& | |
| KW, g | 0.238 ± 0.03 | 0.359 ± 0.04 * | 0.509 ± 0.04 *# | 0.402 ± 0.04 *£ |
| plasma BUN, mg/dL | 20.88 ± 4.73 | 42.66 ± 3.96 * | 45.76 ± 4.13 * | 30.98 ± 2.96 *#£ |
| plasma Cr, mg/dL | 0.18 ± 0.02 | 0.31 ± 0.03 * | 0.33 ± 0.03 * | 0.24 ± 0.02 *#£ |
| Urine Levels | NC–22wk (n = 8) | db/db–14wk (n = 9) | db/db–22wk (n = 9) | db/db + NCS–22wk (n = 9) |
|---|---|---|---|---|
| TNF-a, pg/24 h | 1.22 ± 0.41 | 20.21 ± 3.17 | 88.53 ± 36.73 | 15.16 ± 1.91 |
| logTNF-a, pg/24 h | −0.004 ± 0.16 | 1.24 ± 0.09 * | 1.76 ± 0.12 *# | 1.16 ± 0.05 *£ |
| MCP-1, pg/24 h | 11.81 ± 2.42 | 205.74 ± 31.05 | 687.97 ± 126.79 | 148.67 ± 13.07 |
| logMCP-1, pg/24 h | 1.04 ± 0.09 | 2.26 ± 0.07 * | 2.79 ± 0.06 *# | 2.16 ± 0.04 *£ |
| MDA, µmol/24 h | 0.022 ± 0.002 | 11.56 ± 1.94 | 37.02 ± 5.62 | 9.69 ± 1.00 |
| logMDA, µmol/24 h | −1.66 ± 0.04 | 1.01 ± 0.06 * | 1.54 ± 0.4 *# | 1.00 ± 0.03 *£ |
| Gene | Primer | Sequence 5′–3′ |
|---|---|---|
| mouse | Forward | CCGTGGGATGTTTGAGACTT |
| FN | Reverse | GGCAAAAGAAAGCAGAGGTG |
| mouse | Forward | ACGTCCTGGTGAAGTTGGTC |
| Col-Ia1 | Reverse | CAGGGAAGCCTCTTTCTCCT |
| mouse | Forward | CACCCATCTCTGGGGACAAC |
| Col-IVa1 | Reverse | GTTAGGGCACTGCGGAATCT |
| mouse | Forward | GTCCAGGACTTCACAATTGAGC |
| Wisp1 | Reverse | CCAGGCTTTGCTTCCATTG |
| mouse | Forward | ACCCAGAAGACTGTGGATGG |
| GAPDH | Reverse | CACATTGGGGGTAGGAACAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, L.; Liu, W.; Tsai, X.-Q.; Outtrim, C.; Tang, A.; Wang, Z.; Huang, Y. Repurposing Niclosamide to Modulate Renal RNA-Binding Protein HuR for the Treatment of Diabetic Nephropathy in db/db Mice. Int. J. Mol. Sci. 2024, 25, 9651. https://doi.org/10.3390/ijms25179651
Zhuang L, Liu W, Tsai X-Q, Outtrim C, Tang A, Wang Z, Huang Y. Repurposing Niclosamide to Modulate Renal RNA-Binding Protein HuR for the Treatment of Diabetic Nephropathy in db/db Mice. International Journal of Molecular Sciences. 2024; 25(17):9651. https://doi.org/10.3390/ijms25179651
Chicago/Turabian StyleZhuang, Lili, Wenjin Liu, Xiao-Qing Tsai, Connor Outtrim, Anna Tang, Zhou Wang, and Yufeng Huang. 2024. "Repurposing Niclosamide to Modulate Renal RNA-Binding Protein HuR for the Treatment of Diabetic Nephropathy in db/db Mice" International Journal of Molecular Sciences 25, no. 17: 9651. https://doi.org/10.3390/ijms25179651
APA StyleZhuang, L., Liu, W., Tsai, X.-Q., Outtrim, C., Tang, A., Wang, Z., & Huang, Y. (2024). Repurposing Niclosamide to Modulate Renal RNA-Binding Protein HuR for the Treatment of Diabetic Nephropathy in db/db Mice. International Journal of Molecular Sciences, 25(17), 9651. https://doi.org/10.3390/ijms25179651






