The Influence of Retinol Ointment on Rabbit Skin (Oryctolagus cuniculus) Ion Transport—An In Vitro Study
Abstract
1. Introduction
2. Results
3. Discussion
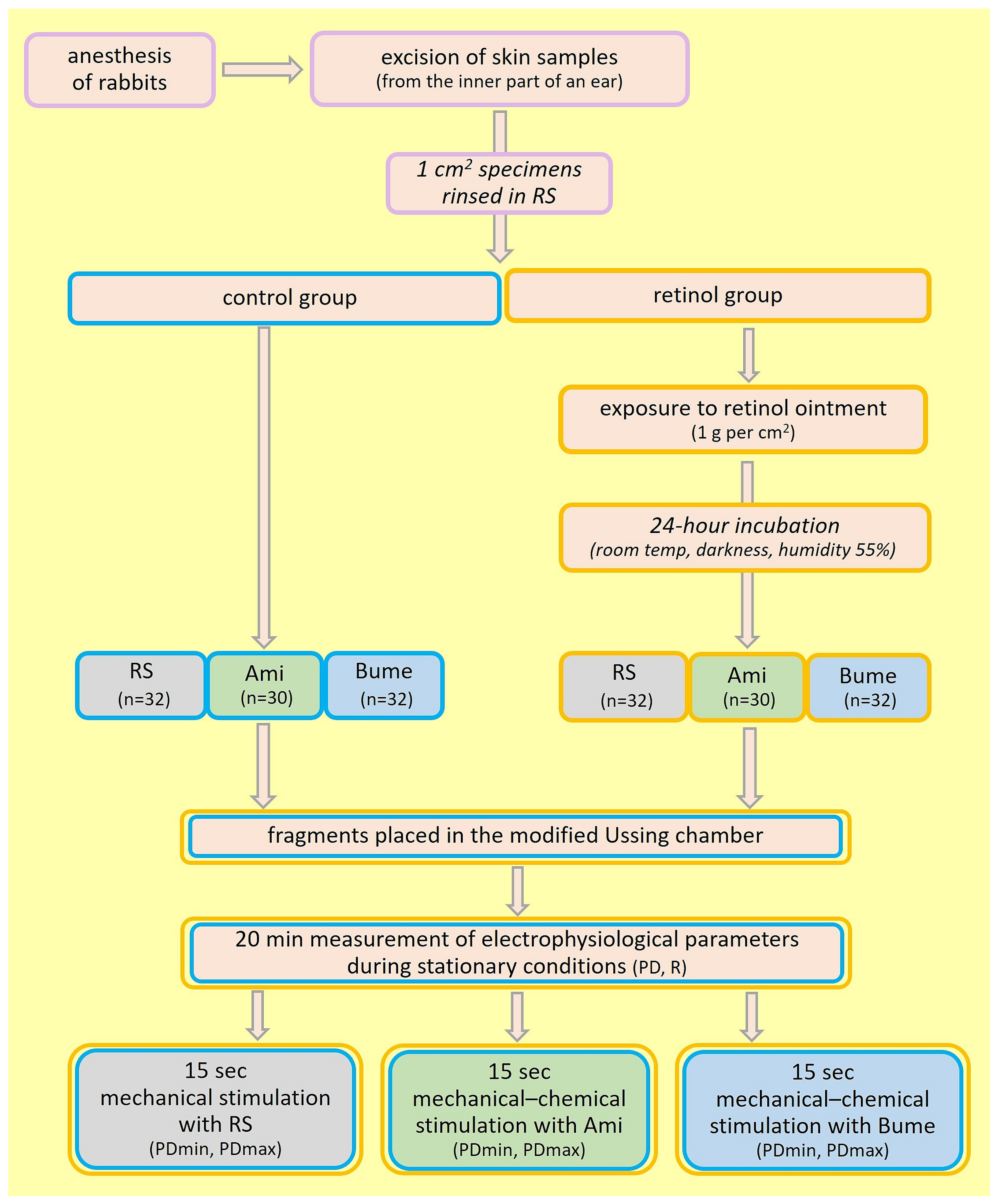
4. Materials and Methods
- -
- RS—Ringer’s solution: K+ 4.0 mM; Na+ 147.2 mM; Ca2+ 2.2 mM; Mg2+ 2.6 mM; Cl− 160.8 mM; 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (Sigma-Aldrich, USA). Iso-osmotic basic solution. Used to incubation and mechanical stimulation.
- -
- Ami—amiloride 0.1 mM (3,5-diamino-6-chloro-2-carboxylic acid) 266.09 g/mol (Sigma-Aldrich, USA). Used as an inhibitor of the sodium ion transport pathway in incubation and mechanical–chemical stimulation tests.
- -
- Bume—bumetanide 0.1 mM (3-butylamino-4-phenoxy-5-sulfamoylbenzoic acid) 364.42 g/mol (Sigma-Aldrich, USA). Used as an inhibitor of the chloride ion transport pathway in incubation and mechanical–chemical stimulation tests.
- -
- Retinol—retinol palmitate. Ointment with retinol at a concentration of 800 mass units/g (Hasco-Lek S.A., Wrocław, Poland). Used in incubation tests.
Experimental Procedure
- -
- PD—transepithelial electric potential measured continuously under stationary conditions (mV);
- -
- PDmin and PDmax—minimal and maximal transepithelial electric potential measured during 15 s of mechanical and/or mechanical–chemical stimulation (mV);
- -
- R—transepithelial resistance measured after applying a stimulus current of ±10 μA to the tissue (after measuring the voltage, the resistance was calculated according to Ohm’s law (Ω/cm2)).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debelo, H.; Novotny, J.A.; Ferruzzi, M.G. Vitamin A. Adv. Nutr. 2017, 8, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef]
- Shao, Y.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Molecular basis of retinol anti-aging properties in naturally aged human skin in vivo. Int. J. Cosmet. Sci. 2017, 39, 56–65. [Google Scholar] [CrossRef]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postep. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Motakis, E.; Artuc, M.; Hazzan, T.; Worm, M.; Forrest, A.R.; Zuberbier, T. Retinoic acid potentiates inflammatory cytokines in human mast cells: Identification of mast cells as prominent constituents of the skin retinoid network. Mol. Cell. Endocrinol. 2015, 406, 49–59. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Silva-Xavier, W.; Monte-Alto-Costa, A. Topical retinol attenuates stress -induced aging signs in human skin ex vivo, through EGFR activation via EGF, but not ERK and AP-1 activation. Exp. Dermatol. 2019, 28, 906–913. [Google Scholar] [CrossRef]
- Rankin, A.C.; Hendry, B.M.; Corcoran, J.P.; Xu, Q. An in vitro model for the pro-fibrotic effects of retinoids: Mechanisms of action. Br. J. Pharmacol. 2013, 170, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.M.; Silva, M.A.; Garcia, P.P.; Silva, L.S.; Costa, G.D.; Araújo, R.M.; Cotta, R.M. Effect of vitamin A suplementation: A systematic review. Cienc. Saude Coletiva 2019, 24, 827–838. [Google Scholar] [CrossRef]
- Sun, M.; Wang, P.; Sachs, D.; Xu, Y.; Xu, Y.; Voorhees, J.J.; Fisher, G.J.; Li, Y. Topical Retinol Restores Type I Collagen Production in Photoaged Forearm Skin within Four weeks. Cosmetics 2016, 3, 35. [Google Scholar] [CrossRef]
- Wang, L.; DeMarco, S.S.; Chen, J.M.; Phillips, C.M.; Bridges, L.C. Retinoids Bias Integrin Expression and Function in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2015, 135, 2102–2108. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J.; Kane, M.A.; Moise, A.R. Modulation of retinoid signaling: Therapeutic opportunities in organ fibrosis and repair. Pharmacol. Ther. 2020, 205, 107415. [Google Scholar] [CrossRef] [PubMed]
- Boehm, N.; Samama, B.; Cribier, B.; Rochette-Egly, C. Retinoic-acid receptor beta expression in melanocytes. Eur. J. Dermatol. 2024, 14, 19–23. [Google Scholar]
- Daly, T.J.; Weston, W.L. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J. Am. Acad. Dermatol. 1986, 15, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, D.; Kielmanowicz, M.G.; Vigodman, S.; Hu, Y.P.; Chen, N.; Nkengne, A.; Oddos, T.; Fischer, D.; Seiberg, M.; Lin, C.B. A novel anti-ageing mechanism for retinol: Induction of dermal elastin synthesis and elastin fibre formation. Int. J. Cosmet. Sci. 2011, 33, 62–69. [Google Scholar] [CrossRef]
- Tajima, S.; Hayashi, A.; Suzuki, T. Elastin expression is up-regulated by retinoic acid but not by retinol in chick embryonic skin fibroblasts. J. Dermatol. Sci. 1997, 15, 166–172. [Google Scholar] [CrossRef]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Skazik, C.; Amann, P.M.; Heise, R.; Marquardt, Y.; Czaja, K.; Kim, A.; Rühl, R.; Kurschat, P.; Merk, H.F.; Bickers, D.R.; et al. Downregulation of STRA6 Expression in Epidermal Keratinocytes Leads to Hyperproliferation-Associated Differentiation in Both In Vitro and In Vivo Skin Models. J. Investig. Dermatol. 2014, 134, 1579–1588. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, W.-H.; Kim, S.; Na, Y.; Choi, J.; Hong, Y.-D.; Park, W.-S.; Shim, S.-M. Bioconversion of retinol and its cell barrier function in human immortalized keratinocytes cells and artificial epidermis-dermis skin. Exp. Dermatol. 2023, 32, 822–830. [Google Scholar] [CrossRef]
- Gruber, J.V.; Stojkoska, V.; Riemer, J. Retinol Has a Skin Dehydrating Effect That Can Be Improved by a Mixture of Water-Soluble Polysaccharides. Cosmetics 2020, 7, 80. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zeitchek, M.; Cooper, G.; Jia, S.; Xie, P.; Quereshi, H.A.; Zhong, A.; Porterfield, M.D.; Galiano, R.D.; et al. Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin. J. Investig. Dermatol. 2015, 135, 796–806. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zhong, A.; Xie, A.; Jia, S.; Xie, Z.; Zeitchek, M.; Niknam-Bienia, S.; Zhao, J.; Porterfield, M.; et al. Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci. Transl. Med. 2015, 7, 312ra177. [Google Scholar] [CrossRef] [PubMed]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Analysis of changes in sodium and chloride ion transport in the skin. Sci. Rep. 2020, 10, 18094. [Google Scholar] [CrossRef] [PubMed]
- Hołyńska-Iwan, I.; Smyk, P.; Chrustek, A.; Olszewska-Słonina, D.; Szewczyk-Golec, K. The influence of hydration status on ion transport in the rabbit (Oryctolagus cuniculus) skin-An in vitro study. PLoS ONE 2021, 16, e0255825. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.C.; Fujii, N.; Meade, R.D.; Kenny, G.P. The roles of the Na+/K+-ATPase, NKCC, and K+ channels in regulating local sweating and cutaneous blood flow during exercise in humans in vivo. Physiol. Rep. 2016, 4, e13024. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Boggula, V.R.; Vaknine, H.; Sharma, S.; Kleyman, T.; Hanukoglu, A. Expression of epithelial sodium channel (ENaC) and CFTR in the human epidermis and epidermal appendages. Histochem. Cell Biol. 2017, 147, 733–748. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Q.; Wang, Z.; Zhang, J.Y.; MacLeod, A.S.; Hall, R.P.; Liedtke, W.B. Transient Receptor Potential Vanilloid 4 Ion Channel Functions as a Pruriceptor in Epidermal Keratinocytes to Evoke Histaminerg icItch. J. Biol. Chem. 2016, 291, 10252–10262. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Sobiesiak, M. Cisplatin influences the skin ion transport—An in vitro study. Biomed. Pharmacol. 2020, 129, 110502. [Google Scholar] [CrossRef]
- Dobrzeniecka, W.; Daca, M.; Nowakowska, B.; Sobiesiak, M.; Szewczyk-Goles, K.; Woźniak, A.; Hołyńska-Iwan, I. The Impact of Diclofenac Gel on Ion Transport in the Rabbit (Oryctolagus cuniculus) Skin: An In Vitro Study. Molecules 2023, 28, 1332. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Escobarm, I.E.; Oanceam, E. A melanosomal two-pore sodium channel regulates pigmentation. Sci. Rep. 2016, 6, 26570. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Silva, C.S.; Souza, M.N. Electrical impedance model for evaluation of skin irritation in rabbits and humans. Ski. Res. Technol. 2007, 13, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, Z.; Cui, H.; Cao, Y.; Zhong, C.; Wang, Y. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 2016, 17, 60. [Google Scholar] [CrossRef]
- Harris, T.A.; Gattu, S.; Propheter, D.C.; Kuang, Z.; Bel, S.; Ruhn, K.A.; Chara, A.L.; Edwards, M.; Zhang, C.; Jo, J.-H.; et al. Resistin-like Molecule a Provides Vitamin-A-Dependent Antimicrobial Protection in the Skin. Cell Host Microbe 2019, 25, 777–788. [Google Scholar] [CrossRef] [PubMed]
| EFFECTS OF VITAMIN A ON HUMAN SKIN | |||
|---|---|---|---|
| Major Cells | Action | ||
| SKIN LAYER | EPIDERMIS | Keratinocytes | Exfoliation of dead cells Proliferation of live cells Strengthening of the epidermal barrier Mitigation of TEWL |
| DERMIS | Fibroblasts | Activation of fibroblast production Stimulation of fibroblasts Elevation of the production of collagen and elastin Protection of collagen destruction by affecting the synthesis of tissue inhibitors of metalloproteinases | |
| Endothelial cells | Synthesis of new capillary networks | ||
| Control | Wilcoxon Test (p) Control | Retinol | Wilcoxon Test (p) Retinol | Mann–Whitney Test (p) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incubation | R Initial (/cm2) | R Final (/cm2) | R Initial vs. R Final | R Initial (/cm2) | R Final (/cm2) | R Initial vs. R Final | R Initial: Control vs. Retinol | R Final: Control vs. Retinol | |
| RS (n = 32) | Median | 11,779 | 12,907 | 0.059 | 34,770 | 30,765 | 0.030 | <0.001 | 0.015 |
| Lower quartile | 5417 | 6565 | 13,786 | 4580 | |||||
| Upper quartile | 28,492 | 28,891 | 68,681 | 61,651 | |||||
| Ami (n = 30) | Median | 3101 | 3108 | 0.594 | 27,525 | 23,368 | <0.001 | 0.004 | 0.005 |
| Lower quartile | 2202 | 1959 | 4473 | 3784 | |||||
| Upper quartile | 6700 | 3989 | 67,306 | 62,322 | |||||
| Bume (n = 32) | Median | 11,759 | 10,904 | 0.629 | 20,941 | 19,773 | 0.002 | 0.296 | 0.390 |
| Lower quartile | 4857 | 4141 | 4991 | 5130 | |||||
| Upper quartile | 31,662 | 31,728 | 43,416 | 35,878 | |||||
| Mann–Whitney test (p) | RS vs. Ami | 0.011 | 0.003 | 0.465 | 0.500 | ||||
| RS vs. Bume | 0.994 | 0.733 | 0.021 | 0.044698 | |||||
| Ami vs. Bume | 0.009 | 0.003 | 0.287 | 0.378340 | |||||
| Control | Wilcoxon Test (p) Control | Retinol | Wilcoxon Test (p) Retinol | Mann–Whitney Test (p) | |||||
| Incubation | PD Initial (mV) | PD Final (mV) | PD Initial vs. PD Final | PD Initial (mV) | PD Final (mV) | PD Initial vs. PD Final | PD Initial: Control vs. Retinol | PD Final: Control vs. Retinol | |
| RS (n = 32) | Median | −0.22 | −0.25 | 0.220473 | 0.11 | 0.18 | 0.798912 | <0.001 | <0.001 |
| Lower quartile | −0.56 | −0.40 | −0.07 | −0.09 | |||||
| Upper quartile | 0 | 0 | 0.56 | 0.49 | |||||
| Ami (n = 30) | Median | 0 | 0 | 0.399309 | −0.14 | −0.04 | 0.037199 | 0.080973 | 0.853131 |
| Lower quartile | −0.21 | −0.24 | −0.34 | −0.18 | |||||
| Upper quartile | 0.18 | 0.13 | 0.05 | 0.07 | |||||
| Bume (n = 32) | Median | 0.32 | 0.37 | 0.127114 | −0.24 | −0.13 | <0.001 | 0.002119 | 0.001038 |
| Lower quartile | −0.15 | 0 | −0.47 | −0.37 | |||||
| Upper quartile | 0.43 | 0.49 | −0.03 | −0.04 | |||||
| Mann–Whitney test (p) | RH vs. Ami | 0.002452 | 0.078170 | < 0.001 | 0.006976 | ||||
| RH vs. Bume | <0.001 | <0.001 | < 0.001 | < 0.001 | |||||
| Ami vs. Bume | 0.137087 | 0.135974 | 0.422000 | 0.220566 | |||||
| Control | Retinol | Mann–Whitney Test (p) | |||||
|---|---|---|---|---|---|---|---|
| Stimulation | PDmax (mV) | PDmin (mV) | PDmax (mV) | PDmin (mV) | PDmax: Control vs. Retinol | PDmin: Control vs. Retinol | |
| RS (n = 32) | Median | 0.87 | −0.5 | 1.60 | −1.2 | 0.038378 | 0.074359 |
| Lower quartile | 0.21 | −1.07 | 0.55 | −2.64 | |||
| Upper quartile | 2.72 | −0.21 | 4.33 | −0.03 | |||
| Ami (n = 30) | Median | 0.21 | −0.29 | 1.575 | −0.77 | <0.001 | <0.001 |
| Lower quartile | 0.00 | −1.1 | 0.34 | −3.11 | |||
| Upper quartile | 1.04 | 0.00 | 4.85 | −0.43 | |||
| Bume (n = 32) | Median | 1.95 | −0.55 | 0.58 | −0.82 | 0.003968 | 0.024506 |
| Lower quartile | 1.07 | −1.83 | 0.46 | −1.63 | |||
| Upper quartile | 6.41 | 0.15 | 5.66 | −0.37 | |||
| Mann–Whitney test (p) | RH vs. Ami | 0.003475 | 0.038445 | 0.646064 | 0.445383 | ||
| RH vs. Bume | 0.004527 | 0.637409 | 0.102272 | 0.335190 | |||
| Ami vs. Bume | <0.001 | 0.140427 | 0.065451 | 0.101114 | |||
| Control | Retinol | |||||
|---|---|---|---|---|---|---|
| Parameters | RS | Ami | Bume | RS | Ami | Bume |
| PD vs. PDmax | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| PD vs. PDmin | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| PDmax vs. PDmin | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dłubała, K.; Wasiek, S.; Pilarska, P.; Szewczyk-Golec, K.; Mila-Kierzenkowska, C.; Łączkowski, K.Z.; Sobiesiak, M.; Gackowski, M.; Tylkowski, B.; Hołyńska-Iwan, I. The Influence of Retinol Ointment on Rabbit Skin (Oryctolagus cuniculus) Ion Transport—An In Vitro Study. Int. J. Mol. Sci. 2024, 25, 9670. https://doi.org/10.3390/ijms25179670
Dłubała K, Wasiek S, Pilarska P, Szewczyk-Golec K, Mila-Kierzenkowska C, Łączkowski KZ, Sobiesiak M, Gackowski M, Tylkowski B, Hołyńska-Iwan I. The Influence of Retinol Ointment on Rabbit Skin (Oryctolagus cuniculus) Ion Transport—An In Vitro Study. International Journal of Molecular Sciences. 2024; 25(17):9670. https://doi.org/10.3390/ijms25179670
Chicago/Turabian StyleDłubała, Klaudia, Sandra Wasiek, Patrycja Pilarska, Karolina Szewczyk-Golec, Celestyna Mila-Kierzenkowska, Krzysztof Z. Łączkowski, Marta Sobiesiak, Marcin Gackowski, Bartosz Tylkowski, and Iga Hołyńska-Iwan. 2024. "The Influence of Retinol Ointment on Rabbit Skin (Oryctolagus cuniculus) Ion Transport—An In Vitro Study" International Journal of Molecular Sciences 25, no. 17: 9670. https://doi.org/10.3390/ijms25179670
APA StyleDłubała, K., Wasiek, S., Pilarska, P., Szewczyk-Golec, K., Mila-Kierzenkowska, C., Łączkowski, K. Z., Sobiesiak, M., Gackowski, M., Tylkowski, B., & Hołyńska-Iwan, I. (2024). The Influence of Retinol Ointment on Rabbit Skin (Oryctolagus cuniculus) Ion Transport—An In Vitro Study. International Journal of Molecular Sciences, 25(17), 9670. https://doi.org/10.3390/ijms25179670










