Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice
Abstract
1. Introduction
2. Results
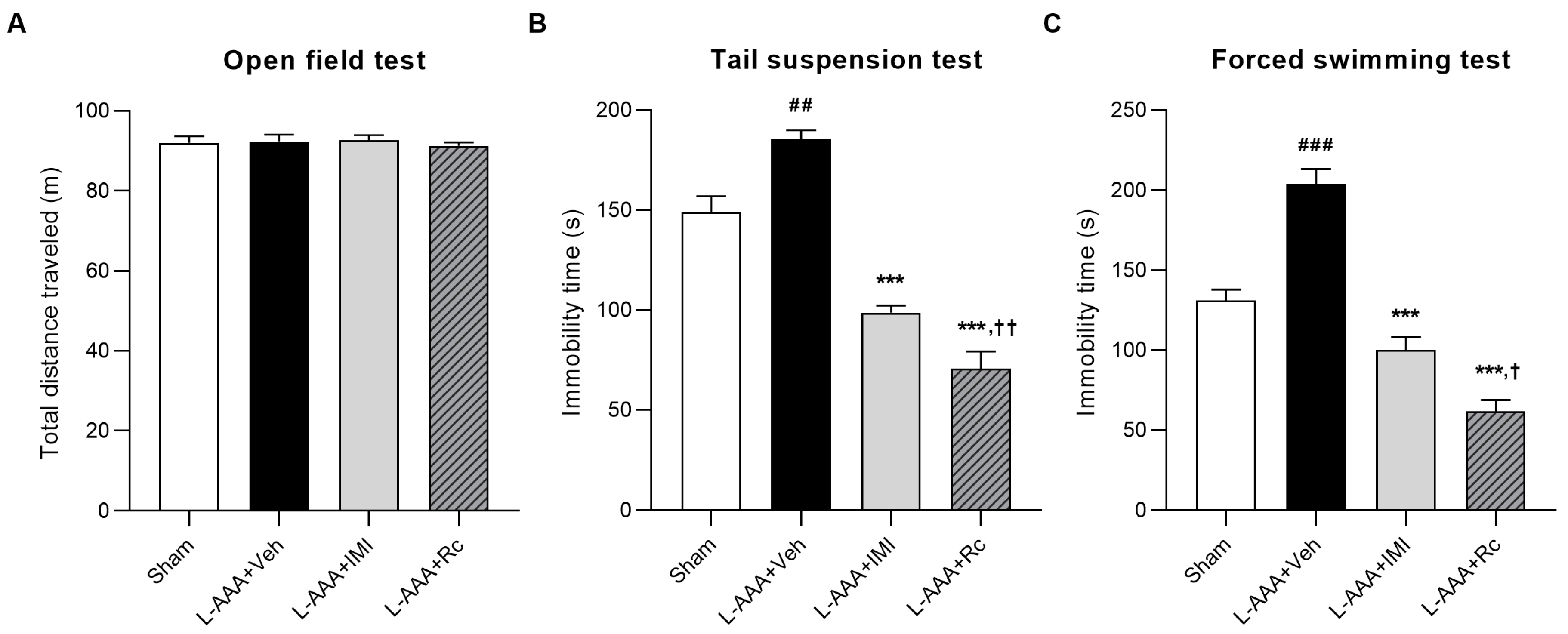
2.1. Effects of G-Rc Administration on the Depression-like Behavior of L-AAA-Induced Depression in Mice
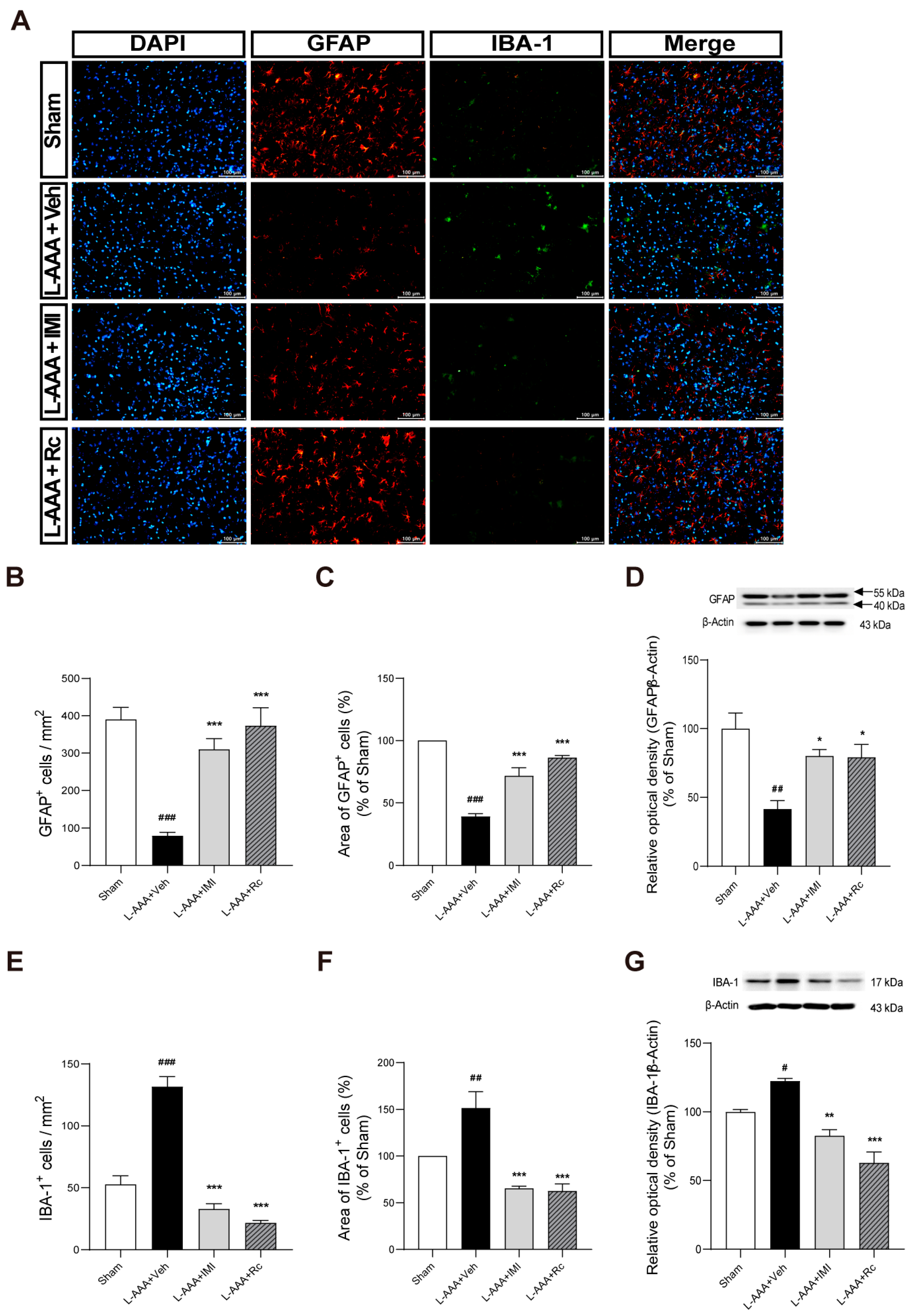
2.2. Anti-Inflammatory Effects of G-Rc on L-AAA-Infused Mice
2.3. Effects of G-Rc on Downregulation of GFAP and Upregulation of IBA-1 Caused by L-AAA Infusion
2.4. Effects of G-Rc on Apoptosis-Related Proteins Following L-AAA Infusion
3. Discussion
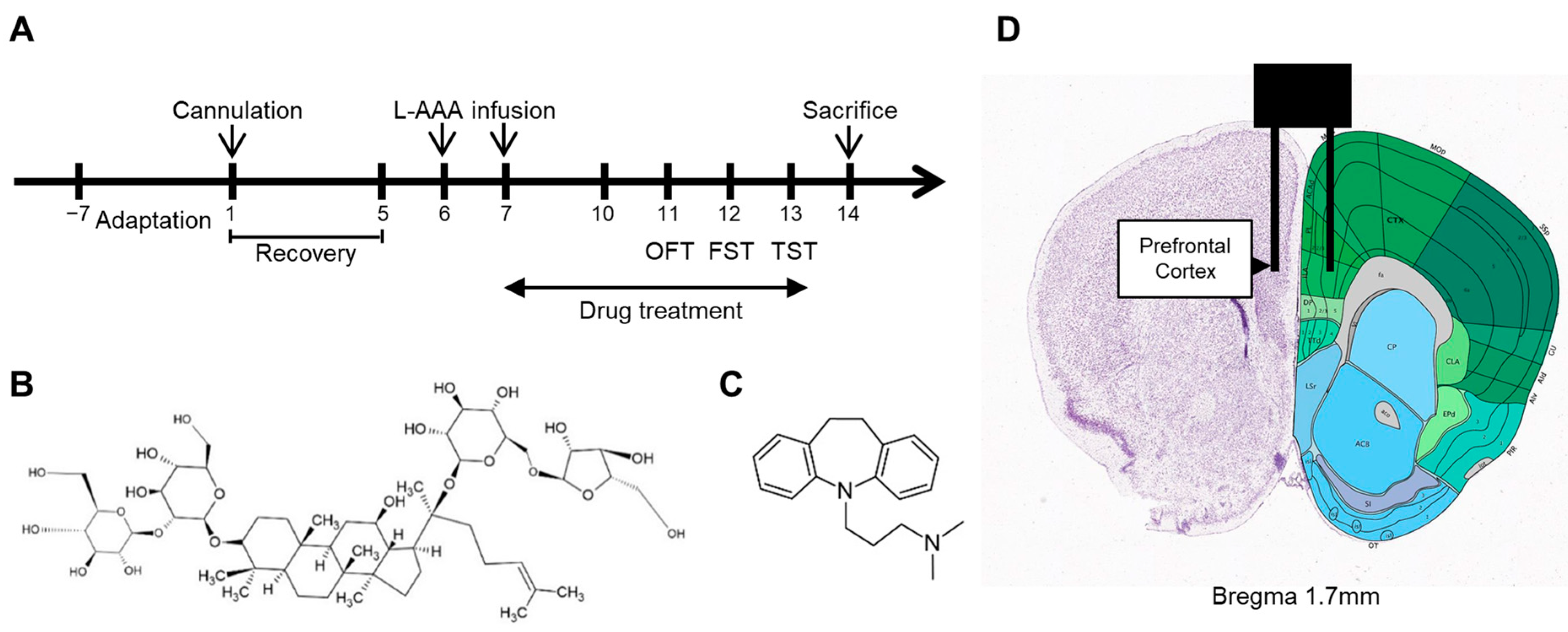
4. Materials and Methods
4.1. Animals
4.2. Drug Administration
4.3. Cannula Implantation and Injection of L-AAA
4.4. Behavioral Tests
4.4.1. Open-Field Test
4.4.2. Tail Suspension Test
4.4.3. Forced Swimming Test
4.5. Tissue Collection
4.6. ELISA
4.7. RNA Extraction and cDNA Synthesis
4.8. Quantitative Real-Time PCR
4.9. Immunofluorescence
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| FST | Forced swimming test |
| GABA | Gamma-aminobutyric acid |
| GFAP | Glial fibrillary acidic protein |
| G-Rc | Ginsenoside Rc |
| IBA-1 | Ionized calcium-binding adapter molecule 1 |
| IL-6 | Interleukin-6 |
| IMI | Imipramine |
| L-AAA | L-alpha-aminoadipic acid |
| LCN2 | Lipocalin-2 |
| MDD | Major depressive disorder |
| NeuN | Neuronal nuclei antigen |
| OCT | Optimal cutting temperature |
| OFT | Open-field test |
| PFC | Prefrontal cortex |
| Real-time PCR | Real-time polymerase chain reaction |
| TBST | Tris-buffered saline with Tween |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
| TST | Tail suspension test |
References
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci Rep 2018, 8, 2861. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Alemi, F.; Min, H.; Yousefi, M.; Becker, L.K.; Hane, C.A.; Nori, V.S.; Wojtusiak, J. Effectiveness of Common Antidepressants: A Post Market Release Study. eClinicalMedicine 2021, 41, 101171. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant Depression: Definition, Prevalence, Detection, Management, and Investigational Interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry 2022, 28, 3243–3256. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Baucom, C.; Dilley, G.; Overholser, J.C.; Meltzer, H.Y.; Stockmeier, C.A.; Rajkowska, G. Glial Fibrillary Acidic Protein Immunoreactivity in the Prefrontal Cortex Distinguishes Younger from Older Adults in Major Depressive Disorder. Biol. Psychiatry 2000, 48, 861–873. [Google Scholar] [CrossRef]
- Rajkowska, G.; A Stockmeier, C. Astrocyte Pathology in Major Depressive Disorder: Insights from Human Postmortem Brain Tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- Wang, Q.; Jie, W.; Liu, J.-H.; Yang, J.-M.; Gao, T.-M. An Astroglial Basis of Major Depressive Disorder? An Overview. Glia 2017, 65, 1227–1250. [Google Scholar] [CrossRef]
- Domin, H.; Szewczyk, B.; Pochwat, B.; Woźniak, M.; Śmiałowska, M. Antidepressant-like Activity of the Neuropeptide Y Y5 Receptor Antagonist Lu AA33810: Behavioral, Molecular, and Immunohistochemical Evidence. Psychopharmacology 2017, 234, 631–645. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yi, S.; Jiang, X.; Qiao, Y.; Zhang, Y.; Xiao, C.; Zhou, T. Mouse Astrocytes Promote Microglial Ramification by Releasing TGF-β and Forming Glial Fibers. Front. Cell. Neurosci. 2020, 14, 195. [Google Scholar] [CrossRef]
- Afridi, R.; Suk, K. Neuroinflammatory Basis of Depression: Learning from Experimental Models. Front. Cell. Neurosci. 2021, 15, 691067. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Gliogenesis and Glial Pathology in Depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Lee, S.; Park, D.H.; Kook, H.; Park, K.-G.; Lee, I.-K.; Suk, K. Diverse Functional Roles of Lipocalin-2 in the Central Nervous System. Neurosci. Biobehav. Rev. 2015, 49, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Huang, Z.; Zang, Z.; Qiao, X.; Yan, J.; Shao, X. Identifying Circulating Biomarkers for Major Depressive Disorder. Front. Psychiatry 2023, 14, 1230246. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, C.; Rao, X.; Wan, W.; Lin, W.; Huang, S.; Ying, J.; Lin, Y.; Hua, F. The Interaction of Lipocalin-2 and Astrocytes in Neuroinflammation: Mechanisms and Therapeutic Application. Front. Immunol. 2024, 15, 1358719. [Google Scholar] [CrossRef]
- Akter, M.S.; Emon, F.A.; Nahar, Z.; Shalahuddin Qusar, M.; Islam, S.M.A.; Shahriar, M.; Bhuiyan, M.A.; Islam, M.R. Altered IL-3 and Lipocalin-2 Levels Are Associated with the Pathophysiology of Major Depressive Disorder: A Case-Control Study. BMC Psychiatry 2023, 23, 830. [Google Scholar] [CrossRef] [PubMed]
- Naudé, P.J.W.; Eisel, U.L.M.; Comijs, H.C.; Groenewold, N.A.; De Deyn, P.P.; Bosker, F.J.; Luiten, P.G.M.; den Boer, J.A.; Oude Voshaar, R.C. Neutrophil Gelatinase-Associated Lipocalin: A Novel Inflammatory Marker Associated with Late-Life Depression. J. Psychosom. Res. 2013, 75, 444–450. [Google Scholar] [CrossRef]
- Stankiewicz, A.M.; Goscik, J.; Majewska, A.; Swiergiel, A.H.; Juszczak, G.R. The Effect of Acute and Chronic Social Stress on the Hippocampal Transcriptome in Mice. PLoS ONE 2015, 10, e0142195. [Google Scholar] [CrossRef]
- Saito-Takatsuji, H.; Yoshitomi, Y.; Yamamoto, R.; Furuyama, T.; Ishigaki, Y.; Kato, N.; Yonekura, H.; Ikeda, T. Transthyretin Is Commonly Upregulated in the Hippocampus of Two Stress-Induced Depression Mouse Models. Int. J. Mol. Sci. 2023, 24, 3736. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, S.-H. The Effect of Ginsenosides on Depression in Preclinical Studies: A Systematic Review and Meta-Analysis. J. Ginseng Res. 2021, 45, 420–432. [Google Scholar] [CrossRef]
- Dang, H.; Chen, Y.; Liu, X.; Wang, Q.; Wang, L.; Jia, W.; Wang, Y. Antidepressant Effects of Ginseng Total Saponins in the Forced Swimming Test and Chronic Mild Stress Models of Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Lee, B. Wild Ginseng Attenuates Anxiety- and Depression-Like Behaviors During Morphine Withdrawal. J. Microbiol. Biotechnol. 2011, 21, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Araki, H.; Yoshimura, H. Identification of Antidepressant-like Ingredients in Ginseng Root (Panax Ginseng CA Meyer) Using a Menopausal Depressive-like State in Female Mice: Participation of 5-HT 2A Receptors. Psychopharmacology 2011, 216, 589–599. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, L.; Xiang, H. Ginsenoside Rb3 Exerts Antidepressant-like Effects in Several Animal Models. J. Psychopharmacol. 2012, 26, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, H.-Y.; Choi, Y.-J.; Cho, S.-H. Antidepressant Effects of Ginsenoside Rf on Behavioral Change in the Glial Degeneration Model of Depression by Reversing Glial Loss. J. Ginseng Res. 2020, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liang, Y.; Kang, A.; Ma, S.-J.; Xing, L.; Zhou, Y.-Y.; Dai, C.; Xie, H.; Xie, L.; Wang, G.-J. Peripheral Immunomodulation with Ginsenoside Rg1 Ameliorates Neuroinflammation-Induced Behavioral Deficits in Rats. Neuroscience 2014, 256, 210–222. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, Y.; Cheng, Z.; Zhu, X.; Fan, C.; Yu, S.Y. The Effects of Ginsenoside Rg1 on Chronic Stress Induced Depression-like Behaviors, BDNF Expression and the Phosphorylation of PKA and CREB in Rats. Neuroscience 2016, 322, 358–369. [Google Scholar] [CrossRef]
- Xu, C.; Teng, J.; Chen, W.; Ge, Q.; Yang, Z.; Yu, C.; Yang, Z.; Jia, W. 20(S)-Protopanaxadiol, an Active Ginseng Metabolite, Exhibits Strong Antidepressant-like Effects in Animal Tests. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1402–1411. [Google Scholar] [CrossRef]
- Park, S.-E.; Na, C.-S.; Yoo, S.-A.; Seo, S.-H.; Son, H.-S. Biotransformation of Major Ginsenosides in Ginsenoside Model Culture by Lactic Acid Bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Y.; Kwak, Y.-S.; Song, G.G.; Kim, M.-Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng Exerts Anti-Inflammatory Activity by Targeting TANK-Binding Kinase 1/Interferon Regulatory Factor-3 and P38/ATF-2. J. Ginseng Res. 2017, 41, 127–133. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, X.; Zhang, X.; Yu, P.; Li, Y.; Fu, W.; Yu, J.; Sui, D. Protective Effects of Ginsenoside Rc against Acute Cold Exposure-Induced Myocardial Injury in Rats. J. Food Sci. 2021, 86, 3252–3264. [Google Scholar] [CrossRef]
- Shi, L.; Fu, W.; Xu, H.; Li, S.; Yang, X.; Yang, W.; Sui, D.; Wang, Q. Ginsenoside Rc Attenuates Myocardial Ischaemic Injury through Antioxidative and Anti-Inflammatory Effects. Pharm. Biol. 2022, 60, 1038–1046. [Google Scholar] [CrossRef]
- Yu, T.; Rhee, M.H.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.-S.; Kim, J.-H.; et al. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) Attenuates Inflammatory Symptoms of Gastritis, Hepatitis and Arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, C.H.; Park, D.; Choi, Y.J.; Park, M.H.; Chung, K.W.; Kim, S.R.; Lee, J.S.; Chung, H.Y. Ginsenoside Rc Modulates Akt/FoxO1 Pathways and Suppresses Oxidative Stress. Arch. Pharmacal. Res. 2014, 37, 813–820. [Google Scholar] [CrossRef]
- Choi, R.J.; Roy, A.; Jung, H.J.; Ali, M.Y.; Min, B.-S.; Park, C.H.; Yokozawa, T.; Fan, T.-P.; Choi, J.S.; Jung, H.A. BACE1 Molecular Docking and Anti-Alzheimer’s Disease Activities of Ginsenosides. J. Ethnopharmacol. 2016, 190, 219–230. [Google Scholar] [CrossRef]
- Banasr, M.; Duman, R.S. Glial Loss in the Prefrontal Cortex Is Sufficient to Induce Depressive-like Behaviors. Biol. Psychiatry 2008, 64, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Son, H.; Kim, G.; Kim, S.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Kim, H.J. Glutamine Deficiency in the Prefrontal Cortex Increases Depressive-like Behaviours in Male Mice. J. Psychiatry Neurosci. 2013, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, C.; Turan, İ.; Özaçmak, H.S. The Effect of Glucagon like Peptide-1 Receptor Agonist on Behavioral Despair and Anxiety-like Behavior in Ovariectomized Rats: Modulation of BDNF/CREB, Nrf2 and Lipocalin 2. Behav. Brain Res. 2022, 435, 114053. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Mechawar, N. Implication of Cerebral Astrocytes in Major Depression: A Review of Fine Neuroanatomical Evidence in Humans. Glia 2021, 69, 2077–2099. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.-L.; Zhang, X.; Wang, H.; Ye, Y.; Song, L.; Wang, Y.-J.; Tu, M.-J.; Wang, W.-W.; Yang, L.; et al. Antidepressant-like Effects of Ginsenoside Rg2 in a Chronic Mild Stress Model of Depression. Brain Res. Bull. 2017, 134, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Z.; Chen, Z.; Zhong, Z.; Li, Z. Ginsenoside Rg3 Exerts Anti-Depressive Effect on an NMDA-Treated Cell Model and a Chronic Mild Stress Animal Model. J. Pharmacol. Sci. 2017, 134, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sang, W.; Yang, X.; Zhai, M.; Wang, L.; Qin, P.; Wu, L.; Zhou, X.; Wang, L.; Li, J.; et al. Antidepressant Effects of Ginsenosides from Panax notoginseng. J. Integr. Agric. 2012, 11, 483–488. [Google Scholar] [CrossRef]
- Yu, H.; Fan, C.; Yang, L.; Yu, S.; Song, Q.; Wang, P.; Mao, X. Ginsenoside Rg1 Prevents Chronic Stress-Induced Depression-Like Behaviors and Neuronal Structural Plasticity in Rats. Cell. Physiol. Biochem. 2018, 48, 2470–2482. [Google Scholar] [CrossRef]
- Huang, Q.; Chu, S.; Zhang, J.; Chen, N. Effects of Ginsenoside Rg1 on Anti-Depression and Synaptic Ultrastructure. Chin. Pharmacol. Bull. 2013, 29, 1124–1127. [Google Scholar]
- Jin, C.; Wang, Z.-Z.; Zhou, H.; Lou, Y.-X.; Chen, J.; Zuo, W.; Tian, M.-T.; Wang, Z.-Q.; Du, G.-H.; Kawahata, I.; et al. Ginsenoside Rg1-Induced Antidepressant Effects Involve the Protection of Astrocyte Gap Junctions within the Prefrontal Cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 183–191. [Google Scholar] [CrossRef]
- Wu, H.-F.; Zhu, C.-H.; Guo, J.-Y. Effect of ginsenoside Rg1 on behaviors and hippocampal amino acids in depressive-like rats. Zhongguo Zhong Yao Za Zhi 2012, 37, 3117–3121. [Google Scholar]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effect of Ginsenoside Re on Depression- and Anxiety-Like Behaviors and Cognition Memory Deficit Induced by Repeated Immobilization in Rats. J. Microbiol. Biotechnol. 2012, 22, 708–720. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Yao, Q.; Shen, J.; Gu, Z.; Xu, H.; Wu, Z.; Chen, C.; Li, L. Antidepressant-like Effects of Ginsenoside Rg3 in Mice via Activation of the Hippocampal BDNF Signaling Cascade. J. Nat. Med. 2017, 71, 367–379. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Zhao, W.; Gao, S.; Cui, Z. Antidepressant-like Effects of Ginsenoside Rg5 in Mice: Involving of Hippocampus BDNF Signaling Pathway. Neurosci. Lett. 2017, 645, 97–105. [Google Scholar] [CrossRef]
- Duong, Q.; Nguyen, P.; Nguyen, H.; Nguyen, D. Effects of Ocotillol-Type Saponins Majonoside-R1 and Vina-Ginsenoside-R2 on Abrogating Depression and Neuronal Oxidative Stress in Socially Isolated Depression Mouse Model. Int. J. Appl. Res. Nat. Prod. 2016, 9, 27–32. [Google Scholar]
- Chen, L.; Qi, Z.; Shao, Z.; Li, S.; Qi, Y.; Gao, K.; Liu, S.; Li, Z.; Sun, Y.; Li, P. Study on Antidepressant Activity of Pseudo-Ginsenoside HQ on Depression-Like Behavior in Mice. Molecules 2019, 24, 870. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Legutko, B.; Moulana, M.; Syed, M.; Romero, D.G.; Stockmeier, C.A.; Miguel-Hidalgo, J.J. Astrocyte Pathology in the Ventral Prefrontal White Matter in Depression. J. Psychiatr. Res. 2018, 102, 150–158. [Google Scholar] [CrossRef]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-Immunoreactive Astrocytes Is Decreased in Left Hippocampi in Major Depressive Disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef]
- Altshuler, L.L.; Abulseoud, O.A.; Foland-Ross, L.; Bartzokis, G.; Chang, S.; Mintz, J.; Hellemann, G.; Vinters, H.V. Amygdala Astrocyte Reduction in Subjects with Major Depressive Disorder but Not Bipolar Disorder. Bipolar Disord. 2010, 12, 541–549. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Belliveau, C.; Davoli, M.A.; Ma, J.C.; Tanti, A.; Turecki, G.; Mechawar, N. Widespread Decrease of Cerebral Vimentin-Immunoreactive Astrocytes in Depressed Suicides. Front. Psychiatry 2021, 12, 640963. [Google Scholar] [CrossRef]
- Öngür, D.; Drevets, W.C.; Price, J.L. Glial Reduction in the Subgenual Prefrontal Cortex in Mood Disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric Evidence for Neuronal and Glial Prefrontal Cell Pathology in Major Depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Q.; Xie, L.; Yang, F.; Wang, L.; Tu, J. Astrocyte, a Promising Target for Mood Disorder Interventions. Front. Mol. Neurosci. 2019, 12, 136. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Z.; Chen, L.; Ouyang, L.; Gu, L.; Chen, F.; Zhang, Q. Hippocampal Astrocyte Atrophy in a Mouse Depression Model Induced by Corticosterone Is Reversed by Fluoxetine Instead of Benzodiazepine Diazepam. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 83, 99–109. [Google Scholar] [CrossRef]
- Tsai, M.; Chang, Y.; Schwarcz, R.; Brookes, N. Characterization of L-Alpha-Aminoadipic Acid Transport in Cultured Rat Astrocytes. Brain Res. 1996, 741, 166–173. [Google Scholar] [CrossRef]
- Bansal, Y.; Codeluppi, S.A.; Banasr, M. Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target. Int. J. Mol. Sci. 2024, 25, 6357. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. De Pharmacodyn. Et De Ther. 1977, 229, 327–336. [Google Scholar]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The Tail Suspension Test as a Model for Assessing Antidepressant Activity: Review of Pharmacological and Genetic Studies in Mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Chenu, F.; Ripoll, N.; David, D.J.P. A Proposal of Decision Tree to Screen Putative Antidepressants Using Forced Swim and Tail Suspension Tests. Behav. Brain Res. 2005, 164, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5166 Patients and 5083 Controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef]
- Hostenbach, S.; Cambron, M.; D’haeseleer, M.; Kooijman, R.; De Keyser, J. Astrocyte Loss and Astrogliosis in Neuroinflammatory Disorders. Neurosci. Lett. 2014, 565, 39–41. [Google Scholar] [CrossRef]
- Davis, N.; Mota, B.C.; Stead, L.; Palmer, E.O.C.; Lombardero, L.; Rodríguez-Puertas, R.; de Paola, V.; Barnes, S.J.; Sastre, M. Pharmacological Ablation of Astrocytes Reduces Aβ Degradation and Synaptic Connectivity in an Ex Vivo Model of Alzheimer’s Disease. J. Neuroinflamm. 2021, 18, 73. [Google Scholar] [CrossRef]
- Gouweleeuw, L.; Naudé, P.J.W.; Rots, M.; DeJongste, M.J.L.; Eisel, U.L.M.; Schoemaker, R.G. The Role of Neutrophil Gelatinase Associated Lipocalin (NGAL) as Biological Constituent Linking Depression and Cardiovascular Disease. Brain Behav. Immun. 2015, 46, 23–32. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; Mommersteeg, P.M.C.; Gouweleeuw, L.; Eisel, U.L.M.; Denollet, J.; Westerhuis, L.W.J.J.M.; Schoemaker, R.G. NGAL and Other Markers of Inflammation as Competitive or Complementary Markers for Depressive Symptom Dimensions in Heart Failure. World J. Biol. Psychiatry 2015, 16, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Huang, C.; Tong, J.; Qiu, G.; Huang, B.; Wu, Q.; Li, F.; Xu, Z.; Bowser, R.; Xia, X.-G.; et al. Reactive Astrocytes Secrete Lcn2 to Promote Neuron Death. PNAS 2013, 110, 4069–4074. [Google Scholar] [CrossRef]
- Lee, S.; Lee, W.-H.; Lee, M.-S.; Mori, K.; Suk, K. Regulation by Lipocalin-2 of Neuronal Cell Death, Migration, and Morphology. J. Neurosci. Res. 2012, 90, 540–550. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; den Boer, J.A.; Comijs, H.C.; Bosker, F.J.; Zuidersma, M.; Groenewold, N.A.; De Deyn, P.P.; Luiten, P.G.M.; Eisel, U.L.M.; Voshaar, R.O. Sex-Specific Associations between Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Cognitive Domains in Late-Life Depression. Psychoneuroendocrinology 2014, 48, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, C.; Ko, Y.-H.; Lee, M.-S.; Park, M.H.; Pae, C.-U.; Yoon, H.-K.; Han, C. Plasminogen Activator Inhibitor-1: Potential Inflammatory Marker in Late-Life Depression. Clin. Psychopharmacol. Neurosci. 2023, 21, 147–161. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, F.; Zhang, L.; Li, J.; Tang, S.; Li, X.; Peng, M.; Zhao, Q.; Zhu, X. A Bioinformatics Approach to Identifying the Biomarkers and Pathogenesis of Major Depressive Disorder Combined with Acute Myocardial Infarction. Am. J. Transl. Res. 2023, 15, 932–948. [Google Scholar]
- Chen, Y.; Zheng, D.; Wang, H.; Zhang, S.; Zhou, Y.; Ke, X.; Chen, G. Lipocalin 2 in the Paraventricular Thalamic Nucleus Contributes to DSS-Induced Depressive-Like Behaviors. Neurosci. Bull. 2023, 39, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Kim, J.-H.; Lee, S.; Kim, J.-H.; Seo, J.-W.; Jin, M.; Lee, M.-G.; Jang, I.-S.; Lee, W.-H.; Suk, K. Phenotypic Polarization of Activated Astrocytes: The Critical Role of Lipocalin-2 in the Classical Inflammatory Activation of Astrocytes. J. Immunol. 2013, 191, 5204–5219. [Google Scholar] [CrossRef]
- Lee, S.; Jha, M.K.; Suk, K. Lipocalin-2 in the Inflammatory Activation of Brain Astrocytes. CRI 2015, 35, 77–84. [Google Scholar] [CrossRef]
- Jung, B.-K.; Park, Y.; Yoon, B.; Bae, J.-S.; Han, S.-W.; Heo, J.-E.; Kim, D.-E.; Ryu, K.-Y. Reduced Secretion of LCN2 (Lipocalin 2) from Reactive Astrocytes through Autophagic and Proteasomal Regulation Alleviates Inflammatory Stress and Neuronal Damage. Autophagy 2023, 19, 2296–2317. [Google Scholar] [CrossRef]
- Afridi, R.; Kim, J.-H.; Bhusal, A.; Lee, W.-H.; Suk, K. Lipocalin-2 as a Mediator of Neuroimmune Communication. J. Leukoc. Biol. 2023, 116, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Tang, X.; Liang, L.; Wang, F.; Du, H. Lipocalin 2 Protects Against Escherichia Coli Infection by Modulating Neutrophil and Macrophage Function. Front. Immunol. 2019, 10, 2594. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-H.; Kim, J.-H.; Seo, J.-W.; Han, H.-S.; Lee, W.-H.; Mori, K.; Nakao, K.; Barasch, J.; Suk, K. Lipocalin-2 Is a Chemokine Inducer in the Central Nervous System. J. Biol. Chem. 2011, 286, 43855–43870. [Google Scholar] [CrossRef]
- Norden, D.M.; Fenn, A.M.; Dugan, A.; Godbout, J.P. TGFβ Produced by IL-10 Redirected Astrocytes Attenuates Microglial Activation. Glia 2014, 62, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Hiew, L.-F.; Poon, C.-H.; You, H.-Z.; Lim, L.-W. TGF-β/Smad Signalling in Neurogenesis: Implications for Neuropsychiatric Diseases. Cells 2021, 10, 1382. [Google Scholar] [CrossRef] [PubMed]
- Mihailova, S.; Ivanova-Genova, E.; Lukanov, T.; Stoyanova, V.; Milanova, V.; Naumova, E. A Study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ Gene Polymorphisms in Patients with Depression. J. Neuroimmunol. 2016, 293, 123–128. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, F.; Li, P.; Song, C. Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression. Int. J. Mol. Sci. 2022, 23, 13856. [Google Scholar] [CrossRef]
- Trojan, E.; Ślusarczyk, J.; Chamera, K.; Kotarska, K.; Głombik, K.; Kubera, M.; Basta-Kaim, A. The Modulatory Properties of Chronic Antidepressant Drugs Treatment on the Brain Chemokine—Chemokine Receptor Network: A Molecular Study in an Animal Model of Depression. Front. Pharmacol. 2017, 8, 779. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Yu, G.-R.; Lee, M.-J.; Lee, S.-Y.; Chu, I.-S.; Leem, S.-H.; Kim, D.-G. Lipocalin-2 Negatively Modulates the Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma through the Epidermal Growth Factor (TGF-Beta1)/Lcn2/Twist1 Pathway. Hepatology 2013, 58, 1349–1361. [Google Scholar] [CrossRef]
- Domin, H.; Konieczny, J.; Cieślik, P.; Pochwat, B.; Wyska, E.; Szafarz, M.; Lenda, T.; Biała, D.; Gąsior, Ł.; Śmiałowska, M.; et al. The Antidepressant-like and Glioprotective Effects of the Y2 Receptor Antagonist SF-11 in the Astroglial Degeneration Model of Depression in Rats: Involvement of Glutamatergic Inhibition. Behav. Brain Res. 2023, 457, 114729. [Google Scholar] [CrossRef]
- Nishimura, R.; Santos, D.; Fu, S.; Dwyer, B. Induction of Cell Death by L-Alpha-Aminoadipic Acid Exposure in Cultured Rat Astrocytes: Relationship to Protein Synthesis. Neurotoxicology 2000, 21, 313–320. [Google Scholar] [PubMed]
- Lee, S.; Park, J.-Y.; Lee, W.-H.; Kim, H.; Park, H.-C.; Mori, K.; Suk, K. Lipocalin-2 Is an Autocrine Mediator of Reactive Astrocytosis. J. Neurosci. 2009, 29, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Simon, H.U. Granulocyte Apoptosis: Death by a Secreted Lipocalin? Cell Death Differ. 2002, 9, 595–597. [Google Scholar] [CrossRef]
- Devireddy, L.R.; Gazin, C.; Zhu, X.; Green, M.R. A Cell-Surface Receptor for Lipocalin 24p3 Selectively Mediates Apoptosis and Iron Uptake. Cell 2005, 123, 1293–1305. [Google Scholar] [CrossRef]
- Tong, Z.; Wu, X.; Kehrer, J.P. Increased Expression of the Lipocalin 24p3 as an Apoptotic Mechanism for MK886. Biochem. J. 2003, 372, 203–210. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hwang, J.-T.; Kim, S.; Yoon, S.; Kim, M.-S.; Yang, H.J.; Kwon, D.Y. Ginsenoside Rc, an Active Component of Panax Ginseng, Stimulates Glucose Uptake in C2C12 Myotubes through an AMPK-Dependent Mechanism. J. Ethnopharmacol. 2010, 127, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, W.; Xue, Y.; Lu, Z.; Li, Y.; Yu, P.; Yu, X.; Xu, H.; Sui, D. Ginsenoside Rc Ameliorates Endothelial Insulin Resistance via Upregulation of Angiotensin-Converting Enzyme 2. Front. Pharmacol. 2021, 12, 620524. [Google Scholar] [CrossRef]
- Yang, J.-W.; Kim, S.S. Ginsenoside Rc Promotes Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Down-Regulating C/EBPα and PPARγ. Molecules 2015, 20, 1293–1303. [Google Scholar] [CrossRef]
- Choi, S.-E.; Choi, S.; Lee, J.-H.; Whiting, P.J.; Lee, S.-M.; Nah, S.-Y. Effects of Ginsenosides on GABA A Receptor Channels Expressed in Xenopus Oocytes. Arch. Pharmacal. Res. 2003, 26, 28–33. [Google Scholar] [CrossRef]
- Xue, Y.; Fu, W.; Yu, P.; Li, Y.; Yu, X.; Xu, H.; Sui, D. Ginsenoside Rc Alleviates Myocardial Ischemia-Reperfusion Injury by Reducing Mitochondrial Oxidative Stress and Apoptosis: Role of SIRT1 Activation. J. Agric. Food Chem. 2023, 71, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Chen, L.-D.; Tsauer, W.; Tsai, C.C.; Chen, B.-C.; Chen, Y.-J. Effects of Ginsenoside Rb2 and Rc on Inferior Human Sperm Motility In Vitro. Am. J. Chin. Med. 2001, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Peng, W.; Xia, Z.-A.; Wang, Y.; Chen, Z.; Su, N.; Wang, Z. The Impact of Ginsenosides on Cognitive Deficits in Experimental Animal Studies of Alzheimer’s Disease: A Systematic Review. BMC Complement. Altern. Med. 2015, 15, 386. [Google Scholar] [CrossRef]
- Du, Y.; Fu, M.; Wang, Y.T.; Dong, Z. Neuroprotective Effects of Ginsenoside Rf on Amyloid-β-Induced Neurotoxicity in Vitro and in Vivo. JAD 2018, 64, 309–322. [Google Scholar] [CrossRef]
- Chu, S.; Gu, J.; Feng, L.; Liu, J.; Zhang, M.; Jia, X.; Liu, M.; Yao, D. Ginsenoside Rg5 Improves Cognitive Dysfunction and Beta-Amyloid Deposition in STZ-Induced Memory Impaired Rats via Attenuating Neuroinflammatory Responses. Int. Immunopharmacol. 2014, 19, 317–326. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xing, Y.; Gong, L.; Li, H.; Wu, Z.; Li, Y.; Wang, J.; Wang, Y.; Dong, L.; et al. Effects of Ginsenoside Rg1 or 17β-Estradiol on a Cognitively Impaired, Ovariectomized Rat Model of Alzheimer’s Disease. Neuroscience 2012, 220, 191–200. [Google Scholar] [CrossRef]
- Nah, S.-Y.; Kim, D.-H.; Rhim, H. Ginsenosides: Are Any of Them Candidates for Drugs Acting on the Central Nervous System? CNS Drug Rev. 2007, 13, 381–404. [Google Scholar] [CrossRef]
- Allen Institute for Brain Science. Allen Mouse Brain Atlas (Dataset). 2004. Available online: https://mouse.brain-map.org/ (accessed on 8 July 2024).
- Allen Institute for Brain Science. Allen Reference Atlas—Mouse Brain (Brain Atlas). 2011. Available online: https://atlas.brain-map.org/ (accessed on 8 July 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Kim, Y.; Cho, S.-H. Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice. Int. J. Mol. Sci. 2024, 25, 9673. https://doi.org/10.3390/ijms25179673
Kwon D, Kim Y, Cho S-H. Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice. International Journal of Molecular Sciences. 2024; 25(17):9673. https://doi.org/10.3390/ijms25179673
Chicago/Turabian StyleKwon, Dohyung, Yunna Kim, and Seung-Hun Cho. 2024. "Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice" International Journal of Molecular Sciences 25, no. 17: 9673. https://doi.org/10.3390/ijms25179673
APA StyleKwon, D., Kim, Y., & Cho, S.-H. (2024). Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice. International Journal of Molecular Sciences, 25(17), 9673. https://doi.org/10.3390/ijms25179673






