Mesocorticolimbic and Cardiometabolic Diseases—Two Faces of the Same Coin?
Abstract
:1. Introduction
2. Results
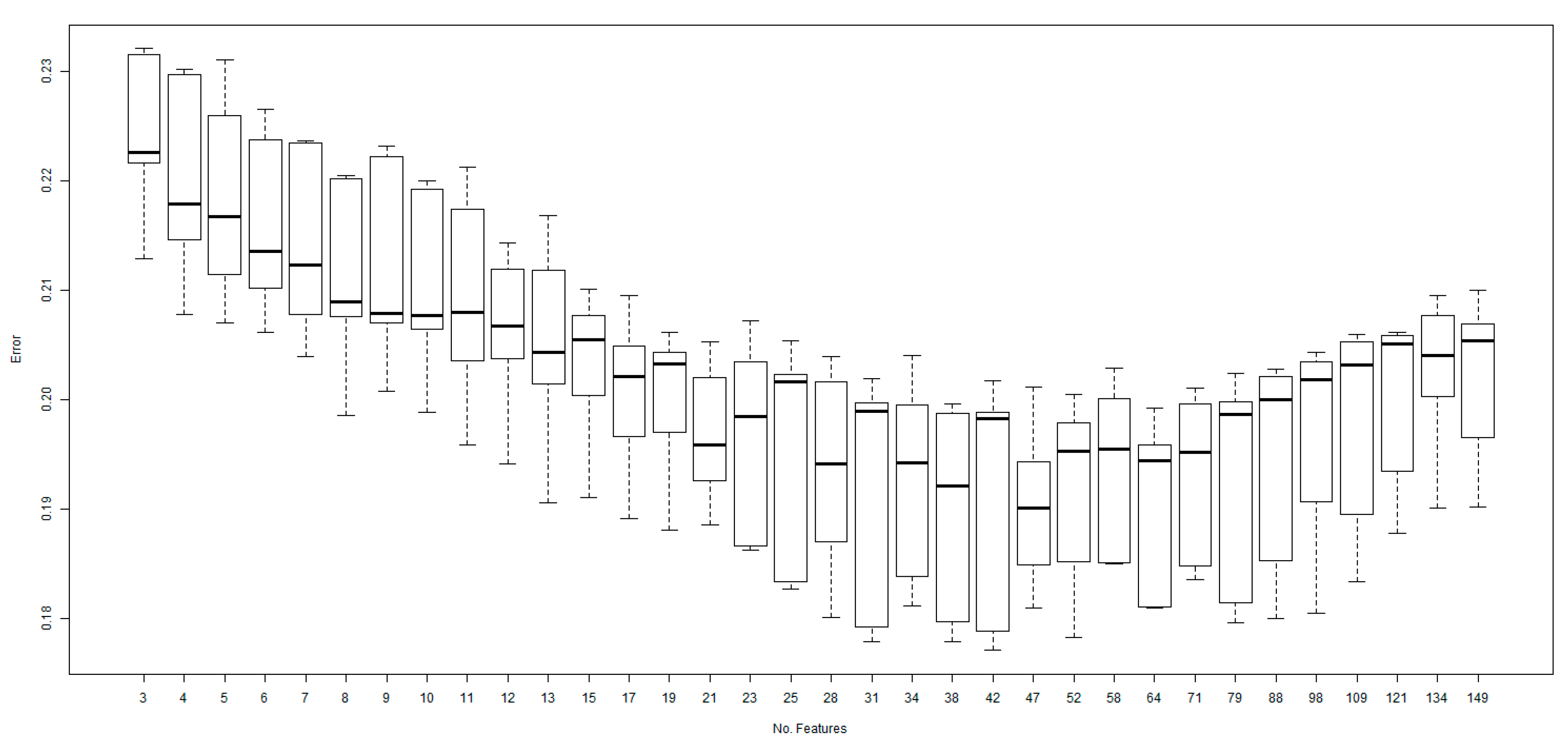
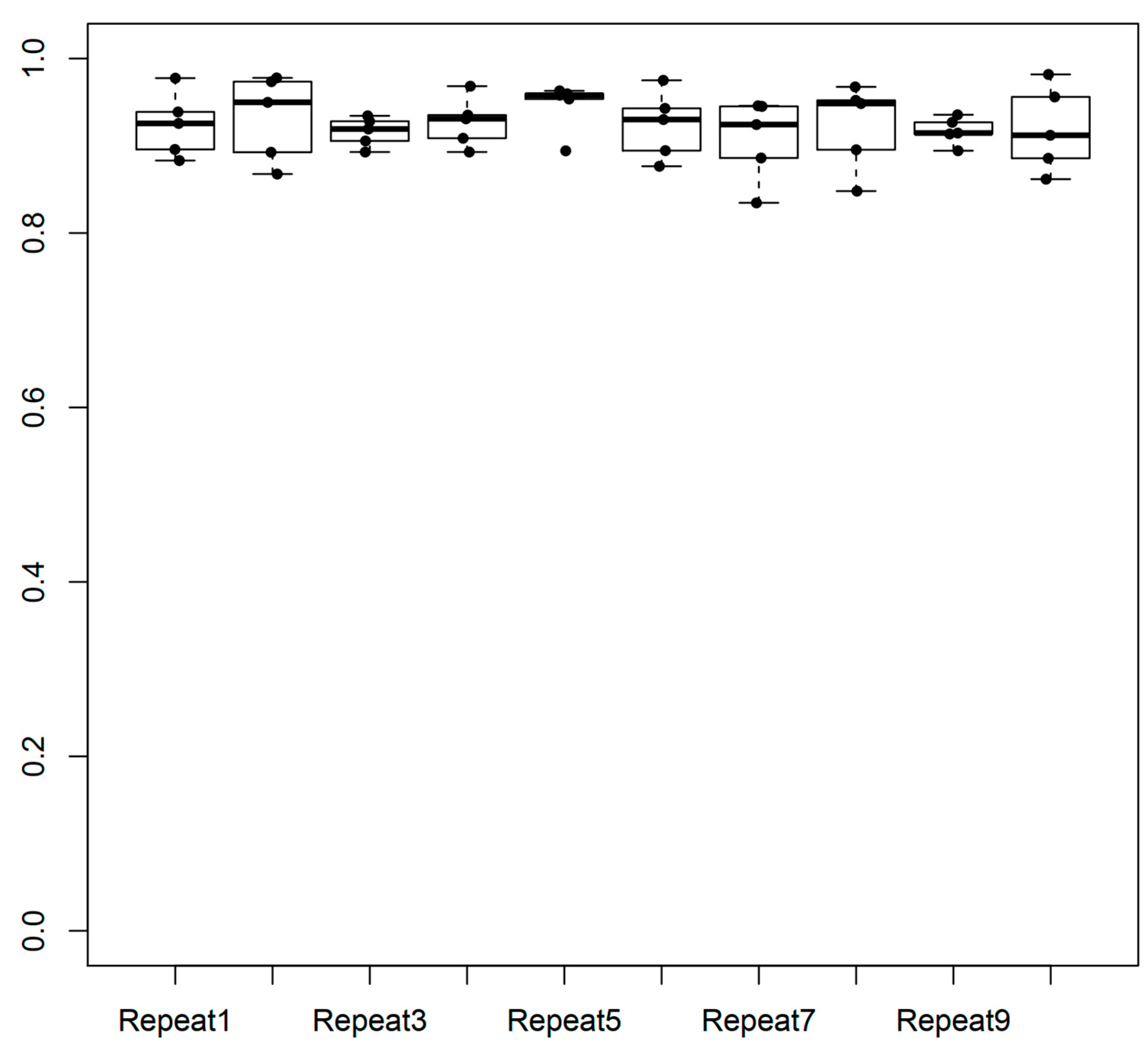
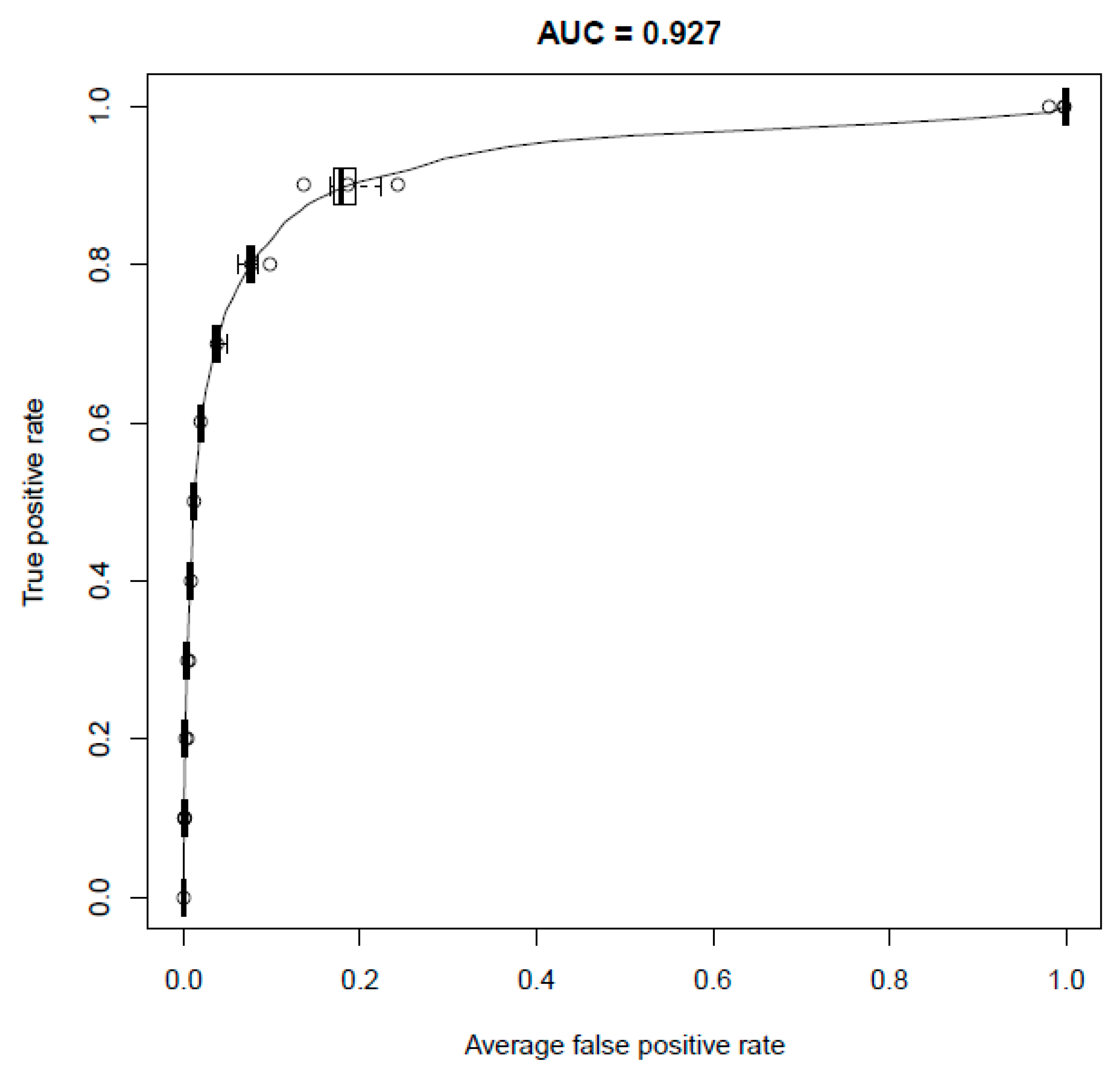
2.1. Performance of the Model
2.2. Targets for Treating MCL Disorders
2.3. Assessment of the Top 25 Control and Novel Targets
2.4. Putative Targets for MCL Disorders
3. Discussion
4. Materials and Methods
4.1. Selection of Disorders and Positive Control Targets
4.2. Knowledge-Based Approaches
4.3. Network-Based Approaches
4.4. Disease Similarity Approach
4.5. Integration of the Evidence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Noncommunicable Diseases: Progress Monitor 2022. World Health Organization. 2022. Available online: https://iris.who.int/handle/10665/353048 (accessed on 25 July 2024).
- Faro, E.; Adeagbo, O.; Mpinganjira, M.G.; Chirwa, T.; Matanje, B.; Mayige, M.; Kavishe, B.B.; Mmbaga, B.; Francis, J.M. Measurement of and training for NCD guideline implementation in LMICs: A scoping review protocol. BMJ Open 2023, 13, e073550. [Google Scholar] [CrossRef] [PubMed]
- Zsuga, J.; Biro, K.; Papp, C.; Tajti, G.; Gesztelyi, R. The “proactive” model of learning: Integrative framework for model-free and model-based reinforcement learning utilizing the associative learning-based proactive brain concept. Behav. Neurosci. 2016, 130, 6–18. [Google Scholar] [CrossRef]
- Zsuga, J.; Biro, K.; Tajti, G.; Szilasi, M.E.; Papp, C.; Juhasz, B.; Gesztelyi, R. ‘Proactive’ use of cue-context congruence for building reinforcement learning’s reward function. BMC Neurosci. 2016, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Jacka, F.N.; Quirk, S.E.; Cocker, F.; Taylor, C.B.; Oldenburg, B.; Berk, M. A shared framework for the common mental disorders and Non-Communicable Disease: Key considerations for disease prevention and control. BMC Psychiatry 2015, 15, 15. [Google Scholar] [CrossRef]
- Piantadosi, P.T.; Halladay, L.R.; Radke, A.K.; Holmes, A. Advances in understanding meso-cortico-limbic-striatal systems mediating risky reward seeking. J. Neurochem. 2021, 157, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Picciotto, M.R. Nicotine addiction: More than just dopamine. Curr. Opin. Neurobiol. 2023, 83, 102797. [Google Scholar] [CrossRef]
- Morales, I.; Berridge, K.C. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef]
- Chase, H.W.; Loriemi, P.; Wensing, T.; Eickhoff, S.B.; Nickl-Jockschat, T. Meta-analytic evidence for altered mesolimbic responses to reward in schizophrenia. Hum. Brain Mapp. 2018, 39, 2917–2928. [Google Scholar] [CrossRef]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef]
- Berry, A.S.; White, R.L.; Furman, D.J.; Naskolnakorn, J.R.; Shah, V.D.; D’Esposito, M.; Jagust, W.J. Dopaminergic Mechanisms Underlying Normal Variation in Trait Anxiety. J. Neurosci. 2019, 39, 2735–2744. [Google Scholar] [CrossRef]
- Tadayonnejad, R.; Majid, D.A.; Tsolaki, E.; Rane, R.; Wang, H.; Moody, T.D.; Pauli, W.M.; Pouratian, N.; Bari, A.A.; Murray, S.B.; et al. Mesolimbic Neurobehavioral Mechanisms of Reward Motivation in Anorexia Nervosa: A Multimodal Imaging Study. Front. Psychiatry 2022, 13, 806327. [Google Scholar] [CrossRef] [PubMed]
- Pettorruso, M.; Zoratto, F.; Miuli, A.; De Risio, L.; Santorelli, M.; Pierotti, A.; Martinotti, G.; Adriani, W.; di Giannantonio, M. Exploring dopaminergic transmission in gambling addiction: A systematic translational review. Neurosci. Biobehav. Rev. 2020, 119, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Leggio, G.M.; Drago, F.; Salomone, S. Therapeutic Challenges of Post-traumatic Stress Disorder: Focus on the Dopaminergic System. Front. Pharmacol. 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Bisi, N.; Rastelli, G. How drug repurposing can advance drug discovery: Challenges and opportunities. Front. Drug Discov. 2024, 4, 1460100. [Google Scholar] [CrossRef]
- Mak, K.K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving personalized medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef]
- Emig, D.; Ivliev, A.; Pustovalova, O.; Lancashire, L.; Bureeva, S.; Nikolsky, Y.; Bessarabova, M. Drug target prediction and repositioning using an integrated network-based approach. PLoS ONE 2013, 8, e60618. [Google Scholar] [CrossRef]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef]
- Kawahata, I.; Finkelstein, D.I.; Fukunaga, K. Dopamine D1–D5 Receptors in Brain Nuclei: Implications for Health and Disease. Receptors 2024, 3, 155–181. [Google Scholar] [CrossRef]
- Ruiz-Tejada, A.; Neisewander, J.; Katsanos, C.S. Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain. Brain Sci. 2022, 12, 333. [Google Scholar] [CrossRef]
- Finberg, J.P.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 inhibitors for treating T2DM—Hype or hope? an analysis based on the current literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Ghorpade, D.S.; Tabas, I. Targeting Soluble DPP-4 for Insulin Resistance: Origin Matters. J. Clin. Endocrinol. Metab. 2021, 106, e1460–e1462. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Fan, S.; Shi, X.; Gong, X.; Zhao, J.; Fan, G. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers on insulin sensitivity in hypertensive patients: A meta-analysis of randomized controlled trials. PLoS ONE 2021, 16, e0253492. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Carlström, M.; Azizi, F.; Ghasemi, A. Circulating markers of nitric oxide homeostasis and cardiometabolic diseases: Insights from population-based studies. Free Radic. Res. 2019, 53, 359–376. [Google Scholar] [CrossRef]
- Smith, N.K.; Grueter, B.A. Hunger-driven adaptive prioritization of behavior. FEBS J. 2022, 289, 922–936. [Google Scholar] [CrossRef]
- Greenberg, D.; St Peter, J.V. Sugars and Sweet Taste: Addictive or Rewarding? Int. J. Environ. Res. Public Health 2021, 18, 9791. [Google Scholar] [CrossRef]
- Allen, B.; Jennings, J.R.; Muldoon, M.F.; Gianaros, P.J. Frontostriatal brain activation is associated with the longitudinal progression of cardiometabolic risk. Psychosom. Med. 2020, 82, 454–460. [Google Scholar] [CrossRef]
- Rossi, A.; Mikail, N.; Bengs, S.; Haider, A.; Treyer, V.; Buechel, R.R.; Wegener, S.; Rauen, K.; Tawakol, A.; Merz, C.N.B.; et al. Heart–brain interactions in cardiac and brain diseases: Why sex matters. Eur. Heart J. 2022, 43, 3971–3980. [Google Scholar] [CrossRef]
- Gong, L.; Ma, T.; He, L.; Lin, G.; Zhang, G.; Cheng, X.; Luo, F.; Bai, Y. Association between single and multiple cardiometabolic diseases and depression: A cross-sectional study of 391,083 participants from the UK biobank. Front. Public Health 2022, 10, 904876. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Mikkelsen, D.H.; Luitva, L.B.; Song, H.; Kasela, S.; Aspelund, T.; Bergstedt, J.; Lu, Y.; Sullivan, P.F.; Ye, W.; et al. Psychiatric disorders and subsequent risk of cardiovascular disease: A longitudinal matched cohort study across three countries. eClinicalMedicine 2023, 61, 102063. [Google Scholar] [CrossRef]
- Deste, G.; Lombardi, C.M. Cardiometabolic disease and psychiatric disorders. Front. Psychiatry 2023, 14, 1174055. [Google Scholar] [CrossRef]
- di Girolamo, G.; Bracco, I.F.; Portigliatti Pomeri, A.; Puglisi, S.; Oliva, F. Prevalence of metabolic syndrome and insulin resistance in a sample of adult ADHD outpatients. Front. Psychiatry 2022, 13, 891479. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, W.; Wang, F.; Tian, L.; Dong, Y. The utility of cognitive screening in asian patients with heart failure: A systematic review. Front. Psychiatry 2022, 13, 930121. [Google Scholar] [CrossRef] [PubMed]
- Raei, M.; Ghasemi, M.; Hushmandi, K.; Shirmohammadi-Khoram, N.; Omolbanin Seyedrezaei, S.; Rostami, H.; Vahedian-Azimi, A. Effectiveness of family-centered empowerment model on psychological improvement of patients with myocardial infarction: A Bayesian multivariate approach. Front. Public Health 2022, 10, 878259. [Google Scholar] [CrossRef]
- Dixit, A.; Mishra, A.K.; Singh, C.V.; Gupta, V.K.; Pandey, D. Drug repositioning: Current scenario and future prospective for rewriting saga of drug development. Int. J. Res. Med. Sci. 2024, 12, 1334. [Google Scholar] [CrossRef]
- Bessarabova, M.; Ishkin, A.; JeBailey, L.; Nikolskaya, T.; Nikolsky, Y. Knowledge-based analysis of proteomics data. BMC Bioinform. 2012, 13 (Suppl. S16), S13. [Google Scholar] [CrossRef]
- Vanunu, O.; Magger, O.; Ruppin, E.; Shlomi, T.; Sharan, R. Associating genes and protein complexes with disease via network propagation. PLoS Comput. Biol. 2010, 6, e1000641. [Google Scholar] [CrossRef]
- Köhler, S.; Bauer, S.; Horn, D.; Robinson, P.N. Walking the interactome for prioritization of candidate disease genes. Am. J. Hum. Genet. 2008, 82, 949–958. [Google Scholar] [CrossRef]
- Hsu, C.L.; Huang, Y.H.; Hsu, C.T.; Yang, U.C. Prioritizing disease candidate genes by a gene interconnectedness-based approach. BMC Genom. 2011, 12 (Suppl. S3), S25. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, D.; Gonçalves, J.P.; Ojeda, F.; de Moor, B.; Moreau, Y. Candidate gene prioritization by network analysis of differential expression using machine learning approaches. BMC Bioinform. 2010, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]

| Entrez ID | Gene Symbol | Gene Name | Score |
|---|---|---|---|
| 1813 | DRD2 | dopamine receptor D2 | 4.2 |
| 4129 | MAOB | monoamine oxidase B | 7.3 |
| 6531 | SLC6A3 | solute carrier family 6 (neurotransmitter transporter), member 3 | 10.5 |
| 4128 | MAOA | monoamine oxidase A | 16.0 |
| 3358 | HTR2C | 5-hydroxytryptamine (serotonin) receptor 2C, G protein-coupled | 18.7 |
| 3351 | HTR1B | 5-hydroxytryptamine (serotonin) receptor 1B, G protein-coupled | 19.6 |
| 1814 | DRD3 | dopamine receptor D3 | 20.4 |
| 6530 | SLC6A2 | solute carrier family 6 (neurotransmitter transporter), member 2 | 21.5 |
| 5970 | RELA | v-rel avian reticuloendotheliosis viral oncogene homolog A | 25.0 |
| 1137 | CHRNA4 | cholinergic receptor, nicotinic, alpha 4 (neuronal) | 26.1 |
| 9177 | HTR3B | 5-hydroxytryptamine (serotonin) receptor 3B, ionotropic | 32.6 |
| 320 | APBA1 | amyloid beta (A4) precursor protein-binding, family A, member 1 | 33.1 |
| 2904 | GRIN2B | glutamate receptor, ionotropic, N-methyl D-aspartate 2B | 39.3 |
| 4803 | NGF | nerve growth factor (beta polypeptide) | 40.5 |
| 3352 | HTR1D | 5-hydroxytryptamine (serotonin) receptor 1D, G protein-coupled | 42.8 |
| 1815 | DRD4 | dopamine receptor D4 | 44.4 |
| 321 | APBA2 | amyloid beta (A4) precursor protein-binding, family A, member 2 | 46.1 |
| 2915 | GRM5 | glutamate receptor, metabotropic 5 | 46.5 |
| 3630 | INS | insulin | 47.7 |
| 3350 | HTR1A | 5-hydroxytryptamine (serotonin) receptor 1A, G protein-coupled | 49.1 |
| 3354 | HTR1E | 5-hydroxytryptamine (serotonin) receptor 1E, G protein-coupled | 50.6 |
| 3357 | HTR2B | 5-hydroxytryptamine (serotonin) receptor 2B, G protein-coupled | 60.7 |
| 170572 | HTR3C | 5-hydroxytryptamine (serotonin) receptor 3C, ionotropic | 73.7 |
| 2906 | GRIN2D | glutamate receptor, ionotropic, N-methyl D-aspartate 2D | 78.4 |
| 2668 | GDNF | glial cell derived neurotrophic factor | 91.2 |
| Molecular Function | r | R | n | N | p Value |
|---|---|---|---|---|---|
| serotonin binding | 7 | 25 | 11 | 15,288 | 4.08 × 10−18 |
| amine binding | 7 | 25 | 13 | 15,288 | 2.12 × 10−17 |
| drug binding | 11 | 25 | 130 | 15,288 | 4.4 × 10−17 |
| serotonin receptor activity | 7 | 25 | 15 | 15,288 | 7.93 × 10−17 |
| G-protein coupled amine receptor activity | 7 | 25 | 42 | 15,288 | 3.23 × 10−13 |
| transmembrane signaling receptor activity | 14 | 25 | 1196 | 15,288 | 5.86 × 10−10 |
| dopamine binding | 4 | 25 | 10 | 15,288 | 1.16 × 10−9 |
| signaling receptor activity | 14 | 25 | 1299 | 15,288 | 1.74 × 10−9 |
| signal transducer activity | 15 | 25 | 1617 | 15,288 | 2.55 × 10−9 |
| molecular transducer activity | 15 | 25 | 1617 | 15,288 | 2.55 × 10−9 |
| dopamine neurotransmitter receptor activity, coupled via Gi/Go | 3 | 25 | 3 | 15,288 | 3.86 × 10−9 |
| catecholamine binding | 4 | 25 | 14 | 15,288 | 5.5 × 10−9 |
| G-protein coupled receptor activity | 11 | 25 | 812 | 15,288 | 1.99 × 10−8 |
| receptor activity | 14 | 25 | 1583 | 15,288 | 2.28 × 10−8 |
| dopamine neurotransmitter receptor activity | 3 | 25 | 5 | 15,288 | 3.85 × 10−8 |
| extracellular ligand-gated ion channel activity | 5 | 25 | 74 | 15,288 | 1.14 × 10−7 |
| excitatory extracellular ligand-gated ion channel activity | 4 | 25 | 49 | 15,288 | 1.12 × 10−6 |
| ligand-gated channel activity | 5 | 25 | 145 | 15,288 | 3.27 × 10−6 |
| ligand-gated ion channel activity | 5 | 25 | 145 | 15,288 | 3.27 × 10−6 |
| neurotransmitter binding | 3 | 25 | 24 | 15,288 | 7.64 × 10−6 |
| Entrez ID | Gene Symbol | Gene Name | Score |
|---|---|---|---|
| 1803 | DPP4 | dipeptidyl-peptidase 4 | 14.8 |
| 3359 | HTR3A | 5-hydroxytryptamine (serotonin) receptor 3A, ionotropic | 17.8 |
| 5468 | PPARG | peroxisome proliferator-activated receptor gamma | 29.5 |
| 1385 | CREB1 | cAMP responsive element binding protein 1 | 45.7 |
| 775 | CACNA1C | calcium channel, voltage-dependent, L type, alpha 1C subunit | 48.1 |
| 5443 | POMC | proopiomelanocortin | 51.9 |
| 2905 | GRIN2C | glutamate receptor, ionotropic, N-methyl D-aspartate 2C | 58.2 |
| 2903 | GRIN2A | glutamate receptor, ionotropic, N-methyl D-aspartate 2A | 61.9 |
| 6616 | SNAP25 | synaptosomal-associated protein, 25 kDa | 65.5 |
| 9900 | SV2A | synaptic vesicle glycoprotein 2A | 69.6 |
| 776 | CACNA1D | calcium channel, voltage-dependent, L type, alpha 1D subunit | 73.2 |
| 781 | CACNA2D1 | calcium channel, voltage-dependent, alpha 2/delta subunit 1 | 73.9 |
| 783 | CACNB2 | calcium channel, voltage-dependent, beta 2 subunit | 74.1 |
| 4842 | NOS1 | nitric oxide synthase 1 (neuronal) | 81.5 |
| 1636 | ACE | angiotensin I converting enzyme | 82.1 |
| 801 | CALM1 | calmodulin 1 (phosphorylase kinase, delta) | 83.0 |
| 784 | CACNB3 | calcium channel, voltage-dependent, beta 3 subunit | 95.1 |
| 322 | APBB1 | amyloid beta (A4) precursor protein-binding, family B, member 1 (Fe65) | 97.4 |
| 59285 | CACNG6 | calcium channel, voltage-dependent, gamma subunit 6 | 97.4 |
| 9254 | CACNA2D2 | calcium channel, voltage-dependent, alpha 2/delta subunit 2 | 98.3 |
| 19 | ABCA1 | ATP-binding cassette, sub-family A (ABC1), member 1 | 98.9 |
| 6285 | S100B | S100 calcium binding protein B | 100.7 |
| 185 | AGTR1 | angiotensin II receptor, type 1 | 101.7 |
| 773 | CACNA1A | calcium channel, voltage-dependent, P/Q type, alpha 1A subunit | 107.0 |
| 6570 | SLC18A1 | solute carrier family 18 (vesicular monoamine transporter), member 1 | 107.7 |
| Molecular Function | r | R | n | N | p-Value |
|---|---|---|---|---|---|
| voltage-gated cation channel activity | 11 | 25 | 138 | 15,288 | 8.66 × 10−17 |
| voltage-gated calcium channel activity | 8 | 25 | 35 | 15,288 | 3.35 × 10−16 |
| voltage-gated ion channel activity | 11 | 25 | 188 | 15,288 | 2.78 × 10−15 |
| voltage-gated channel activity | 11 | 25 | 188 | 15,288 | 2.78 × 10−15 |
| cation channel activity | 12 | 25 | 289 | 15,288 | 6.93 × 10−15 |
| ion gated channel activity | 12 | 25 | 323 | 15,288 | 2.63 × 10−14 |
| gated channel activity | 12 | 25 | 323 | 15,288 | 2.63 × 10−14 |
| calcium channel activity | 9 | 25 | 109 | 15,288 | 6.32 × 10−14 |
| calcium ion transmembrane transporter activity | 9 | 25 | 128 | 15,288 | 2.77 × 10−13 |
| ion channel activity | 12 | 25 | 415 | 15,288 | 5.17 × 10−13 |
| substrate-specific channel activity | 12 | 25 | 425 | 15,288 | 6.85 × 10−13 |
| high voltage-gated calcium channel activity | 5 | 25 | 9 | 15,288 | 9.58 × 10−13 |
| passive transmembrane transporter activity | 12 | 25 | 450 | 15,288 | 1.34 × 10−12 |
| channel activity | 12 | 25 | 450 | 15,288 | 1.34 × 10−12 |
| divalent inorganic cation transmembrane transporter activity | 9 | 25 | 155 | 15,288 | 1.59 × 10−12 |
| transmembrane transporter activity | 15 | 25 | 972 | 15,288 | 1.81 × 10−12 |
| cation transmembrane transporter activity | 13 | 25 | 618 | 15,288 | 2.26 × 10−12 |
| ion transmembrane transporter activity | 14 | 25 | 825 | 15,288 | 4.08 × 10−12 |
| metal ion transmembrane transporter activity | 11 | 25 | 401 | 15,288 | 1.13 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, C.; Mikaczo, A.; Szabo, J.; More, C.E.; Viczjan, G.; Gesztelyi, R.; Zsuga, J. Mesocorticolimbic and Cardiometabolic Diseases—Two Faces of the Same Coin? Int. J. Mol. Sci. 2024, 25, 9682. https://doi.org/10.3390/ijms25179682
Papp C, Mikaczo A, Szabo J, More CE, Viczjan G, Gesztelyi R, Zsuga J. Mesocorticolimbic and Cardiometabolic Diseases—Two Faces of the Same Coin? International Journal of Molecular Sciences. 2024; 25(17):9682. https://doi.org/10.3390/ijms25179682
Chicago/Turabian StylePapp, Csaba, Angela Mikaczo, Janos Szabo, Csaba E. More, Gabor Viczjan, Rudolf Gesztelyi, and Judit Zsuga. 2024. "Mesocorticolimbic and Cardiometabolic Diseases—Two Faces of the Same Coin?" International Journal of Molecular Sciences 25, no. 17: 9682. https://doi.org/10.3390/ijms25179682







