Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches
Abstract
1. The Importance of the Gut Microbiota for Human Health
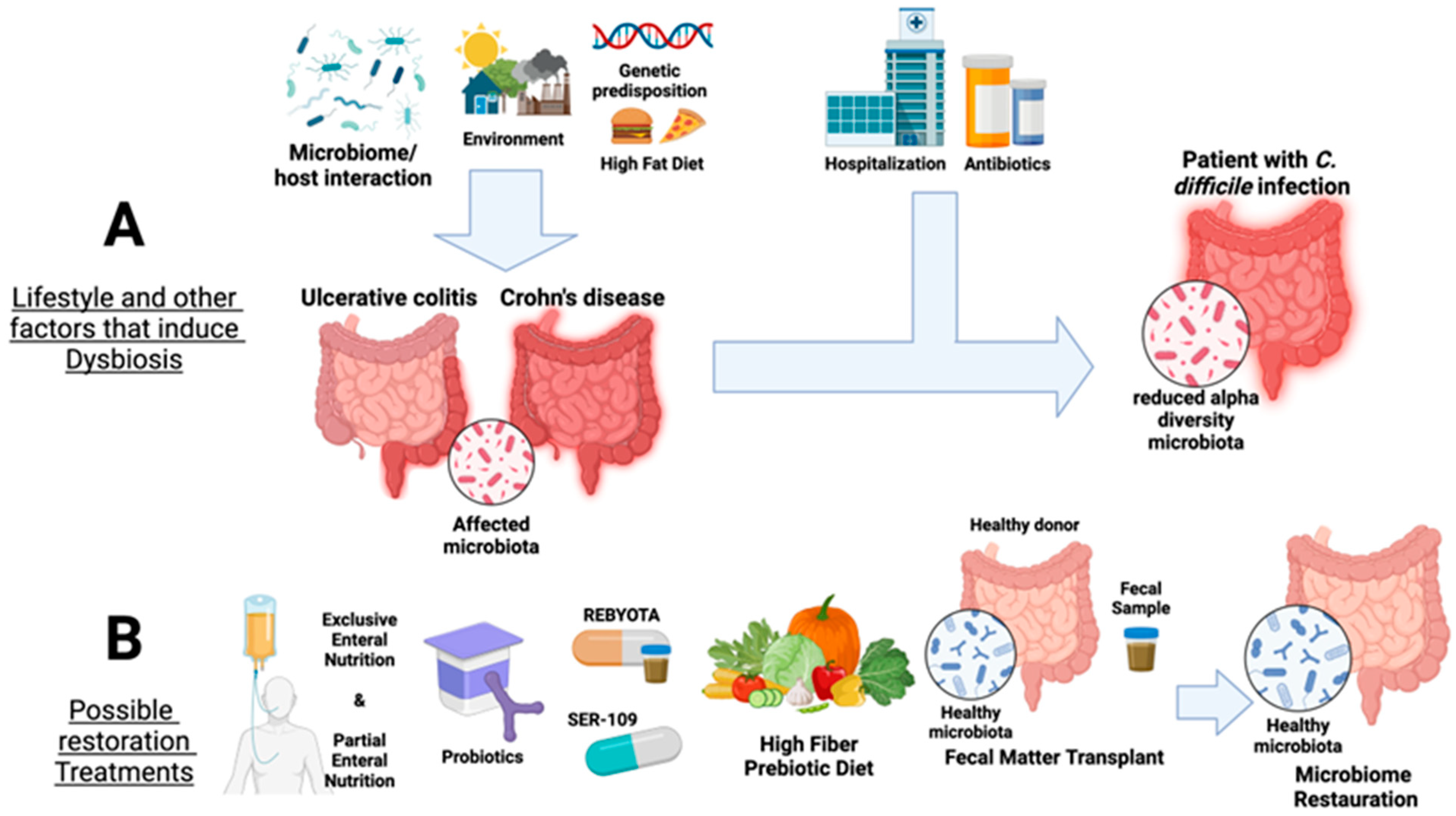
2. Inflammatory Bowel Diseases: Ulcerative Colitis and Crohn’s Disease
2.1. Crohn’s Disease Etiology and Pathogenesis
2.2. Clinical Significance of Crohn’s Disease and Diagnostic Procedures
2.3. Treatment Options for Crohn’s Disease
2.4. An Overview of Ulcerative Colitis and the Microbiota
| Gut Health and Disease/Dysbiotic Phenotypes | Significant Taxa | Country | References |
|---|---|---|---|
| Normal gut microbiome | ↑Firmicutes ↑Streptococcus ↑Veillonella ↑Clostridium ↑Faecalibactrium prausnitzii ↑Blautia faecis ↑Roseburia inulinivorans ↑Ruminococcus torques ↑Clostridium lavalense ↑Bacteroidetes ↑Proteobacteria | Korea Germany/Lithuania/India | [3,55,56] |
| Ulcerative colitis | ↑Proteobacteria ↑Escherichia ↑Klebsiella ↑Bacteroidetes ↓Firmicutes ↓ Roseburia ↓ Faecalibacterium ↓ Eubacterium hallii ↓ Gemmiger formicilis ↓ Eubacterium rectale ↓ Ruminococcus bromii ↓Tenericutes | Netherlands Italy China Germany/Lithuania/India | [33,35,38,43,56,57,58] |
| ↓Actinobacteria ↓Bifidobacterium longum | Netherlands | [58] | |
| ↓Cyanobacteria ↓Fusobactera ↑Verrucomicrobia | Italy | [59] | |
| Chron’s disease | ↑Fusobacteria ↑Fusobacteriaaceae | Southern China Israel | [17] |
| ↓Firmicutes ↓Eubacterium rectale ↓Faecalibacterium prausnitzii ↓Roseburia intestinalis ↓Roseburia inulinivorans ↓Blautia faecis ↑Bacteroidetes ↑Bacteroides fragilis | Netherlands Korea Germany/Lithuania/India | [55,56,58] | |
| Clostridioides difficile infection | ↑Clostridioides difficile ↓Bacteroidetes ↓Bacteroides ↓Firmicutes ↓Lactobacilllus ↓Enterococcus ↓Bacillus ↓Faecalibacterium ↓Ruminococcus | France | [60] |
| Obesity | ↓Actinobacteria ↓Bifidobacterium longum subsp. longum ↓Bifidobacterium bifidum | Italy Brazil | [61,62] |
| ↑Firmicutes ↑Eubacterium ↑Roseburia ↓Faecalibaterium ↓Clostridiaceae ↑Bacteroides | Japan Korea Mexico | [63,64,65,66] |
3. Clostridioides Difficile—A Special Case of Antibiotic-Driven Dysbiosis
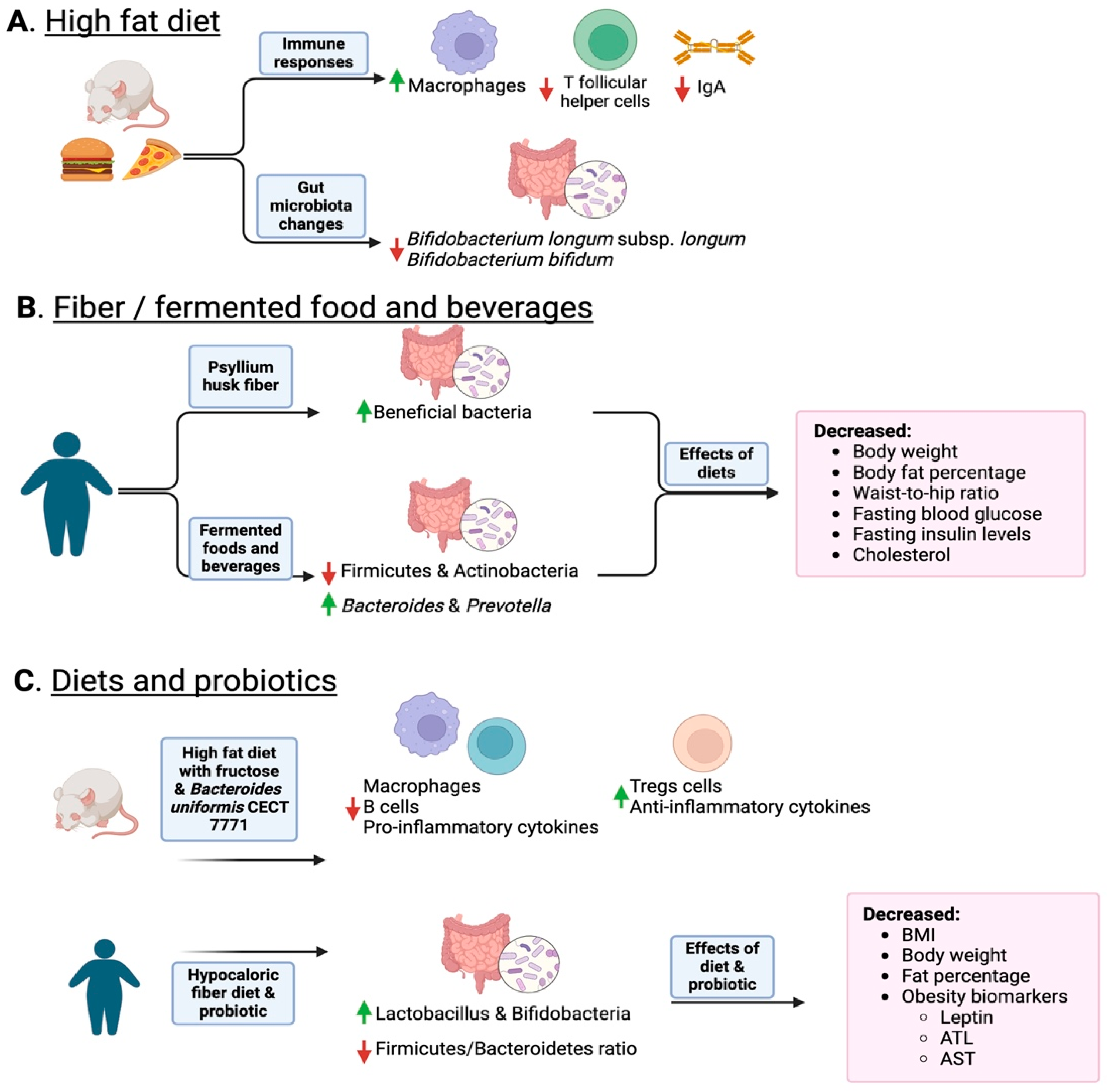
4. Inflammation Is a Matter of Microbial Dysbiosis Resulting in Obesity
5. Restoration Procedures for Gut Dysbiosis
5.1. Fecal Microbiota Transplantation (FMT)
5.2. FMT Safety Concerns and Alternative Therapies
| Probiotic/Prebiotic | Functions | Outcomes | References |
|---|---|---|---|
| Psyllium | Increased levels of Lachnospira, Roseburia, and Faecalibacterium | Improved abdominal discomfort, epigastric pain, and constipation symptoms | [132,133] |
| Kimchi | Increased levels of Actinobacteria | Improved cholesterol levels, insulin levels, body weight, body fat percentage, and BMI | [65,134] |
| Lacticaseibacillus paracasei YIT 9029 and Bifidobacterium breve YIT 12272 | Increased levels of Bifidobacteriaceae and Lactobacillus | Improved glucose metabolism | [136] |
| Yogurt | Increased levels of Bacteroides, Streptococcus, Blautia, and Saccharomyces; NK cells, B cells, IL-5, and Th2 | Improved constipation symptoms and immune responses | [138,139] |
| Kefir | Increased levels of Lactobacillus | Improved constipation symptoms, cholesterol levels, and obesity risks | [141,142,143] |
| Kombucha | Increased levels of beneficial E. coli, Bacteroidetes and Lactobacillus | Improved glucose levels, obesity risks, and cholesterol levels. | [145,150,151] |
| Kiwi | Increased levels of Lactobacilli, and Bifidobacteria | Improved the growth of intestinal lactid acid bacteria and perturbation of Clostridium | [156] |
| Black raspberries | Increased levels of Bacteroidetes | Improved colon microbial α-diversity | [157,158] |
| Coffee spent grounds | Increased levels of Bifidobacterium, Jamie Quinton, and Ruminococcus | Improved levels of microbial α-diversity in fecal samples | [152,153] |
| Bifidobacterium animalis and Lactobacillus paracasei | Increased levels of Bacteroidetes | Improved gut microbiota and risk for metabolic disorder | [159,160] |
6. Conclusions/Summary
Author Contributions
Funding
Conflicts of Interest
References
- Godoy-Vitorino, F. Human Microbial Ecology and the Rising New Medicine. Ann. Transl. Med. 2019, 7, 342. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the Microbiome in Human Development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Kastl, A.J., Jr.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef]
- Adamberg, S.; Adamberg, K. Prevotella Enterotype Associates with Diets Supporting Acidic Faecal pH and Production of Propionic Acid by Microbiota. Heliyon 2024, 10, e31134. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Cheng, X.; Xing, C. The Gut Microbial Diversity of Colon Cancer Patients and the Clinical Significance. Bioengineered 2021, 12, 7046–7060. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Nishida, A.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. The Efficacy of Fecal Microbiota Transplantation for Patients with Chronic Pouchitis: A Case Series. Clin. Case Rep. 2019, 7, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.E. Microbiota in Inflammatory Bowel Disease Pathogenesis and Therapy: Is It All About Diet? Nutr. Clin. Prac. 2015, 30, 760–779. [Google Scholar] [CrossRef]
- Kassinen, A.; Krogius-Kurikka, L.; Makivuokko, H.; Rinttila, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The Fecal Microbiota of Irritable Bowel Syndrome Patients Differs Significantly from That of Healthy Subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, J.-P.; Deng, K.; Li, X.; Yuan, Y.; Xuan, Q.; Xie, J.; He, X.-M.; Wang, Q.; Li, J.-J.; et al. Prophylactic Effects of Bifidobacterium Adolescentis on Anxiety and Depression-Like Phenotypes After Chronic Stress: A Role of the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A Review of the Diagnosis, Prevention, and Treatment Methods of Inflammatory Bowel Disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Cushing, K.; Higgins, P.D.R. Management of Crohn Disease: A Review. JAMA 2021, 325, 69–80. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Bao, J.-C.; Liao, X.-Y.; Chen, Y.-J.; Wang, L.-W.; Fan, Y.-Y.; Xu, Q.-Y.; Hao, L.-X.; Li, K.-J.; Liang, M.-X.; et al. Trends and Projections of Inflammatory Bowel Disease at the Global, Regional and National Levels, 1990–2050: A Bayesian Age-Period-Cohort Modeling Study. BMC Public Health 2023, 23, 2507. [Google Scholar] [CrossRef]
- Kaczmarek-Ryś, M.; Hryhorowicz, S.T.; Lis, E.; Banasiewicz, T.; Paszkowski, J.; Borejsza-Wysocki, M.; Walkowiak, J.; Cichy, W.; Krokowicz, P.; Czkwianianc, E.; et al. Crohn’s Disease Susceptibility and Onset Are Strongly Related to Three NOD2 Gene Haplotypes. J. Clin. Med. 2021, 10, 3777. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Feng, R.; Amir, A.; Levhar, N.; Shacham, H.; Mao, R.; Hadar, R.; Toren, I.; Algavi, Y.; Abu-Saad, K.; et al. Diet-Omics in the Study of Urban and Rural Crohn Disease Evolution (SOURCE) Cohort. Nat. Commun. 2024, 15, 3764. [Google Scholar] [CrossRef]
- Metwaly, A.; Dunkel, A.; Waldschmitt, N.; Raj, A.C.D.; Lagkouvardos, I.; Corraliza, A.M.; Mayorgas, A.; Martinez-Medina, M.; Reiter, S.; Schloter, M.; et al. Integrated Microbiota and Metabolite Profiles Link Crohn’s Disease to Sulfur Metabolism. Nat. Commun. 2020, 11, 4322. [Google Scholar] [CrossRef]
- Zheng, L.; Wen, X.-L.; Duan, S.-L. Role of Metabolites Derived from Gut Microbiota in Inflammatory Bowel Disease. World J. Clin. Cases 2022, 10, 2660–2677. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Vandana, U.K.; Barlaskar, N.H.; Gulzar, A.B.M.; Laskar, I.H.; Kumar, D.; Paul, P.; Pandey, P.; Mazumder, P.B. Linking Gut Microbiota with the Human Diseases. Bioinformation 2020, 16, 196–208. [Google Scholar] [CrossRef]
- Vakadaris, G.; Stefanis, C.; Giorgi, E.; Brouvalis, M.; Voidarou, C.; Kourkoutas, Y.; Tsigalou, C.; Bezirtzoglou, E. The Role of Probiotics in Inducing and Maintaining Remission in Crohn’s Disease and Ulcerative Colitis: A Systematic Review of the Literature. Biomedicines 2023, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.-N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Lopez, R.N. Exclusive Enteral Nutrition in Children with Crohn’s Disease. World J. Gastroenterol. 2015, 21, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R. Update on Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 31–34. [Google Scholar]
- Kahn, S.A. A Phase I/II, Double Blinded, Placebo Controlled, Single-Center Study of Fecal Microbiota Transplant (FMT) for the Treatment of Active Pediatric Ulcerative Colitis and Pediatric Active Crohn’s Colitis. 2023. Available online: https://clinicaltrials.gov/ (accessed on 24 May 2024).
- Voelker, R. What Is Ulcerative Colitis? JAMA 2024, 331, 716. [Google Scholar] [CrossRef]
- Definition & Facts of Ulcerative Colitis—NIDDK; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Goldinova, A.; Tan, C.X.W.; Bouma, G.; Duijvestein, M.; Brand, H.S.; de Boer, N.K. Oral Health and Salivary Function in Ulcerative Colitis Patients. United Eur. Gastroenterol. J. 2020, 8, 1067–1075. [Google Scholar] [CrossRef]
- Zhu, L.; Qiao, L.; Dou, X.; Song, X.; Chang, J.; Zeng, X.; Xu, C. Lactobacillus Casei ATCC 393 Combined with Vasoactive Intestinal Peptide Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis in C57BL/6 Mice via NF-κB and Nrf2 Signaling Pathways. Biomed. Pharmacother. 2023, 165, 115033. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Liu, F.; Zhu, X.-R.; Suo, F.-Y.; Jia, Z.; Yao, S.-K. Altered Profiles of Fecal Bile Acids Correlate with Gut Microbiota and Inflammatory Responses in Patients with Ulcerative Colitis. World J. Gastroenterol. 2021, 27, 3609–3629. [Google Scholar] [CrossRef] [PubMed]
- Inciuraite, R.; Gedgaudas, R.; Lukosevicius, R.; Tilinde, D.; Ramonaite, R.; Link, A.; Kasetiene, N.; Malakauskas, M.; Kiudelis, G.; Jonaitis, L.V.; et al. Constituents of Stable Commensal Microbiota Imply Diverse Colonic Epithelial Cell Reactivity in Patients with Ulcerative Colitis. Gut Pathog. 2024, 16, 16. [Google Scholar] [CrossRef]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. The Interplay of Host Genetics and the Gut Microbiota Underlying the Onset and Clinical Presentation of Inflammatory Bowel Disease. Gut 2019, 67, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zou, G.; Li, B.; Du, X.; Sun, Z.; Sun, Y.; Jiang, X. Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation. J. Microbiol. Biotechnol. 2020, 30, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces Cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Zhou, Y.-L.; Sun, H.; Zhang, Y.; Shen, C.; Wang, Z.; Xuan, B.; Zhao, Y.; Ma, Y.; Yan, Y.; et al. Microbiome and Metabolome Features in Inflammatory Bowel Disease via Multi-Omics Integration Analyses across Cohorts. Nat. Commun. 2023, 14, 7135. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Zhang, D.; Wang, J.; Tan, Y.; Feng, W.; Peng, C. Ginsenoside Rg1 Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Microbial Tryptophan Metabolism. Front. Immunol. 2022, 13, 817600. [Google Scholar] [CrossRef]
- Jia, D.-J.-C.; Wang, Q.-W.; Hu, Y.-Y.; He, J.-M.; Ge, Q.-W.; Qi, Y.-D.; Chen, L.-Y.; Zhang, Y.; Fan, L.-N.; Lin, Y.-F.; et al. Lactobacillus Johnsonii Alleviates Colitis by TLR1/2-STAT3 Mediated CD206+ macrophagesIL-10 Activation. Gut Microbes 2022, 14, 2145843. [Google Scholar] [CrossRef]
- Sovran, B.; Planchais, J.; Jegou, S.; Straube, M.; Lamas, B.; Natividad, J.M.; Agus, A.; Dupraz, L.; Glodt, J.; Da Costa, G.; et al. Enterobacteriaceae Are Essential for the Modulation of Colitis Severity by Fungi. Microbiome 2018, 6, 152. [Google Scholar] [CrossRef]
- Wang, M.-X.; Lin, L.; Chen, Y.-D.; Zhong, Y.-P.; Lin, Y.-X.; Li, P.; Tian, X.; Han, B.; Xie, Z.-Y.; Liao, Q.-F. Evodiamine Has Therapeutic Efficacy in Ulcerative Colitis by Increasing Lactobacillus Acidophilus Levels and Acetate Production. Pharmacol. Res. 2020, 159, 104978. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, Y.; He, W.; Tian, Z.; Lin, J.; Liu, Z.; Li, Y.; Chen, M.; Han, S.; Liang, J.; et al. Gut Microbiota and Metabolite Changes in Patients With Ulcerative Colitis and Clostridioides difficile Infection. Front. Microbiol. 2022, 13, 802823. [Google Scholar] [CrossRef]
- Dong, Y.; Liao, W.; Tang, J.; Fei, T.; Gai, Z.; Han, M. Bifidobacterium BLa80 Mitigates Colitis by Altering Gut Microbiota and Alleviating Inflammation. AMB Express 2022, 12, 67. [Google Scholar] [CrossRef]
- Kawade, Y.; Sakai, M.; Okamori, M.; Morita, M.; Mizushima, K.; Ueda, T.; Takagi, T.; Naito, Y.; Itoh, Y.; Shimada, T. Administration of Live, but Not Inactivated, Faecalibacterium Prausnitzii Has a Preventive Effect on Dextran Sodium Sulfate-induced Colitis in Mice. Mol. Med. Rep. 2019, 20, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A Bacterial Genus with Promising Human Health Applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.H.; Dulai, P.S.; Vázquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Ochoa, M.M.; Zhu, Q.; Weldon, K.; et al. Multi-Omics Analyses of the Ulcerative Colitis Gut Microbiome Link Bacteroides Vulgatus Proteases with Disease Severity. Nat. Microbiol. 2022, 7, 262–276. [Google Scholar] [CrossRef]
- Khorsand, B.; Asadzadeh Aghdaei, H.; Nazemalhosseini-Mojarad, E.; Nadalian, B.; Nadalian, B.; Houri, H. Overrepresentation of Enterobacteriaceae and Escherichia Coli Is the Major Gut Microbiome Signature in Crohn’s Disease and Ulcerative Colitis; a Comprehensive Metagenomic Analysis of IBDMDB Datasets. Front. Cell Infect. Microbiol. 2022, 12, 1015890. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, C.; Feng, J.; Zhou, S.; Feng, X.; Yang, Z.; Lu, H.; Tao, H.; Li, L.; Xv, H.; et al. Muc2 Mucin O-Glycosylation Interacts with Enteropathogenic Escherichia Coli to Influence the Development of Ulcerative Colitis Based on the NF-kB Signaling Pathway. J. Transl. Med. 2023, 21, 793. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, A.; Jayawardena, D.; Priyamvada, S.; Anbazhagan, A.N.; Alrefai, W.A.; Gill, R.K.; Dudeja, P.K.; Saksena, S. Citrobacter Rodentium Infection Inhibits Colonic P-Glycoprotein Expression. Gene Rep. 2020, 18, 100549. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhao, Y.; Wu, W.; Meng, W.; Zhou, Y.; Qiu, Y.; Li, C. Protection against Ulcerative Colitis and Colorectal Cancer by Evodiamine via Anti-Inflammatory Effects. Mol. Med. Rep. 2022, 25, 188. [Google Scholar] [CrossRef]
- Meisner, J.; Bransford, T.; Miller, K.; Lee, J.; Jose, A.; Giuggio, M.; McComb, M.; Humphries, E.; Rosini, M.; Wingertzahn, M.; et al. The Synthetic Glycan KB295 Optimizes Microbiome Composition and Function in Ulcerative Colitis—Results from a Proof of Principle Human Study. Gastroenterology 2022, 162, S70–S71. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The Ugly Duckling of Energy Metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Luzentales-Simpson, M.; Pang, Y.C.F.; Zhang, A.; Sousa, J.A.; Sly, L.M. Vedolizumab: Potential Mechanisms of Action for Reducing Pathological Inflammation in Inflammatory Bowel Diseases. Front. Cell Dev. Biol. 2021, 9, 612830. [Google Scholar] [CrossRef] [PubMed]
- Eun, C.S.; Kwak, M.-J.; Han, D.S.; Lee, A.R.; Park, D.I.; Yang, S.-K.; Kim, Y.S.; Kim, J.F. Does the Intestinal Microbial Community of Korean Crohn’s Disease Patients Differ from That of Western Patients? BMC Gastroenterol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Rehman, A.; Rausch, P.; Wang, J.; Skieceviciene, J.; Kiudelis, G.; Bhagalia, K.; Amarapurkar, D.; Kupcinskas, L.; Schreiber, S.; Rosenstiel, P.; et al. Geographical Patterns of the Standing and Active Human Gut Microbiome in Health and IBD. Gut 2016, 65, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2017, 36, 56–65. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut Microbiota Composition and Functional Changes in Inflammatory Bowel Disease and Irritable Bowel Syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross Sectional Evaluation of the Gut-Microbiome Metabolome Axis in an Italian Cohort of IBD Patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef]
- Martinez, E.; Crevecoeur, S.; Thirion, C.; Grandjean, J.; Fall, P.A.; Hayette, M.-P.; Michel, M.; Taminiau, B.; Louis, E.; Daube, G. Gut Microbiota Associated with Clostridioides difficile Carriage in Three Clinical Groups (Inflammatory Bowel Disease, C. difficile Infection and Healthcare Workers) in Hospital Field. Microorganisms 2023, 11, 2527. [Google Scholar] [CrossRef] [PubMed]
- Nobili, A.; Pane, M.; Skvortsova, M.; Salem, M.B.; Morgenthaler, S.; Jamieson, E.; Stefano, M.D.; Bathrellou, E.; Mamalaki, E.; Ramos-Garcia, V.; et al. Innovative Biomarkers for Obesity and Type 1 Diabetes Based on Bifidobacterium and Metabolomic Profiling. Microorganisms 2024, 12, 931. [Google Scholar] [CrossRef]
- Teixeira, T.F.S.; Grześkowiak, Ł.M.; Salminen, S.; Laitinen, K.; Bressan, J.; Peluzio, M.D.C.G. Faecal Levels of Bifidobacterium and Clostridium Coccoides but Not Plasma Lipopolysaccharide Are Inversely Related to Insulin and HOMA Index in Women. Clin. Nutr. 2013, 32, 1017–1022. [Google Scholar] [CrossRef]
- Kubinak, J.L.; Petersen, C.; Stepens, W.Z.; Soto, R.; Bake, E.; O’Connell, R.M.; Round, J.L. MyD88 Signaling in T Cells Directs IgA-Mediated Control of the Microbiota to Promote Health. Cell Host Microbe 2015, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Env. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bose, S.; Wang, J.; Kim, B.-S.; Kim, M.J.; Kim, E.-J.; Kim, H. Contrasting Effects of Fresh and Fermented Kimchi Consumption on Gut Microbiota Composition and Gene Expression Related to Metabolic Syndrome in Obese Korean Women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the Gut Microbial Community between Obese and Lean Peoples Using 16S Gene Sequencing in a Japanese Population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Shen, A.; Sorg, J.A. Clostridium Difficile Spore Biology: Sporulation, Germination, and Spore Structural Proteins. Trends Microbiol. 2014, 22, 406–416. [Google Scholar] [CrossRef]
- Lyerly, D.M.; Krivan, H.C.; Wilkins, T.D. Clostridium Difficile: Its Disease and Toxins. Clin. Microbiol. Rev. 1988, 1, 1–18. [Google Scholar] [CrossRef]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium Difficile Binary Toxin CDT: Mechanism, Epidemiology, and Potential Clinical Importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef]
- Young, M.K.; Leslie, J.L.; Madden, G.R.; Lyerly, D.M.; Carman, R.J.; Lyerly, M.W.; Stewart, D.B.; Abhyankar, M.M.; Petri, W.A. Binary Toxin Expression by Clostridioides difficile Is Associated With Worse Disease. Open Forum Infect. Dis. 2022, 9, ofac001. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; You, P.; Shi, Q.; Hu, H.; Zhang, L.; Chen, L.; Wu, Z.; Lin, S.; Song, X.; Luo, Y.; et al. Gut Microbiome Changes in Mouse, Mongolian Gerbil, and Hamster Models Following Clostridioides difficile Challenge. Front. Microbiol. 2024, 15, 1368194. [Google Scholar] [CrossRef]
- Rao, K.; Malani, P.N. Diagnosis and Treatment of Clostridioides ( Clostridium ) Difficile Infection in Adults in 2020. JAMA 2020, 323, 1403. [Google Scholar] [CrossRef]
- Gawey, B.J.; Khanna, S. Clostridioides difficile Infection: Landscape and Microbiome Therapeutics. Gastroenterol. Hepatol. 2023, 19, 319–328. [Google Scholar]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef]
- Yunita, B.; Fauzi, A. Current Diagnostic and Treatment Approach of Clostridioides difficile Infection. Acta Medica Indones. 2023, 55, 231. [Google Scholar]
- Pike, C.M.; Theriot, C.M. Mechanisms of Colonization Resistance Against Clostridioides difficile. J. Infect. Dis. 2020, 223, S194–S200. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, K.R.; Carr, A.; Diener, C.; Locey, K.J.; Gibbons, S.M. Island Biogeography Theory Provides a Plausible Explanation for Why Larger Vertebrates and Taller Humans Have More Diverse Gut Microbiomes. ISME J. 2024, 18, wrae114. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut Microbiota Phenotypes of Obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Fuchs, R.; Müller, R.L.; Keller, L.; Baumann, Z.; Bosch, A.J.T.; Schneider, R.; Labes, D.; Langer, I.; Pilz, J.B.; et al. Obesity in Humans Is Characterized by Gut Inflammation as Shown by Pro-Inflammatory Intestinal Macrophage Accumulation. Front. Immunol. 2021, 12, 668654. [Google Scholar] [CrossRef]
- Djalalinia, S.; Qorbani, M.; Peykari, N.; Kelishadi, R. Health Impacts of Obesity. Pak. J. Med. Sci. 2015, 31, 239–242. [Google Scholar]
- Fabersani, E.; Portune, K.; Campillo, I.; López-Almela, I.; la Paz, S.M.; Romaní-Pérez, M.; Benítez-Páez, A.; Sanz, Y. Bacteroides Uniformis CECT 7771 Alleviates Inflammation within the Gut-adipose Tissue Axis Involving TLR5 Signaling in Obese Mice. Nat. Portf. 2021, 11, 11788. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.-H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T Cell–Mediated Regulation of the Microbiota Protects against Obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef] [PubMed]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Chan, Y.T.; Nøhr, M.K.; et al. Gut-Associated IgA+ Immune Cells Regulate Obesity-Related Insulin Resistance. Nat. Commun. 2019, 10, 3650. [Google Scholar] [CrossRef]
- Tran, H.Q.; Ley, R.E.; Gewirtz, A.T.; Chassaing, B. Flagellin-Elicited Adaptive Immunity Suppresses Flagellated Microbiota and Vaccinates against Chronic Inflammatory Diseases. Nat. Commun. 2019, 10, 5650. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Baptista, B.G.; Martins, L.F.; Borland, G.; Shiels, P.G.; Stenvinkel, P. What Can the Gut Microbiota of Animals Teach Us about the Relationship between Nutrition and Burden of Lifestyle Diseases? Nutrients 2024, 16, 1789. [Google Scholar] [CrossRef]
- Gogarten, J.F.; Davies, T.J.; Benjamino, J.; Gogarten, J.P.; Graf, J.; Mielke, A.; Mundry, R.; Nelson, M.C.; Wittig, R.M.; Leendertz, F.H.; et al. Factors Influencing Bacterial Microbiome Composition in a Wild Non-Human Primate Community in Taï National Park, Côte d’Ivoire. ISME J. 2018, 12, 2559–2574. [Google Scholar] [CrossRef]
- McKenzie, V.J.; Song, S.J.; Delsuc, F.; Prest, T.L.; Oliverio, A.M.; Korpita, T.M.; Alexiev, A.; Amato, K.R.; Metcalf, J.L.; Kowalewski, M.; et al. The Effects of Captivity on the Mammalian Gut Microbiome. Integr. Comp. Biol. 2017, 57, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Lagkouvardos, I.; Blaut, M.; Stecher, B. The Mouse Gut Microbiome Revisited: From Complex Diversity to Model Ecosystems—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1438422116300170?returnurl=null&referrer=null (accessed on 29 August 2024).
- Pei, L.; Ke, Y.; Zhao, H.; Wang, L.; Jia, C.; Liu, W.; Fu, Q.; Shi, M.; Cui, J.; Li, S. Role of Colonic Microbiota in the Pathogenesis of Ulcerative Colitis. BMC Gastroenterol. 2019, 19, 10. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A Novel Method in the Induction of Reliable Experimental Acute and Chronic Ulcerative Colitis in Mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Gray, S.M.; Moss, A.D.; Herzog, J.W.; Kashiwagi, S.; Liu, B.; Young, J.B.; Sun, S.; Bhatt, A.P.; Fodor, A.A.; Balfour Sartor, R. Mouse Adaptation of Human Inflammatory Bowel Diseases Microbiota Enhances Colonization Efficiency and Alters Microbiome Aggressiveness Depending on the Recipient Colonic Inflammatory Environment. Microbiome 2024, 12, 147. [Google Scholar] [CrossRef]
- Rashed, R.; Valcheva, R.; Dieleman, L.A. Manipulation of Gut Microbiota as a Key Target for Crohn’s Disease. Front. Med. 2022, 9, 887044. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; Melgar, S.; O’Donoghue, K.; Martínez-Sánchez, M.A.; Fernández-Ruiz, V.E.; Ferrer-Gómez, M.; Ruiz-Alcaraz, A.J.; Ramos-Molina, B. Crohn’s Disease, Host–Microbiota Interactions, and Immunonutrition: Dietary Strategies Targeting Gut Microbiome as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 8361. [Google Scholar] [CrossRef] [PubMed]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic Gut Microbiota Causes Transmissible Crohn’s Disease-like Ileitis Independent of Failure in Antimicrobial Defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Jarchum, I.; Equinda, M.; Lipuma, L.; Gobourne, A.; Viale, A.; Ubeda, C.; Xavier, J.; Pamer, E.G. Profound Alterations of Intestinal Microbiota Following a Single Dose of Clindamycin Results in Sustained Susceptibility to Clostridium Difficile-Induced Colitis. Infect. Immun. 2012, 80, 62–73. [Google Scholar] [CrossRef]
- Antonopoulos, D.A.; Huse, S.M.; Morrison, H.G.; Schmidt, T.M.; Sogin, M.L.; Young, V.B. Reproducible Community Dynamics of the Gastrointestinal Microbiota Following Antibiotic Perturbation. Infect. Immun. 2009, 77, 2367–2375. [Google Scholar] [CrossRef]
- Piccioni, A.; Rosa, F.; Manca, F.; Pignataro, G.; Zanza, C.; Savioli, G.; Covino, M.; Ojetti, V.; Gasbarrini, A.; Franceschi, F.; et al. Gut Microbiota and Clostridium Difficile: What We Know and the New Frontiers. Int. J. Mol. Sci. 2022, 23, 13323. [Google Scholar] [CrossRef]
- Sehgal, K.; Khanna, S. Gut Microbiome and Clostridioides difficile Infection: A Closer Look at the Microscopic Interface. Ther. Adv. Gastroenterol. 2021, 14, 1756284821994736. [Google Scholar] [CrossRef]
- van der Vossen, E.W.J.; de Goffau, M.C.; Levin, E.; Nieuwdorp, M. Recent Insights into the Role of Microbiome in the Pathogenesis of Obesity. Ther. Adv. Gastroenterol. 2022, 15, 17562848221115320. [Google Scholar] [CrossRef]
- Chauhan, S.; Jena, K.K.; Mehto, S.; Chauhan, N.R.; Sahu, R.; Dhar, K.; Yadav, R.; Krishna, S.; Jaiswal, P.; Chauhan, S. Innate Immunity and Inflammophagy: Balancing the Defence and Immune Homeostasis. FEBS J. 2022, 289, 4112–4131. [Google Scholar] [CrossRef]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of High-Fat Diet on Gut Microbiota: A Driving Force for Chronic Disease Risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Allegretti, J.R.; Dubberke, E.R.; Guo, A.; Harvey, A.; Yang, M.; Garcia-Horton, V.; Fillbrunn, M.; Tillotson, G.; Bancke, L.L.; et al. Efficacy and Health-Related Quality of Life Impact of Fecal Microbiota, Live-Jslm: A Post Hoc Analysis of PUNCH CD3 Patients at First Recurrence of Clostridioides difficile Infection. Infect. Dis. Ther. 2024, 13, 221–236. [Google Scholar] [CrossRef]
- Binyamin, D.; Nitzan, O.; Azrad, M.; Hamo, Z.; Koren, O.; Peretz, A. The Microbial Diversity Following Antibiotic Treatment of Clostridioides difficile Infection. BMC Gastroenterol. 2021, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Dicksved, J.; Jansson, J.K.; Sadowsky, M.J. Changes in the Composition of the Human Fecal Microbiome After Bacteriotherapy for Recurrent Clostridium Difficile-Associated Diarrhea. J. Clin. Gastroenterol. 2010, 44, 354. [Google Scholar] [CrossRef] [PubMed]
- Roshan, N.; Clancy, A.K.; Borody, T.J. Faecal Microbiota Transplantation Is Effective for the Initial Treatment of Clostridium Difficile Infection: A Retrospective Clinical Review. Infect. Dis. Ther. 2020, 9, 935–942. [Google Scholar] [CrossRef]
- Zou, B.; Liu, S.; Li, X.; He, J.; Dong, C.; Ruan, M.; Huang, Z.; Shu, S. Repeated and Multiple Fecal Microbiota Transplantations plus Partial Enteral Nutrition as the First-Line Treatment in Active Pediatric Crohn’s Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1083236. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Khanna, S.; Assi, M.; Lee, C.; Yoho, D.; Louie, T.; Knapple, W.; Aguilar, H.; Garcia-Diaz, J.; Wang, G.P.; Berry, S.M.; et al. Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection. Drugs 2022, 82, 1527–1538. [Google Scholar] [CrossRef]
- Lee, C.; Louie, T.; Bancke, L.; Guthmueller, B.; Harvey, A.; Feuerstadt, P.; Khanna, S.; Orenstein, R.; Dubberke, E.R. Safety of Fecal Microbiota, Live-Jslm (REBYOTATM) in Individuals with Recurrent Clostridioides difficile Infection: Data from Five Prospective Clinical Trials. Ther. Adv. Gastroenterol. 2023, 16, 17562848231174277. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.L.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N. Engl. J. Med. 2022, 386, 220–229. [Google Scholar] [CrossRef]
- Berenson, C.S.; Lashner, B.; Korman, L.Y.; Hohmann, E.; Deshpande, A.; Louie, T.J.; Sims, M.; Pardi, D.; Kraft, C.S.; Wang, E.E.L.; et al. Prevalence of Comorbid Factors in Patients With Recurrent Clostridioides difficile Infection in ECOSPOR III, a Randomized Trial of an Oral Microbiota–Based Therapeutic. Clin. Infect. Dis. 2023, 77, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.; Lee, K.C.; Wang, Y.P.; Lee, P.C.; Chang, T.E.; Huang, Y.H.; Lin, Y.T.; Hou, M.C. High Carriage Rate of Extended-Spectrum Beta-Lactamase Enterobacterales and Diarrheagenic Escherichia Coli in Healthy Donor Screening for Fecal Microbiota Transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.A.; Baig, M.; Puli, S.R. Adverse Events in Fecal Microbiota Transplantation: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2022, 35, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Merrick, B.; Allen, L.; Masirah, M.Z.N.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Prac. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity Influences the Gut Microbiota of Individuals Sharing a Geographical Location: A Cross-Sectional Study from a Middle-Income Country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef]
- van Leeuwen, P.T.; Brul, S.; Zhang, J.; Wortel, M.T. Synthetic Microbial Communities (SynComs) of the Human Gut: Design, Assembly, and Applications. FEMS Microbiol. Rev. 2023, 47, fuad012. [Google Scholar] [CrossRef]
- Dillon, K.M.; Morrison, H.A.; Powell, C.R.; Carrazzone, R.J.; Ringel-Scaia, V.M.; Winckler, E.W.; Council-Troche, R.M.; Allen, I.C.; Matson, J.B. Targeted Delivery of Persulfides to the Gut: Effects on the Microbiome. Angew. Chem. Int. Ed. Engl. 2021, 60, 6061–6067. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.B.; Way, J.C.; Silver, P.A. Stable Neutralization of a Virulence Factor in Bacteria Using Temperate Phage in the Mammalian Gut. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; English translation edited by P. Chalmers Mitchell; Heinemann: London, UK, 1907. [Google Scholar]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef]
- Lau, C.S.M.; Chamberlain, R.S. Probiotics Are Effective at Preventing Clostridium Difficile-Associated Diarrhea: A Systematic Review and Meta-Analysis. Int. J. Gen. Med. 2016, 9, 27–37. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Fleur, A.S.; Galen, J.; Yang, Z.; Feng, H. A Probiotic Yeast-Based Immunotherapy against Clostridioides difficile Infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I. Probiotics in Functional Gastrointestinal Disorders. Adv. Exp. Med. Biol. 2019, 1125, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera-deGuise, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and Prebiotics. J. Clin. Gastroenterol. 2024, 58, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, L.; Cai, Y.; Kang, Y. The Development of Probiotics and Prebiotics Therapy to Ulcerative Colitis: A Therapy That Has Gained Considerable Momentum. Cell Commun. Signal 2024, 22, 268. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Wang, B.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Suo, H.; Chen, W.; Zhai, Q. Stachyose Modulates Gut Microbiota and Alleviates DSS-Induced Ulcerative Colitis in Mice. Food Sci. Hum. Wellness 2023, 12, 2211–2220. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Bacha, A.A.; Suhail, M.; Awwad, F.A.; Ismail, E.A.A.; Ahmad, H. Role of Dietary Fiber and Lifestyle Modification in Gut Health and Sleep Quality. Front. Nutr. 2024, 11, 1324793. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef]
- Kim, E.K.; An, S.-Y.; Lee, M.-S.; Kim, T.H.; Lee, H.-K.; Hwang, W.S.; Choe, S.J.; Kim, T.-Y.; Han, S.J.; Kim, H.J.; et al. Fermented Kimchi Reduces Body Weight and Improves Metabolic Parameters in Overweight and Obese Patients. Nutr. Res. 2011, 31, 436–443. [Google Scholar] [CrossRef]
- Hassan, N.E.; El-Masry, S.A.; Shebini, S.M.E.; Ahmed, N.H.; Mehanna, N.S.; Wahed, M.M.A.; Amine, D.; Hashish, A.; Selim, M.; Afify, M.A.S.; et al. Effect of Weight Loss Program Using Prebiotics and Probiotics on Body Composition, Physique, and Metabolic Products: Longitudinal Intervention Study. Sci. Rep. 2024, 14, 10960. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Atazadegan, M.A.; Heidari-Beni, M.; Entezari, M.H.; Sharifianjazi, F.; Kelishadi, R. Effects of Synbiotic Supplementation on Anthropometric Indices and Body Composition in Overweight or Obese Children and Adolescents: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. World J. Pediatr. WJP 2023, 19, 356–365. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wu, X.; Liu, B.; Ma, H.; Zhao, X.; Cao, S.; Ding, S.; Li, T.; Wang, X.; et al. Specially Designed Yogurt Supplemented with Combination of Pro- and Prebiotics Relieved Constipation in Mice and Humans. Nutrition 2022, 103–104, 111802. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Casquete, M.; Castro, M.J.; Redondo Del Rio, P.; Gutierrez, E.; Mayo-Iscar, A.; Nocito, M.; Corell, A. Prospective, Randomized, Double-Blind Parallel Group Nutritional Study to Evaluate the Effects of Routine Intake of Fresh vs. Pasteurized Yogurt on the Immune System in Healthy Adults. Nutrients 2024, 16, 1969. [Google Scholar] [CrossRef] [PubMed]
- Lazda, I.; Krūmiņa, A.; Zeltiņa, I.; Krūmiņa, N.; Ķibilds, J.; Siksna, I.; Vīksna, L.; Derovs, A. Microbial Community of Kefir and Its Impact on the Gastrointestinal Microbiome in Health and Disease. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2020, 74, 58–64. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Ju, T.; Fouhse, J.M.; Forgie, A.J.; Sergi, C.; Cotter, P.D.; Willing, B.P. Kefir Microbial Composition Is a Deciding Factor in the Physiological Impact of Kefir in a Mouse Model of Obesity. Br. J. Nutr. 2021, 125, 129–138. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Cotter, P.D.; Willing, B.P. Traditional Kefir Reduces Weight Gain and Improves Plasma and Liver Lipid Profiles More Successfully than a Commercial Equivalent in a Mouse Model of Obesity. J. Funct. Foods 2018, 46, 29–37. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rajendraprasad, S.; Ozkan, M.; Ramachandran, D.; Ahmad, S.; Bakken, J.S.; Laudanski, K.; Gajic, O.; Bauer, B.; Zec, S.; et al. Safety, Feasibility, and Impact on the Gut Microbiome of Kefir Administration in Critically Ill Adults. BMC Med. 2024, 22, 80. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Nuirat, A.; Zihlif, M.A.; Taha, M.O. Exploring the Influence of Culture Conditions on Kefir’s Anticancer Properties. J. Dairy Sci. 2018, 101, 3771–3777. [Google Scholar] [CrossRef]
- Fraiz, G.M.; Costa, M.A.C.; Cardoso, R.R.; Hébert, J.R.; Zhao, L.; Corich, V.; Giacomini, A.; Milagro, F.I.; Barros, F.A.R. Fermentation | Free Full-Text | Black Tea Kombucha Consumption: Effect on Cardiometabolic Parameters and Diet Quality of Individuals with and without Obesity. Fermentation 2024, 10, 384. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bhattarai, U.; Adhikari, K. The Healthy Eater’s Idea and Related Behavior of a Healthy Diet—A Case Study with Kombucha Drinkers. Beverages 2022, 8, 25. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Alaei, Z.; Doudi, M.; Setorki, M. The Protective Role of Kombucha Extract on the Normal Intestinal Microflora, High-Cholesterol Diet Caused Hypercholesterolemia, and Histological Structures Changes in New Zealand White Rabbits. Avicenna J. Phytomed. 2020, 10, 604–614. [Google Scholar] [PubMed]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.-S. Effect of Kombucha on Gut-Microbiota in Mouse Having Non-Alcoholic Fatty Liver Disease. Food Sci. Biotechnol. 2018, 28, 261–267. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Fernández-Arteaga, A.; Luzón, G.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.Á. Spent Coffee Grounds Extract, Rich in Mannooligosaccharides, Promotes a Healthier Gut Microbial Community in a Dose-Dependent Manner. J. Agric. Food Chem. 2019, 67, 2500–2509. [Google Scholar] [CrossRef]
- Godoy-Vitorino, F.; Vilanova-Cuevas, B. Probiotic Microbiota of Fermented Tropical Wood Drinks: Mauby and Tepache; ResearchGate: Santiago, Chile, 2019. [Google Scholar]
- de la Fuente-Salcido, N.; Castañeda-Ramirez, J.; García-Almendárez, B.; Bideshi, D.; Salcedo-Hernández, R.; Barbosa-Corona, J. Isolation and Characterization of Bacteriocinogenic Lactic Bacteria from M-Tuba and Tepache, Two Traditional Fermented Beverages in México. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/fsn3.236 (accessed on 27 August 2024).
- Lee, Y.K.; Low, K.Y.; Siah, K.; Drummond, L.M.; Gwee, K.A. Kiwifruit (Actinidia Deliciosa) Changes Intestinal Microbial Profile. Microb. Ecol. Health Dis. 2012, 23, 18572. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary Black Raspberries Impact the Colonic Microbiome and Phytochemical Metabolites in Mice. Mol. Nutr. Food Res. 2019, 63, 1800636. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Ke, X.; Walker, A.; Haange, S.-B.; Lagkouvardos, I.; Liu, Y.; Schmitt-Kopplin, P.; von Bergen, M.; Jehmlich, N.; He, X.; Clavel, T.; et al. Synbiotic-Driven Improvement of Metabolic Disturbances Is Associated with Changes in the Gut Microbiome in Diet-Induced Obese Mice. Mol. Metab. 2019, 22, 96–109. [Google Scholar] [CrossRef] [PubMed]
| Gut Health and Disease/Dysbiotic Phenotypes | Animal Studies | References | Human Studies | References |
|---|---|---|---|---|
| Normal gut microbiome | In mammals, opportunistic bacteria that can thrive in dysbiotic habitats displace the microbes that contribute to a healthy ecosystem, influencing the environmental metagenome; as such, a clear difference in microbial populations is seen between captive and wild animals. In the mouse research model, Firmicutes, Bacteroidetes, Proteobacteria, Deferribacteres, Actinobacteria, Tenericutes, and Verrucomicrobia are shown to be commensal inhabitants of the gut microbiota, with the first three being the most abundant taxa. | [88,89,90,91] | An abundance of microbes such as Bacteroides, Lactobacillus, Bacillus, Enterococcus, Faecalibacterium, Bifidobacterium and Ruminococcus spp. are characteristic of a healthy and diverse gut microbiota. | [24,25,60] |
| Ulcerative colitis | In mice with UC, dysbiosis in their microbiota is closely related to disease pathogenesis and symptomatology. Increased populations of Proteobacteria, Actinobacteria, and Clostridium spp. were observed in mice with mucosal inflammation correlating to a UC profile. Enterobacteriaceae taxa, R. gnavus, and especially E. coli are shown to drive colonization of pathobionts and inflammation in colitis mice models. | [92,93,94] | The interactions of the host and their microbiome, a decrease in Firmicutes, an increase in Proteobacteria, reduction in microbial diversity, and alterations in homeostasis are associated with UC pathogenesis. The connection of fungi with certain intestinal bacteria could lead to developing UC. S. boulardii and C. albicans are seen to confer positive or negative effects, respectively, depending on the bacterial diversity present in the gut microbiota. | [31,33,34,41,95] |
| Crohn’s disease | The role of the microbiota can be seen in how the transference of microbes from a dysbiotic animal model can influence a healthy system. The transferrence of an altered microbiota from actively diseased mice induced CD symptomatology in healthy mice. Research by Schaubeck et al. points to Bacteroidaceae, Erysipelotrichaceae, Peptostreptococcaceae and Verrucomicrobiaceae taxa in mice that simulate a CD profile. Specifically, B. acidifaciens and B. sartorii were found in greater abundance. Bacteroides spp. are more prevalent when transferring human microbiota into mice. | [94,96,97] | Reduction in bacterial exposure and lower microbial diversity correlates with increased disease incidence. Increase in Fusobacteriaceae-like taxa is linked with the Crohn’s disease profile. CD is also characterized by a decrease in fungal and bacterial interactions. An abundance of fungi, like C. tropicalis, can give rise to more opportunistic pathogenic bacteria. | [17,95] |
| Clostridioides difficile | Antibiotics can diminish microbial communities for prolonged periods and eliminate species that could provide colonization resistance against C. difficile. A combination of antibiotics and CDI can further alter the intestinal microbiota and increase mucosal inflammation in mice, demonstrating increased abundance in Proteobacteria and Enterococci and a loss of microbial commensal diversity, such as Enterobacteriaceae. Even after the removal of antibiotics, their dysbiotic microbial communities remain susceptible to recurrence of CDI. | [98,99,100] | Antibiotics are known to generate dysbiosis in the human microbiome; in the same manner, they are heavily linked to the pathogenesis of CDI. Beneficial bacteria such as Bacteroides, Lactobacillus, and Ruminococcus, among related taxa, provide resistance for the gut microbiota against developing CDI in a perturbed intestinal system. | [60,76,101] |
| Obesity | There exist links between the immune system and the gut microbiome. NOD2, as well as TLR5-deficient, mice are prone to increased adiposity. Dysbiotic microbiota transference into germ-free mice resulted in inflammation and gain in adipose tissue. | [78,102,103] | Diet has been proven to influence the microbes in the gut and modulate the levels of inflammation and obesity in a human host, especially in a Westernized high-fat diet selecting for less beneficial bacteria. The unbalanced ratio between Firmicutes and Bacteroidetes in dysbiosis correlates with increased adiposity, weight gain, and BMI. | [10,102,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. https://doi.org/10.3390/ijms25179715
Acevedo-Román A, Pagán-Zayas N, Velázquez-Rivera LI, Torres-Ventura AC, Godoy-Vitorino F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. International Journal of Molecular Sciences. 2024; 25(17):9715. https://doi.org/10.3390/ijms25179715
Chicago/Turabian StyleAcevedo-Román, Andy, Natalia Pagán-Zayas, Liz I. Velázquez-Rivera, Aryanne C. Torres-Ventura, and Filipa Godoy-Vitorino. 2024. "Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches" International Journal of Molecular Sciences 25, no. 17: 9715. https://doi.org/10.3390/ijms25179715
APA StyleAcevedo-Román, A., Pagán-Zayas, N., Velázquez-Rivera, L. I., Torres-Ventura, A. C., & Godoy-Vitorino, F. (2024). Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. International Journal of Molecular Sciences, 25(17), 9715. https://doi.org/10.3390/ijms25179715






