The Underlying Molecular Mechanisms of the Placenta Accreta Spectrum: A Narrative Review
Abstract
:1. Introduction
2. Methodology
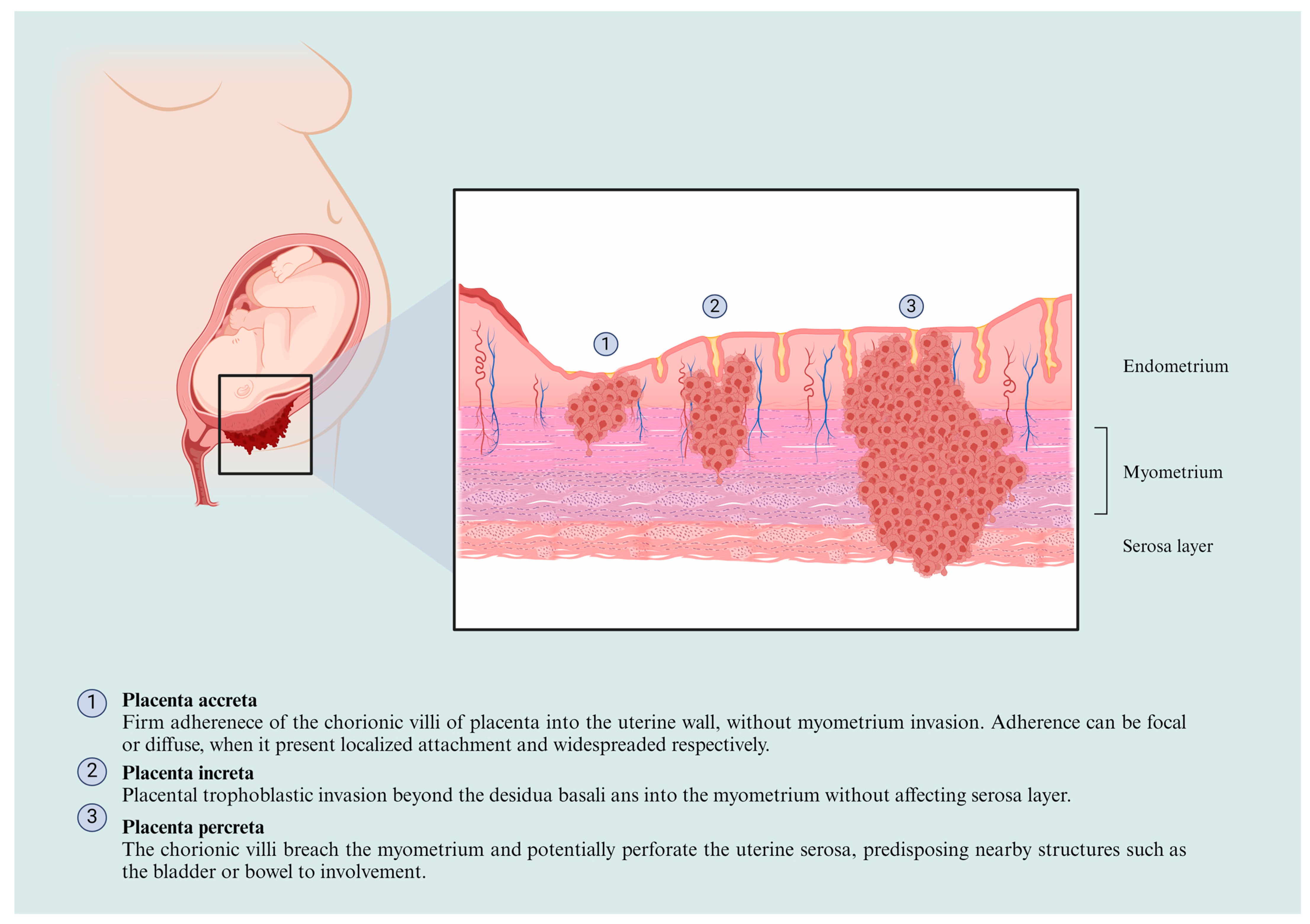
3. The PAS Classification and Physiopathological Features
4. Biomarkers Associated with Placenta Accreta Development
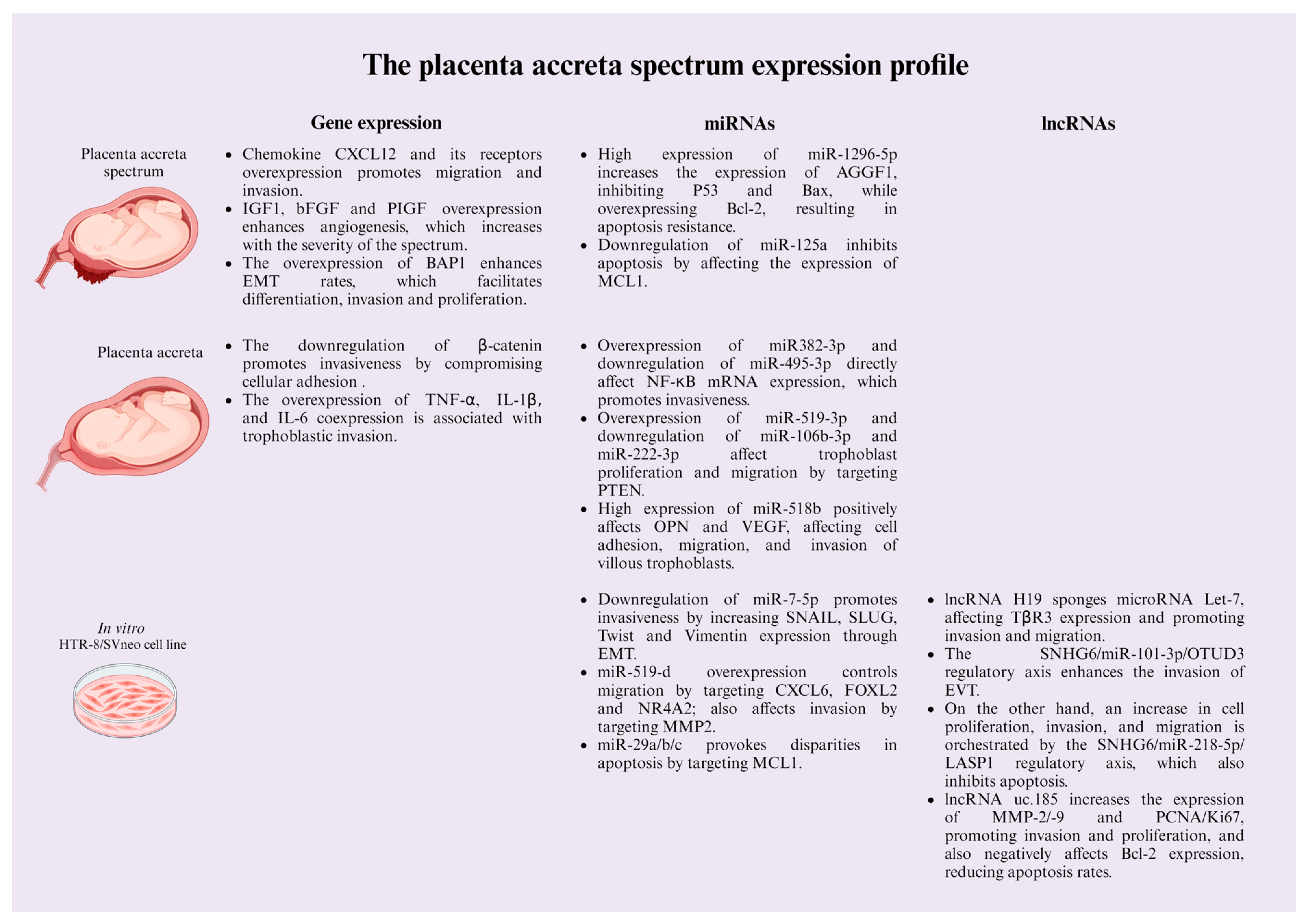
5. Molecular Mechanisms Involved in the Placenta Acreta Spectrum
5.1. Gene Expression
5.2. The Roles of Non-Coding RNAs in the PAS
5.2.1. microRNAs
5.2.2. Long Non-Coding RNAs
6. Aberrant Signaling Pathways in the PAS
7. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Markfeld Erol, F.; Häußler, J.A.; Medl, M.; Juhasz-Boess, I.; Kunze, M. Placenta Accreta Spectrum (PAS): Diagnosis, Clinical Presentation, Therapeutic Approaches, and Clinical Outcomes. Medicina 2024, 60, 1180. [Google Scholar] [CrossRef] [PubMed]
- Piñas Carrillo, A.; Chandraharan, E. Placenta accreta spectrum: Risk factors, diagnosis and management with special reference to the Triple P procedure. Women’s Health 2019, 15, 1745506519878081. [Google Scholar] [CrossRef]
- Cahill, A.G.; Beigi, R.; Heine, P.; Silver, R.M.; Wax, J.R. Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstet. Gynecol. 2018, 132, e259–e275. [Google Scholar] [CrossRef]
- Bowman, Z.S.; Eller, A.G.; Bardsley, T.R.; Greene, T.; Varner, M.W.; Silver, R.M. Risk factors for placenta accreta: A large prospective cohort. Am. J. Perinatol. 2014, 31, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Fu, R.; Guise, J.M. Impact of multiple cesarean deliveries on maternal morbidity: A systematic review. Am. J. Obstet. Gynecol. 2011, 205, 262.e1–262.e8. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D. Placenta accreta: Pathogenesis of a 20th century iatrogenic uterine disease. Placenta 2012, 33, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Chantraine, F.; Silver, R.M.; Langhoff-Roos, J. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int. J. Gynecol. Obstet. 2018, 140, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Betrán, A.P.; Ye, J.; Moller, A.B.; Zhang, J.; Gülmezoglu, A.M.; Torloni, M.R. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990–2014. PLoS ONE 2016, 11, e0148343. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, J.; Mikolajczyk, R.; Torloni, M.R.; Gülmezoglu, A.M.; Betran, A.P. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: A worldwide population-based ecological study with longitudinal data. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 745–753. [Google Scholar] [CrossRef]
- Gloria, C.; Salvatore, P.; Francesco, L.; Anna, G.R.; Francesca, M.; Chiara, B.; Gaspare, C.; Giuseppe, C. Placenta Accreta Spectrum Disorder in a Patient with Six Previous Caesarean Deliveries: Step by Step Management. Case Rep. Obstet. Gynecol. 2021, 2021, 2105248. [Google Scholar] [CrossRef]
- van Beekhuizen, H.J.; Stefanovic, V.; Schwickert, A.; Henrich, W.; Fox, K.A.; MHallem Gziri, M.; Sentilhes, L.; Gronbeck, L.; Chantraine, F.; Morel, O.; et al. A multicenter observational survey of management strategies in 442 pregnancies with suspected placenta accreta spectrum. Acta Obstet. Gynecol. Scand. 2021, 100 (Suppl. S1), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, H.; Ma, J.; Dou, R.; Zhao, X.; Yan, J.; Yang, H. Validation of a scoring system for prediction of obstetric complications in placenta accreta spectrum disorders. J. Matern.-Fetal Neonatal Med. 2022, 35, 4149–4155. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; Kastelein, A.W.; Kleinrouweler, C.E.; Van Leeuwen, E.; De Jong, K.H.; Pajkrt, E.; Van Noorden, C.J.F. Development of placental abnormalities in location and anatomy. Acta Obstet. Gynecol. Scand. 2020, 99, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lorca, R.A.; Su, E.J. Molecular and cellular underpinnings of normal and abnormal human placental blood flows. J. Mol. Endocrinol. 2018, 60, R9–R22. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.A.M.; Mesbah, Y.; Soliman, M.F.M.; Firgany, A.E.L. Dominance of Pro-Inflammatory Cytokines Over Anti-Inflammatory Ones in Placental Bed of Creta Cases. J. Microsc. Ultrastruct. 2024, 12, 14–20. [Google Scholar] [CrossRef]
- Tantbirojn, P.; Crum, C.P.; Parast, M.M. Pathophysiology of Placenta Creta: The Role of Decidua and Extravillous Trophoblast. Placenta 2008, 29, 639–645. [Google Scholar] [CrossRef]
- Wang, S.C.; Yu, M.; Li, Y.H.; Piao, H.L.; Tang, C.L.; Sun, C.; Zhu, R.; Li, M.Q.; Jin, L.P.; Li, D.J.; et al. Cyclosporin A promotes proliferating cell nuclear antigen expression and migration of human cytotrophoblast cells via the mitgen-activated protein kinase-3/1-mediated nuclear factor-κB signaling pathways. Int. J. Clin. Exp. Pathol. 2013, 6, 1999–2010. [Google Scholar]
- Gualdoni, G.; Gomez Castro, G.; Hernández, R.; Barbeito, C.; Cebral, E. Comparative matrix metalloproteinase-2 and -9 expression and activity during endotheliochorial and hemochorial trophoblastic invasiveness. Tissue and Cell 2022, 74, 101698. [Google Scholar] [CrossRef]
- Arakaza, A.; Liu, X.; Zhu, J.; Zou, L. Assessment of serum levels and placental bed tissue expression of IGF-1, bFGF, and PLGF in patients with placenta previa complicated with placenta accreta spectrum disorders. J. Matern.-Fetal Neonatal Med. 2024, 37, 2305264. [Google Scholar] [CrossRef]
- Schwickert, A.; Chantraine, F.; Ehrlich, L.; Henrich, W.; Muallem, M.Z.; Nonnenmacher, A.; Petit, P.; Weizsäcker, K.; Braun, T. Maternal Serum VEGF Predicts Abnormally Invasive Placenta Better than NT-proBNP: A Multicenter Case-Control Study. Reprod. Sci. 2021, 28, 361–370. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, M.; Chen, P.; Wan, S.; Zhou, Q.; Lu, Y.; Li, L. Distinguishing placenta accreta from placenta previa via maternal plasma levels of sFlt-1 and PLGF and the sFlt-1/PLGF ratio. Placenta 2022, 124, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Verde, F.; Sarno, L.; Migliorini, S.; Petretta, M.; Mainenti, P.P.; D’Armiento, M.; Guida, M.; Brunetti, A.; Maurea, S. Prediction of placenta accreta spectrum in patients with placenta previa using clinical risk factors, ultrasound and magnetic resonance imaging findings. La Radiol. Medica 2021, 126, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Wu, Y.; Zeng, J.; Yuan, X.; Tong, C.; Qi, H. What we know about placenta accreta spectrum (PAS). Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Baergen, R.; Ernst, L.M.; Katzman, P.J.; Jacques, S.M.; Jauniaux, E.; Khong, T.Y.; Metlay, L.A.; Poder, L.; Qureshi, F.; et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: Recommendations from an expert panel. Mod. Pathol. 2020, 33, 2382–2396. [Google Scholar] [CrossRef]
- Shainker, S.A.; Silver, R.M.; Modest, A.M.; Hacker, M.R.; Hecht, J.L.; Salahuddin, S.; Dillon, S.T.; Ciampa, E.J.; D’Alton, M.E.; Otu, H.H.; et al. Placenta accreta spectrum: Biomarker discovery using plasma proteomics. Am. J. Obstet. Gynecol. 2020, 223, 433.e1–433.e14. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S. Potential Serum Biomarkers in Prenatal Diagnosis of Placenta Accreta Spectrum. Front. Med. 2022, 9, 860186. [Google Scholar] [CrossRef]
- Berezowsky, A.; Pardo, J.; Ben-Zion, M.; Wiznitzer, A.; Aviram, A. Second Trimester Biochemical Markers as Possible Predictors of Pathological Placentation: A Retrospective Case-Control Study. Fetal Diagn. Ther. 2019, 46, 187–192. [Google Scholar] [CrossRef]
- Oztas, E.; Ozler, S.; Caglar, A.T.; Yucel, A. Analysis of first and second trimester maternal serum analytes for the prediction of morbidly adherent placenta requiring hysterectomy. Kaohsiung J. Med. Sci. 2016, 32, 579–585. [Google Scholar] [CrossRef]
- Lumbanraja, S.; Yaznil, M.R.; Siahaan, A.M.; Berry Eka Parda, B. Soluble FMS-Like Tyrosine Kinase-1: Role in placenta accreta spectrum disorder. F1000Research 2022, 10, 618. [Google Scholar] [CrossRef]
- Büke, B.; Akkaya, H.; Demir, S.; Sağol, S.; Şimşek, D.; Başol, G.; Barutçuoğlu, B. Relationship between first trimester aneuploidy screening test serum analytes and placenta accreta. J. Matern.-Fetal Neonatal Med. 2018, 31, 59–62. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Yan, P.; Ye, Y.H.; Peng, W.; Wang, S.; Wang, X.T. Maternal plasma levels of cell-free β-HCG mRNA as a prenatal diagnostic indicator of placenta accrete. Placenta 2014, 35, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.; Akbarzadeh-Jahromi, M.; Bahrami, S.; Gharamani, S.; Raeisi Shahraki, H.; Kasraeian, M.; Vafaei, H.; Zare, M.; Asadi, N. Predictive value of vascular endothelial growth factor and placenta growth factor for placenta accreta spectrum. J. Obstet. Gynaecol. 2022, 42, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, S.; Wang, J.; Wang, Y.; Ruan, F.; Shu, H.; Zhu, L.; Man, D. First trimester serum PAPP-A is associated with placenta accreta: A retrospective study. Arch. Gynecol. Obstet. 2021, 303, 645–652. [Google Scholar] [CrossRef]
- Desai, N.; Krantz, D.; Roman, A.; Fleischer, A.; Boulis, S.; Rochelson, B. Elevated first trimester PAPP-A is associated with increased risk of placenta accreta. Prenat. Diagn. 2014, 34, 159–162. [Google Scholar] [CrossRef]
- Thompson, O.; Otigbah, C.; Nnochiri, A.; Sumithran, E.; Spencer, K. First trimester maternal serum biochemical markers of aneuploidy in pregnancies with abnormally invasive placentation. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1370–1376. [Google Scholar] [CrossRef]
- Lyell, D.J.; Faucett, A.M.; Baer, R.J.; Blumenfeld, Y.J.; Druzin, M.L.; El-Sayed, Y.Y.; Shaw, G.M.; Currier, R.J.; Jelliffe-Pawlowski, L.L. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J. Perinatol. 2015, 35, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Penzhoyan, G.A.; Makukhina, T.B. Significance of the routine first-trimester antenatal screening program for aneuploidy in the assessment of the risk of placenta accreta spectrum disorders. J. Perinat. Med. 2019, 48, 21–26. [Google Scholar] [CrossRef]
- Kawashima, A.; Sekizawa, A.; Ventura, W.; Koide, K.; Hori, K.; Okai, T.; Masashi, Y.; Furuya, K.; Mizumoto, Y. Increased levels of cell-free human placental lactogen mRNA at 28-32 gestational weeks in plasma of pregnant women with placenta previa and invasive placenta. Reprod. Sci. 2014, 21, 215–220. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Zhang, Y.; Hu, X.; Gao, G.; Ye, Y.; Peng, W.; Zhou, J. Human placental lactogen mRNA in maternal plasma play a role in prenatal diagnosis of abnormally invasive placenta: Yes or no? Gynecol. Endocrinol. 2019, 35, 631–634. [Google Scholar] [CrossRef]
- Shainker, S.A.; Dannheim, K.; Gerson, K.D.; Neo, D.; Zsengeller, Z.K.; Pernicone, E.; Karumanchi, S.A.; Hacker, M.R.; Hecht, J.L. Down-regulation of soluble fms-like tyrosine kinase 1 expression in invasive placentation. Arch. Gynecol. Obstet. 2017, 296, 257–262. [Google Scholar] [CrossRef]
- Goh, W.; Yamamoto, S.Y.; Thompson, K.S.; Bryant-Greenwood, G.D. Relaxin, its receptor (RXFP1), and insulin-like peptide 4 expression through gestation and in placenta accreta. Reprod. Sci. 2013, 20, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Bartels, H.C.; Postle, J.D.; Downey, P.; Brennan, D.J. Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis. Markers 2018, 2018, 1507674. [Google Scholar] [CrossRef]
- Illsley, N.P.; DaSilva-Arnold, S.C.; Zamudio, S.; Alvarez, M.; Al-Khan, A. Trophoblast invasion: Lessons from abnormally invasive placenta (placenta accreta). Placenta 2020, 102, 61–66. [Google Scholar] [CrossRef]
- Long, Y.; Jiang, Y.; Zeng, J.; Dang, Y.; Chen, Y.; Lin, J.; Wei, H.; Xia, H.; Long, J.; Luo, C.; et al. The expression and biological function of chemokine CXCL12 and receptor CXCR4/CXCR7 in placenta accreta spectrum disorders. J. Cell. Mol. Med. 2020, 24, 3167–3182. [Google Scholar] [CrossRef]
- Heidari, S.; Kolahdouz-Mohammadi, R.; Khodaverdi, S.; Tajik, N.; Delbandi, A.-A. Expression levels of MCP-1, HGF, and IGF-1 in endometriotic patients compared with non-endometriotic controls. BMC Women’s Health 2021, 21, 422. [Google Scholar] [CrossRef]
- Shigematsu, S.; Yamauchi, K.; Nakajima, K.; Iijima, S.; Aizawa, T.; Hashizume, K. IGF-1 Regulates Migration and Angiogenesis of Human Endothelial Cells. Endocr. J. 1999, 46, S59–S62. [Google Scholar] [CrossRef]
- He, X.; Zhao, L.; Yue, L.; Zhang, W.; Wang, W.; Fu, Y.; Feng, Y.; Fu, F. The relationship between IGF1 and the expression spectrum of miRNA in the placenta of preeclampsia patients. Ginekol. Pol. 2019, 90, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Pedone, E.; Marucci, L. Role of β-Catenin Activation Levels and Fluctuations in Controlling Cell Fate. Genes 2019, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, L.; Liu, Z.; Luo, J.; Chen, R.; Yan, J. Expression of β-catenin in human trophoblast and its role in placenta accreta and placenta previa. J. Int. Med. Res. 2019, 47, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, V.; Lea, G.; Lopez-Jimenez, P.; Okkenhaug, H.; Burton, G.J.; Moffett, A.; Turco, M.Y.; Hemberger, M. BAP1/ASXL complex modulation regulates epithelial-mesenchymal transition during trophoblast differentiation and invasion. eLife 2021, 10, e63254. [Google Scholar] [CrossRef]
- Cheng, S.B.; Nakashima, A.; Huber, W.J.; Davis, S.; Banerjee, S.; Huang, Z.; Saito, S.; Sadovsky, Y.; Sharma, S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019, 10, 927. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.J.; Peñailillo, R.; Sánchez, M.; Acuña-Gallardo, S.; Mönckeberg, M.; Ong, J.; Choolani, M.; Illanes, S.E.; Nardocci, G. The Role of Long Non-Coding RNAs in Trophoblast Regulation in Preeclampsia and Intrauterine Growth Restriction. Genes 2021, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Kannampuzha, S.; Ravichandran, M.; Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; Iyer, M.; Dey, A.; George, A.; Gopalakrishnan, A.V. The mechanism of action of non-coding RNAs in placental disorders. Biomed. Pharmacother. 2022, 156, 113964. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-S.; Su, J.-L.; Hung, M.-C. Dysregulation of MicroRNAs in cancer. J. Biomed. Sci. 2012, 19, 90. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Liang, B.; Lin, J.; Kim, T.K.; Yu, H.; Hang, H.; Wang, K. A Systematic Study of Dysregulated MicroRNA in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2017, 18, 456. [Google Scholar] [CrossRef]
- Shih, J.C.; Lin, H.H.; Hsiao, A.C.; Su, Y.T.; Tsai, S.; Chien, C.L.; Kung, H.N. Unveiling the role of microRNA-7 in linking TGF-β-Smad-mediated epithelial-mesenchymal transition with negative regulation of trophoblast invasion. FASEB J. 2019, 33, 6281–6295. [Google Scholar] [CrossRef]
- Xie, L.; Sadovsky, Y. The function of miR-519d in cell migration, invasion, and proliferation suggests a role in early placentation. Placenta 2016, 48, 34–37. [Google Scholar] [CrossRef]
- Murrieta-Coxca, J.M.; Barth, E.; Fuentes-Zacarias, P.; Gutiérrez-Samudio, R.N.; Groten, T.; Gellhaus, A.; Köninger, A.; Marz, M.; Markert, U.R.; Morales-Prieto, D.M. Identification of altered miRNAs and their targets in placenta accreta. Front. Endocrinol. 2023, 14, 1021640. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, J.; Liu, C.; Li, S.; Liu, W.; Cao, Q. Decreased AGGF1 facilitates the progression of placenta accreta spectrum via mediating the P53 signaling pathway under the regulation of miR-1296-5p. Reprod. Biol. 2023, 23, 100735. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Chen, Y.; Fu, X.Q.; Yang, F.; Chen, Z.W.; Mo, G.L.; Lao, D.Y.; Li, M.J. Research on the expression of MRNA-518b in the pathogenesis of placenta accreta. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 23–28. [Google Scholar] [CrossRef]
- Gu, Y.; Meng, J.; Zuo, C.; Wang, S.; Li, H.; Zhao, S.; Huang, T.; Wang, X.; Yan, J. Downregulation of MicroRNA-125a in Placenta Accreta Spectrum Disorders Contributes Antiapoptosis of Implantation Site Intermediate Trophoblasts by Targeting MCL1. Reprod. Sci. 2019, 26, 1582–1589. [Google Scholar] [CrossRef]
- Gu, Y.; Bian, Y.; Xu, X.; Wang, X.; Zuo, C.; Meng, J.; Li, H.; Zhao, S.; Ning, Y.; Cao, Y.; et al. Downregulation of miR-29a/b/c in placenta accreta inhibits apoptosis of implantation site intermediate trophoblast cells by targeting MCL1. Placenta 2016, 48, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Zuckerwise, L.; Li, J.; Lu, L.; Men, Y.; Geng, T.; Buhimschi, C.S.; Buhimschi, I.A.; Bukowski, R.; Guller, S.; Paidas, M.; et al. H19 long noncoding RNA alters trophoblast cell migration and invasion by regulating TβR3 in placentae with fetal growth restriction. Oncotarget 2016, 7, 38398–38407. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Ren, C.; Zhang, H.; Gao, L. Silencing of LncRNA SNHG6 protects trophoblast cells through regulating miR-101-3p/OTUD3 axis in unexplained recurrent spontaneous abortion. Histochem. J. 2022, 53, 871–882. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Y.; Zheng, H.; Gao, Q.; Wang, H. LncRNA SNHG16 regulates trophoblast functions by the miR-218-5p/LASP1 axis. Histochem. J. 2021, 52, 1021–1033. [Google Scholar] [CrossRef]
- Cao, C.; Li, J.; Li, J.; Liu, L.; Cheng, X.; Jia, R. Long Non-Coding RNA Uc.187 Is Upregulated in Preeclampsia and Modulates Proliferation, Apoptosis, and Invasion of HTR-8/SVneo Trophoblast Cells. J. Cell. Biochem. 2017, 118, 1462–1470. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Li, R.; Wang, W.; Qiu, X.; He, M.; Tang, X.; Zhong, M. Periostin promotes extensive neovascularization in placenta accreta spectrum disorders via Notch signaling. J. Matern.-Fetal Neonatal Med. 2023, 36, 2264447. [Google Scholar] [CrossRef] [PubMed]
- Calì, G.; D’Antonio, F.; Forlani, F.; Timor-Tritsch, I.E.; Palacios-Jaraquemada, J.M. Ultrasound Detection of Bladder-Uterovaginal Anastomoses in Morbidly Adherent Placenta. Fetal Diagn. Ther. 2017, 41, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, R.; Liu, S.; Yin, X.; Huo, Y.; Zhang, R.; Li, J. YKL-40 promotes proliferation and invasion of HTR-8/SVneo cells by activating akt/MMP9 signalling in placenta accreta spectrum disorders. J. Obstet. Gynaecol. 2023, 43, 2211681. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Su, C.; Zhu, L.; Dong, F.; Shu, H.; Zhang, H.; Wang, M.; Wang, F.; Man, D. Highly expressed FYN promotes the progression of placenta accreta by activating STAT3, p38, and JNK signaling pathways. Acta Histochem. 2023, 125, 151991. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Zhao, J.; Liu, L.; Li, S.; Duan, Y.; Huo, Y. Overexpressed LAMC2 promotes trophoblast over-invasion through the PI3K/Akt/MMP2/9 pathway in placenta accreta spectrum. J. Obstet. Gynaecol. Res. 2023, 49, 548–559. [Google Scholar] [CrossRef]
- Duan, L.; Schimmelmann, M.; Wu, Y.; Reisch, B.; Faas, M.; Kimmig, R.; Winterhager, E.; Köninger, A.; Gellhaus, A. CCN3 Signaling Is Differently Regulated in Placental Diseases Preeclampsia and Abnormally Invasive Placenta. Front. Endocrinol. 2020, 11, 597549. [Google Scholar] [CrossRef]
- Badary, D.M.; Elsaied, H.; Abdel-Fadeil, M.R.; Ali, M.K.; Abou-Taleb, H.; Iraqy, H.M. Possible Role of Netrin-1/Deleted in Colorectal Cancer/Vascular Endothelial Growth Factor Signaling Pathway in the Pathogenesis of Placenta Accreta Spectrum: A Case-control Study. Int. J. Gynecol. Pathol. 2024. [Google Scholar] [CrossRef]
- Hashimoto, K.; Miyagawa, Y.; Watanabe, S.; Takasaki, K.; Nishizawa, M.; Yatsuki, K.; Takahashi, Y.; Kamata, H.; Kihira, C.; Hiraike, H.; et al. The TGF-β/UCHL5/Smad2 Axis Contributes to the Pathogenesis of Placenta Accreta. Int. J. Mol. Sci. 2023, 24, 13706. [Google Scholar] [CrossRef]
- Li, R.; Weng, X.; Hu, X.; Wang, J.; Zheng, L. Pigment epithelium-derived factor inhibits proliferation, invasion and angiogenesis, and induces ferroptosis of extravillous trophoblasts by targeting Wnt-β-catenin/VEGF signaling in placenta accreta spectrum. Mol. Med. Rep. 2024, 29, 75. [Google Scholar] [CrossRef]
- Morlando, M.; Collins, S. Placenta accreta spectrum disorders: Challenges, risks, and management strategies. Int. J. Women’s Health 2020, 12, 1033–1045. [Google Scholar] [CrossRef]
| Biomarker | Source | Findings | References |
|---|---|---|---|
| Alpha-fetoprotein (AFP) | Maternal serum | APF showed sensitivity and specificity of 71 and 46%, respectively, to serve as a biomarker for pathological placentation, specifically in women with placenta previa and acreta in the second trimester. Thus, a high level of AFP can be used as a cause for suspicion in high-risk pathological placentation. | [27] |
| Maternal serum AFP levels were associated with PAS patients; it was established as a predictor for PAS patients that require hysterectomy with 85.94% sensitivity and 71.43% specificity. | [28] | ||
| Soluble fms-like tyrosine kinase-1 (sFlt-1) | Maternal serum | Third trimester sFlt-1 serum levels were decreased in PAS-affected women, respectively, with pathological severity. | [29] |
| Maternal plasma | Concentrations of sFlt-1 were lower in patients with PAS than those with normal placentation, with 90.0% sensitivity and 82.0% specificity. The lower concentrations were also associated with intraoperative blood loss. | [21] | |
| β human chorionic gonadotrophin (β-hCG) | Maternal plasma or serum | The elevated concentration of β-HCG in serum may be appropriate for the prenatal diagnosis of placenta accreta, which suggests the relationship between the risk of PAS and the first trimester. | [30] |
| Maternal serum | hCG showed a sensitivity and specificity of 53 and 68%, respectively, to serve as a biomarker for pathological placentation. Higher levels of hCG can be used as a cause for suspicion in high-risk pathological placentation. | [27] | |
| Maternal plasma cell-free β-hCG mRNA | Cell-free β-hCG mRNA concentrations were significantly elevated in women with placenta accreta. This suggests that β-hCG mRNA levels might be a marker for identifying women with placenta accreta likely to require hysterectomy. | [31] | |
| Placental growth factor (PlGF) | Maternal plasma | Concentrations of PlGF were higher in patients with PAS than those with normal placentation, with 86.0% sensitivity and 93.0% specificity. Higher concentrations were also associated with intraoperative bleeding. | [21] |
| Maternal serum | PIGF serum levels were higher in PAS severity groups than in normal placentation patients, including placenta previa patients, suggesting these levels are a predictor criterion exclusive for PAS patients with 83% sensitivity and 82% specificity. | [32] | |
| Maternal serum and placental bed tissues | High serum levels and high placental bed expression in placenta previa patients with PAS disorders were explored. PlGF serum levels might predict PAS affection, excepting the severity grade based on FIGO. | [19] | |
| Pregnancy-associated plasma protein-A (PAPP-A) | Maternal serum | Increased first-trimester serum was positively associated with placenta accreta, suggesting the potential role of PAPP-A as a biomarker in identifying pregnancies at high risk for placenta accreta. | [30,33,34,35,36] |
| A significant correlation was found between PAPP-A levels and blood loss volume. This suggests that first-trimester PAPP-A levels may be useful for the early prediction of pathological blood loss at delivery in pregnant women with PAS and for recognizing a high-risk group for PAS. | [37] | ||
| Human placental lactogen mRNA (hPL mRNA) | Maternal plasma | The expression of hPL mRNA is elevated in the plasma of women diagnosed with placenta previa and invasive placenta between 28 and 32 weeks of gestation. | [38] |
| The multiple of the median (MoM) for hPL mRNA was significantly higher in the placenta accreta group compared to the control and placenta previa groups. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizárraga-Verdugo, E.; Beltrán-Ontiveros, S.A.; Gutiérrez-Grijalva, E.P.; Montoya-Moreno, M.; Gutiérrez-Arzapalo, P.Y.; Avendaño-Félix, M.; Gutiérrez-Castro, K.P.; Cuén-Lazcano, D.E.; González-Quintero, P.; Mora-Palazuelos, C.E. The Underlying Molecular Mechanisms of the Placenta Accreta Spectrum: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9722. https://doi.org/10.3390/ijms25179722
Lizárraga-Verdugo E, Beltrán-Ontiveros SA, Gutiérrez-Grijalva EP, Montoya-Moreno M, Gutiérrez-Arzapalo PY, Avendaño-Félix M, Gutiérrez-Castro KP, Cuén-Lazcano DE, González-Quintero P, Mora-Palazuelos CE. The Underlying Molecular Mechanisms of the Placenta Accreta Spectrum: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(17):9722. https://doi.org/10.3390/ijms25179722
Chicago/Turabian StyleLizárraga-Verdugo, Erik, Saúl Armando Beltrán-Ontiveros, Erick Paul Gutiérrez-Grijalva, Marisol Montoya-Moreno, Perla Y. Gutiérrez-Arzapalo, Mariana Avendaño-Félix, Karla Paola Gutiérrez-Castro, Daniel E. Cuén-Lazcano, Paul González-Quintero, and Carlos Ernesto Mora-Palazuelos. 2024. "The Underlying Molecular Mechanisms of the Placenta Accreta Spectrum: A Narrative Review" International Journal of Molecular Sciences 25, no. 17: 9722. https://doi.org/10.3390/ijms25179722







