8-Prenylgenistein Isoflavone in Cheonggukjang Acts as a Novel AMPK Activator Attenuating Hepatic Steatosis by Enhancing the SIRT1-Mediated Pathway
Abstract
:1. Introduction
2. Results
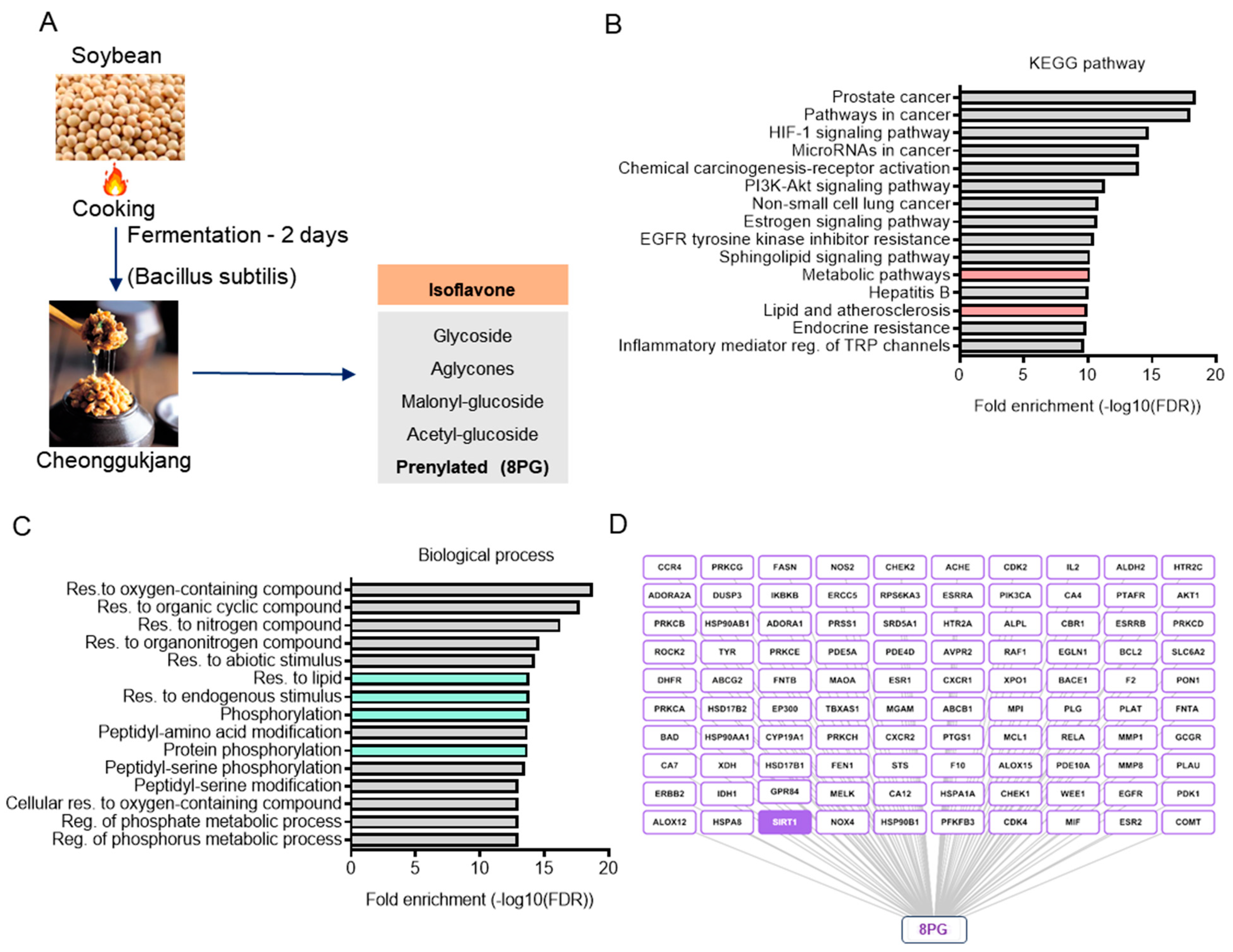
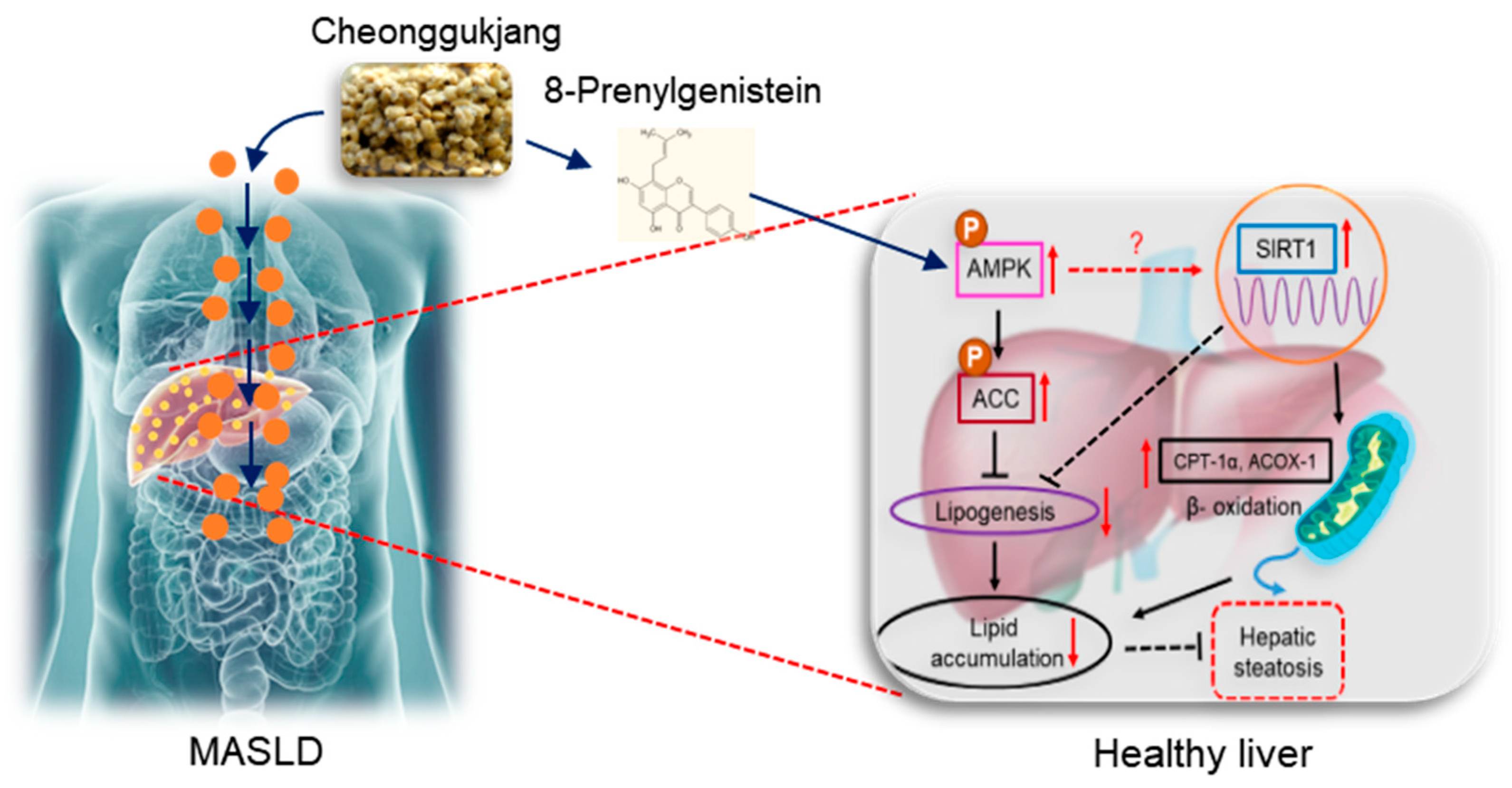
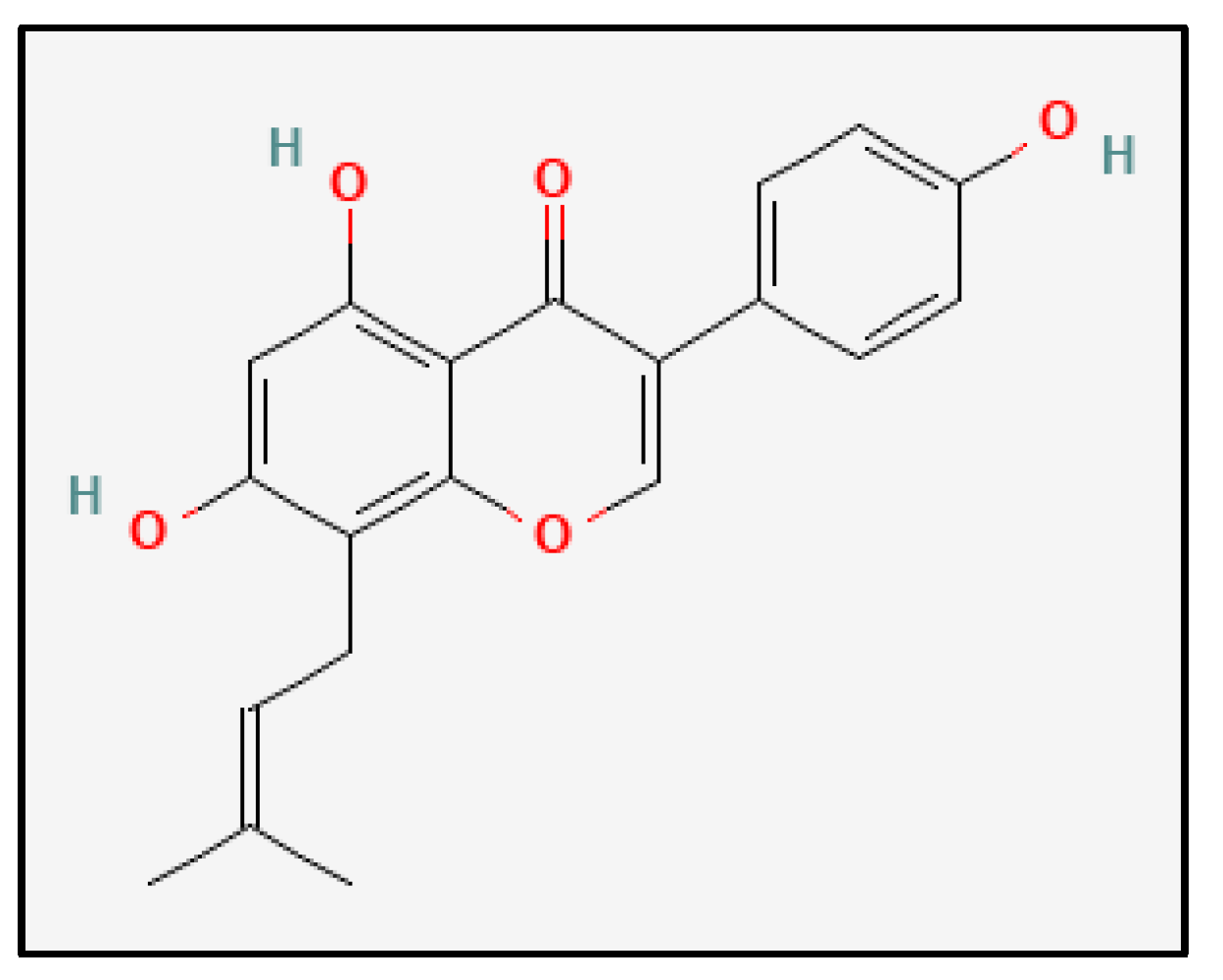
2.1. Identification and Characterization of Bioactive Metabolites from Cheonggukjang (CGJ)
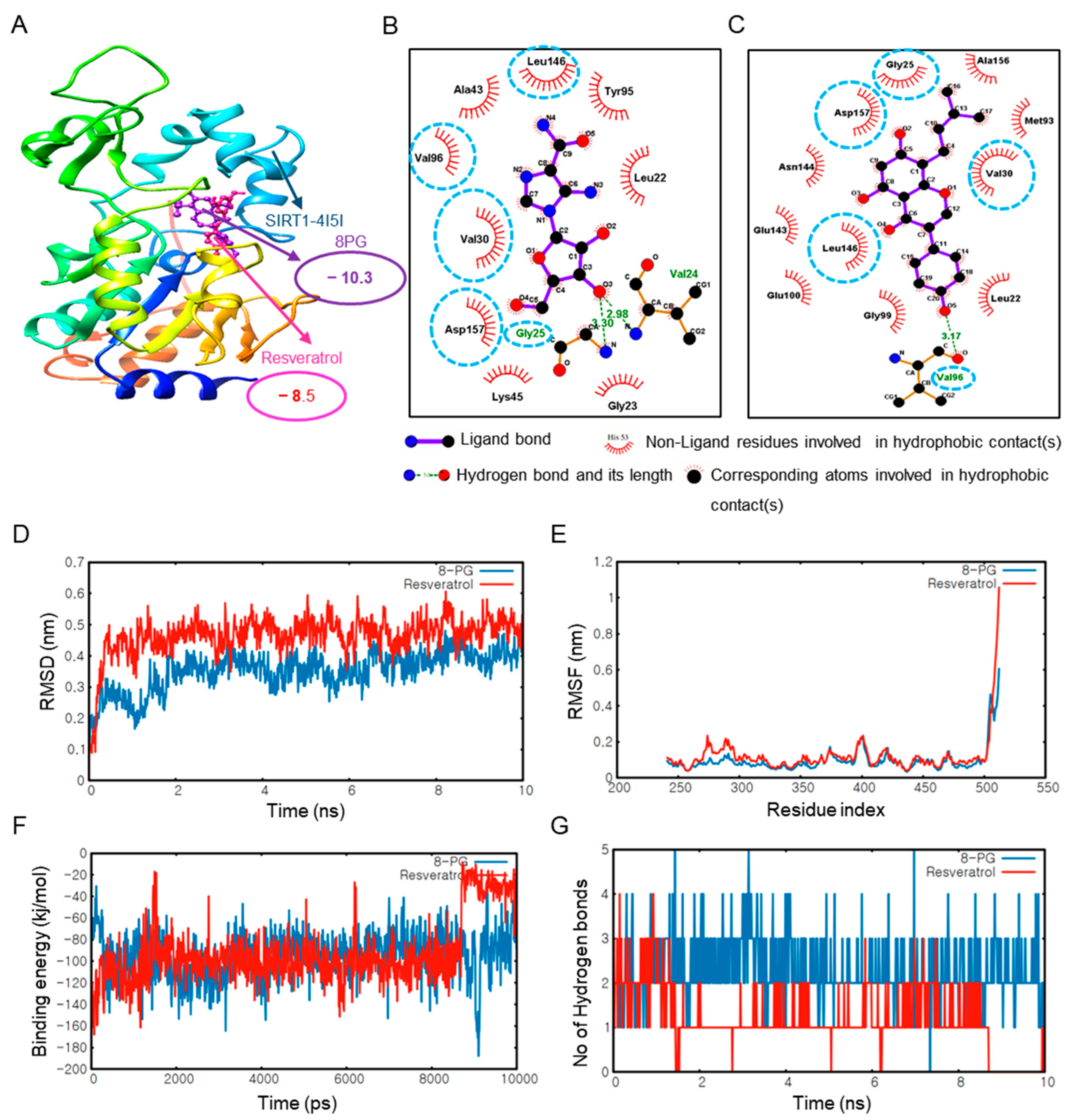
2.2. 8PG Has the Highest Affinity with AMPK and SIRT1 as Validated by Molecular Dynamics (MD) Simulation In Silico
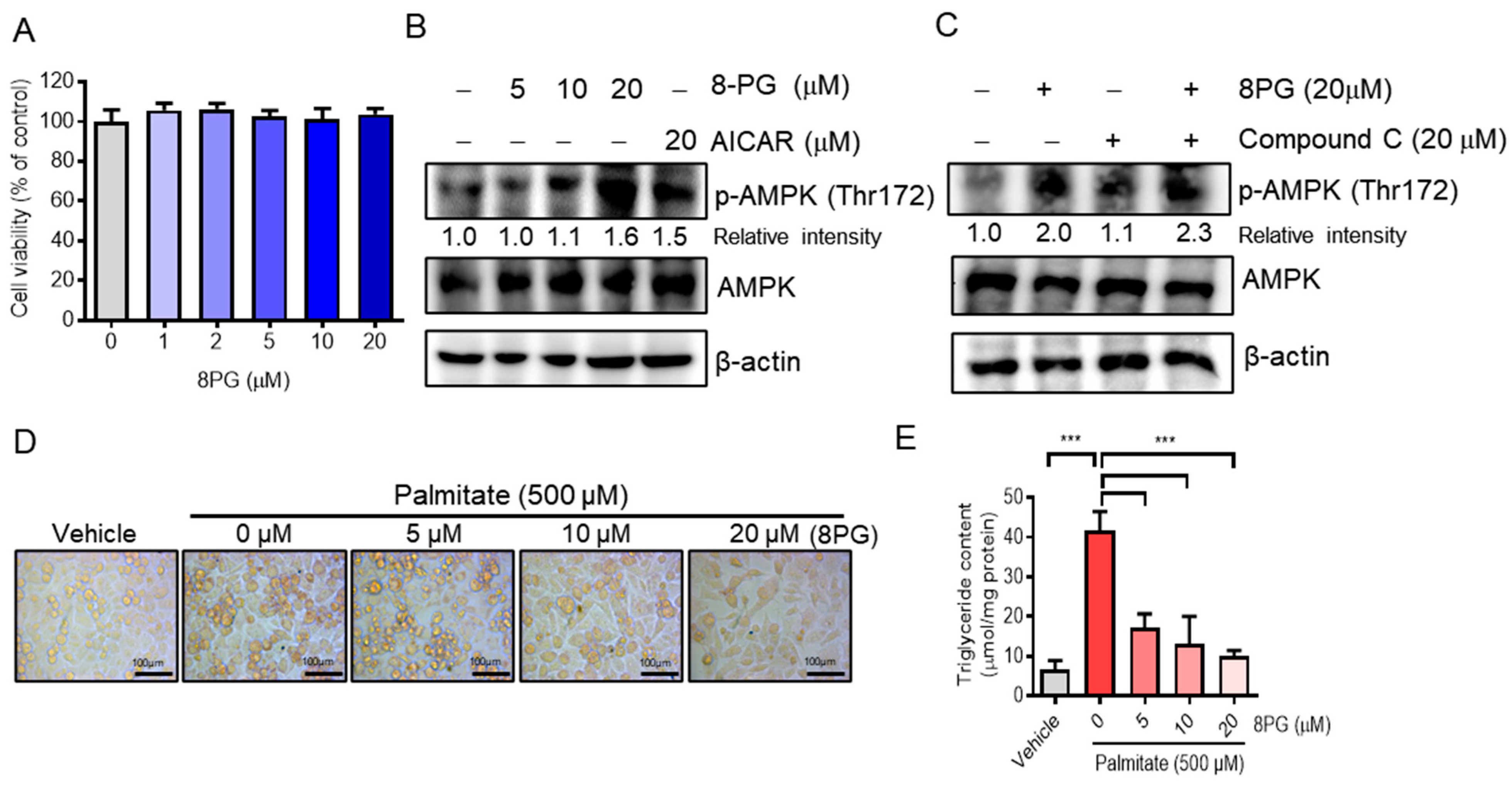
2.3. 8PG Acts as an Activator of AMPK and Attenuates Lipid Accumulation in HepG2 Cells
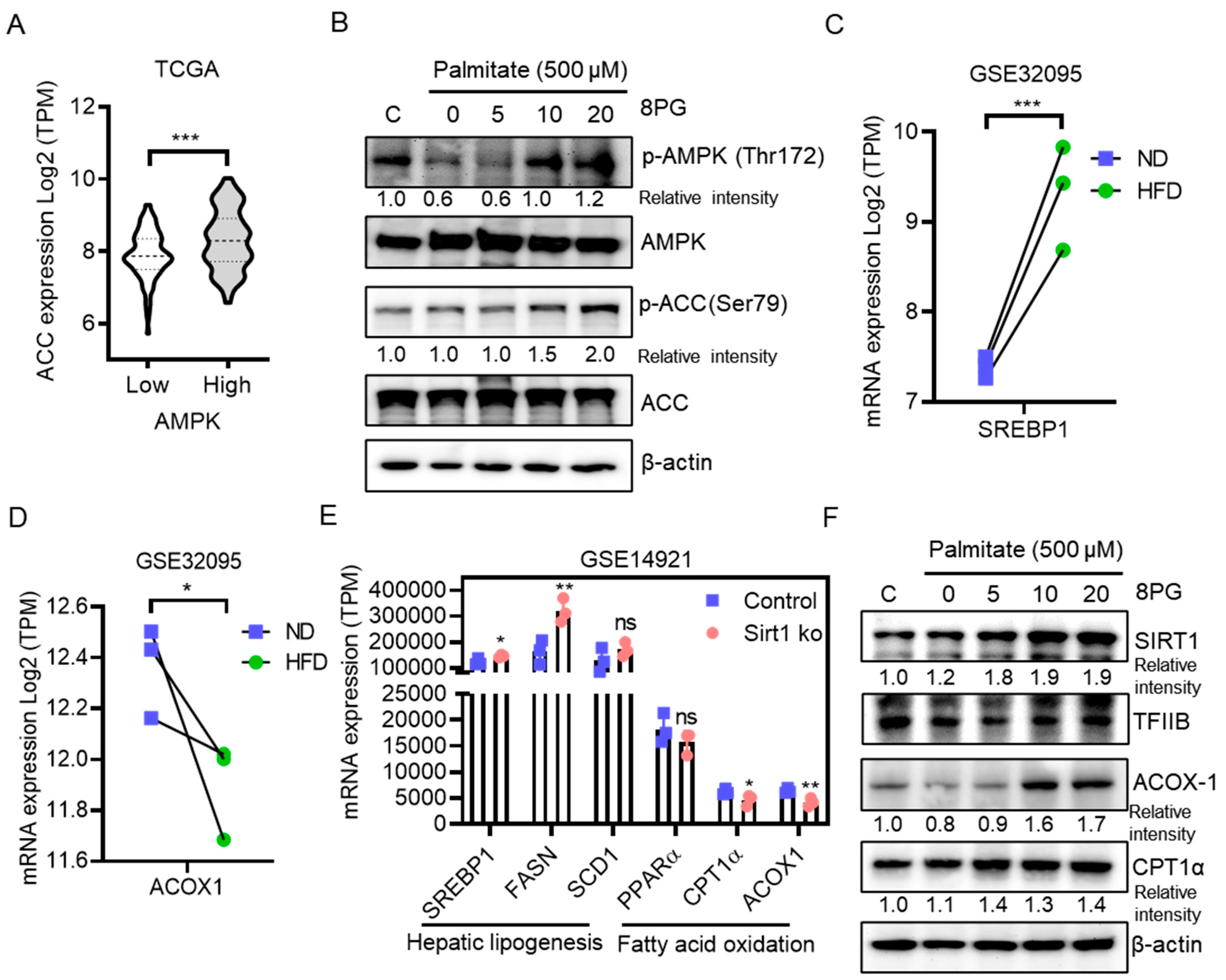
2.4. 8PG Mitigates Fatty Acid Synthesis and Enhances β-Oxidation by Activating the SIRT1-Mediated Pathway in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Selection of Active Metabolites in Cheonggukjang (CGJ)
4.2. Network Pharmacology Analysis
4.3. Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Property Prediction
4.4. Molecular Docking
4.5. Molecular Dynamics Simulations
4.6. Public Datasets and Clinical Data Analysis
4.7. Reagents and Antibodies
4.8. Cell Culture and Treatment
4.9. Cell Viability
4.10. Preparation of Sodium Palmitate and Its Administration to HepG2 Cells
4.11. Oil Red O Staining
4.12. Measurements of Intracellular Triglyceride Contents
4.13. Western Blotting
4.14. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, J.G.; Saibara, T.; Chitturi, S.; Kim, B.I.; Sung, J.J.Y.; Chutaputti, A.; Party, A.P.W. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J. Gastroenterol. Hepatol. 2007, 22, 794–800. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008, 34 Pt 2, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Fingas, C.D.; Best, J.; Sowa, J.P.; Canbay, A. Epidemiology of nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin. Liver Dis. 2016, 8, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Valanejad, L.; Cast, A.; Wright, M.; Garcia, J.M.; El-Serag, H.B.; Karns, R.; Timchenko, N.A. Elimination of Age-Associated Hepatic Steatosis and Correction of Aging Phenotype by Inhibition of cdk4-C/EBPalpha-p300 Axis. Cell Rep. 2018, 24, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Ahn, Y.C.; Wang, J.H.; Lee, M.M.; Son, C.G. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101526. [Google Scholar] [CrossRef]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef]
- Arad, Y.; Newstein, D.; Cadet, F.; Roth, M.; Guerci, A.D. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 2051–2058. [Google Scholar] [CrossRef]
- Tayama, K.; Inukai, T.; Shimomura, Y. Preperitoneal fat deposition estimated by ultrasonography in patients with non-insulin-dependent diabetes mellitus. Diabetes Res. Clin. Pract. 1999, 43, 49–58. [Google Scholar] [CrossRef]
- Laakso, M.; Lehto, S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis 1998, 137, S65–S73. [Google Scholar] [CrossRef]
- Kim, N.Y.; Song, E.J.; Kwon, D.Y.; Kim, H.P.; Heo, M.Y. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food Chem. Toxicol. 2008, 46, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Pichiah, P.B.; Kim, M.J.; Cha, Y.S. Cheonggukjang, a soybean paste fermented with B. licheniformis-67 prevents weight gain and improves glycemic control in high fat diet induced obese mice. J. Clin. Biochem. Nutr. 2016, 59, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.N.; Choi, J.H.; Cha, Y.S.; Muthaiya, M.J.; Lee, C.H. Effect of fermented soybean product (Cheonggukjang) intake on metabolic parameters in mice fed a high-fat diet. Mol. Nutr. Food Res. 2013, 57, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Omura, K.; Hitosugi, M.; Zhu, X.; Ikeda, M.; Maeda, H.; Tokudome, S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J. Pharmacol. Sci. 2005, 99, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, C.E.; Lee, C.K.; Lee, J.H.; Kim, G.M.; Jeong, S.H.; Shin, J.H.; Kim, J.S.; Cho, K.M. Characteristics and antioxidant effect of garlic in the fermentation of Cheonggukjang by Bacillus amyloliquefaciens MJ1-4. J. Microbiol. Biotechnol. 2014, 24, 959–968. [Google Scholar] [CrossRef]
- D’Adamo, C.R.; Sahin, A. Soy foods and supplementation: A review of commonly perceived health benefits and risks. Altern. Ther. Health Med. 2014, 20 (Suppl. S1), 39–51. [Google Scholar]
- Pyo, Y.H.; Lee, T.C. The potential antioxidant capacity and angiotensin I-converting enzyme inhibitory activity of Monascus-fermented soybean extracts: Evaluation of Monascus-fermented soybean extracts as multifunctional food additives. J. Food Sci. 2007, 72, S218–S223. [Google Scholar] [CrossRef]
- Champagne, C.P.; Tompkins, T.A.; Buckley, N.D.; Green-Johnson, J.M. Effect of fermentation by pure and mixed cultures of Streptococcus thermophilus and Lactobacillus helveticus on isoflavone and B-vitamin content of a fermented soy beverage. Food Microbiol. 2010, 27, 968–972. [Google Scholar] [CrossRef]
- Bakheet, T.M.; Doig, A.J. Properties and identification of human protein drug targets. Bioinformatics 2009, 25, 451–457. [Google Scholar] [CrossRef]
- Yamanishi, Y.; Araki, M.; Gutteridge, A.; Honda, W.; Kanehisa, M. Prediction of drug-target interaction networks from the integration of chemical and genomic spaces. Bioinformatics 2008, 24, i232–i240. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Zhang, F.X.; Tang, Z.L.; Qiu, Z.C. A novel strategy for exploring food originated anti-adipogenesis substances and mechanism by structural similarity evaluation, ADME prediction, network pharmacology and experimental validation. Food Funct. 2021, 12, 7081–7091. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.F.; Takase, M.; Kawarasaki, S.; Ni, Z.; Mohri, S.; Hayashi, K.; Izumi, A.; Sasaki, K.; Shinyama, Y.; Kwon, J.; et al. 8-Prenyl daidzein and 8-prenyl genistein from germinated soybean modulate inflammatory response in activated macrophages. Biosci. Biotechnol. Biochem. 2023, 87, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Lee, S.; Lee, S.H.; Kim, H.J.; Lee, C.H. Comparative Evaluation of Six Traditional Fermented Soybean Products in East Asia: A Metabolomics Approach. Metabolites 2019, 9, 183. [Google Scholar] [CrossRef]
- Chacko, B.K.; Chandler, R.T.; D’Alessandro, T.L.; Mundhekar, A.; Khoo, N.K.; Botting, N.; Barnes, S.; Patel, R.P. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell PPARgamma. J. Nutr. 2007, 137, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef]
- Rodriguez-Roque, M.J.; Rojas-Grau, M.A.; Elez-Martinez, P.; Martin-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Mijiti, N.; Someya, A.; Nagaoka, I. Effects of isoflavone derivatives on the production of inflammatory cytokines by synovial cells. Exp. Ther. Med. 2021, 22, 1300. [Google Scholar] [CrossRef]
- Moesgaard, L.; Pedersen, M.L.; Uhd Nielsen, C.; Kongsted, J. Structure-based discovery of novel P-glycoprotein inhibitors targeting the nucleotide binding domains. Sci. Rep. 2023, 13, 21217. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Ouchi, N.; Shibata, R.; Walsh, K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ. Res. 2005, 96, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M. Hypothalamic AMPK and energy balance. Eur. J. Clin. Investig. 2018, 48, e12996. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Karimi, G. The role of SIRT1 in diabetic retinopathy. Biomed. Pharmacother. 2018, 97, 190–194. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Fiorino, E.; Giudici, M.; Ferrari, A.; Mitro, N.; Caruso, D.; De Fabiani, E.; Crestani, M. The sirtuin class of histone deacetylases: Regulation and roles in lipid metabolism. IUBMB Life 2014, 66, 89–99. [Google Scholar] [CrossRef]
- Engin, A. Non-Alcoholic Fatty Liver Disease. Adv. Exp. Med. Biol. 2017, 960, 443–467. [Google Scholar]
- Schmucker, D.L. Age-related changes in liver structure and function: Implications for disease ? Exp.Gerontol. 2005, 40, 650–659. [Google Scholar] [CrossRef]
- Kewalramani, G.; An, D.; Kim, M.S.; Ghosh, S.; Qi, D.; Abrahani, A.; Pulinilkunnil, T.; Sharma, V.; Wambolt, R.B.; Allard, M.F.; et al. AMPK control of myocardial fatty acid metabolism fluctuates with the intensity of insulin-deficient diabetes. J. Mol. Cell Cardiol. 2007, 42, 333–342. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Francini, F.; Schinella, G.R.; Rios, J.L. Activation of AMPK by Medicinal Plants and Natural Products: Its Role in Type 2 Diabetes Mellitus. Mini Rev. Med. Chem. 2019, 19, 880–901. [Google Scholar] [CrossRef]
- Tiao, M.M.; Lin, Y.J.; Yu, H.R.; Sheen, J.M.; Lin, I.C.; Lai, Y.J.; Tain, Y.L.; Huang, L.T.; Tsai, C.C. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Weiqiang, K.; Qing, Z.; Pengju, Z.; Yi, L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp. Mol. Med. 2007, 39, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233. [Google Scholar] [CrossRef]
- Xu, H.E.; Lambert, M.H.; Montana, V.G.; Plunket, K.D.; Moore, L.B.; Collins, J.L.; Oplinger, J.A.; Kliewer, S.A.; Gampe, R.T., Jr.; McKee, D.D.; et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 13919–13924. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss Target Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. Medchemcomm 2019, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Hou, T.J.; Zhang, W.; Xia, K.; Qiao, X.B.; Xu, X.J. ADME evaluation in drug discovery. 5. Correlation of Caco-2 permeation with simple molecular properties. J. Chem. Inf. Comput. Sci. 2004, 44, 1585–1600. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.P.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Yao, X.; Li, D.; Xu, L.; Li, Y.; Tian, S.; Hou, T. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Shin, T.H.; Lee, G. A Molecular Dynamics Approach to Explore the Intramolecular Signal Transduction of PPAR-alpha. Int. J. Mol. Sci. 2019, 20, 1666. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.C.; Chen, C.Y. In silico design for adenosine monophosphate-activated protein kinase agonist from traditional chinese medicine for treatment of metabolic syndromes. Evid. Based Complement. Altern. Med. 2014, 2014, 928589. [Google Scholar] [CrossRef] [PubMed]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef]
- Racine, J. gnuplot 4.0: A portable interactive plotting utility. J. Appl. Econom. 2006, 21, 133–141. [Google Scholar] [CrossRef]
- Park, S.J.; Yoon, B.H.; Kim, S.K.; Kim, S.Y. GENT2: An updated gene expression database for normal and tumor tissues. BMC Med. Genomics 2019, 12 (Suppl. S5), 101. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, Y.; Im, J.A.; Lee, H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitate-induced in HepG2 hepatocytes as a cellular steatosis model. BMC Complement. Altern. Med. 2015, 15, 185. [Google Scholar] [CrossRef]
- Habib, A.; Créminon, C.; Frobert, Y.; Grassi, J.; Pradelles, P.; Maclouf, J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J. Biol. Chem. 1993, 268, 23448–23454. [Google Scholar] [CrossRef]

| Compound | AMPK (PDB ID: 2Y94) | ||
|---|---|---|---|
| AutoDock Vina | AutoDock4 | LeDock | |
| AICAR (Control) | −6 | −7.54 | −6.01 |
| 8PG | −8.7 | −9.58 | −5.02 |
| Commonly shared residues | LEU22, GLY25, VAL30, VAL 96, LEU146, and ASP 157 | ||
| Compound | SIRT1 (PDB ID: 4I5I) | ||
|---|---|---|---|
| AutoDock Vina | AutoDock4 | LeDock | |
| Resveratrol (Control) | −8.5 | −7.69 | −4.89 |
| 8PG | −10.3 | −11.11 | −5.39 |
| Commonly shared residues | GLY25, VAL30, VAL96, LEU146, and ASP157 | ||
| Properties | AICAR | Resveratrol | 8PG |
| Absorption | |||
| Human intestinal absorption (HIA %) | 18.271181 | 88.479404 | 90.773093 |
| Caco-2 cell Permeability (nm s−1) | 6.79648 | 5.19172 | 10.5061 |
| MDCK cell permeability (nm s−1) | 0.583302 | 76.7444 | 0.188383 |
| Skin permeability (logKp, cm h−1) | −5.17298 | −3.15256 | −2.95147 |
| Distribution | |||
| Plasma protein binding (%) | 5.116658 | 100 | 98.929110 |
| Blood–brain barrier penetration (Cb/Cb) | 0.627516 | 1.73812 | 0.856453 |
| Metabolism | |||
| CYP2C19 inhibition | Non | Inhibitor | Inhibitor |
| CYP2C9 inhibition | Non | Inhibitor | Inhibitor |
| CYP2D6 inhibition | Non | Non | Non |
| CYP2D6 substrate | Non | Non | Non |
| CYP3A4 inhibition | Inhibitor | Inhibitor | Inhibitor |
| CYP3A4 substrate | Weakly | Non | Non |
| Excretion | |||
| P-GP inhibition | Non | Non | Inhibitor |
| Toxicity | |||
| Ames test | Mutagen | Mutagen | Mutagen |
| Carcino_rat | Non | Carcinogen | Non |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arulkumar, R.; Jung, H.J.; Noh, S.G.; Kim, H.W.; Chung, H.Y. 8-Prenylgenistein Isoflavone in Cheonggukjang Acts as a Novel AMPK Activator Attenuating Hepatic Steatosis by Enhancing the SIRT1-Mediated Pathway. Int. J. Mol. Sci. 2024, 25, 9730. https://doi.org/10.3390/ijms25179730
Arulkumar R, Jung HJ, Noh SG, Kim HW, Chung HY. 8-Prenylgenistein Isoflavone in Cheonggukjang Acts as a Novel AMPK Activator Attenuating Hepatic Steatosis by Enhancing the SIRT1-Mediated Pathway. International Journal of Molecular Sciences. 2024; 25(17):9730. https://doi.org/10.3390/ijms25179730
Chicago/Turabian StyleArulkumar, Radha, Hee Jin Jung, Sang Gyun Noh, Hyun Woo Kim, and Hae Young Chung. 2024. "8-Prenylgenistein Isoflavone in Cheonggukjang Acts as a Novel AMPK Activator Attenuating Hepatic Steatosis by Enhancing the SIRT1-Mediated Pathway" International Journal of Molecular Sciences 25, no. 17: 9730. https://doi.org/10.3390/ijms25179730






