Role of CD44-Positive Extracellular Vesicles Derived from Highly Metastatic Mouse Mammary Carcinoma Cells in Pre-Metastatic Niche Formation
Abstract
:1. Introduction
2. Results
2.1. Determination of the Intratumor Microenvironment Suitable for Extracellular Vesicle Secretion and Formation of a Pre-Metastatic Niche
2.2. EVs Purified under Normoxic Conditions Were Advantageous for Pre-Metastatic Niche Formation
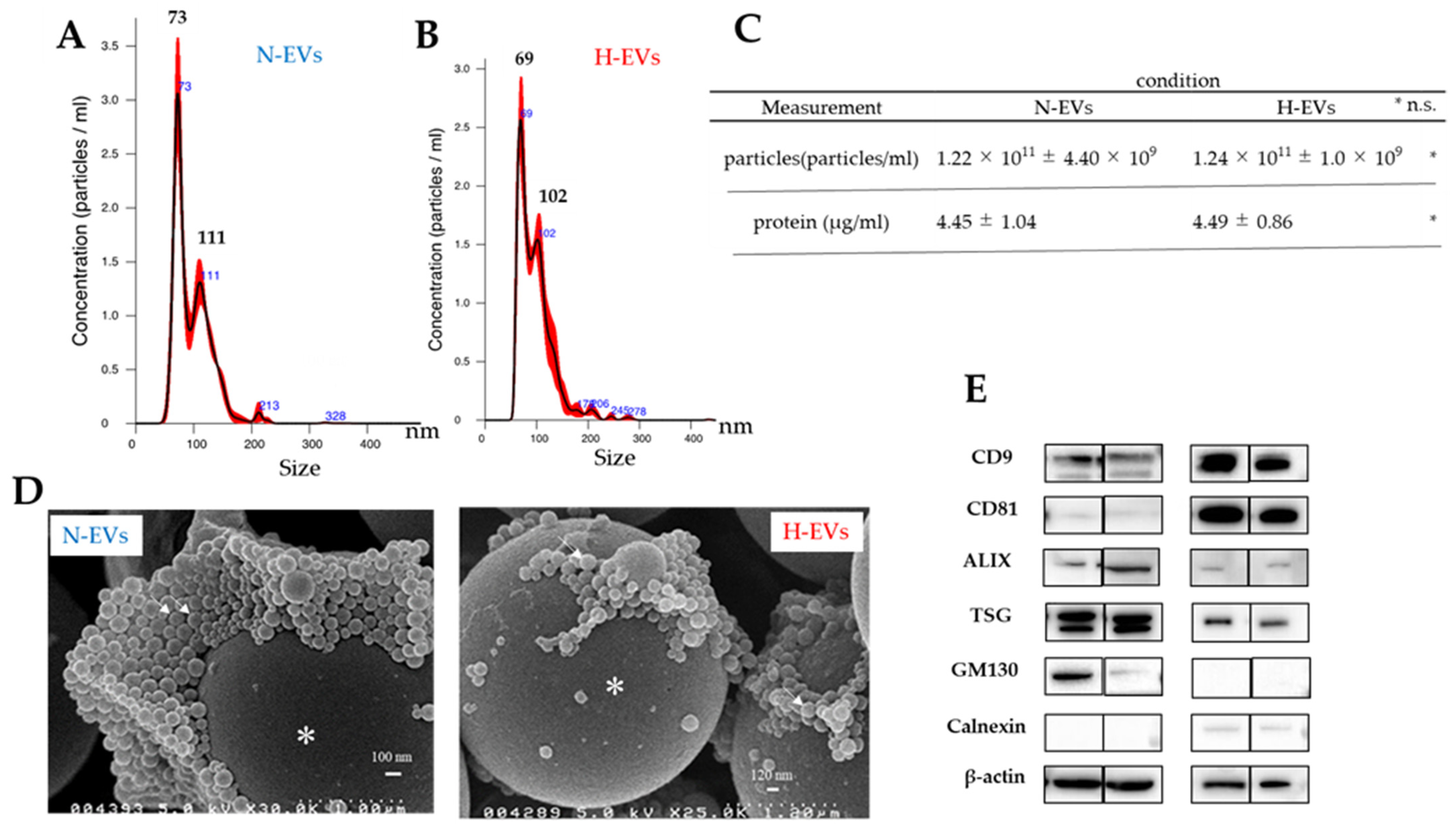
2.3. EVs Secreted by Luc2 Cells Meet the Exosome Requirements
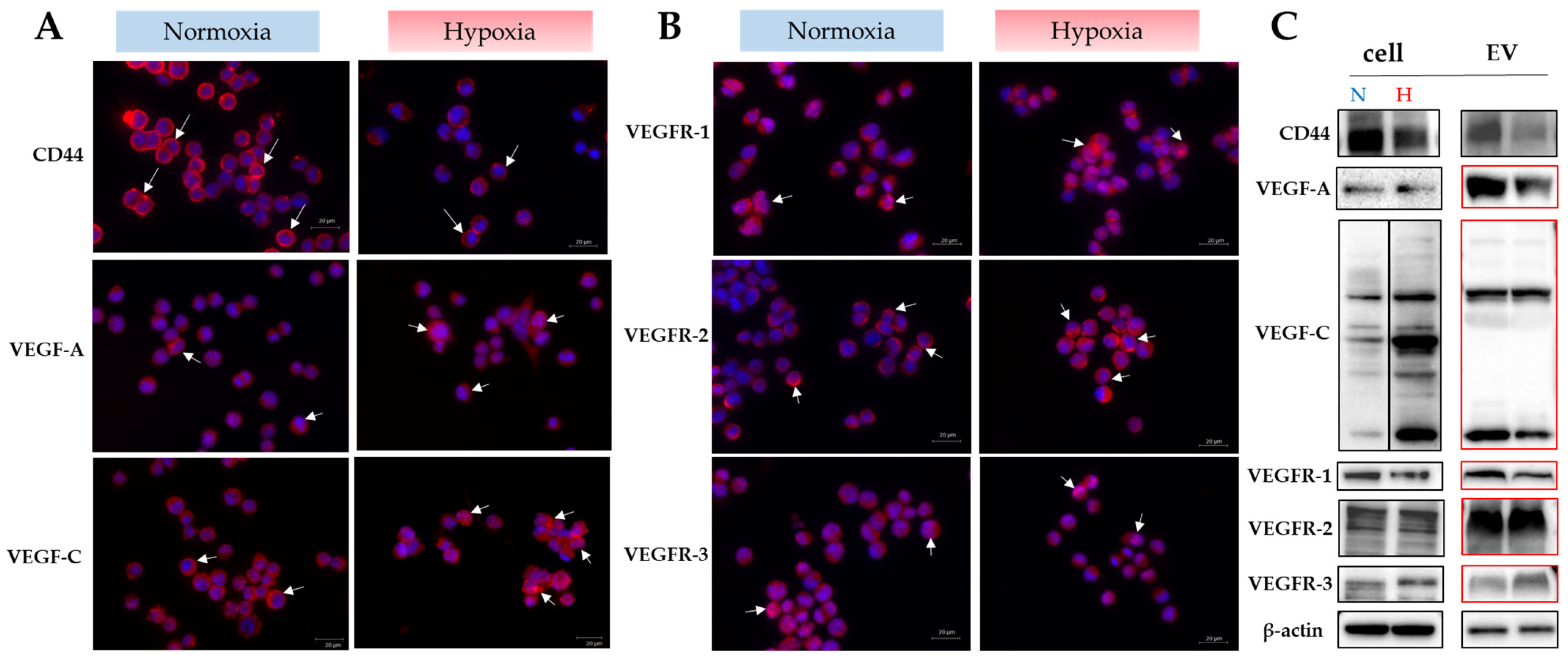
2.4. Role of CD44 on Extracellular Vesicles Derived from Luc2 Cells
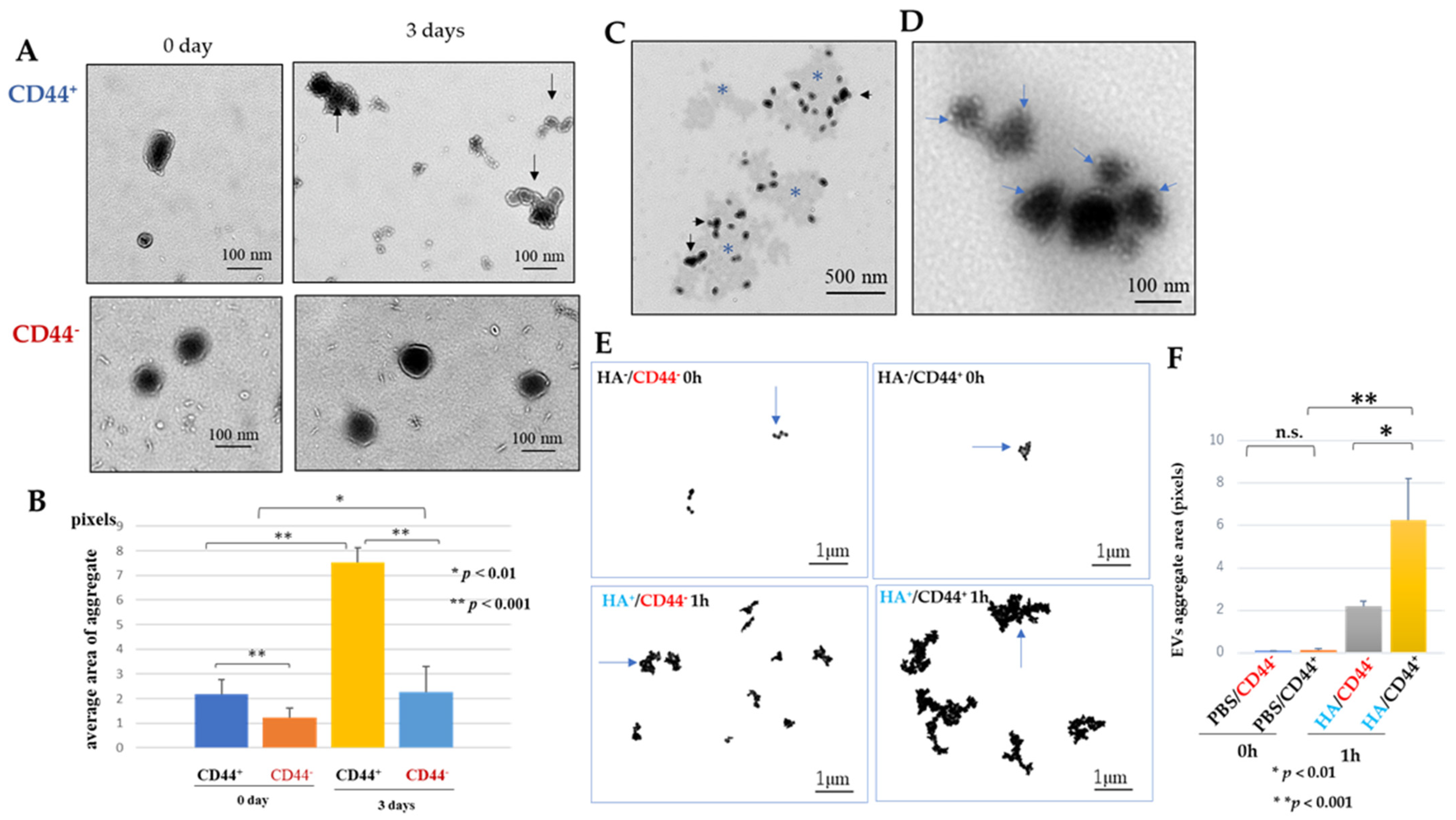
2.4.1. CD44 on the Luc2-EV Forms Aggregates among Luc2-EVs by Homophilic Protein Interaction (CD44-CD44)
2.4.2. Hyaluronan (Ligand for CD44) Contributes to Forming Small Clusters of Luc2-EVs
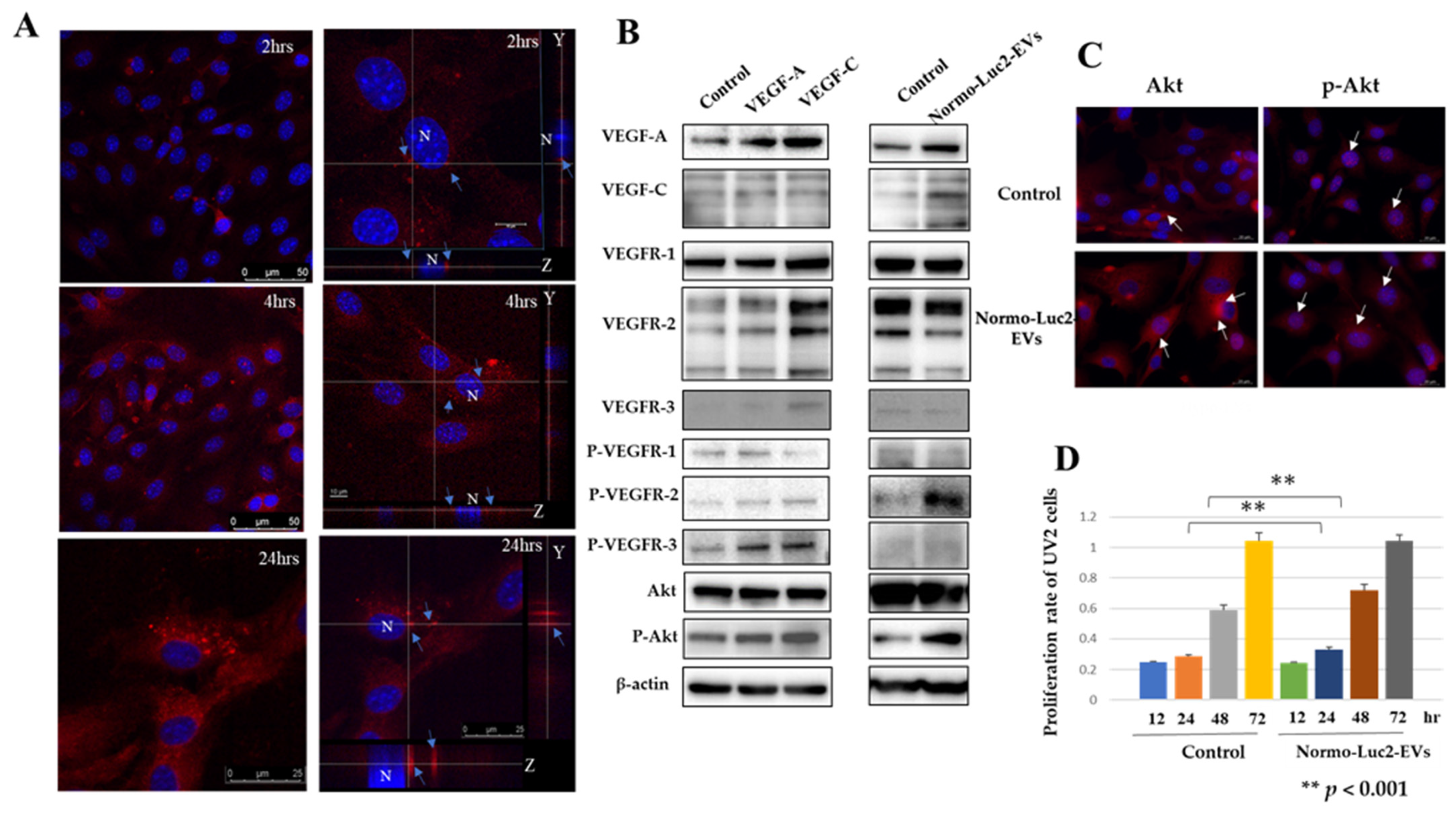
2.5. Were Luc2-EVs Internalized by Cells of the Endothelial Niche Cells?
2.5.1. VEGFs Internalized by Luc2 Extracellular Vesicles May Act on UV2 Endothelial Cell Metabolism
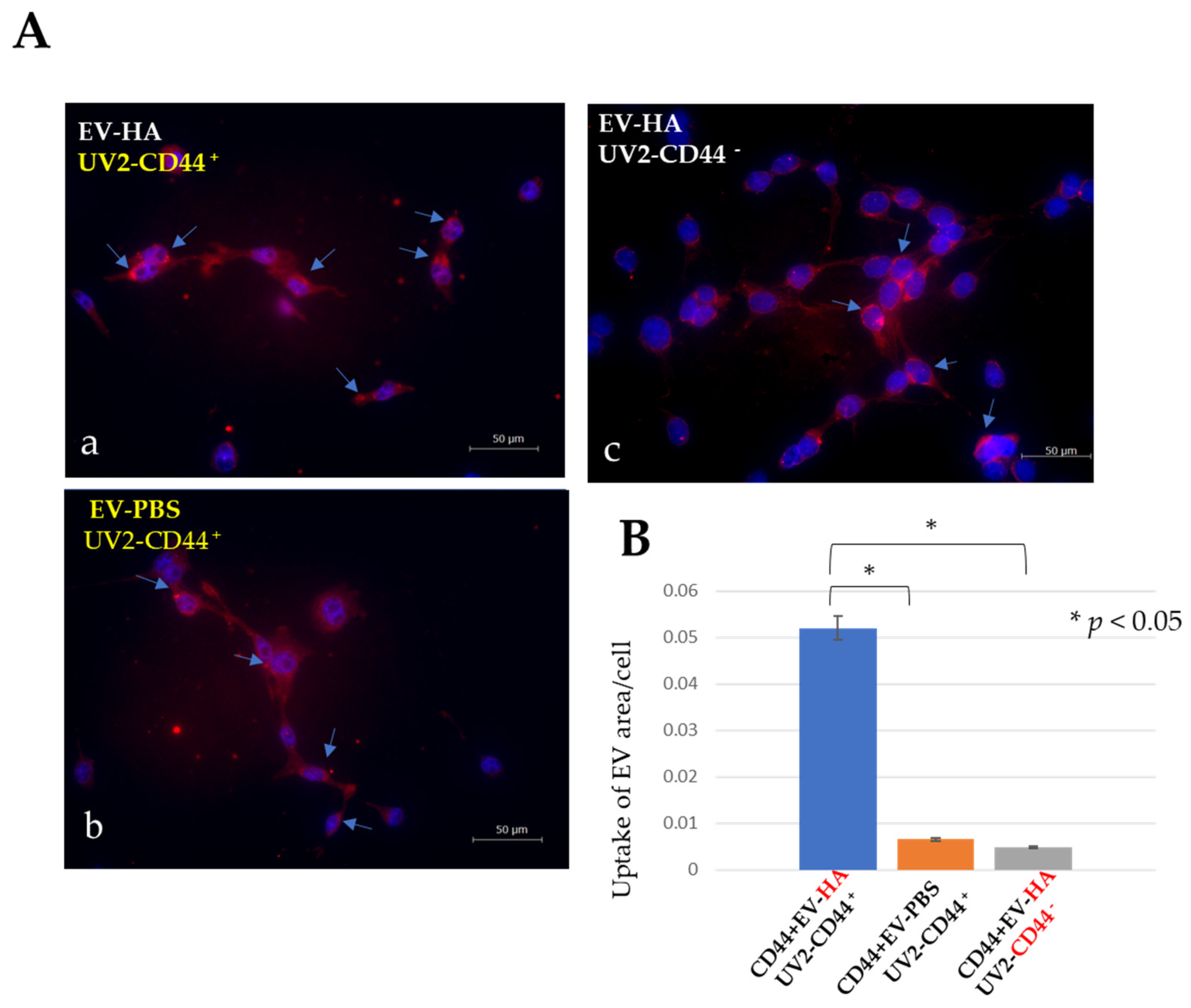
2.5.2. Some Extracellular Vesicles Are Readily Internalized by CD44-Positive UV2 Endothelial Cells, Hyaluronan-Coated or Non-Coated EVs
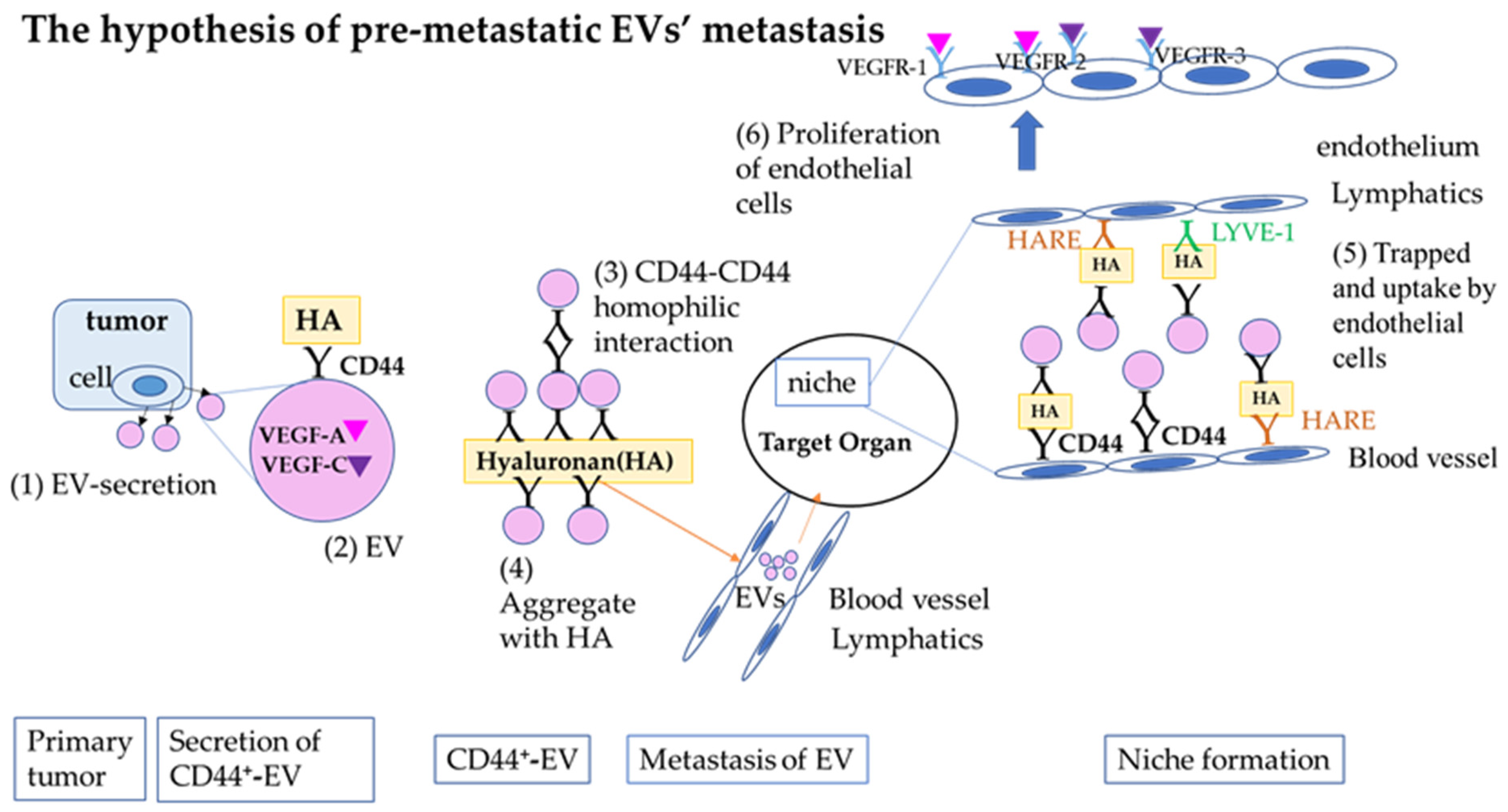
3. Discussion
3.1. Luc2-EVs Collected Met the Criteria for Extracellular Vesicles Defined in the Minimal Information for the Studies of Extracellular Vesicles (MISEV) Guidelines
3.2. Extracellular Vesicles, Normally Secreted under Oxygenated Conditions, Facilitated Pre-Metastatic Niche Formation
3.3. Luc2 Extracellular Vesicles Were Observed to Form CD44-Mediated Clumps, and Hyaluronan Was Found to Promote Clump Growth
3.4. Hyaluronan Receptors (CD44, HARE, and LYVE-1) on Vascular Endothelial Cells Facilitate the Take-Up of EVs
3.5. UV2 Endothelial Cells Take Up Normoxic EVs and Proliferate
3.6. Extracellular Vesicles Expressing CD44 May Serve as a Potential Therapeutic Agent for Breast Cancer
3.7. Limitations
4. Materials and Methods
4.1. Cell Culture and Cell Preparation
4.2. Extracellular Vesicle Isolation
4.3. Nano Tracking Analysis (NTA)
4.4. Scanning Electron Microscopy (SEM)
4.5. Western Blotting Analysis
4.6. Single and Double-Labeling Immunofluorescent Study
4.7. Analyzing CD44’s Ability to Bind with HA and Homophilic Interactions (CD44-CD44) on Luc2-EVs and UV2 Endothelial Cells
4.8. Detecting the Vascular Endothelial Growth Factors and HA Receptor and Their Receptor-Expression Profile of UV2 Cells
4.9. Uptake of EVs by UV2 Cells
4.10. Effect of EVs Uptake on UV2 Cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 2003, 100, 3983–3988, Erratum in Proc. Natl Acad. Sci. USA 2003, 100, 6890. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional mediator of cancer progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Godar, S.; Ince, T.A.; Bell, G.W.; Feldser, D.; Donaher, J.L.; Bergh, J.; Liu, A.; Miu, K.; Watnick, R.S.; Reinhardt, F.; et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell 2008, 134, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10158–10163. [Google Scholar] [CrossRef]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 Interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M. CD44 and tumor-derived extracellular vesicles (TEVs). Possible gateway to cancer metastasis. Int. J. Mol. Sci. 2021, 22, 1463. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawada, K.; Kinose, Y.; Yoshimura, A.; Toda, A.; Nakatsuka, E.; Hashimoto, K.; Mabuchi, S.; Morishige, K.I.; Kurachi, H.; et al. Exosomes promote ovarian cancer cell invasion through transfer of CD44 to peritoneal mesothelial cells. Mol. Cancer Res. 2017, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; von Au, A.; Schnölzer, M.; Hackert, T.; Zöller, M. CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget 2016, 7, 55409–55436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, T.; Mizutani, K.; Kawakami, K.; Fujita, Y.; Ehara, H.; Ito, M. CD44v8-10 mRNA contained in serum exosomes as a diagnostic marker for docetaxel resistance in prostate cancer patients. Heliyon 2020, 6, e04138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Yu, W.; Cao, X.; Gu, H.; Huang, J.; Wu, C.; Wang, L.; Sha, X.; Shen, B.; Wang, T.; et al. Exosomal CD44 transmits lymph node metastatic capacity between gastric cancer cells via YAP-CPT1A-Mediated FAO reprogramming. Front. Oncol. 2022, 12, 860175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, X.; Wang, C.; Zhu, H.; Wang, Y.; Wang, X.; Cheng, X.; Ge, W.; Lu, W. Exosome-mediated transfer of CD44 from high-metastatic ovarian cancer cells promotes migration and invasion of low-metastatic ovarian cancer cells. J. Ovarian Res. 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Cheng, K.; Zhang, G.; Jia, Z.; Yu, Y.; Guo, J.; Hua, Y.; Guo, F.; Li, X.; Zou, W.; et al. Enrichment of CD44 in exosomes from breast cancer cells treated with doxorubicin promotes chemoresistance. Front. Oncol. 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, L.; Zhou, H.; Zhang, S.; Wang, C.; Fu, K.; Ma, C.; Zhang, Y.; Peng, C.; Li, Y. CD44-targeting drug delivery system of exosomes loading forsythiaside A combats liver fibrosis via regulating NLRP3-mediated pyroptosis. Adv. Healthc. Mater. 2023, 12, e2202228. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gong, L.; Lin, H.; Yao, S.; Yin, Y.; Zhou, Z.; Shi, J.; Wu, Z.; Huang, Z. Hyaluronic acid-coated bovine milk exosomes for achieving tumor-specific intracellular delivery of miRNA-204. Cells 2022, 11, 3065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef]
- Valachová, K.; Hassan, M.E.; Šoltés, L. Hyaluronan: Sources, structure, features and applications. Molecules 2024, 29, 739. [Google Scholar] [CrossRef]
- Jackson, D.G.; Prevo, R.; Clasper, S.; Banerji, S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001, 22, 317–321. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar]
- Fidler, I.J.; Nicolson, G.L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J. Natl Cancer Inst. 1976, 57, 1199–1202. [Google Scholar] [CrossRef]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. npj Precis. Oncol. 2018, 2, 4. [Google Scholar] [CrossRef]
- Hu, M.; Kenific, C.M.; Boudreau, N.; Lyden, D. Tumor-derived nanoseeds condition the soil for metastatic organotropism. Semin. Cancer Biol. 2023, 93, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Kodama, S.; Kunstfeld, R.; Kajiya, K.; Brown, L.F.; Detmar, M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005, 201, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Fertig, E.J.; Jin, K.; Sukumar, S.; Pandey, N.B.; Popel, A.S. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat. Commun. 2014, 5, 4715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillot, L.; Baudin, L.; Rouaud, L.; Kridelka, F.; Noël, A. The pre-metastatic niche in lymph nodes: Formation and characteristics. Cell. Mol. Life Sci. 2021, 78, 5987–6002. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.; Willms, E.; Hill, A.F. Understanding extracellular vesicle and nanoparticle heterogeneity: Novel methods and considerations. Proteomics 2021, 21, e2000118. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891, Erratum in: Nat. Med. 2016, 22, 1502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Tan, X.; Du, Y.; Wei, Y.; Liu, S. Extracellular vesicle-mediated pre-metastatic niche formation via altering host microenvironments. Front. Immunol. 2024, 15, 1367373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clares-Pedrero, I.; Rocha-Mulero, A.; Palma-Cobo, M.; Cardeñes, B.; Yáñez-Mó, M.; Cabañas, C. Molecular determinants involved in the docking and uptake of tumor-derived extracellular vesicles: Implications in cancer. Int. J. Mol. Sci. 2024, 25, 3449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF family members, their receptors and cell death in the neoplastic transformation of colorectal cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef]
- Ito, Y.; Shibata, M.A.; Eid, N.; Morimoto, J.; Otsuki, Y. Lymphangiogenesis and axillary lymph node metastases correlated with VEGF-C expression in two immunocompetent mouse mammary carcinoma models. Int. J. Breast Cancer 2011, 2011, 867152. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Man, G.C.W.; May, K.E.; Becker, C.M.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wong, A.W.Y.; et al. Vascular endothelial growth factor C is increased in endometrium and promotes endothelial functions, vascular permeability and angiogenesis and growth of endometriosis. Angiogenesis 2013, 16, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Kurenova, E.V.; Hunt, D.L.; He, D.; Fu, A.D.; Massoll, N.A.; Golubovskaya, V.M.; Garces, C.A.; Cance, W.G. Vascular endothelial growth factor receptor-3 promotes breast cancer cell proliferation, motility and survival in vitro and tumor formation in vivo. Cell Cycle 2009, 8, 2266–2280. [Google Scholar] [CrossRef]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into extracellular vesicle-cell communication: From cell recognition to intracellular fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Kotrbová, A.; Štěpka, K.; Maška, M.; Pálenik, J.J.; Ilkovics, L.; Klemová, D.; Kravec, M.; Hubatka, F.; Dave, Z.; Hampl, A.; et al. TEM ExosomeAnalyzer: A computer-assisted software tool for quantitative evaluation of extracellular vesicles in transmission electron microscopy images. J. Extracell. Vesicles 2019, 8, 1560808. [Google Scholar] [CrossRef]
- Vestad, B.; Llorente, A.; Neurauter, A.; Phuyal, S.; Kierulf, B.; Kierulf, P.; Skotland, T.; Sandvig, K.; Haug, K.B.F.; Øvstebø, R. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: A variation study. J. Extracell. Vesicles 2017, 6, 1344087. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Han, C.; Kang, H.; Yi, J.; Kang, M.; Lee, H.; Kwon, Y.; Jung, J.; Lee, J.; Park, J. Single-vesicle imaging and co-localization analysis for tetraspanin profiling of individual extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12047. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Wang, C.A.; Chang, I.H.; Hou, P.C.; Tai, Y.J.; Li, W.N.; Hsu, P.L.; Wu, S.R.; Chiu, W.T.; Li, C.F.; Shan, Y.S.; et al. DUSP2 regulates extracellular vesicle-VEGF-C secretion and pancreatic cancer early dissemination. J. Extracell. Vesicles 2020, 9, 1746529. [Google Scholar] [CrossRef]
- Lin, S.C.; Chien, C.W.; Lee, J.C.; Yeh, Y.C.; Hsu, K.F.; Lai, Y.Y.; Lin, S.C.; Tsai, S.J. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J. Clin. Investig. 2011, 121, 1905–1916. [Google Scholar] [CrossRef]
- Shibata, M.A.; Shiraoka, C.; Kondo, Y. Lymphatic sinus hyperplasia in sentinel lymph nodes prior to mouse mammary cancer metastasis and its mechanism. In Proceedings of the 40th Annual Meeting of the Japanese Society of Toxicologic Pathology, Tokyo, Japan, 23–24 January 2024; p. 193. Available online: https://www.japantoxpath.org/ja/event/jstp40_abstract.pdf (accessed on 15 July 2024).
- Mäkinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Mattila, M.M.T.; Ruohola, J.K.; Karpanen, T.; Jackson, D.G.; Alitalo, K.; Härkönen, P.L. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int. J. Cancer 2002, 98, 946–951. [Google Scholar] [CrossRef]
- Ramos, E.K.; Tsai, C.F.; Jia, Y.; Cao, Y.; Manu, M.; Taftaf, R.; Hoffmann, A.D.; El-Shennawy, L.; Gritsenko, M.A.; Adorno-Cruz, V.; et al. Machine learning-assisted elucidation of CD81-CD44 interactions in promoting cancer stemness and extracellular vesicle integrity. eLife 2022, 11, e82669. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.S.; Harris, E.N.; Weigel, J.A.; Weigel, P.H. The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J. Biol. Chem. 2008, 283, 21453–21461. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Milasan, A.; Tessandier, N.; Tan, S.; Brisson, A.; Boilard, E.; Martel, C. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J. Extracell. Vesicles 2016, 5, 31427. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Bonsergent, E.; Grisard, E.; Buchrieser, J.; Schwartz, O.; Théry, C.; Lavieu, G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat. Commun. 2021, 12, 1864. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rupp, U.; Schoendorf-Holland, E.; Eichbaum, M.; Schuetz, F.; Lauschner, I.; Schmidt, P.; Staab, A.; Hanft, G.; Huober, J.; Sinn, H.P.; et al. Safety and pharmacokinetics of Bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: Final results of a phase I study. Anticancer Drugs 2007, 18, 477–485. [Google Scholar] [CrossRef]
- Goldfarb, S.B.; Traina, T.A.; Dickler, M.N. Bevacizumab for advanced breast cancer. Womens Health (Lond.) 2010, 6, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Dagur, R.S.; Liao, K.; Peeples, E.S.; Hu, G.; Periyasamy, P.; Buch, S. Strategies for the use of extracellular vesicles for the delivery of therapeutics. J. Neuroimmune Pharmacol. 2020, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E. Extracellular vesicles in cancer therapy. Semin. Cancer Biol. 2022, 86, 296–309. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Rani, S.; Corcoran, C.; Wallace, R.; Hughes, L.; Friel, A.M.; McDonnell, S.; Crown, J.; Radomski, M.W.; O’Driscoll, L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur. J. Cancer 2013, 49, 1845–1859. [Google Scholar] [CrossRef]
- Li, W.N.; Hsiao, K.Y.; Wang, C.A.; Chang, N.; Hsu, P.L.; Sun, C.H.; Wu, S.R.; Wu, M.H.; Tsai, S.J. Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis. Proc. Natl Acad. Sci. USA 2020, 117, 25859–25868. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, T.; Wan, X.; Gao, Y.; Wang, H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012, 279, 2047–2059. [Google Scholar] [CrossRef]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef]
- Xie, Q.; Hao, Y.; Li, N.; Song, H.; Chen, X.; Zhou, Z.; Wang, J.; Zhang, Y.; Li, H.; Han, P.; et al. Cellular uptake of engineered extracellular vesicles: Biomechanisms, engineered strategies, and disease treatment. Adv. Healthc. Mater. 2024, 13, e2302280. [Google Scholar] [CrossRef]
- Shibata, M.A.; Shibata, E.; Morimoto, J.; Eid, N.A.S.; Tanaka, Y.; Watanabe, M.; Otsuki, Y. An immunocompetent murine model of metastatic mammary cancer accessible to bioluminescence imaging. Anticancer Res. 2009, 29, 4389–4395. [Google Scholar] [PubMed]
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013, 288, 34343–34351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikari, A.; Ito, Y.; Taniguchi, K.; Shibata, M.-A.; Kimura, K.; Iwamoto, M.; Lee, S.-W. Role of CD44-Positive Extracellular Vesicles Derived from Highly Metastatic Mouse Mammary Carcinoma Cells in Pre-Metastatic Niche Formation. Int. J. Mol. Sci. 2024, 25, 9742. https://doi.org/10.3390/ijms25179742
Ikari A, Ito Y, Taniguchi K, Shibata M-A, Kimura K, Iwamoto M, Lee S-W. Role of CD44-Positive Extracellular Vesicles Derived from Highly Metastatic Mouse Mammary Carcinoma Cells in Pre-Metastatic Niche Formation. International Journal of Molecular Sciences. 2024; 25(17):9742. https://doi.org/10.3390/ijms25179742
Chicago/Turabian StyleIkari, Ayana, Yuko Ito, Kohei Taniguchi, Masa-Aki Shibata, Kosei Kimura, Mitsuhiko Iwamoto, and Sang-Woong Lee. 2024. "Role of CD44-Positive Extracellular Vesicles Derived from Highly Metastatic Mouse Mammary Carcinoma Cells in Pre-Metastatic Niche Formation" International Journal of Molecular Sciences 25, no. 17: 9742. https://doi.org/10.3390/ijms25179742






