Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight
Abstract
1. Introduction
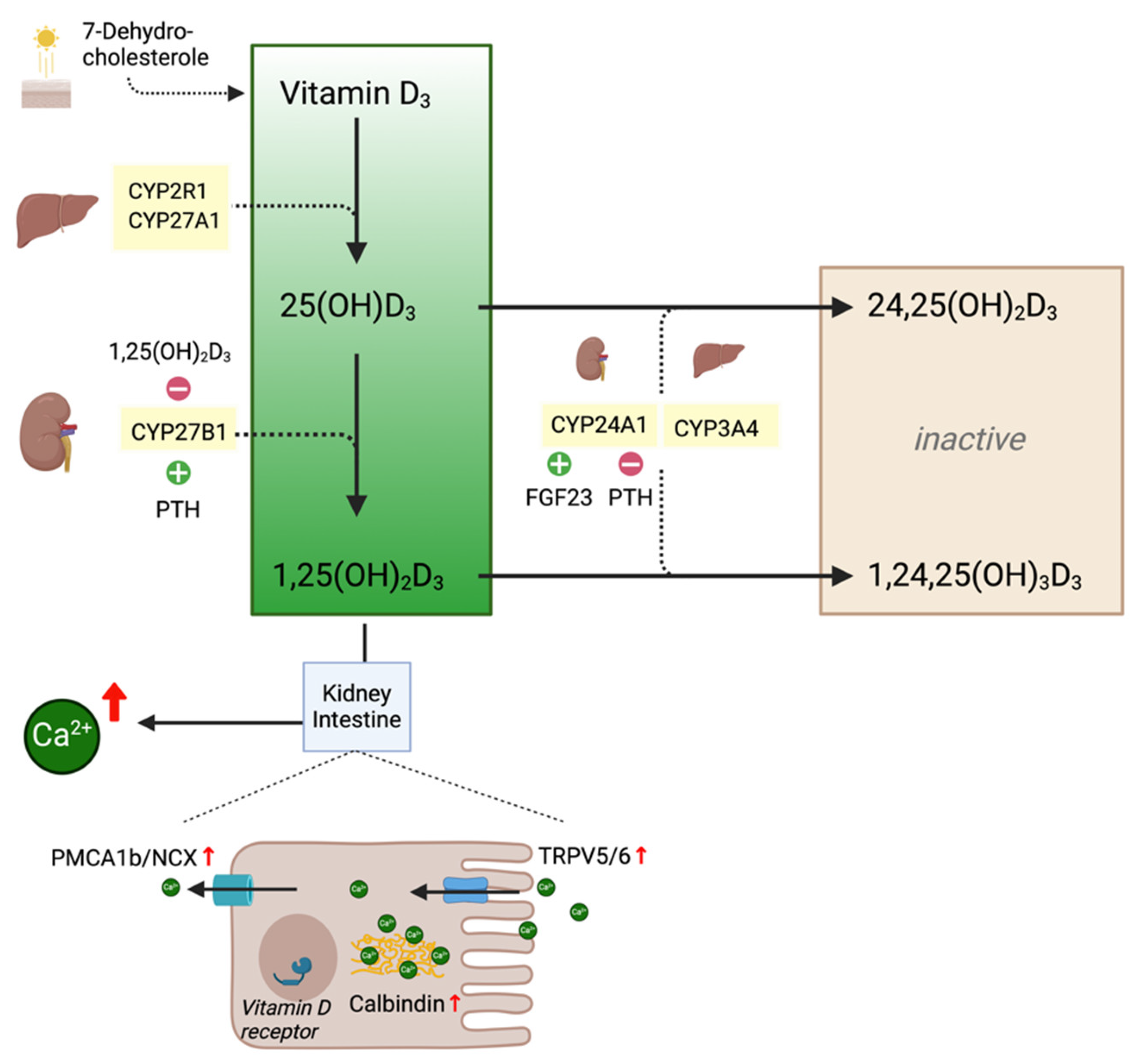
1.1. Physiological Background
1.2. Clinical Background
2. Methods
3. Review—Targeting Calcitriol Metabolism
3.1. Degradation of Vitamin D Metabolites
3.2. Therapeutic Targets
3.2.1. Glucocorticoids
3.2.2. CYP27B1 Inhibition
3.2.3. CYP3A4 Induction
3.2.4. VDR Antagonists
3.3. Case Presentation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, A.N.; Tan, B.H.; Pan, Y.; Ong, C.E. The CYP2R1 Enzyme: Structure, Function, Enzymatic Properties and Genetic Polymorphism. J. Pharm. Pharm. Sci. 2021, 24, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Sakaki, T.; Ohta, M.; Inouye, K. Metabolism of Vitamin D3 by Human CYP27A1. Biochem. Biophys. Res. Commun. 2000, 273, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; Deluca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.; Chain, B.M.; Coussens, A.K.; Khoo, B.; Noursadeghi, M. Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur. J. Immunol. 2014, 44, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Monkawa, T.; Yoshida, T.; Hayashi, M.; Saruta, T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000, 58, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Spiwak, E.; Goswami, S.; Lay, S.E.; Nailescu, C. Case report: Histoplasmosis presenting as asymptomatic hypercalcemia detected on routine laboratory testing in a pediatric kidney transplant recipient. Front. Pediatr. 2022, 10, 1058832. [Google Scholar] [CrossRef] [PubMed]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. Biomed. Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582–586. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22, 64–68. [Google Scholar] [CrossRef]
- Deluca, H.F.; Prahl, J.M.; Plum, L.A. 1,25-Dihydroxyvitamin D is not responsible for toxicity caused by vitamin D or 25-hydroxyvitamin D. Arch. Biochem. Biophys. 2011, 505, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D Stored in Fat Tissue During a 5-Year Intervention Affects Serum 25-Hydroxyvitamin D Levels the Following Year. J. Clin. Endocrinol. Metab. 2017, 2017, 3731–3738. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Grant, W.B. Preventing the Adverse Effects of SARS-CoV-2 Infection and COVID-19 through Diet, Supplements, and Lifestyle. Nutrients 2021, 14, 115. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougère, B.; Guérin, O.; Houvet, M.; et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Pilz, S. Long-term supplementation with 3200 to 4000 IU of vitamin D daily and adverse events: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2023, 62, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Aloizou, A.M.; Zafiriou, E.; Bargiota, A.; Skaperda, Z.; Kouretas, D.; Roussaki-Schulze, A.V. Non-Melanoma Skin Cancer and Vitamin D: The “Lost Sunlight” Paradox and the Oxidative Stress Explanation. Antioxidants 2023, 15, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies of Sciences Press: Washington, DC, USA, 2011. [Google Scholar]
- Rooney, M.R.; Harnack, L.; Michos, E.D.; Ogilvie, R.P.; Sempos, C.T.; Lutsey, P.L. Trends in Use of High-Dose Vitamin D Supplements Exceeding 1000 or 4000 International Units Daily 1999-2014. JAMA 2017, 317, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, K.; Menon, K.; Weiler, H.A.; Amrein, K.; Fergusson, D.; Gunz, A.; Bustos, R.; Campos, R.; Catalan, V.; Roedl, S.; et al. A phase II dose evaluation pilot feasibility randomized controlled trial of cholecalciferol in critically ill children with vitamin D deficiency (VITdAL-PICU study). BMC Pediatr. 2023, 23, 397. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Adoni, T.; Damasceno, A.; de Albuquerque Damasceno, C.A.; Ferreira, M.L.B.; Finkelzstejn, A.; Gomes, S.; Goncalves, M.V.M.; Grzesiuk, A.K.; Lins, S.; et al. Unfavorable outcomes during treatment of multiple sclerosis with high doses of vitamin D. J. Neurol. Sci. 2014, 346, 341–342. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Purkart, T.U.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 2014, 2014, 1520–1530. [Google Scholar] [CrossRef]
- Barry, E.L.; Rees, J.R.; Peacock, J.L.; Mott, L.A.; Amos, C.I.; Bostick, R.M.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Burke, C.A.; et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 2133–2137. [Google Scholar] [CrossRef]
- Haridas, K.; Holick, M.F.; Burmeister, L.A. Hypercalcemia, nephrolithiasis, and hypervitaminosis D precipitated by supplementation in a susceptible individual. Nutrition 2020, 74, 110754. [Google Scholar] [CrossRef]
- Krasniqi, E.; Boshnjaku, A.; Wagner, K.H.; Wessner, B. Association between Polymorphisms in Vitamin D Pathway-Related Genes, Vitamin D Status, Muscle Mass and Function: A Systematic Review. Nutrients 2021, 13, 3109. [Google Scholar] [CrossRef]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Cormier, C. Genetic hypercalcemia. Jt. Bone Spine 2019, 86, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Irwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Kuwertz-Bröking, E.; Fehrenbach, H.; et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011, 365, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Dickens, L.T.; Derman, B.; Alexander, J.T. Endocrine Society Hypercalcemia of Malignancy Guidelines. JAMA Oncol. 2023, 9, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Koutkia, P.; Chen, T.C.; Holick, M.F. Vitamin D intoxication associated with an over-the-counter supplement. N. Engl. J. Med. 2001, 345, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Pike, J.W. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J. Steroid Biochem. Mol. Biol. 2020, 196, 105500. [Google Scholar] [CrossRef] [PubMed]
- Kocełak, P.; Olszanecka-Glinianowicz, M.; Chudek, J. Fibroblast growth factor 23-structure, function and role in kidney diseases. Adv. Clin. Exp. Med. 2012, 21, 391–401. [Google Scholar] [PubMed]
- Wang, Z.; Lin, Y.S.; Zheng, X.E.; Senn, T.; Hashizume, T.; Scian, M.; Dickmann, L.J.; Nelson, S.D.; Baillie, T.A.; Hebert, M.F.; et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol. Pharmacol. 2012, 81, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Marks, B.E.; Doyle, D.A. Idiopathic infantile hypercalcemia: Case report and review of the literature. J. Pediatr. Endocrinol. Metab. 2016, 29, 127–132. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Pludowski, P.; Zelzer, S.; Meinitzer, A.; Karras, S.N.; Misiorowski, W.; Zittermann, A.; März, W.; Trummer, C. Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature. Nutrients 2022, 14, 2518. [Google Scholar] [CrossRef]
- Dhayat, N.A.; Mattmann, C.; Seeger, H.; Ritter, A.; Ernandez, T.; Stoermann-Chopard, C.; Buchkremer, F.; Segerer, S.; Roth, B.; Wuerzner, G.; et al. The Vitamin D Metabolite Diagnostic Ratio Associates With Phenotypic Traits of Idiopathic Hypercalciuria. Kidney Int. Rep. 2024, 9, 1072–1082. [Google Scholar] [CrossRef]
- Azer, S.M.; Vaughan, L.E.; Tebben, P.J.; Sas, D.J. 24-Hydroxylase Deficiency Due to CYP24A1 Sequence Variants: Comparison With Other Vitamin D-mediated Hypercalcemia Disorders. J. Endocr. Soc. 2021, 5, bvab119. [Google Scholar] [CrossRef]
- Wang, J.; Shu, B.; Li, C.G.; Xie, X.W.; Liang, D.; Chen, B.L.; Lin, X.C.; Wei, X.; Wang, L.; Leng, X.Y.; et al. Polymorphisms of genes related to vitamin D metabolism and transportation and its relationship with the risk of osteoporosis: Protocol for a multicentre prospective cohort study in China. BMJ Open 2019, 9, e028084. [Google Scholar] [CrossRef] [PubMed]
- Huybers, S.; Naber, T.H.; Bindels, R.J.; Hoenderop, J.G. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, I.; Matsunuma, A.; Kawane, T.; Abe, M.; Horiuchi, N. Dexamethasone enhances vitamin D-24-hydroxylase expression in osteoblastic (UMR-106) and renal (LLC-PK1) cells treated with 1alpha,25-dihydroxyvitamin D3. Endocrine 2002, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Akeno, N.; Matsunuma, A.; Maeda, T.; Kawane, T.; Horiuchi, N. Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J. Endocrinol. 2000, 164, 339–348. [Google Scholar] [CrossRef]
- Dhawan, P.; Christakos, S. Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase) transcription by glucocorticoids: Cooperative effects of the glucocorticoid receptor, C/EBP beta, and the Vitamin D receptor in 24(OH)ase transcription. J. Cell Biochem. 2010, 110, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.B.; Shin, J.H.; Kwon, S.; Kim, J.; Seok, J.M.; Kim, B.J.; Min, J.H. Effects of Vitamin D and Dexamethasone on Lymphocyte Proportions and Their Associations With Serum Concentrations of 25-Hydroxyvitamin D(3)In Vitro in Patients With Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder. Front. Immunol. 2021, 12, 677041. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Phillips, P.J.; Pannall, P.R.; Cain, H.J.; Leckie, W.J. Effect of orally administered beclomethasone dipropionate on calcium absorption from the gut in normal subjects. Thorax 1993, 48, 890–893. [Google Scholar] [CrossRef][Green Version]
- Edsbäcker, S.; Bengtsson, B.; Larsson, P.; Lundin, P.; Nilsson, Å.; Ulmius, J.; Wollmer, P. A pharmacoscintigraphic evaluation of oral budesonide given as controlled-release (Entocort) capsules. Aliment. Pharmacol. Ther. 2003, 17, 525–536. [Google Scholar] [CrossRef]
- Miller, W.L. Genetic disorders of Vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2017, 165, 101–108. [Google Scholar] [CrossRef]
- Smith, S.J.; Rucka, A.K.; Berry, J.L.; Davies, M.; Mylchreest, S.; Paterson, C.R.; Heath, D.A.; Tassabehji, M.; Read, A.P.; Mee, A.P.; et al. Novel mutations in the 1alpha-hydroxylase (P450c1) gene in three families with pseudovitamin D-deficiency rickets resulting in loss of functional enzyme activity in blood-derived macrophages. J. Bone Miner. Res. 1999, 14, 730–739. [Google Scholar] [CrossRef]
- Bland, R.; Walker, E.A.; Hughes, S.V.; Stewart, P.M.; Hewison, M. Constitutive expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in a transformed human proximal tubule cell line: Evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology 1999, 140, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Bia, M.J.; Insogna, K. Treatment of sarcoidosis-associated hypercalcemia with ketoconazole. Am. J. Kidney Dis. 1991, 18, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Sharma, O.P.; Diz, M.M.; Endres, D.B. Ketoconazole decreases the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. J. Clin. Endocrinol. Metab. 1990, 70, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Barré, P.E.; Gascon-Barré, M.; Meakins, J.L.; Goltzman, D. Hydroxychloroquine treatment of hypercalcemia in a patient with sarcoidosis undergoing hemodialysis. Am. J. Med. 1987, 1987, 1259–1262. [Google Scholar] [CrossRef]
- Nguyen, M.; Boutignon, H.; Mallet, E.; Linglart, A.; Guillozo, H.; Jehan, F.; Garabedian, M. Infantile hypercalcemia and hypercalciuria: New insights into a vitamin D-dependent mechanism and response to ketoconazole treatment. J. Pediatr. 2010, 157, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sayers, J.; Hynes, A.M.; Srivastava, S.; Dowen, F.; Quinton, R.; Datta, H.K.; Sayer, J.A. Successful treatment of hypercalcaemia associated with a CYP24A1 mutation with fluconazole. Clin. Kidney J. 2015, 8, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Gomez-Alonso, C.; Cannata-Andia, J.B. The hypercalcaemia of CYP24A1 inactivation: New ways to improve diagnosis and treatment. Clin. Kidney J. 2015, 8, 456–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roizen, J.D.; Li, D.; O’Lear, L.; Javaid, M.K.; Shaw, N.J.; Ebeling, P.R.; Nguyen, H.H.; Rodda, C.P.; Thummel, K.E.; Thacher, T.D.; et al. CYP3A4 mutation causes vitamin D-dependent rickets type 3. J. Clin. Invest. 2018, 128, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivistö, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003, 42, 819–850. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Dooley, K.E.; Zhang, Y.; Back, D.J.; Owen, A. Induction of influx and efflux transporters and cytochrome P450 3A4 in primary human hepatocytes by rifampin, rifabutin, and rifapentine. Antimicrob. Agents Chemother. 2013, 57, 6366–6369. [Google Scholar] [CrossRef]
- Hawkes, C.P.; Li, D.; Hakonarson, H.; Meyers, K.E.; Thummel, K.E.; Levine, M.A. CYP3A4 Induction by Rifampin: An Alternative Pathway for Vitamin D Inactivation in Patients With CYP24A1 Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Rochel, N. Vitamin D and Its Receptor from a Structural Perspective. Nutrients 2022, 14, 2847. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, Y.; Iwaki, M.; Sawada, T.; Nojiri, K.; Kurokawa, T.; Tsutsumi, R.; Nagasawa, K.; Kato, S. Advances in the Administration of Vitamin D Analogues to Support Bone Health and Treat Chronic Diseases. J. Bone Metab. 2023, 30, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rovito, D.; Belorusova, A.Y.; Chalhoub, S.; Rerra, A.I.; Guiot, E.; Molin, A.; Linglart, A.; Rochel, N.; Laverny, G.; Metzger, D. Cytosolic sequestration of the vitamin D receptor as a therapeutic option for vitamin D-induced hypercalcemia. Nat. Commun. 2020, 11, 6942. [Google Scholar] [CrossRef]
- Lee, J.P.; Tansey, M.; Jetton, J.G.; Krasowski, M.D. Vitamin D Toxicity: A 16-Year Retrospective Study at an Academic Medical Center. Lab. Med. 2018, 49, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef]
- Ketha, H.; Wadams, H.; Lteif, A.; Singh, R.J. Iatrogenic vitamin D toxicity in an infant--a case report and review of literature. J. Steroid Biochem. Mol. Biol. 2015, 148, 14–18. [Google Scholar] [CrossRef]
- Burton, J.M.; Kimball, S.; Vieth, R.; Bar-Or, A.; Dosch, H.M.; Cheung, R.; Gagne, D.; D‘souza, C.; Ursell, M.; O‘connor, P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010, 74, 1852–1859. [Google Scholar] [CrossRef]
- Walker, M.D.; Shane, E. Hypercalcemia: A Review. JAMA 2022, 328, 1624–1636. [Google Scholar] [CrossRef]
- Shultz, T.D.; Bollman, S.; Kumar, R. Decreased intestinal calcium absorption in vivo and normal brush border membrane vesicle calcium uptake in cortisol-treated chickens: Evidence for dissociation of calcium absorption from brush border vesicle uptake. Proc. Natl. Acad. Sci. USA 1982, 79, 3542–3546. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.; Ejlertsen, B.; Holmegaard, S.N.; Krarup-Hansen, A.; Transbøl, I.; Mouridsen, H. Prednisolone in the treatment of severe malignant hypercalcaemia in metastatic breast cancer: A randomized study. J. Intern. Med. 1992, 232, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Goischke, H.K. Comorbidities in multiple sclerosis-a plea for interdisciplinary collaboration to improve the quality of life of MS patients. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 39–53. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aberger, S.; Schreiber, N.; Pilz, S.; Eller, K.; Rosenkranz, A.R.; Kirsch, A.H. Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight. Int. J. Mol. Sci. 2024, 25, 10003. https://doi.org/10.3390/ijms251810003
Aberger S, Schreiber N, Pilz S, Eller K, Rosenkranz AR, Kirsch AH. Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight. International Journal of Molecular Sciences. 2024; 25(18):10003. https://doi.org/10.3390/ijms251810003
Chicago/Turabian StyleAberger, Simon, Nikolaus Schreiber, Stefan Pilz, Kathrin Eller, Alexander R. Rosenkranz, and Alexander H. Kirsch. 2024. "Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight" International Journal of Molecular Sciences 25, no. 18: 10003. https://doi.org/10.3390/ijms251810003
APA StyleAberger, S., Schreiber, N., Pilz, S., Eller, K., Rosenkranz, A. R., & Kirsch, A. H. (2024). Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight. International Journal of Molecular Sciences, 25(18), 10003. https://doi.org/10.3390/ijms251810003







