WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD
Abstract
1. Introduction
2. Background
2.1. WISP1
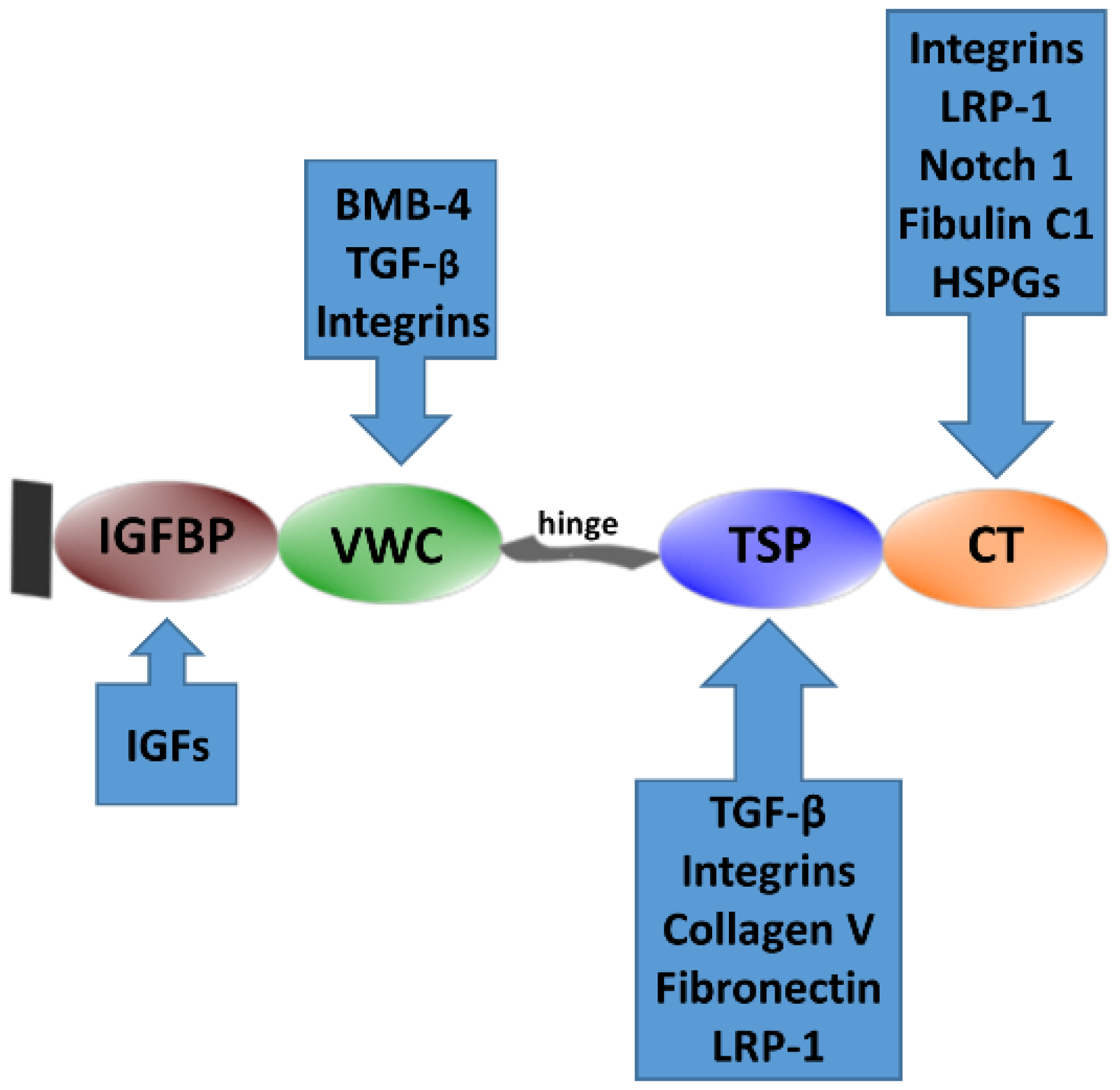
2.1.1. WISP1 Structure, Expression, and Regulation
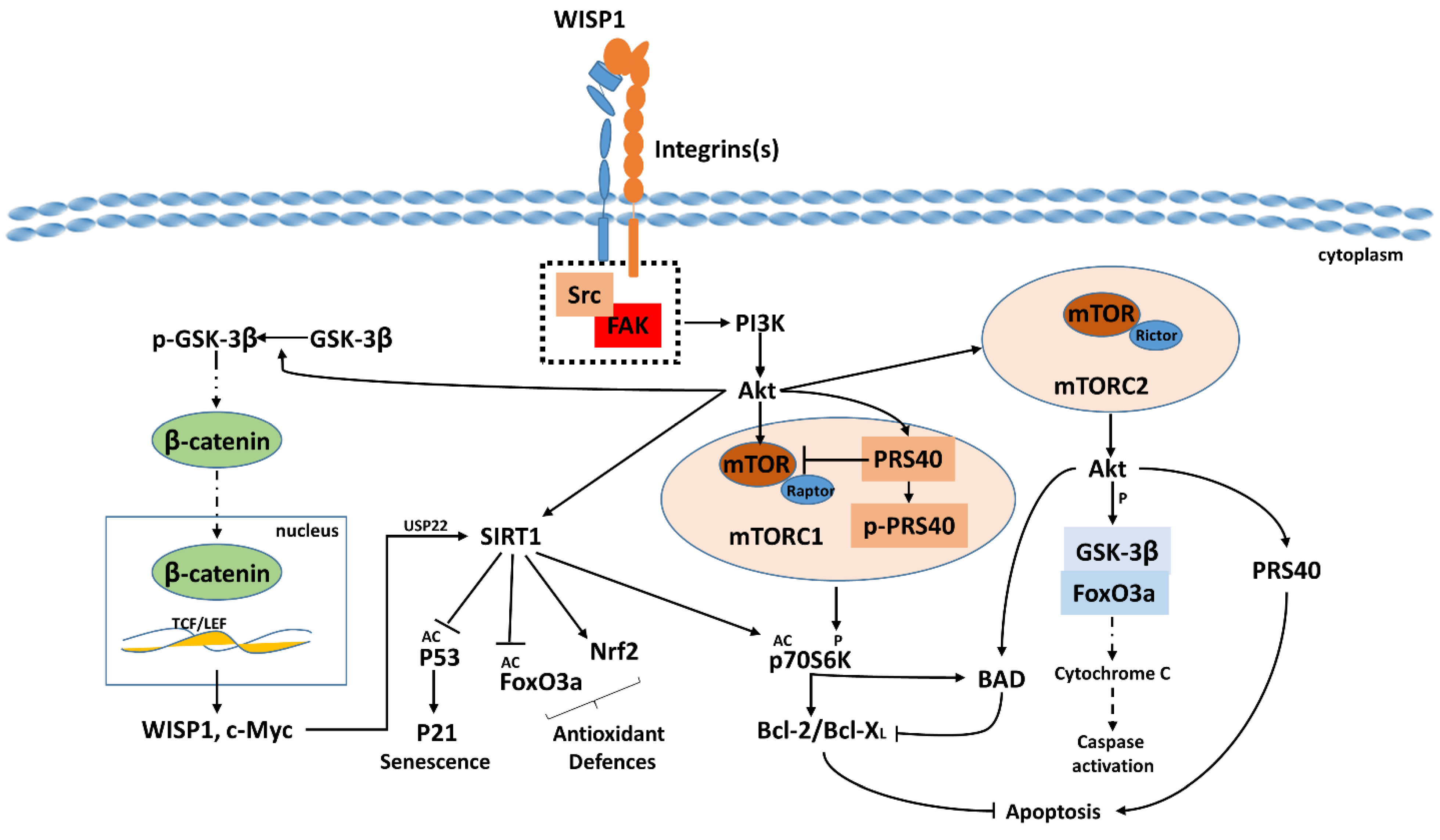
2.1.2. Roles of WISP1 in Signaling Pathways and Cellular Processes
2.2. MIF
2.2.1. Roles of MIF in Signaling Pathways and Cellular Processes
2.2.2. Involvement of MIF in Immune Responses and Inflammation
3. Molecular Mechanisms Involving WISP/MIF in Asthma and COPD
3.1. WISP1 in Asthma
3.2. MIF in Asthma
3.3. WISP1 in COPD
3.4. MIF in COPD
4. WISP–MIF Axis: A Novel Regulatory Pathway in Immunity and Inflammation?
5. Clinical Implications and Future Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 2521–2522. [Google Scholar] [CrossRef] [PubMed]
- Harker, J.A.; Lloyd, C.M. T helper 2 cells in asthma. J. Exp. Med. 2023, 220, e20221094. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.; Gosens, R. Revisiting asthma therapeutics: Focus on WNT signal transduction. Drug Discov. Today 2018, 23, 49–62. [Google Scholar] [CrossRef]
- Kumawat, K.; Menzen, M.H.; Bos, I.S.; Baarsma, H.A.; Borger, P.; Roth, M.; Tamm, M.; Halayko, A.J.; Simoons, M.; Prins, A.; et al. Noncanonical WNT-5A signaling regulates TGF-beta-induced extracellular matrix production by airway smooth muscle cells. FASEB J. 2013, 27, 1631–1643. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef]
- Mercado, N.; Ito, K.; Barnes, P.J. Accelerated ageing of the lung in COPD: New concepts. Thorax 2015, 70, 482–489. [Google Scholar] [CrossRef]
- Barker, D.J.; Godfrey, K.M.; Fall, C.; Osmond, C.; Winter, P.D.; Shaheen, S.O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991, 303, 671–675. [Google Scholar] [CrossRef]
- Todisco, T.; de Benedictis, F.M.; Iannacci, L.; Baglioni, S.; Eslami, A.; Todisco, E.; Dottorini, M. Mild prematurity and respiratory functions. Eur. J. Pediatr. 1993, 152, 55–58. [Google Scholar] [CrossRef]
- Shi, W.; Chen, F.; Cardoso, W.V. Mechanisms of lung development: Contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 558–563. [Google Scholar] [CrossRef]
- Park, S.; Ra, S.W.; Kang, S.Y.; Kim, H.C.; Lee, S.W. Effect of particulate matter exposure on patients with COPD and risk reduction through behavioural interventions: The protocol of a prospective panel study. BMJ Open 2020, 10, e039394. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, J.; Hu, Y.; Tian, Y. Air pollution and risk of chronic obstructed pulmonary disease: The modifying effect of genetic susceptibility and lifestyle. EBioMedicine 2022, 79, 103994. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.S.; Warner, L.; Cascagnette, P.; Victor, J.C.; To, T. Lifetime risk of developing chronic obstructive pulmonary disease: A longitudinal population study. Lancet 2011, 378, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Beran, D.; Zar, H.J.; Perrin, C.; Menezes, A.M.; Burney, P.; Forum of International Respiratory Societies Working Group Collaboration. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir. Med. 2015, 3, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.E.; Sherrill, D.L.; Guerra, S.; Barbee, R.A. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004, 126, 59–65. [Google Scholar] [CrossRef] [PubMed]
- de Marco, R.; Accordini, S.; Marcon, A.; Cerveri, I.; Anto, J.M.; Gislason, T.; Heinrich, J.; Janson, C.; Jarvis, D.; Kuenzli, N.; et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am. J. Respir. Crit. Care Med. 2011, 183, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Repine, J.E.; Bast, A.; Lankhorst, I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am. J. Respir. Crit. Care Med. 1997, 156, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Meyer, M.; Jaspers, I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1189–L1201. [Google Scholar] [CrossRef]
- Lin, C.R.; Bahmed, K.; Kosmider, B. Dysregulated Cell Signaling in Pulmonary Emphysema. Front. Med. 2021, 8, 762878. [Google Scholar] [CrossRef]
- Van Pottelberge, G.R.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. The role of dendritic cells in the pathogenesis of COPD: Liaison officers in the front line. J. Chronic Obstr. Pulm. Dis. 2009, 6, 284–290. [Google Scholar] [CrossRef]
- Chong, D.L.W.; Rebeyrol, C.; Jose, R.J.; Williams, A.E.; Brown, J.S.; Scotton, C.J.; Porter, J.C. ICAM-1 and ICAM-2 Are Differentially Expressed and Up-Regulated on Inflamed Pulmonary Epithelium, but Neither ICAM-2 nor LFA-1: ICAM-1 Are Required for Neutrophil Migration into the Airways In Vivo. Front. Immunol. 2021, 12, 691957. [Google Scholar] [CrossRef]
- Huang, X.; Guan, W.; Xiang, B.; Wang, W.; Xie, Y.; Zheng, J. MUC5B regulates goblet cell differentiation and reduces inflammation in a murine COPD model. Respir. Res. 2022, 23, 11. [Google Scholar] [CrossRef]
- Rose, M.C.; Voynow, J.A. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Gunther, A.; Bracke, K.R.; Dagouassat, M.; et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 143–163, Erratum in J. Exp. Med. 2017, 214, 565. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Morty, R.E.; Konigshoff, M.; Eickelberg, O. Transforming growth factor-beta signaling across ages: From distorted lung development to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 607–613. [Google Scholar] [CrossRef]
- Mahmood, M.Q.; Reid, D.; Ward, C.; Muller, H.K.; Knight, D.A.; Sohal, S.S.; Walters, E.H. Transforming growth factor (TGF) beta(1) and Smad signalling pathways: A likely key to EMT-associated COPD pathogenesis. Respirology 2017, 22, 133–140. [Google Scholar] [CrossRef]
- Lo, H.W.; Hsu, S.C.; Xia, W.; Cao, X.; Shih, J.Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67, 9066–9076. [Google Scholar] [CrossRef]
- Shujaat, A.; Bajwa, A.A.; Cury, J.D. Pulmonary Hypertension Secondary to COPD. Pulm. Med. 2012, 2012, 203952. [Google Scholar] [CrossRef]
- Roland, M.; Bhowmik, A.; Sapsford, R.J.; Seemungal, T.A.; Jeffries, D.J.; Warner, T.D.; Wedzicha, J.A. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax 2001, 56, 30–35. [Google Scholar] [CrossRef]
- Tan, X.; Chai, J.; Bi, S.C.; Li, J.J.; Li, W.W.; Zhou, J.Y. Involvement of matrix metalloproteinase-2 in medial hypertrophy of pulmonary arterioles in broiler chickens with pulmonary arterial hypertension. Vet. J. 2012, 193, 420–425. [Google Scholar] [CrossRef]
- Koutsokera, A.; Stolz, D.; Loukides, S.; Kostikas, K. Systemic biomarkers in exacerbations of COPD: The evolving clinical challenge. Chest 2012, 141, 396–405. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Chi, S.Y.; Shin, H.J.; Kim, E.Y.; Yoon, B.K.; Ban, H.J.; Oh, I.J.; Kim, K.S.; Kim, Y.C.; Lim, S.C. Plasma C-reactive protein and endothelin-1 level in patients with chronic obstructive pulmonary disease and pulmonary hypertension. J. Korean Med. Sci. 2010, 25, 1487–1491. [Google Scholar] [CrossRef]
- Minai, O.A.; Chaouat, A.; Adnot, S. Pulmonary hypertension in COPD: Epidemiology, significance, and management: Pulmonary vascular disease: The global perspective. Chest 2010, 137, 39S–51S. [Google Scholar] [CrossRef]
- Hao, S.; Yan, K.K.; Ding, L.; Qian, C.; Chi, H.; Yu, J. Network Approaches for Dissecting the Immune System. iScience 2020, 23, 101354. [Google Scholar] [CrossRef]
- Rankin, L.C.; Artis, D. Beyond Host Defense: Emerging Functions of the Immune System in Regulating Complex Tissue Physiology. Cell 2018, 173, 554–567. [Google Scholar] [CrossRef]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell. Mol. Immunol. 2023, 20, 1101–1113. [Google Scholar] [CrossRef]
- Riley, H.J.; Bradshaw, A.D. The Influence of the Extracellular Matrix in Inflammation: Findings from the SPARC-Null Mouse. Anat Rec 2020, 303, 1624–1629. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef]
- Schiller, H.B.; Fernandez, I.E.; Burgstaller, G.; Schaab, C.; Scheltema, R.A.; Schwarzmayr, T.; Strom, T.M.; Eickelberg, O.; Mann, M. Time-and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol. Syst. Biol. 2015, 11, 819. [Google Scholar] [CrossRef]
- Hussain, M.; Xu, C.; Lu, M.; Wu, X.; Tang, L.; Wu, X. Wnt/beta-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3226–3242. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Konigshoff, M. ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax 2017, 72, 746–759. [Google Scholar] [CrossRef]
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Florez-Sampedro, L.; Soto-Gamez, A.; Poelarends, G.J.; Melgert, B.N. The role of MIF in chronic lung diseases: Looking beyond inflammation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L1183–L1197. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 945–963. [Google Scholar] [CrossRef]
- Stephens, S.; Palmer, J.; Konstantinova, I.; Pearce, A.; Jarai, G.; Day, E. A functional analysis of Wnt inducible signalling pathway protein -1 (WISP-1/CCN4). J. Cell Commun. Signal 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Perbal, B.; Tweedie, S.; Bruford, E. The official unified nomenclature adopted by the HGNC calls for the use of the acronyms, CCN1-6, and discontinuation in the use of CYR61, CTGF, NOV and WISP 1-3 respectively. J. Cell Commun. Signal 2018, 12, 625–629. [Google Scholar] [CrossRef]
- Holbourn, K.P.; Acharya, K.R.; Perbal, B. The CCN family of proteins: Structure-function relationships. Trends Biochem. Sci. 2008, 33, 461–473. [Google Scholar] [CrossRef]
- Xia, Y.F.; Chang, J.; Yang, J.F.; Ouyang, W.; Pitt, B.; Billiar, T.; Zhang, L.M. Non-canonical Wnt signaling contributes to ventilator-induced lung injury through upregulation of WISP1 expression. Int. J. Mol. Med. 2019, 43, 1217–1228. [Google Scholar] [CrossRef]
- Monsen, V.T.; Attramadal, H. Structural insights into regulation of CCN protein activities and functions. J. Cell Commun. Signal 2023, 17, 371–390. [Google Scholar] [CrossRef]
- Birkeness, L.B.; Banerjee, S.; Quadir, M.; Banerjee, S.K. The role of CCNs in controlling cellular communication in the tumor microenvironment. J. Cell Commun. Signal 2023, 17, 35–45. [Google Scholar] [CrossRef]
- Berschneider, B.; Konigshoff, M. WNT1 inducible signaling pathway protein 1 (WISP1): A novel mediator linking development and disease. Int. J. Biochem. Cell Biol. 2011, 43, 306–309. [Google Scholar] [CrossRef]
- Perbal, B. Alternative splicing of CCN mRNAs …. it has been upon us. J. Cell Commun. Signal 2009, 3, 153–157. [Google Scholar] [CrossRef]
- Tanaka, S.; Sugimachi, K.; Kameyama, T.; Maehara, S.; Shirabe, K.; Shimada, M.; Wands, J.R.; Maehara, Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology 2003, 37, 1122–1129. [Google Scholar] [CrossRef]
- Tanaka, S.; Sugimachi, K.; Saeki, H.; Kinoshita, J.; Ohga, T.; Shimada, M.; Maehara, Y.; Sugimachi, K. A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene 2001, 20, 5525–5532. [Google Scholar] [CrossRef][Green Version]
- Pennica, D.; Swanson, T.A.; Welsh, J.W.; Roy, M.A.; Lawrence, D.A.; Lee, J.; Brush, J.; Taneyhill, L.A.; Deuel, B.; Lew, M.; et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl. Acad. Sci. USA 1998, 95, 14717–14722. [Google Scholar] [CrossRef]
- Inkson, C.A.; Ono, M.; Kuznetsov, S.A.; Fisher, L.W.; Robey, P.G.; Young, M.F. TGF-beta1 and WISP-1/CCN-4 can regulate each other’s activity to cooperatively control osteoblast function. J. Cell Biochem. 2008, 104, 1865–1878. [Google Scholar] [CrossRef]
- Soon, L.L.; Yie, T.A.; Shvarts, A.; Levine, A.J.; Su, F.; Tchou-Wong, K.M. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J. Biol. Chem. 2003, 278, 11465–11470. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Conner, E.A.; Ladu, S.; Lemmer, E.R.; Factor, V.M.; Thorgeirsson, S.S. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J. Hepatol. 2005, 42, 842–849. [Google Scholar] [CrossRef]
- Konigshoff, M.; Kramer, M.; Balsara, N.; Wilhelm, J.; Amarie, O.V.; Jahn, A.; Rose, F.; Fink, L.; Seeger, W.; Schaefer, L.; et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 2009, 119, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Wen, S.; McCafferty, D.M.; Beck, P.L.; MacNaughton, W.K. Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J. Mol. Med. 2009, 87, 435–445. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes. Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Fiedler, M.; Mendoza-Topaz, C.; Rutherford, T.J.; Mieszczanek, J.; Bienz, M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating beta-catenin. Proc. Natl. Acad. Sci. USA 2011, 108, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Romond, T.; Metcalfe, C.; Bienz, M. Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 2007, 120, 2402–2412. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The beta-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Y.; Su, Y.; Li, R.; Lin, Y.; Zhou, X.; Ye, L. C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells. PLoS ONE 2013, 8, e69440. [Google Scholar] [CrossRef]
- Kuhl, M.; Sheldahl, L.C.; Park, M.; Miller, J.R.; Moon, R.T. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000, 16, 279–283. [Google Scholar] [CrossRef]
- Rao, T.P.; Kuhl, M. An updated overview on Wnt signaling pathways: A prelude for more. Circ. Res. 2010, 106, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Corcoran, R.B.; Welsh, J.W.; Pennica, D.; Levine, A.J. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes. Dev. 2000, 14, 585–595. [Google Scholar] [CrossRef]
- Yang, M.; Du, Y.; Xu, Z.; Jiang, Y. Functional Effects of WNT1-Inducible Signaling Pathway Protein-1 on Bronchial Smooth Muscle Cell Migration and Proliferation in OVA-Induced Airway Remodeling. Inflammation 2016, 39, 16–29. [Google Scholar] [CrossRef]
- Koopmans, T.; Crutzen, S.; Menzen, M.H.; Halayko, A.J.; Hackett, T.L.; Knight, D.A.; Gosens, R. Selective targeting of CREB-binding protein/beta-catenin inhibits growth of and extracellular matrix remodelling by airway smooth muscle. Br. J. Pharmacol. 2016, 173, 3327–3341. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.; Hesse, L.; Nawijn, M.C.; Kumawat, K.; Menzen, M.H.; Sophie, T.B.I.; Smits, R.; Bakker, E.R.M.; van den Berge, M.; Koppelman, G.H.; et al. Smooth-muscle-derived WNT5A augments allergen-induced airway remodelling and Th2 type inflammation. Sci. Rep. 2020, 10, 6754. [Google Scholar] [CrossRef]
- Bartis, D.; Csongei, V.; Weich, A.; Kiss, E.; Barko, S.; Kovacs, T.; Avdicevic, M.; D’Souza, V.K.; Rapp, J.; Kvell, K.; et al. Down-regulation of canonical and up-regulation of non-canonical Wnt signalling in the carcinogenic process of squamous cell lung carcinoma. PLoS ONE 2013, 8, e57393. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Zhu, H.; Feng, J.; Ni, S.; Huang, J. Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer. Oncotarget 2015, 6, 24912–24921. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Chan, R.W.S.; Cheng, F.H.C.; Li, J.; Li, T.; Pang, R.T.K.; Lee, C.L.; Li, R.H.W.; Ng, E.H.Y.; Chiu, P.C.N.; et al. Myometrial Cells Stimulate Self-Renewal of Endometrial Mesenchymal Stem-Like Cells Through WNT5A/beta-Catenin Signaling. Stem Cells 2019, 37, 1455–1466. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, X.; Jin, S.; Pitt, B.; Zhang, L.; Billiar, T.; Li, Q. WISP1-alphavbeta3 integrin signaling positively regulates TLR-triggered inflammation response in sepsis induced lung injury. Sci. Rep. 2016, 6, 28841. [Google Scholar] [CrossRef]
- Li, H.H.; Li, Q.; Liu, P.; Liu, Y.; Li, J.; Wasserloos, K.; Chao, W.; You, M.; Oury, T.D.; Chhinder, S.; et al. WNT1-inducible signaling pathway protein 1 contributes to ventilator-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 528–535. [Google Scholar] [CrossRef]
- Villar, J.; Cabrera, N.E.; Valladares, F.; Casula, M.; Flores, C.; Blanch, L.; Quilez, M.E.; Santana-Rodriguez, N.; Kacmarek, R.M.; Slutsky, A.S. Activation of the Wnt/beta-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS ONE 2011, 6, e23914. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.E.; Zhou, B.; Solomon, N.; Sunohara, M.; Li, C.; Nguyen, C.; Liu, Y.; Pan, J.H.; Minoo, P.; Crandall, E.D.; et al. p300/beta-Catenin Interactions Regulate Adult Progenitor Cell Differentiation Downstream of WNT5a/Protein Kinase C (PKC). J. Biol. Chem. 2016, 291, 6569–6582. [Google Scholar] [CrossRef]
- Torii, K.; Nishizawa, K.; Kawasaki, A.; Yamashita, Y.; Katada, M.; Ito, M.; Nishimoto, I.; Terashita, K.; Aiso, S.; Matsuoka, M. Anti-apoptotic action of Wnt5a in dermal fibroblasts is mediated by the PKA signaling pathways. Cell Signal 2008, 20, 1256–1266. [Google Scholar] [CrossRef]
- Brown, B.A.; Connolly, G.M.; Mill, C.E.J.; Williams, H.; Angelini, G.D.; Johnson, J.L.; George, S.J. Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells. Aging Cell 2019, 18, e12844. [Google Scholar] [CrossRef]
- Kumawat, K.; Gosens, R. WNT-5A: Signaling and functions in health and disease. Cell Mol. Life Sci. 2016, 73, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tang, Z.; Gong, H.; Zhu, L.; Liu, X. Wnt5a promotes epithelial-to-mesenchymal transition and metastasis in non-small-cell lung cancer. Biosci. Rep. 2017, 37, BSR20171092. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Fuerer, C.; Mizutani, M.; Nusse, R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev. Biol. 2012, 369, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Mill, C.; Monk, B.A.; Williams, H.; Simmonds, S.J.; Jeremy, J.Y.; Johnson, J.L.; George, S.J. Wnt5a-induced Wnt1-inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2449–2456. [Google Scholar] [CrossRef]
- Henderson, W.R., Jr.; Chi, E.Y.; Ye, X.; Nguyen, C.; Tien, Y.T.; Zhou, B.; Borok, Z.; Knight, D.A.; Kahn, M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14309–14314. [Google Scholar] [CrossRef]
- Tong, Y.; Yu, Z.; Zhang, R.; Ding, X.; Chen, Z.; Li, Q. WISP1 mediates lung injury following hepatic ischemia reperfusion dependent on TLR4 in mice. BMC Pulm. Med. 2018, 18, 189. [Google Scholar] [CrossRef]
- Colston, J.T.; de la Rosa, S.D.; Koehler, M.; Gonzales, K.; Mestril, R.; Freeman, G.L.; Bailey, S.R.; Chandrasekar, B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1839–H1846. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Venkatesan, B.; Valente, A.J.; Melby, P.C.; Nandish, S.; Reusch, J.E.; Clark, R.A.; Chandrasekar, B. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J. Biol. Chem. 2009, 284, 14414–14427. [Google Scholar] [CrossRef]
- Chang, K.S.; Chen, S.T.; Sung, H.C.; Hsu, S.Y.; Lin, W.Y.; Hou, C.P.; Lin, Y.H.; Feng, T.H.; Tsui, K.H.; Juang, H.H. WNT1 Inducible Signaling Pathway Protein 1 Is a Stroma-Specific Secreting Protein Inducing a Fibroblast Contraction and Carcinoma Cell Growth in the Human Prostate. Int. J. Mol. Sci. 2022, 23, 11437. [Google Scholar] [CrossRef]
- Berschneider, B.; Ellwanger, D.C.; Baarsma, H.A.; Thiel, C.; Shimbori, C.; White, E.S.; Kolb, M.; Neth, P.; Konigshoff, M. miR-92a regulates TGF-beta1-induced WISP1 expression in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2014, 53, 432–441. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, T.; Zhang, L.; Yang, X.; Li, Q.; Ding, X. WISP1 and TLR4 on Macrophages Contribute to Ventilator-Induced Lung Injury. Inflammation 2020, 43, 425–432, Erratum in Inflammation 2021, 44, 1205. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Overholtzer, M.; Besser, D.; Levine, A.J. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes. Dev. 2002, 16, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Heise, R.L.; Stober, V.; Cheluvaraju, C.; Hollingsworth, J.W.; Garantziotis, S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J. Biol. Chem. 2011, 286, 17435–17444. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, M.-E.; Skandalis, S.S.; Papakonstantinou, E.; Stolz, D.; Aletras, A.J. WISP1 induces the expression of macrophage migration inhibitory factor in human lung fibroblasts through Src kinases and EGFR-activated signaling pathways. Am. J. Physiol.-Cell Physiol. 2024, 326, C850–C865. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Picking a bone with WISP1 (CCN4): New strategies against degenerative joint disease. J. Transl. Sci. 2016, 1, 83–85. [Google Scholar] [CrossRef]
- Klee, S.; Lehmann, M.; Wagner, D.E.; Baarsma, H.A.; Konigshoff, M. WISP1 mediates IL-6-dependent proliferation in primary human lung fibroblasts. Sci. Rep. 2016, 6, 20547. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, S.; Wang, X.; Zhou, J.; Cao, Y.; Wang, F.; Duan, E. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell 2011, 10, 661–674. [Google Scholar] [CrossRef]
- Lau, L.F. Cell surface receptors for CCN proteins. J. Cell Commun. Signal 2016, 10, 121–127. [Google Scholar] [CrossRef]
- Rosano, L.; Spinella, F.; Bagnato, A. Endothelin 1 in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2013, 13, 637–651. [Google Scholar] [CrossRef]
- Deng, W.; Fernandez, A.; McLaughlin, S.L.; Klinke, D.J., 2nd. WNT1-inducible signaling pathway protein 1 (WISP1/CCN4) stimulates melanoma invasion and metastasis by promoting the epithelial-mesenchymal transition. J. Biol. Chem. 2019, 294, 5261–5280. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jia, S. Dual effect of WISP-1 in diverse pathological processes. Chin. J. Cancer Res. 2016, 28, 553–560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, X.; Tong, Y.; Jin, S.; Chen, Z.; Li, T.; Billiar, T.R.; Pitt, B.R.; Li, Q.; Zhang, L.-M. Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: Role of WISP1–αvβ5 integrin pathway in TLR4-mediated inflammation and injury. Crit. Care 2018, 22, 302. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, Q.C.; Yang, X.F.; Mu, W.D. TGF-beta1/WISP1/Integrin-alpha interaction mediates human chondrocytes dedifferentiation. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8675–8684. [Google Scholar] [CrossRef]
- Ono, M.; Masaki, A.; Maeda, A.; Kilts, T.M.; Hara, E.S.; Komori, T.; Pham, H.; Kuboki, T.; Young, M.F. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via alpha5beta1 and TNFalpha. Matrix Biol. 2018, 68–69, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; LaCanna, R.; Ma, H.Y.; N’Diaye, E.N.; Gierke, S.; Caplazi, P.; Sagolla, M.; Huang, Z.; Lucio, L.; Arlantico, A.; et al. A WISP1 antibody inhibits MRTF signaling to prevent the progression of established liver fibrosis. Cell Metab. 2022, 34, 1377–1393.e1378. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H. Adhesion signaling-crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef]
- Olson, E.N.; Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010, 11, 353–365. [Google Scholar] [CrossRef]
- Tao, W.; Chu, C.; Zhou, W.; Huang, Z.; Zhai, K.; Fang, X.; Huang, Q.; Zhang, A.; Wang, X.; Yu, X.; et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 2020, 11, 3015. [Google Scholar] [CrossRef]
- Blalock, T.D.; Gibson, D.J.; Duncan, M.R.; Tuli, S.S.; Grotendorst, G.R.; Schultz, G.S. A connective tissue growth factor signaling receptor in corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3387–3394. [Google Scholar] [CrossRef]
- Chen, P.C.; Cheng, H.C.; Yang, S.F.; Lin, C.W.; Tang, C.H. The CCN family proteins: Modulators of bone development and novel targets in bone-associated tumors. Biomed. Res. Int. 2014, 2014, 437096. [Google Scholar] [CrossRef] [PubMed]
- Case, N.; Ma, M.; Sen, B.; Xie, Z.; Gross, T.S.; Rubin, J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J. Biol. Chem. 2008, 283, 29196–29205. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Inkson, C.A.; Kilts, T.M.; Young, M.F. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J. Bone Miner. Res. 2011, 26, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Ye, M.; Hu, X.; Wang, Z.P.; Zhu, X. The emerging role of WISP proteins in tumorigenesis and cancer therapy. J. Transl. Med. 2019, 17, 28. [Google Scholar] [CrossRef]
- Tsai, H.C.; Tzeng, H.E.; Huang, C.Y.; Huang, Y.L.; Tsai, C.H.; Wang, S.W.; Wang, P.C.; Chang, A.C.; Fong, Y.C.; Tang, C.H. WISP-1 positively regulates angiogenesis by controlling VEGF-A expression in human osteosarcoma. Cell Death Dis. 2017, 8, e2750. [Google Scholar] [CrossRef]
- Sun, C.; Ma, Q.; Yin, J.; Zhang, H.; Liu, X. WISP-1 induced by mechanical stress contributes to fibrosis and hypertrophy of the ligamentum flavum through Hedgehog-Gli1 signaling. Exp. Mol. Med. 2021, 53, 1068–1079. [Google Scholar] [CrossRef]
- Wang, S.; Zhong Chong, Z.; Chen Shang, Y.; Maiese, K. Wnt1 Inducible Signaling Pathway Protein 1 (WISP1) Blocks Neurodegeneration through Phosphoinositide 3 Kinase/Akt1 and Apoptotic Mitochondrial Signaling Involving Bad, Bax, Bim, and Bcl-xL. Curr. Neurovascular Res. 2012, 9, 20–31. [Google Scholar] [CrossRef]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr. Neurovasc. Res. 2012, 9, 91–101. [Google Scholar] [CrossRef]
- Gurbuz, I.; Chiquet-Ehrismann, R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): A focus on its role in cancer. Int. J. Biochem. Cell Biol. 2015, 62, 142–146. [Google Scholar] [CrossRef]
- Maiese, K. The impact of aging and oxidative stress in metabolic and nervous system disorders: Programmed cell death and molecular signal transduction crosstalk. Front. Immunol. 2023, 14, 1273570. [Google Scholar] [CrossRef]
- Liu, H.; Dong, W.; Lin, Z.; Lu, J.; Wan, H.; Zhou, Z.; Liu, Z. CCN4 regulates vascular smooth muscle cell migration and proliferation. Mol. Cells 2013, 36, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A.; Nielsen, H.C. Targeting Airway Smooth Muscle Hypertrophy in Asthma: An Approach Whose Time Has Come. J. Asthma Allergy 2021, 14, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Prabhu, S.D.; Venkatachalam, K.; Mummidi, S.; Valente, A.J.; Clark, R.A.; Delafontaine, P.; Chandrasekar, B. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal 2010, 22, 809–820. [Google Scholar] [CrossRef]
- Schlegelmilch, K.; Keller, A.; Zehe, V.; Hondke, S.; Schilling, T.; Jakob, F.; Klein-Hitpass, L.; Schutze, N. WISP 1 is an important survival factor in human mesenchymal stromal cells. Gene 2014, 551, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, B. MiR-128-3p Post-Transcriptionally Inhibits WISP1 to Suppress Apoptosis and Inflammation in Human Articular Chondrocytes via the PI3K/AKT/NF-kappaB Signaling Pathway. Cell Transplant. 2020, 29, 963689720939131. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Li, P.; Li, L. A novel adipokine WISP1 attenuates lipopolysaccharide-induced cell injury in 3T3-L1 adipocytes by regulating the PI3K/Akt pathway. Obes. Res. Clin. Pract. 2022, 16, 122–129. [Google Scholar] [CrossRef]
- Arunachalam, G.; Samuel, S.M.; Marei, I.; Ding, H.; Triggle, C.R. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014, 171, 523–535. [Google Scholar] [CrossRef]
- Maiese, K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr. Neurovasc. Res. 2014, 11, 378–389. [Google Scholar] [CrossRef]
- Lai, C.S.; Tsai, M.L.; Badmaev, V.; Jimenez, M.; Ho, C.T.; Pan, M.H. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agric. Food Chem. 2012, 60, 1094–1101. [Google Scholar] [CrossRef]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr. Neurovasc. Res. 2013, 10, 54–69. [Google Scholar] [CrossRef]
- Blom, A.B.; Brockbank, S.M.; van Lent, P.L.; van Beuningen, H.M.; Geurts, J.; Takahashi, N.; van der Kraan, P.M.; van de Loo, F.A.; Schreurs, B.W.; Clements, K.; et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: Prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009, 60, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Lawson, W.E.; Blackwell, T.S. beta-Catenin and CCNs in lung epithelial repair. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L579–L581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Jia, S.; Qu, T.; Feng, M.; Ji, K.; Li, Z.; Jiang, W.; Ji, J. Association of Wnt1-inducible signaling pathway protein-1 with the proliferation, migration and invasion in gastric cancer cells. Tumour Biol. 2017, 39, 1010428317699755. [Google Scholar] [CrossRef]
- Nivison, M.P.; Meier, K.E. The role of CCN4/WISP-1 in the cancerous phenotype. Cancer Manag. Res. 2018, 10, 2893–2903. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, K.; Kim, K.; Lee, Y.J.; Lee, S.; Ahn, S.Y.; Ahn, Y.H.; Kang, J.L. Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via Notch1-WISP-1 signaling. Cell. Mol. Immunol. 2022, 19, 1373–1391. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Wang, Y.; Zou, T.; Li, X.P.; Cao, L.; Chen, J. The Effects of WISP1 Polymorphisms on the Prognosis of Lung Cancer Patients with Platinum-Based Chemotherapy. Pharmgenomics Pers. Med. 2021, 14, 1193–1203. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Luo, M.; Wei, J.; Liu, X. Distinct Roles of Wnt/beta-Catenin Signaling in the Pathogenesis of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Mediators Inflamm. 2017, 2017, 3520581. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, T.; Wu, Y.; Yuan, Z.; Dong, J.; Li, X.; An, J.; Liao, Z.; Zhang, X.; Xu, D.; et al. WNT/beta-catenin signaling regulates cigarette smoke-induced airway inflammation via the PPARdelta/p38 pathway. Lab. Invest. 2016, 96, 218–229. [Google Scholar] [CrossRef]
- Gueugnon, F.; Thibault, V.C.; Kearley, J.; Petit-Courty, A.; Vallet, A.; Guillon, A.; Si-Tahar, M.; Humbles, A.A.; Courty, Y. Altered expression of the CCN genes in the lungs of mice in response to cigarette smoke exposure and viral and bacterial infections. Gene 2016, 586, 176–183. [Google Scholar] [CrossRef]
- Grieb, G.; Merk, M.; Bernhagen, J.; Bucala, R. Macrophage migration inhibitory factor (MIF): A promising biomarker. Drug News Perspect. 2010, 23, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Leyton-Jaimes, M.F.; Kahn, J.; Israelson, A. Macrophage migration inhibitory factor: A multifaceted cytokine implicated in multiple neurological diseases. Exp. Neurol. 2018, 301, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Benedek, G.; Bucala, R.; Matejuk, S.; Offner, H.; Vandenbark, A.A. MIF contribution to progressive brain diseases. J. Neuroinflammation 2024, 21, 8. [Google Scholar] [CrossRef]
- Basile, M.S.; Battaglia, G.; Bruno, V.; Mangano, K.; Fagone, P.; Petralia, M.C.; Nicoletti, F.; Cavalli, E. The Dichotomic Role of Macrophage Migration Inhibitory Factor in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3023. [Google Scholar] [CrossRef]
- Adamali, H.; Armstrong, M.E.; McLaughlin, A.M.; Cooke, G.; McKone, E.; Costello, C.M.; Gallagher, C.G.; Leng, L.; Baugh, J.A.; Fingerle-Rowson, G.; et al. Macrophage migration inhibitory factor enzymatic activity, lung inflammation, and cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 162–169. [Google Scholar] [CrossRef]
- Calandra, T.; Spiegel, L.A.; Metz, C.N.; Bucala, R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 1998, 95, 11383–11388. [Google Scholar] [CrossRef]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by endothelial cells-partnering up with the immune system? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Calandra, T.; Bucala, R. Regulation of the immune response by macrophage migration inhibitory factor: Biological and structural features. J. Mol. Med. 1998, 76, 151–161. [Google Scholar] [CrossRef]
- Karsten, E.; Hill, C.J.; Herbert, B.R. Red blood cells: The primary reservoir of macrophage migration inhibitory factor in whole blood. Cytokine 2018, 102, 34–40. [Google Scholar] [CrossRef]
- Voss, S.; Kruger, S.; Scherschel, K.; Warnke, S.; Schwarzl, M.; Schrage, B.; Girdauskas, E.; Meyer, C.; Blankenberg, S.; Westermann, D.; et al. Macrophage Migration Inhibitory Factor (MIF) Expression Increases during Myocardial Infarction and Supports Pro-Inflammatory Signaling in Cardiac Fibroblasts. Biomolecules 2019, 9, 38. [Google Scholar] [CrossRef]
- Maricchiolo, E.; Panfili, E.; Pompa, A.; De Marchis, F.; Bellucci, M.; Pallotta, M.T. Unconventional Pathways of Protein Secretion: Mammals vs. Plants. Front. Cell Dev. Biol. 2022, 10, 895853. [Google Scholar] [CrossRef] [PubMed]
- Ndreu, L.; Sasse, S.; Karlberg, A.T.; Karlsson, I. Haptenation of Macrophage Migration Inhibitory Factor: A Potential Biomarker for Contact Hypersensitivity. Front. Toxicol. 2022, 4, 856614. [Google Scholar] [CrossRef] [PubMed]
- Merk, M.; Baugh, J.; Zierow, S.; Leng, L.; Pal, U.; Lee, S.J.; Ebert, A.D.; Mizue, Y.; Trent, J.O.; Mitchell, R.; et al. The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J. Immunol. 2009, 182, 6896–6906. [Google Scholar] [CrossRef] [PubMed]
- Flieger, O.; Engling, A.; Bucala, R.; Lue, H.; Nickel, W.; Bernhagen, J. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett. 2003, 551, 78–86. [Google Scholar] [CrossRef]
- Jia, X.; Xi, J.; Tian, B.; Zhang, Y.; Wang, Z.; Wang, F.; Li, Z.; Long, J.; Wang, J.; Fan, G.H.; et al. The Tautomerase Activity of Tumor Exosomal MIF Promotes Pancreatic Cancer Progression by Modulating MDSC Differentiation. Cancer Immunol. Res. 2024, 12, 72–90. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Leng, L.; Metz, C.N.; Fang, Y.; Xu, J.; Donnelly, S.; Baugh, J.; Delohery, T.; Chen, Y.; Mitchell, R.A.; Bucala, R. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003, 197, 1467–1476. [Google Scholar] [CrossRef]
- Shi, X.; Leng, L.; Wang, T.; Wang, W.; Du, X.; Li, J.; McDonald, C.; Chen, Z.; Murphy, J.W.; Lolis, E.; et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006, 25, 595–606. [Google Scholar] [CrossRef]
- Yoo, S.A.; Leng, L.; Kim, B.J.; Du, X.; Tilstam, P.V.; Kim, K.H.; Kong, J.S.; Yoon, H.J.; Liu, A.; Wang, T.; et al. MIF allele-dependent regulation of the MIF coreceptor CD44 and role in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2016, 113, E7917–E7926. [Google Scholar] [CrossRef]
- Gore, Y.; Starlets, D.; Maharshak, N.; Becker-Herman, S.; Kaneyuki, U.; Leng, L.; Bucala, R.; Shachar, I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J. Biol. Chem. 2008, 283, 2784–2792. [Google Scholar] [CrossRef]
- Zernecke, A.; Bernhagen, J.; Weber, C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation 2008, 117, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Fingerle-Rowson, G.; Petrenko, O.; Metz, C.N.; Forsthuber, T.G.; Mitchell, R.; Huss, R.; Moll, U.; Muller, W.; Bucala, R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc. Natl. Acad. Sci. USA 2003, 100, 9354–9359. [Google Scholar] [CrossRef] [PubMed]
- Becker-Herman, S.; Arie, G.; Medvedovsky, H.; Kerem, A.; Shachar, I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol. Biol. Cell 2005, 16, 5061–5069. [Google Scholar] [CrossRef] [PubMed]
- Beisner, D.R.; Langerak, P.; Parker, A.E.; Dahlberg, C.; Otero, F.J.; Sutton, S.E.; Poirot, L.; Barnes, W.; Young, M.A.; Niessen, S.; et al. The intramembrane protease Sppl2a is required for B cell and DC development and survival via cleavage of the invariant chain. J. Exp. Med. 2013, 210, 23–30. [Google Scholar] [CrossRef]
- Schneppenheim, J.; Dressel, R.; Huttl, S.; Lullmann-Rauch, R.; Engelke, M.; Dittmann, K.; Wienands, J.; Eskelinen, E.L.; Hermans-Borgmeyer, I.; Fluhrer, R.; et al. The intramembrane protease SPPL2a promotes B cell development and controls endosomal traffic by cleavage of the invariant chain. J. Exp. Med. 2013, 210, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Gil-Yarom, N.; Radomir, L.; Sever, L.; Kramer, M.P.; Lewinsky, H.; Bornstein, C.; Blecher-Gonen, R.; Barnett-Itzhaki, Z.; Mirkin, V.; Friedlander, G.; et al. CD74 is a novel transcription regulator. Proc. Natl. Acad. Sci. USA 2017, 114, 562–567. [Google Scholar] [CrossRef]
- Skandalis, S.S. CD44 Intracellular Domain: A Long Tale of a Short Tail. Cancers 2023, 15, 5041. [Google Scholar] [CrossRef]
- Lang, T.; Lee, J.P.W.; Elgass, K.; Pinar, A.A.; Tate, M.D.; Aitken, E.H.; Fan, H.; Creed, S.J.; Deen, N.S.; Traore, D.A.K.; et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat. Commun. 2018, 9, 2223. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Shin, M.S.; Kang, Y.; Wahl, E.R.; Park, H.J.; Lazova, R.; Leng, L.; Mamula, M.; Krishnaswamy, S.; Bucala, R.; Kang, I. Macrophage Migration Inhibitory Factor Regulates U1 Small Nuclear RNP Immune Complex-Mediated Activation of the NLRP3 Inflammasome. Arthritis Rheumatol. 2019, 71, 109–120. [Google Scholar] [CrossRef]
- Harris, J.; Borg, N.A. The multifaceted roles of NLRP3-modulating proteins in virus infection. Front. Immunol. 2022, 13, 987453. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, W.S.; Kim, D.O.; Byun, J.E.; Huy, H.; Lee, S.Y.; Song, H.Y.; Park, Y.J.; Kim, T.D.; Yoon, S.R.; et al. Macrophage migration inhibitory factor interacts with thioredoxin-interacting protein and induces NF-kappaB activity. Cell Signal 2017, 34, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Alibashe-Ahmed, M.; Roger, T.; Serre-Beinier, V.; Berishvili, E.; Reith, W.; Bosco, D.; Berney, T. Macrophage migration inhibitory factor regulates TLR4 expression and modulates TCR/CD3-mediated activation in CD4+ T lymphocytes. Sci. Rep. 2019, 9, 9380. [Google Scholar] [CrossRef]
- Harris, J.; VanPatten, S.; Deen, N.S.; Al-Abed, Y.; Morand, E.F. Rediscovering MIF: New Tricks for an Old Cytokine. Trends Immunol. 2019, 40, 447–462. [Google Scholar] [CrossRef]
- Filip, A.M.; Klug, J.; Cayli, S.; Frohlich, S.; Henke, T.; Lacher, P.; Eickhoff, R.; Bulau, P.; Linder, M.; Carlsson-Skwirut, C.; et al. Ribosomal protein S19 interacts with macrophage migration inhibitory factor and attenuates its pro-inflammatory function. J. Biol. Chem. 2009, 284, 7977–7985. [Google Scholar] [CrossRef]
- Kleemann, R.; Hausser, A.; Geiger, G.; Mischke, R.; Burger-Kentischer, A.; Flieger, O.; Johannes, F.J.; Roger, T.; Calandra, T.; Kapurniotu, A.; et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 2000, 408, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Chamovitz, D.A.; Segal, D. JAB1/CSN5 and the COP9 signalosome. A complex situation. EMBO Rep. 2001, 2, 96–101. [Google Scholar] [CrossRef]
- Song, S.; Xiao, Z.; Dekker, F.J.; Poelarends, G.J.; Melgert, B.N. Macrophage migration inhibitory factor family proteins are multitasking cytokines in tissue injury. Cell Mol. Life Sci. 2022, 79, 105. [Google Scholar] [CrossRef]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Groning, S.; Schmitz, C.; Zierow, S.; Drucker, N.; Bakou, M.; Kohl, K.; Mertens, A.; Lue, H.; Weber, C.; et al. Macrophage Migration Inhibitory Factor-CXCR4 Receptor Interactions: Evidence for Partial Allosteric Agonism In Comparison with CXCL12 Chemokine. J. Biol. Chem. 2016, 291, 15881–15895. [Google Scholar] [CrossRef]
- Lourenco, S.; Teixeira, V.H.; Kalber, T.; Jose, R.J.; Floto, R.A.; Janes, S.M. Macrophage Migration Inhibitory Factor–CXCR4 Is the Dominant Chemotactic Axis in Human Mesenchymal Stem Cell Recruitment to Tumors. J. Immunol. 2015, 194, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Fingerle-Rowson, G.; Koch, P.; Bikoff, R.; Lin, X.; Metz, C.N.; Dhabhar, F.S.; Meinhardt, A.; Bucala, R. Regulation of macrophage migration inhibitory factor expression by glucocorticoids in vivo. Am. J. Pathol. 2003, 162, 47–56. [Google Scholar] [CrossRef]
- Kong, Y.Z.; Chen, Q.; Lan, H.Y. Macrophage Migration Inhibitory Factor (MIF) as a Stress Molecule in Renal Inflammation. Int. J. Mol. Sci. 2022, 23, 4908. [Google Scholar] [CrossRef]
- Breidung, D.; Megas, I.F.; Freytag, D.L.; Bernhagen, J.; Grieb, G. The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective. Biomedicines 2023, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Calandra, T.; Bucala, R.; Meylan, P.R. Macrophage migration inhibitory factor reduces the growth of virulent Mycobacterium tuberculosis in human macrophages. Infect. Immun. 2005, 73, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Roger, T.; Glauser, M.P.; Calandra, T. Macrophage migration inhibitory factor (MIF) modulates innate immune responses induced by endotoxin and Gram-negative bacteria. J. Endotoxin Res. 2001, 7, 456–460. [Google Scholar] [CrossRef]
- Ghosh, S.; Jiang, N.; Farr, L.; Ngobeni, R.; Moonah, S. Parasite-Produced MIF Cytokine: Role in Immune Evasion, Invasion, and Pathogenesis. Front. Immunol. 2019, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Farr, L.; Ghosh, S.; Moonah, S. Role of MIF Cytokine/CD74 Receptor Pathway in Protecting Against Injury and Promoting Repair. Front. Immunol. 2020, 11, 1273. [Google Scholar] [CrossRef]
- Bucala, R.; Donnelly, S.C. Macrophage migration inhibitory factor: A probable link between inflammation and cancer. Immunity 2007, 26, 281–285. [Google Scholar] [CrossRef]
- Smith, C.A.; Tyrell, D.J.; Kulkarni, U.A.; Wood, S.; Leng, L.; Zemans, R.L.; Bucala, R.; Goldstein, D.R. Macrophage migration inhibitory factor enhances influenza-associated mortality in mice. JCI Insight 2019, 4, e128034. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Leopold, S.J.; Cranendonk, D.R.; van der Poll, T. Host innate immune responses to sepsis. Virulence 2014, 5, 36–44. [Google Scholar] [CrossRef]
- Tilstam, P.V.; Schulte, W.; Holowka, T.; Kim, B.S.; Nouws, J.; Sauler, M.; Piecychna, M.; Pantouris, G.; Lolis, E.; Leng, L.; et al. MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model. J. Clin. Invest. 2021, 131, e127171. [Google Scholar] [CrossRef]
- Hoffmann, A.; Bernhagen, J. Revisiting the secretion mechanism(s) of macrophage migration inhibitory factor-welcome to the "UPS club". Immunol. Cell Biol. 2020, 98, 704–708. [Google Scholar] [CrossRef]
- Dankers, W.; Hasnat, M.A.; Swann, V.; Alharbi, A.; Lee, J.P.; Cristofaro, M.A.; Gantier, M.P.; Jones, S.A.; Morand, E.F.; Flynn, J.K.; et al. Necrotic cell death increases the release of macrophage migration inhibitory factor by monocytes/macrophages. Immunol. Cell Biol. 2020, 98, 782–790. [Google Scholar] [CrossRef]
- Bloom, B.R.; Bennett, B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 1966, 153, 80–82. [Google Scholar] [CrossRef] [PubMed]
- David, J.R. Delayed hypersensitivity in vitro: Its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 1966, 56, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Bacher, M.; Metz, C.N.; Calandra, T.; Mayer, K.; Chesney, J.; Lohoff, M.; Gemsa, D.; Donnelly, T.; Bucala, R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 1996, 93, 7849–7854. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.C.; Haslett, C.; Reid, P.T.; Grant, I.S.; Wallace, W.A.; Metz, C.N.; Bruce, L.J.; Bucala, R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat. Med. 1997, 3, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Bernhagen, J.; Mitchell, R.A.; Bucala, R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 1994, 179, 1895–1902. [Google Scholar] [CrossRef]
- Calandra, T.; Bucala, R. Macrophage migration inhibitory factor: A counter-regulator of glucocorticoid action and critical mediator of septic shock. J. Inflamm. 1995, 47, 39–51. [Google Scholar]
- Makita, H.; Nishimura, M.; Miyamoto, K.; Nakano, T.; Tanino, Y.; Hirokawa, J.; Nishihira, J.; Kawakami, Y. Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharide-induced pulmonary neutrophil accumulation. Am. J. Respir. Crit. Care Med. 1998, 158, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Allam, V.; Pavlidis, S.; Liu, G.; Kermani, N.Z.; Simpson, J.; To, J.; Donnelly, S.; Guo, Y.K.; Hansbro, P.M.; Phipps, S.; et al. Macrophage migration inhibitory factor promotes glucocorticoid resistance of neutrophilic inflammation in a murine model of severe asthma. Thorax 2023, 78, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Cutrullis, R.A.; Petray, P.B.; Corral, R.S. MIF-driven activation of macrophages induces killing of intracellular Trypanosoma cruzi dependent on endogenous production of tumor necrosis factor, nitric oxide and reactive oxygen species. Immunobiology 2017, 222, 423–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Chen, S.; Hu, Y.; Zhu, Z.; Wang, Y.; Du, N.; Song, T.; Yang, Y.; Guo, A.; et al. Macrophage migration inhibitory factor facilitates prostaglandin E(2) production of astrocytes to tune inflammatory milieu following spinal cord injury. J. Neuroinflammation 2019, 16, 85. [Google Scholar] [CrossRef]
- Mitchell, R.A.; Metz, C.N.; Peng, T.; Bucala, R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem. 1999, 274, 18100–18106. [Google Scholar] [CrossRef]
- Stathas, T.; Athanassiou, S.D.; Drakouli, S.; Giannopoulou, E.; Mastronikolis, N.S.; Naxakis, S.; Aletras, A.J. MIF attenuates the suppressive effect of dexamethasone on IL-6 production by nasal polyp. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1455–1466. [Google Scholar]
- Yang, M.; Zhao, X.; Liu, Y.; Tian, Y.; Ran, X.; Jiang, Y. A role for WNT1-inducible signaling protein-1 in airway remodeling in a rat asthma model. Int. Immunopharmacol. 2013, 17, 350–357. [Google Scholar] [CrossRef]
- Jia, X.X.; Zhu, T.T.; Huang, Y.; Zeng, X.X.; Zhang, H.; Zhang, W.X. Wnt/beta-catenin signaling pathway regulates asthma airway remodeling by influencing the expression of c-Myc and cyclin D1 via the p38 MAPK-dependent pathway. Exp. Ther. Med. 2019, 18, 3431–3438. [Google Scholar] [CrossRef]
- Knobloch, J.; Lin, Y.; Konradi, J.; Jungck, D.; Behr, J.; Strauch, J.; Stoelben, E.; Koch, A. Inflammatory responses of airway smooth muscle cells and effects of endothelin receptor antagonism. Am. J. Respir. Cell Mol. Biol. 2013, 49, 114–127. [Google Scholar] [CrossRef]
- Zhang, Q.N.; Xiao, H.; Fang, L.T.; Sun, Q.X.; Li, L.D.; Xu, S.Y.; Li, C.Q. Aerosol inhalation of Mycobacterium vaccae ameliorates airway structural remodeling in chronic asthma mouse model. Exp. Lung Res. 2022, 48, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tantisira, K.; Carey, V.; Murphy, A.J.; Lasky-Su, J.; Celedon, J.C.; Lazarus, R.; Klanderman, B.; Rogers, A.; Soto-Quiros, M.; et al. A role for Wnt signaling genes in the pathogenesis of impaired lung function in asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Luis, A.; Corrales, A.; Acosta-Herrera, M.; Gonzalez-Colino, C.; Cumplido, J.; Martinez-Tadeo, J.; Carracedo, A.; Villar, J.; Carrillo, T.; Pino-Yanes, M.; et al. A pathway-based association study reveals variants from Wnt signalling genes contributing to asthma susceptibility. Clin. Exp. Allergy 2017, 47, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Nishihira, J.; Shimizu, T.; Takahashi, T.; Kitashiro, N.; Hizawa, N.; Kamishima, K.; Kawakami, Y. Macrophage migration inhibitory factor (MIF) in bronchial asthma. Clin. Exp. Allergy 2000, 30, 1244–1249. [Google Scholar] [CrossRef]
- Li, R.; Wang, F.; Wei, J.; Lin, Y.; Tang, G.; Rao, L.; Ma, L.; Xu, Q.; Wu, J.; Lv, Q.; et al. The Role of Macrophage Migration Inhibitory Factor (MIF) in Asthmatic Airway Remodeling. Allergy Asthma Immunol. Res. 2021, 13, 88–105. [Google Scholar] [CrossRef]
- Rossi, A.G.; Haslett, C.; Hirani, N.; Greening, A.P.; Rahman, I.; Metz, C.N.; Bucala, R.; Donnelly, S.C. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J. Clin. Invest. 1998, 101, 2869–2874. [Google Scholar] [CrossRef]
- Mizue, Y.; Ghani, S.; Leng, L.; McDonald, C.; Kong, P.; Baugh, J.; Lane, S.J.; Craft, J.; Nishihira, J.; Donnelly, S.C.; et al. Role for macrophage migration inhibitory factor in asthma. Proc. Natl. Acad. Sci. USA 2005, 102, 14410–14415. [Google Scholar] [CrossRef]
- Magalhaes, E.S.; Mourao-Sa, D.S.; Vieira-de-Abreu, A.; Figueiredo, R.T.; Pires, A.L.; Farias-Filho, F.A.; Fonseca, B.P.; Viola, J.P.; Metz, C.; Martins, M.A.; et al. Macrophage migration inhibitory factor is essential for allergic asthma but not for Th2 differentiation. Eur. J. Immunol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nasuhara, Y.; Kamachi, A.; Tanino, Y.; Betsuyaku, T.; Yamaguchi, E.; Nishihira, J.; Nishimura, M. Role of macrophage migration inhibitory factor in ovalbumin-induced airway inflammation in rats. Eur. Respir. J. 2006, 27, 726–734. [Google Scholar] [CrossRef]
- Chen, P.F.; Luo, Y.L.; Wang, W.; Wang, J.X.; Lai, W.Y.; Hu, S.M.; Cheng, K.F.; Al-Abed, Y. ISO-1, a macrophage migration inhibitory factor antagonist, inhibits airway remodeling in a murine model of chronic asthma. Mol. Med. 2010, 16, 400–408. [Google Scholar] [CrossRef]
- Amano, T.; Nishihira, J.; Miki, I. Blockade of macrophage migration inhibitory factor (MIF) prevents the antigen-induced response in a murine model of allergic airway inflammation. Inflamm. Res. 2007, 56, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Chen, H.; Wang, Y.; Li, D.; Zhang, Q.; Chai, L.; Qiu, Y.; Zhang, J.; Shen, N.; et al. Macrophage migration inhibitory factor exacerbates asthmatic airway remodeling via dynamin-related protein 1-mediated autophagy activation. Respir. Res. 2023, 24, 216. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Tortori, C.A.; Lintomen, L.; Figueiredo, R.T.; Bernardazzi, C.; Leng, L.; Bucala, R.; Madi, K.; Buongusto, F.; Elia, C.C.; et al. Macrophage migration inhibitory factor promotes eosinophil accumulation and tissue remodeling in eosinophilic esophagitis. Mucosal Immunol. 2015, 8, 1154–1165. [Google Scholar] [CrossRef]
- Lan, H.; Wang, N.; Chen, Y.; Wang, X.; Gong, Y.; Qi, X.; Luo, Y.; Yao, F. Macrophage migration inhibitory factor (MIF) promotes rat airway muscle cell proliferation and migration mediated by ERK1/2 and FAK signaling. Cell Biol. Int. 2018, 42, 75–83. [Google Scholar] [CrossRef]
- Lan, H.; Luo, L.; Chen, Y.; Wang, M.; Yu, Z.; Gong, Y. MIF signaling blocking alleviates airway inflammation and airway epithelial barrier disruption in a HDM-induced asthma model. Cell Immunol. 2020, 347, 103965. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, R.; Song, S.; Ma, L.; Xue, H. The Role of MIF-173G/C Gene Polymorphism in the Susceptibility of Autoimmune Diseases. Mediators Inflamm. 2020, 2020, 7825072. [Google Scholar] [CrossRef]
- Wang, F.F.; Huang, X.F.; Shen, N.; Leng, L.; Bucala, R.; Chen, S.L.; Lu, L.J. A genetic role for macrophage migration inhibitory factor (MIF) in adult-onset Still’s disease. Arthritis Res. Ther. 2013, 15, R65. [Google Scholar] [CrossRef]
- Illescas, O.; Gomez-Verjan, J.C.; Garcia-Velazquez, L.; Govezensky, T.; Rodriguez-Sosa, M. Macrophage Migration Inhibitory Factor -173 G/C Polymorphism: A Global Meta-Analysis across the Disease Spectrum. Front. Genet. 2018, 9, 55. [Google Scholar] [CrossRef]
- El-Adly, T.Z.; Kamal, S.; Selim, H.; Botros, S. Association of macrophage migration inhibitory factor promoter polymorphism -173G/C with susceptibility to childhood asthma. Cent. Eur. J. Immunol. 2016, 41, 268–272. [Google Scholar] [CrossRef]
- Wu, J.; Fu, S.; Ren, X.; Jin, Y.; Huang, X.; Zhang, X.; Bai, J.; Fu, S. Association of MIF promoter polymorphisms with childhood asthma in a northeastern Chinese population. Tissue Antigens 2009, 73, 302–306. [Google Scholar] [CrossRef]
- Boucherat, O.; Morissette, M.C.; Provencher, S.; Bonnet, S.; Maltais, F. Bridging Lung Development with Chronic Obstructive Pulmonary Disease. Relevance of Developmental Pathways in Chronic Obstructive Pulmonary Disease Pathogenesis. Am. J. Respir. Crit. Care Med. 2016, 193, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ahmed, J.; Wang, G.; Hassan, I.; Strulovici-Barel, Y.; Hackett, N.R.; Crystal, R.G. Down-regulation of the canonical Wnt beta-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PLoS ONE 2011, 6, e14793. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Mutze, K.; Baarsma, H.A.; Bracke, K.R.; Alsafadi, H.N.; Lehmann, M.; Costa, R.; Stornaiuolo, M.; Novellino, E.; Brusselle, G.G.; et al. Reduced Frizzled Receptor 4 Expression Prevents WNT/beta-Catenin-driven Alveolar Lung Repair in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 172–185. [Google Scholar] [CrossRef]
- Qu, J.; Yue, L.; Gao, J.; Yao, H. Perspectives on Wnt Signal Pathway in the Pathogenesis and Therapeutics of Chronic Obstructive Pulmonary Disease. J. Pharmacol. Exp. Ther. 2019, 369, 473–480. [Google Scholar] [CrossRef]
- Kneidinger, N.; Yildirim, A.O.; Callegari, J.; Takenaka, S.; Stein, M.M.; Dumitrascu, R.; Bohla, A.; Bracke, K.R.; Morty, R.E.; Brusselle, G.G.; et al. Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am. J. Respir. Crit. Care Med. 2011, 183, 723–733. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, Z.; Zhang, P.; Qu, J.; Zheng, C.; Mo, X.; Zhou, W.; Xu, L.; Yao, H.; Gao, J. Nrf2 attenuates inflammatory response in COPD/emphysema: Crosstalk with Wnt3a/beta-catenin and AMPK pathways. J. Cell Mol. Med. 2018, 22, 3514–3525. [Google Scholar] [CrossRef]
- Funato, Y.; Michiue, T.; Asashima, M.; Miki, H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat. Cell Biol. 2006, 8, 501–508. [Google Scholar] [CrossRef]
- Kajla, S.; Mondol, A.S.; Nagasawa, A.; Zhang, Y.; Kato, M.; Matsuno, K.; Yabe-Nishimura, C.; Kamata, T. A crucial role for Nox 1 in redox-dependent regulation of Wnt-beta-catenin signaling. FASEB J. 2012, 26, 2049–2059. [Google Scholar] [CrossRef]
- Rharass, T.; Lemcke, H.; Lantow, M.; Kuznetsov, S.A.; Weiss, D.G.; Panakova, D. Ca2+-mediated mitochondrial reactive oxygen species metabolism augments Wnt/beta-catenin pathway activation to facilitate cell differentiation. J. Biol. Chem. 2014, 289, 27937–27951. [Google Scholar] [CrossRef]
- Thimraj, T.A.; Birru, R.L.; Mitra, A.; Schulz, H.; Leikauf, G.D.; Ganguly, K. Homeobox, Wnt, and Fibroblast Growth Factor Signaling is Augmented During Alveogenesis in Mice Lacking Superoxide Dismutase 3, Extracellular. Lung 2017, 195, 263–270. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Gosens, R.; Konigshoff, M.; Baarsma, H.A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 2018, 187, 150–166. [Google Scholar] [CrossRef]
- Bryja, V.; Schulte, G.; Rawal, N.; Grahn, A.; Arenas, E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J. Cell Sci. 2007, 120, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Carlier, F.M.; Dupasquier, S.; Ambroise, J.; Detry, B.; Lecocq, M.; Bietry-Claudet, C.; Boukala, Y.; Gala, J.L.; Bouzin, C.; Verleden, S.E.; et al. Canonical WNT pathway is activated in the airway epithelium in chronic obstructive pulmonary disease. EBioMedicine 2020, 61, 103034. [Google Scholar] [CrossRef]
- Husebo, G.R.; Bakke, P.S.; Gronseth, R.; Hardie, J.A.; Ueland, T.; Aukrust, P.; Eagan, T.M. Macrophage migration inhibitory factor, a role in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1–L7. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.E.; Chung, K.F.; Clarke, C.J.; Durham, A.L.; Mallia, P.; Footitt, J.; Johnston, S.L.; Barnes, P.J.; Hall, S.R.; Simpson, K.D.; et al. The MIF Antagonist ISO-1 Attenuates Corticosteroid-Insensitive Inflammation and Airways Hyperresponsiveness in an Ozone-Induced Model of COPD. PLoS ONE 2016, 11, e0146102. [Google Scholar] [CrossRef] [PubMed]
- Fallica, J.; Boyer, L.; Kim, B.; Serebreni, L.; Varela, L.; Hamdan, O.; Wang, L.; Simms, T.; Damarla, M.; Kolb, T.M.; et al. Macrophage migration inhibitory factor is a novel determinant of cigarette smoke-induced lung damage. Am. J. Respir. Cell Mol. Biol. 2014, 51, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sauler, M.; Leng, L.; Trentalange, M.; Haslip, M.; Shan, P.; Piecychna, M.; Zhang, Y.; Andrews, N.; Mannam, P.; Allore, H.; et al. Macrophage migration inhibitory factor deficiency in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L487–L496. [Google Scholar] [CrossRef]
- Marsh, L.M.; Cakarova, L.; Kwapiszewska, G.; von Wulffen, W.; Herold, S.; Seeger, W.; Lohmeyer, J. Surface expression of CD74 by type II alveolar epithelial cells: A potential mechanism for macrophage migration inhibitory factor-induced epithelial repair. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L442–L452. [Google Scholar] [CrossRef]
- Sauler, M.; Zhang, Y.; Min, J.N.; Leng, L.; Shan, P.; Roberts, S.; Jorgensen, W.L.; Bucala, R.; Lee, P.J. Endothelial CD74 mediates macrophage migration inhibitory factor protection in hyperoxic lung injury. FASEB J. 2015, 29, 1940–1949. [Google Scholar] [CrossRef]
- Florez-Sampedro, L.; Brandsma, C.-A.; de Vries, M.; Timens, W.; Bults, R.; Vermeulen, C.J.; van den Berge, M.; Obeidat, M.e.; Joubert, P.; Nickle, D.C.; et al. Genetic regulation of gene expression of MIF family members in lung tissue. Sci. Rep. 2020, 10, 16980. [Google Scholar] [CrossRef]
- Zhang, C.; Ramsey, C.; Berical, A.; Yu, L.; Leng, L.; McGinnis, K.A.; Song, Y.; Michael, H.; McCormack, M.C.; Allore, H.; et al. A functional macrophage migration inhibitory factor promoter polymorphism is associated with reduced diffusing capacity. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L400–L405. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; MacIntyre, N.R.; Henderson, R.J.; Jensen, R.L.; Kinney, G.; Stringer, W.W.; Hersh, C.P.; Bowler, R.P.; Casaburi, R.; Han, M.K.; et al. Diffusing Capacity of Carbon Monoxide in Assessment of COPD. Chest 2019, 156, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef]
- Ito, J.T.; Lourenco, J.D.; Righetti, R.F.; Tiberio, I.; Prado, C.M.; Lopes, F. Extracellular Matrix Component Remodeling in Respiratory Diseases: What Has Been Found in Clinical and Experimental Studies? Cells 2019, 8, 342. [Google Scholar] [CrossRef]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Nizamoglu, M.; Fan, Y.; Nemani, S.S.P.; Weckmann, M.; Pouwels, S.D.; Heijink, I.H.; Melgert, B.N.; Pillay, J.; Burgess, J.K. Highway to heal: Influence of altered extracellular matrix on infiltrating immune cells during acute and chronic lung diseases. Front. Pharmacol. 2022, 13, 995051. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.K.; Weiss, D.J.; Westergren-Thorsson, G.; Wigen, J.; Dean, C.H.; Mumby, S.; Bush, A.; Adcock, I.M. Extracellular Matrix as a Driver of Chronic Lung Diseases. Am. J. Respir. Cell Mol. Biol. 2024, 70, 239–246. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Deng, Z.; Fear, M.W.; Suk Choi, Y.; Wood, F.M.; Allahham, A.; Mutsaers, S.E.; Prele, C.M. The extracellular matrix and mechanotransduction in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2020, 126, 105802. [Google Scholar] [CrossRef]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, R.; García-Alamán, A.; Esteban, Y.; Mir-Coll, J.; Serra-Navarro, B.; Fontcuberta-PiSunyer, M.; Broca, C.; Armanet, M.; Wojtusciszyn, A.; Kram, V.; et al. Wisp1 is a circulating factor that stimulates proliferation of adult mouse and human beta cells. Nat. Commun. 2020, 11, 5982. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.C.; Ray, A.; Ag Edgar, P. Biological characteristics of CCN proteins in tumor development. J. BUON 2016, 21, 1359–1367. [Google Scholar]
- Perbal, B. CCN proteins: A centralized communication network. J. Cell Commun. Signal 2013, 7, 169–177. [Google Scholar] [CrossRef]
- Tank, J.; Lindner, D.; Wang, X.; Stroux, A.; Gilke, L.; Gast, M.; Zietsch, C.; Skurk, C.; Scheibenbogen, C.; Klingel, K.; et al. Single-target RNA interference for the blockade of multiple interacting proinflammatory and profibrotic pathways in cardiac fibroblasts. J. Mol. Cell Cardiol. 2014, 66, 141–156. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Targeting disease through novel pathways of apoptosis and autophagy. Expert. Opin. Ther. Targets 2012, 16, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Deng, W.; McLaughlin, S.L.; Pirkey, A.C.; Rellick, S.L.; Razazan, A.; Klinke, D.J., 2nd. Cell Communication Network factor 4 promotes tumor-induced immunosuppression in melanoma. EMBO Rep. 2022, 23, e54127. [Google Scholar] [CrossRef]
- Liao, X.; Bu, Y.; Xu, Z.; Jia, F.; Chang, F.; Liang, J.; Jia, Q.; Lv, Y. WISP1 Predicts Clinical Prognosis and Is Associated With Tumor Purity, Immunocyte Infiltration, and Macrophage M2 Polarization in Pan-Cancer. Front. Genet. 2020, 11, 502. [Google Scholar] [CrossRef]
- Bergholt, N.L.; Demirel, A.; Pedersen, M.; Ding, M.; Kragstrup, T.W.; Andersen, T.; Deleuran, B.W.; Foldager, C.B. Intermittent Hypoxic Therapy Inhibits Allogenic Bone-Graft Resorption by Inhibition of Osteoclastogenesis in a Mouse Model. Int. J. Mol. Sci. 2021, 23, 323. [Google Scholar] [CrossRef]
- Quiros, M.; Nishio, H.; Neumann, P.A.; Siuda, D.; Brazil, J.C.; Azcutia, V.; Hilgarth, R.; O’Leary, M.N.; Garcia-Hernandez, V.; Leoni, G.; et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J. Clin. Invest. 2017, 127, 3510–3520. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal 2019, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/beta-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, R.G.M.; Blom, A.B.; Bloks, N.G.C.; Nelissen, R.; van der Linden, E.; van der Kraan, P.M.; Meulenbelt, I.; Ramos, Y.F.M.; van den Bosch, M.H.J. CCN4/WISP1 Promotes Migration of Human Primary Osteoarthritic Chondrocytes. Cartilage 2023, 14, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.; Blom, A.B.; Kram, V.; Maeda, A.; Sikka, S.; Gabet, Y.; Kilts, T.M.; van den Berg, W.B.; van Lent, P.L.; van der Kraan, P.M.; et al. WISP1/CCN4 aggravates cartilage degeneration in experimental osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1900–1911. [Google Scholar] [CrossRef]
- Hou, C.H.; Tang, C.H.; Hsu, C.J.; Hou, S.M.; Liu, J.F. CCN4 induces IL-6 production through alphavbeta5 receptor, PI3K, Akt, and NF-kappaB singling pathway in human synovial fibroblasts. Arthritis Res. Ther. 2013, 15, R19. [Google Scholar] [CrossRef]
- Cheng, C.; Tian, J.; Zhang, F.; Deng, Z.; Tu, M.; Li, L.; Yang, H.; Xiao, K.; Guo, W.; Yang, R.; et al. WISP1 Protects Against Chondrocyte Senescence and Apoptosis by Regulating alphavbeta3 and PI3K/Akt Pathway in Osteoarthritis. DNA Cell Biol. 2021, 40, 629–637. [Google Scholar] [CrossRef]
- Arenberg, D.; Luckhardt, T.R.; Carskadon, S.; Zhao, L.; Amin, M.A.; Koch, A.E. Macrophage migration inhibitory factor promotes tumor growth in the context of lung injury and repair. Am. J. Respir. Crit. Care Med. 2010, 182, 1030–1037. [Google Scholar] [CrossRef]
- Thiele, M.; Donnelly, S.C.; Mitchell, R.A. OxMIF: A druggable isoform of macrophage migration inhibitory factor in cancer and inflammatory diseases. J. Immunother. Cancer 2022, 10, e005475. [Google Scholar] [CrossRef] [PubMed]
- Baugh, J.A.; Donnelly, S.C. Macrophage migration inhibitory factor: A neuroendocrine modulator of chronic inflammation. J. Endocrinol. 2003, 179, 15–23. [Google Scholar] [CrossRef]
- Zhang, P.L.; Liu, J.; Xu, L.; Sun, Y.; Sun, X.C. Synovial Fluid Macrophage Migration Inhibitory Factor Levels Correlate with Severity of Self-Reported Pain in Knee Osteoarthritis Patients. Med. Sci. Monit. 2016, 22, 2182–2186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.-R.; Kim, K.-W.; Jung, H.G.; Yoon, K.-S.; Oh, H.-J.; Cho, M.-L.; Lee, S.-H. Macrophage migration inhibitory factor enhances osteoclastogenesis through upregulation of RANKL expression from fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R43. [Google Scholar] [CrossRef] [PubMed]
- Onodera, S.; Nishihira, J.; Iwabuchi, K.; Koyama, Y.; Yoshida, K.; Tanaka, S.; Minami, A. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. J. Biol. Chem. 2002, 277, 7865–7874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, S.; Fan, S.; Xu, L.; Jiang, X.; Wang, K.; Cai, B. Macrophage migration inhibitory factor activates the inflammatory response in joint capsule fibroblasts following post-traumatic joint contracture. Aging 2021, 13, 5804–5823. [Google Scholar] [CrossRef]
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert. Opin. Ther. Targets 2019, 23, 733–744. [Google Scholar] [CrossRef]
- Oliver, J.; Marquez, A.; Gomez-Garcia, M.; Martinez, A.; Mendoza, J.L.; Vilchez, J.R.; Lopez-Nevot, M.A.; Pinero, A.; de la Concha, E.G.; Nieto, A.; et al. Association of the macrophage migration inhibitory factor gene polymorphisms with inflammatory bowel disease. Gut 2007, 56, 150–151. [Google Scholar] [CrossRef]
- Tilstam, P.V.; Qi, D.; Leng, L.; Young, L.; Bucala, R. MIF family cytokines in cardiovascular diseases and prospects for precision-based therapeutics. Expert. Opin. Ther. Targets 2017, 21, 671–683. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef]
- Koga, K.; Kenessey, A.; Powell, S.R.; Sison, C.P.; Miller, E.J.; Ojamaa, K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid. Redox Signal 2011, 14, 1191–1202. [Google Scholar] [CrossRef]
- Qi, D.; Hu, X.; Wu, X.; Merk, M.; Leng, L.; Bucala, R.; Young, L.H. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J. Clin. Invest. 2009, 119, 3807–3816. [Google Scholar] [CrossRef]
- Kleemann, R.; Kapurniotu, A.; Frank, R.W.; Gessner, A.; Mischke, R.; Flieger, O.; Juttner, S.; Brunner, H.; Bernhagen, J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J. Mol. Biol. 1998, 280, 85–102. [Google Scholar] [CrossRef] [PubMed]
- de Llano, L.P.; Cosio, B.G.; Iglesias, A.; de Las Cuevas, N.; Soler-Cataluna, J.J.; Izquierdo, J.L.; Lopez-Campos, J.L.; Calero, C.; Plaza, V.; Miravitlles, M.; et al. Mixed Th2 and non-Th2 inflammatory pattern in the asthma-COPD overlap: A network approach. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 591–601. [Google Scholar] [CrossRef] [PubMed]
| Disease | Roles of WISP1 | References |
|---|---|---|
| Asthma | Induces airway remodeling, promotes pro-inflammatory factors, facilitates fibroblast migration, and causes hypertrophy and hyperplasia of airway smooth muscle cells | [76,132] |
| Pulmonary Fibrosis | Promotes fibroblast activation and myofibroblast differentiation, leading to excessive fibrosis | [61,104,106] |
| Lung Cancer | Enhances cell proliferation and survival and influences the tumor microenvironment | [145,146,147] |
| COPD | Contributes to inflammation and airway remodeling | [148,149,150] |
| Acute Lung Injury/ARDS | Exacerbates inflammatory responses and abnormal tissue repair | [85,113] |
| Action | WISP1 | MIF | Common Actions | References | |
|---|---|---|---|---|---|
| Role in Airway Remodeling | Asthma | Promotes ASM hypertrophy and proliferation via the PI3K/Akt/GSK-3β pathway, leading to collagen deposition and ECM remodeling. Stimulates fibroblast proliferation and ECM component production (e.g., Col1alpha1, FN1). | Promotes ASM proliferation and eosinophil migration via ERK1/2/Drp1 axis, leading to ECM deposition and airway remodeling. Enhances TGF-β1 accumulation and collagen deposition. | Both WISP1 and MIF enhance airway remodeling by promoting ASM proliferation and ECM deposition through distinct pathways: PI3K/Akt (WISP1) and ERK1/2 (MIF). | [76,216,225,231,234,235] |
| COPD | Promotes ASM hypertrophy and ECM remodeling, exacerbated by oxidative stress and impaired epithelial repair, leading to chronic inflammation. | Promotes ASM proliferation and ECM deposition, exacerbated by oxidative stress and cigarette smoke exposure, contributing to chronic inflammation. | [45,254,255,257] | ||
| Interaction with TGF-β Signaling | Asthma | Upregulated by TGF-β through the PI3K/Akt/GSK-3β pathway, enhancing remodeling processes. Promotes sustained expression and remodeling effects through non-canonical TGF-β signaling. | Promotes TGF-β signaling by enhancing autophagy and collagen deposition via ERK1/2. MIF inhibition reduces TGF-β1 accumulation. | Both WISP1 and MIF modulate TGF-β signaling pathways, enhancing airway remodeling. WISP1 acts through PI3K/Akt, while MIF acts through ERK1/2. | [76,225] |
| COPD | Upregulated by TGF-β through the PI3K/Akt/GSK-3β pathway, contributing to sustained remodeling and inflammation. | Enhances TGF-β signaling, leading to increased collagen deposition and inflammation. MIF inhibition reduces TGF-β1 levels. | |||
| Impact on Inflammation | Asthma | Increased by TNF-α through WNT/β-catenin signaling, linking to chronic inflammation and remodeling in asthma. | Enhances inflammation by promoting ASMC proliferation and eosinophil migration via ERK1/2 and NF-κB pathways. Contributes to glucocorticoid resistance. Involved in Th2-related inflammation, promoting eosinophil migration. Also linked to glucocorticoid resistance in severe neutrophilic asthma by reducing annexin-A1 activity and promoting NLRP3 inflammasome activation. | Both WISP1 and MIF are involved in pro-inflammatory signaling, contributing to chronic inflammation. | [42,165,204,213,215,216,225,231,232,233] |
| COPD | Increased by TNF-α and exacerbated by oxidative stress, contributing to chronic inflammation and impaired repair. | Increases inflammation by promoting ASMC proliferation and neutrophil accumulation. Contributes to glucocorticoid resistance and NLRP3 inflammasome activation. | |||
| Effect on Glucocorticoid Resistance | Asthma | Not directly associated with glucocorticoid resistance; may indirectly influence steroid responsiveness through its effects on inflammation and remodeling. | Promotes glucocorticoid resistance in severe neutrophilic asthma by inhibiting annexin-A1, enhancing NLRP3 inflammasome activation, and reducing the effectiveness of glucocorticoids. | While WISP1 does not directly induce glucocorticoid resistance, MIF’s role suggests that targeting both could improve glucocorticoid responsiveness by reducing inflammation and remodeling. | [208,211,223,224,225,226] |
| COPD | May indirectly influence glucocorticoid resistance through its effects on remodeling and inflammation. | Induces glucocorticoid resistance by inhibiting annexin-A1, increasing neutrophil accumulation and inflammation. | |||
| Response to Environmental Factors | Asthma | Modulated by cigarette smoke and oxidative stress, impacting WNT signaling and epithelial repair. | Elevated by cigarette smoke exposure, contributing to inflammation and disease progression. | Both WISP1 and MIF respond to environmental factors such as cigarette smoke, exacerbating inflammation and remodeling. | [25,230,231,232,234,235,242,243,244,245] |
| COPD | Modulated by environmental factors such as oxidative stress and cigarette smoke, affecting inflammation and repair. | Elevated in response to cigarette smoke, influencing inflammation and disease severity in COPD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, M.-E.; Aletras, A.J.; Papakonstantinou, E.; Stolz, D.; Skandalis, S.S. WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD. Int. J. Mol. Sci. 2024, 25, 10049. https://doi.org/10.3390/ijms251810049
Christopoulou M-E, Aletras AJ, Papakonstantinou E, Stolz D, Skandalis SS. WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD. International Journal of Molecular Sciences. 2024; 25(18):10049. https://doi.org/10.3390/ijms251810049
Chicago/Turabian StyleChristopoulou, Maria-Elpida, Alexios J. Aletras, Eleni Papakonstantinou, Daiana Stolz, and Spyros S. Skandalis. 2024. "WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD" International Journal of Molecular Sciences 25, no. 18: 10049. https://doi.org/10.3390/ijms251810049
APA StyleChristopoulou, M.-E., Aletras, A. J., Papakonstantinou, E., Stolz, D., & Skandalis, S. S. (2024). WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD. International Journal of Molecular Sciences, 25(18), 10049. https://doi.org/10.3390/ijms251810049







