Abstract
The first example of sonodynamic therapy (SDT) with a cyanine dye–antibody conjugate is reported. The aim of this study was to evaluate the sonodynamic efficacy of a trastuzumab-guided diiodinated heptamethine cyanine-based sensitizer, 2ICy7–Ab, versus its non-iodinated counterpart, Cy7–Ab, in a human epidermal growth factor receptor 2-positive (HER2+) xenograft model. In addition, the combined sonodynamic and photodynamic (PDT) effects were investigated. A single intravenous injection of 2ICy7–Ab followed by sonication or combined sonication and photoirradiation in mice resulted in complete tumor growth suppression compared with the nontreated control and showed no detectable toxicity to off-target tissues. In contrast, Cy7–Ab provided only a moderate therapeutic effect (~1.4–1.6-fold suppression). SDT with 2ICy7–Ab resulted in a 3.5-fold reduction in tumor volume within 45 days and exhibited 13-fold greater tumor suppression than PDT alone. In addition, 2ICy7–Ab showed more durable sonostability than photostability. The sonotoxicity of the iodinated versus noniodinated counterparts is attributed to the increased generation of hydroxyl radicals, superoxide, and singlet oxygen. We observed no significant contribution of PDT to the efficacy of the combined SDT and PDT, indicating that SDT with 2ICy7–Ab is superior to PDT alone. These new findings set the stage for the application of cyanine–antibody conjugates for fluorescently monitored targeted sonodynamic treatment of cancer.
1. Introduction
Sonodynamic (SDT) and photodynamic (PDT) therapies are established non-invasive therapeutic modalities [1] for treating cancer [2,3] and various other diseases [4,5,6,7]. Both methods utilize sensitizers, which are, in general, organic dye molecules or nanoparticles [8,9,10] that act as sonosensitizers or photosensitizers, depending on the nature of the excitation energy, ultrasound (US), or light irradiation, respectively. Sensitizers are typically administered intravenously (IV) [11,12] to reach malignant cells and, upon US or light irradiation, produce cytotoxic species, such as reactive oxygen species (ROS) [8,13], that kill those cells. The mechanism underlying SDT and a comparison with PDT have been described in detail elsewhere [13]. Despite the generally similar principles of action (excitation of sensitizer followed by the generation of cytotoxic species) [13], ultrasound penetrates much deeper into the body (20–30 cm) [13,14,15,16] than NIR light (~2 cm) [17,18], making SDT more effective than PDT, particularly for primary tumors and metastatic cancers that are located deep within the body [11,12,19]. However, SDT is considerably less developed than PDT, and only a few photosensitizers, specifically porphyrins [10,20,21,22], phthalocyanines [23,24,25], xanthenes [4,26,27,28,29], and only two cyanines (indocyanine green, ICG [30,31,32], and IR780 [33,34]), have been successfully employed for treatment with ultrasound excitation [35,36,37].
Importantly, it is not evident that a dye molecule acting as a photosensitizer can be used effectively as a sonosensitizer. This can be attributed to the lack of dye stability under sonication or the insufficient quantum yield of reactive species production upon sonication compared with light [38].
A major limitation for the therapeutic utility of cyanine-based sono- and photosensitizers is the low yield of generated reactive species and, as a result, insufficient treatment efficacy. The incorporation of heavy atoms, such as iodine, bromine, sulfur, and selenium, was recently proven to enhance the PDT efficiency of cyanines due to increased spin-orbit coupling and, therefore, intersystem crossing from the singlet to the triplet state, which results in elevated rates of cytotoxic species generation [39,40]. Thus, the introduction of iodine atoms causes a noticeable increase in the ability of cyanine dyes to photokill cancer cells [41,42,43], as well as Gram-positive and Gram-negative pathogenic bacteria [44,45,46,47].
However, currently available sonosensitizers, including cyanines, suffer from the same limitation as photosensitizers: their targeting specificity to malignant cells is insufficient, leading to side effects, which hinders their prevalent clinical use. This issue can be resolved by equipping the sensitizer with target-specific carriers, such as antibodies [32,48,49,50,51,52]. Despite pronounced progress in the development and application of cyanine dyes in PDT that has been achieved in recent years [53], the potential of cyanines for use in SDT has still been poorly explored, whereas cyanine–antibody conjugates for SDT applications have yet to be investigated.
Recently, we developed an antibody-guided diiodinated heptamethine cyanine-based 2ICy7–Ab conjugate, where the antibody (Ab) is trastuzumab, and demonstrated the high efficiency of this conjugate in the PDT of human epidermal growth factor receptor 2-positive (HER2+) human breast cancer in a xenograft mouse model [41]. In this work, we explored the ability of this 2ICy7–Ab conjugate for SDT and combined PDT + SDT to suppress tumor growth, and we compared the performance of this conjugate with its non-iodinated Cy7–Ab counterpart.
 |
2. Results and Discussion
2.1. Photostability and Sonostability
Organic dyes can degrade upon light and ultrasound irradiation [54,55,56], which can reduce their photo- and sonodynamic efficacy. Therefore, we evaluated the photo- and sonostability of the iodinated vs. non-iodinated dyes and their Ab conjugates. To this end, we irradiated solutions of these compounds in phosphate buffer saline (PBS) (pH 7.4), measured the time-dependent absorption and emission spectra of the solutions vs. the applied light (Figure S1) and ultrasound (US, Figure S2) doses, and plotted the corresponding decay curves (Figure 1). Then, the stabilities of the dyes and conjugates toward the light and US irradiation were quantified through their degradation half-lives (τ1/2,Light and τ1/2,US; Table 1). On the basis of τ1/2,Light and τ1/2,US, we calculated the irradiation light (D1/2,Light) and US (D1/2,US) doses required for 50% degradation of the dyes and Ab conjugates.
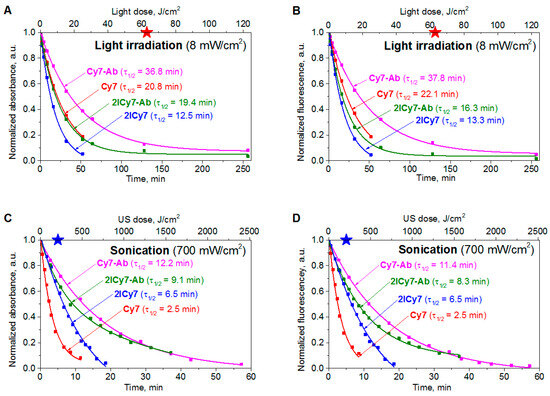
Figure 1.
The normalized absorption (A,C) and fluorescence intensities (B,D) of Cy7, 2ICy7, Cy7–Ab, and 2ICy7–Ab (c = 1 μM) upon light (A,B) and US (C,D) irradiation in 0.1 M PBS, pH 7.4. Light irradiation: 730 nm light-emitting diode (LED), power density 8 mW/cm2. Sonication: frequency (f) 1 MHz, 0.7 W/cm2. The red and blue stars show the irradiation light (63 J/cm2) and US (210 J/cm2) doses applied for PDT and SDT, respectively, in the mouse model.

Table 1.
The degradation half-lives of the dyes and conjugates with different dye-to-antibody ratios (DAR) (c = 1 μM) upon light (τ1/2,Light) and US (τ1/2,US) irradiation, obtained from the absorption/fluorescence (Ab/Fl) intensities, corresponding light (D1/2,Light) and US (D1/2,US) doses, and the quantum yields of photodynamic (ΦΔ,Light) and sonodynamic (φΔ,US) singlet oxygen generation, measured in 0.1 M PBS, pH 7.4.
In our further experiments with mice (see below), we applied a US power density of 0.7 W/cm2 (frequency f = 1 MHz), which is safe for animals [57], and a light irradiance of 8 mW/cm2 (730 nm LED) [41]. These conditions were used to investigate the sono- and photostabilities of the dyes and conjugates. It can be seen from the presented data (Figure 1 and Table 1) that the sonostability of the dyes and conjugates at 0.7 W/cm2 is higher than their photostability at 8 mW/cm2: a much higher (~10–46 time) US dose (D1/2,US) is required for their decomposition compared to the light dose (D1/2,Light). Dye conjugation to an antibody increases photostability and sonostability by approximately 1.2–4.9-fold. Iodination may have different effects: 2ICy7 and 2ICy7–Ab are less photostable than their non-iodinated analogs; the sonostability of 2ICy7–Ab is also lower than that of Cy7–Ab, whereas 2ICy7 is more stable toward sonication than Cy7. As a result, no clear correlation between the sono- and photostability of the investigated compounds was found (Figure S3).
2.2. Singlet Oxygen Generation
Recently, we reported on the quantum yields of singlet oxygen (1O2) generated from Cy7 and 2ICy7 dyes and their Ab conjugates under light irradiation (ΦΔ,Light) [41]. It was found that ΦΔ,Light noticeably (~3-fold) increased upon iodination but decreased 1.7-fold upon conjugation to the Ab (Table 1). The decrease in the ΦΔ,Light of 2ICy7–Ab compared with that of Cy7–Ab was attributed to the aggregation of the iodinated dye on the antibody [41].
In this work, we investigated the efficacy of US-induced 1O2 generation by these sensitizers. Determination of the absolute quantum yields upon US excitation (ΦΔ,US) is problematic [58]. We were also unable to find in the literature any dyes with known ΦΔ,US values to be used as a reference for our measurements. Therefore, the sonodynamic efficacies were estimated in this work through the relative quantum yields (φΔ,US) in comparison with those of Cy7, where φΔ,US of Cy7 was taken as 1 a.u. The φΔ,US measurements were carried out in PBS using the same singlet oxygen sensor green (SOSG)-based method as we applied for the ΦΔ,Light measurements [41], with sonication rather than light excitation being used.
The obtained quantum yields of 1O2 generation (φΔ,US) are shown in Table 1. Upon iodination, φΔ,US increased 1.6-fold for the free dyes and 2.1-fold for the conjugates. Consequently, the magnitudes of these intensifications are less prominent than those upon light excitation (ΦΔ,Light). Additionally, while the ΦΔ,Light of the free dyes decreased 1.7-fold after conjugation, the φΔ,US increased 3.2- and 4.2-fold for Cy7/Cy7–Ab and 2ICy7/2ICy7–Ab, respectively. As a result, while the ΦΔ,Light of 2ICy7–Ab was only 1.8-fold greater than that of Cy7, its φΔ,US was 6.7 times higher. Although the singlet oxygen generation quantum yield does not dramatically increase upon iodination (only 2.1-fold for the conjugate), it contributes to a large increase in SDT efficacy in the mouse model, as demonstrated below.
2.3. Detection and Quantification of Radicals
The ability of 2ICy7 vs. Cy7 to produce free radicals upon sonication and light irradiation and the types of generated reactive species were studied by electron paramagnetic resonance (EPR) spectroscopy (Figure 2, Figures S4 and S5). The measurements were performed under 30 min of light irradiation (730 nm, 30 W LED, 8 mW/cm2) or after 15 min of sonication (40 kHz, 50 W) of 20 μM aqueous dye solutions in the presence of a 25 mM 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) spin trap in double distilled water. The EPR spectra of 2ICy7 (black traces) and Cy7 (red traces) upon light irradiation and sonication are displayed in Figure 2A,B, respectively. The EPR spectrum of the BMPO spin trap is centered at approximately g = 2 and exhibits four lines, which is typical of reactions with hydroxyl radicals (•OH).
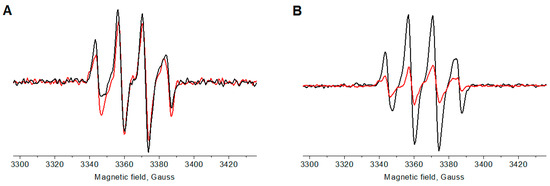
Figure 2.
Electron paramagnetic resonance (EPR) spectra of 2ICy7 (black traces) and Cy7 (red traces) (dye concentration cDye = 20 µM) upon 30 min of light irradiation (A) and after 15 min of sonication (B).
Since the EPR signal intensity is directly related to the dye concentration, Figure 2B shows that, upon sonication, 2ICy7 generates approximately three times more oxygen radicals than Cy7. Under light irradiation, however, both dyes can be seen to produce a similar amount of these reactive species (Figure 2A). Using a calibration curve, we calculated the molar concentrations of the generated radicals (cR) and confirmed that, upon sonication, 2ICy7 indeed produced three times more radicals than Cy7, whereas upon light irradiation, this difference was only approximately 10% (Table 2).

Table 2.
Concentration (cR, μM) and percentage of generated radicals a.
To quantify the generated hydroxyl radicals, the measurements were carried out in the presence of 10% dimethyl sulfoxide (DMSO), which acts as an •OH scavenger. Figures S4 and S5 show a comparison of the EPR spectra recorded with and without DMSO in aqueous solution. It can be seen that the signal intensity decreased in the presence of DMSO but not entirely, indicating that the EPR signal only partially originated from the direct formation of •OH or the spontaneous conversion of BMPO/•OOH (superoxide or hydroperoxyl radicals) to BMPO/•OH (hydroxyl radical) [59]. The data in Table 2 show that upon sonication, 2ICy7 generates approximately 2.7-fold more hydroxyl radicals (72% •OH) than superoxide (28% •O2–),whereas upon light irradiation, it mostly produces superoxide (40% •OH and 60% •O2–). Upon sonication, Cy7 forms ~57% of •OH and ~43% •O2– but approximately 40% •OH and 60% •O2– upon light irradiation.
Importantly, upon sonication and light irradiation, no other radicals except hydroxyl radicals (•OH) and superoxide (•O2–) were identified by EPR spectroscopy, and only singlet oxygen (1O2) was detected among non-radical-based reactive oxygen species by the SOSG-based method (Table 1).
In summary, after sonication, 2ICy7 produces approximately 3.5-fold more radical-based and non-radical-based reactive oxygen species (ROS) than Cy7. Among these ROS, ~1.6-fold greater amounts of 1O2 (Table 1) and ~1.3-fold greater amounts of •OH are generated, but surprisingly ~1.6-fold fewer •O2– radicals are formed (Table 2). Upon light irradiation, 2ICy7 forms only approximately 10% more ROS than Cy7, which consists of ~3-fold increased concentrations of 1O2 but approximately the same amount of •OH and •O2–. Moreover, the relative concentration of radicals and the percentage of light-generated hydroxyl radicals vs. superoxide for 2ICy7 and Cy7 are less pronounced (Table 2). Importantly, hydroxyl radicals are classified as the most active free radicals; they can react directly with biomolecules and are much more cytotoxic than other ROS, particularly superoxide and singlet oxygen [60,61]. Superoxide radicals are less reactive to biological molecules, but they can cause indirect cell damage. Singlet oxygen has a very short diffusion distance, limiting its direct cytotoxicity [61]. Hydroxyl radicals are, therefore, the most important radicals for PDT and SDT applications, although in practice, all these cytotoxic species can work together. We can anticipate, therefore, that 2ICy7 will show increased sonotoxicity in cells compared to Cy7.
2.4. Photothermal and Sonothermal Effects
Cytotoxic effects caused by light or US irradiation can be due not only to the generation of singlet oxygen and/or free radicals, but also to heating. The thermal effect is used in photothermal (PTT) and sonothermal (STT) therapies [62]. We investigated this effect by irradiating 20 µM dye solutions in DMSO, similar to previously reported procedures [63]. The dyes were spectrophotometrically proven to not aggregate at these concentrations. The samples at the same initial temperature (25 °C) were light irradiated (730 nm LED, 40 mW/cm2) or sonicated (1 MHz) at powers of 0.7 W/cm2, 1.2 W/cm2, and 2.0 W/cm2. Consequently, the time-dependent temperature increase for the dye solutions vs. DMSO (without dye) was measured (Figure 3). Both dyes exhibited almost equal photothermal effects (~7 °C), while the solvent itself was heated by only ~2 °C (Figure 3A). The corresponding thermal curves reached saturation after ~10–12 min of irradiation. Moreover, upon sonication at 0.7 W/cm2 and 1.2 W/cm2, no thermal effect was detected (Figure 3B). Only after 10 min of sonication at 2.0 W/cm2 was a temperature increase of approximately 5 °C detected for both dyes compared with the solvent alone. In our further animal experiments, we applied a power of 0.7 W/cm2, at which the sonothermal effect was negligible. In contrast, the light irradiation power in our in vivo PDT experiments was 70 mW/cm2, which was higher than that in the photothermal experiment (40 mW/cm2, Figure 3A); therefore, light irradiation is expected to induce pronounced photothermal cytotoxicity.
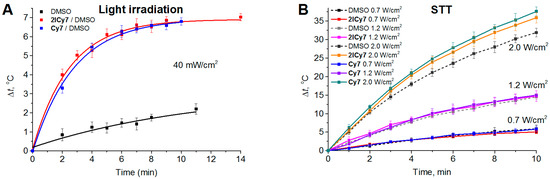
Figure 3.
Temperature increase for the Cy7 and 2ICy7 solutions in DMSO (cDye = 20 µM) vs. dimethyl sulfoxide (DMSO) upon light irradiation (A) and sonication (B). Light irradiation: 730 nm LED, 40 mW/cm2. Sonication: 1 MHz, 0.7 W/cm2, 1.2 W/cm2, and 2.0 W/cm2.
2.5. Comparison of Cytotoxic Factors
The relative contributions of the different cytotoxic effects described above are shown in Figure 4. The presented diagrams demonstrate that 2ICy7, compared to Cy7, more efficiently produces singlet oxygen upon light and US irradiation (Figure 4A,B), but also hydroxyl radicals and superoxide upon sonication (Figure 4C), while the photoactivated generation of hydroxyl radicals and superoxide is approximately equal. Remarkably, both dyes exhibit almost the same photothermal effect (~4.5 °C at ~25 J/cm2), but no reliable temperature increase under sonication even at ~420 J/cm2 (Figure 4D).
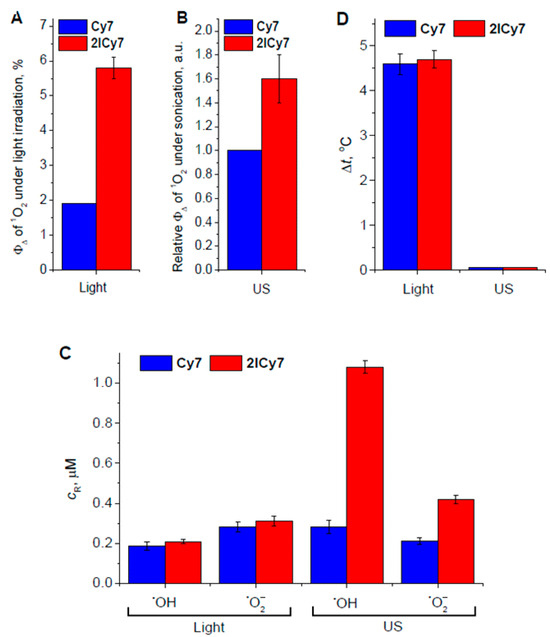
Figure 4.
Quantum yield of singlet oxygen (1O2) upon light irradiation (A) and sonication (B); relative concentrations of hydroxyl radicals (•OH) and superoxide (•O2–) (C); photothermal and sonothermal effects (D). cR is the concentration of free radicals; ΦΔ is the quantum yield of 1O2 generation.
In summary, 2ICy7, compared with Cy7, should have stronger phototoxic and sonotoxic effects because of the more efficient production of singlet oxygen. In addition, the higher sonotoxicity of 2ICy7 vs. Cy7 is due to the more pronounced generation of hydroxyl radicals and superoxide. While singlet oxygen is generated under both photo and US excitations, the mechanisms of the photo- and sonotoxicity of 2ICy7 are suggested to differ to a certain extent. During PDT, the generation of singlet oxygen and thermal effects were observed. In SDT, however, a lack of heating was observed, accompanied by noticeable contributions of hydroxyl radicals and superoxide.
2.6. Mouse Tumor Treatment via SDT and Mutual SDT + PDT vs. PDT
We recently investigated PDT with Cy7–Ab and 2ICy7–Ab conjugates in a mouse xenograft model and demonstrated that the introduction of iodine atoms drastically improves the photo-eradication of tumors, with no detectable side effects on healthy organs [41]. In the present study, we investigated the SDT and combined SDT + PDT effects of these conjugates and compared the obtained data with those for the above-described PDT approach. A schematic representation of these experiments is shown in Figure 5.
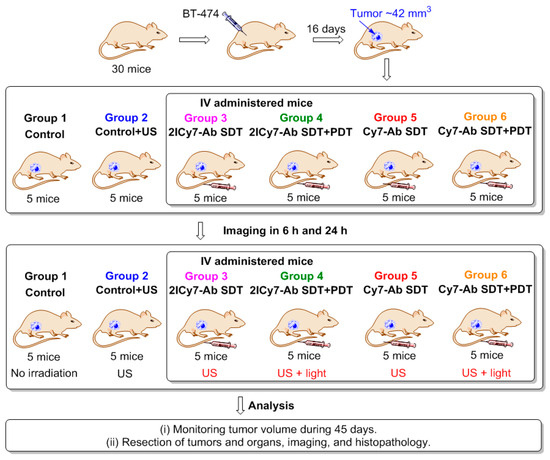
Figure 5.
Mouse experiment. Thirty mice were inoculated with BT-474 cells. Group 1 was used as the control for tumor growth and background autofluorescence upon imaging. Group 2 was used to evaluate the effect of ultrasound alone. After 15 days, the mice were intravenously (IV) administered (tail) 2ICy7–Ab (groups 3 and 4) or Cy7–Ab (groups 5 and 6). The administered dose was 100 μg (5 mg/kg) of conjugate in 200 μL of PBS. Twenty-four hours post-injection, the mice were exposed to US (SDT, f = 1 MHz, 0.7 W/cm2, 1 min irradiation/1 min rest, 5 times) (groups 2–6) followed by NIR light irradiation (PDT, groups 4 and 6 only) for 15 min (730 nm LED, 70 mW/cm2, 63 J/cm2). The tumor volumes for all the groups were subsequently monitored for 45 days. Tumors and organs were then resected and analyzed by imaging. PDT is photodynamic therapy; SDT is sonodynamic therapy; US is ultrasound.
In brief, 30 human breast cancer-bearing mice (BT-474 cell line, tumor volume ~42 mm3) were randomly separated into six groups (5 mice per group). Group 1 was used as the control for tumor growth and background autofluorescence in imaging experiments. Group 2 was used to evaluate the effect of ultrasound alone. Groups 3 and 4 were intravenously (IV) administered (tail) the 2ICy7–Ab conjugate (100 μg in 200 μL of PBS), and groups 5 and 6 were IV administered the same dose of Cy7–Ab to evaluate the effects of SDT and the combination of SDT and PDT. The doses of the conjugates selected for the in vivo study (100 μg) were the same as those used in our previous work [41].
The distribution of the conjugates in the mouse whole body was monitored via fluorescence imaging at 6 h and 24 h post-injection. After 6 h, an intense fluorescence signal originating from the sensitizer was observed, mostly in the lungs. After 24 h, complete accumulation was achieved (the ratio between the bright fluorescence signal in the tumor and the weak signal from the benign tissues exhibited no detectable change; Figure 6). At this time point (24 h), groups 3 and 4 (administered 2ICy7–Ab) and groups 5 and 6 (administered Cy7–Ab) were exposed to ultrasound (US dose 210 J/cm2), whereas groups 4 and 6 were subjected to light irradiation. The light dose was 63 J/cm2 at 70 mW/cm2, which was the same as that used in our previous work [41]. The light power was within the range applied by other researchers (50–100 mW/cm2) [64]. Notably, the applied US dose was two times lower than the safe dose (420 W/cm2 at 2 W/cm2, 1 MHz) previously reported in the literature [57]. The instrument settings for the mouse experiments, including the applied US and light irradiation doses, were the same as those for the above sono- and photostability measurements, which allowed us to estimate the effect of dye degradation on the sensitizing properties (Figure 1). The dyes almost completely decomposed at a light dose of 63 J/cm2 (Figure 1A,B); therefore, increasing the applied light dose to improve photodynamic treatment without additional administration of the sensitizer is not reasonable. Moreover, we used a much higher US dose (210 J/cm2) at which at least 60% of the Ab-conjugated sensitizers remained undamaged (Figure 1C,D), and this dose could still be increased.
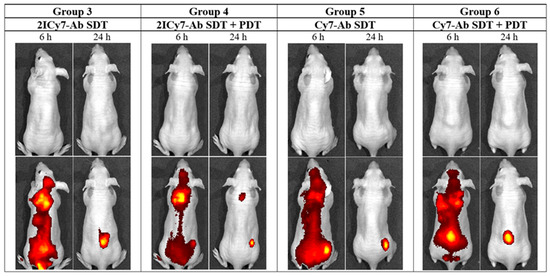
Figure 6.
In vivo whole-body images of representative tumor-bearing mice (groups 3–6) captured at 6 h and 24 h after intravenous (IV) injection of the Cy7–Ab and 2ICy7–Ab conjugates. 1st row: white light; 2nd row: overlay of the fluorescence channel with white light. PDT is photodynamic therapy; SDT is sonodynamic therapy.
The tumor sizes were monitored for 45 days using the hands-on digital caliper method (Figure 6) [65]. On the 45th day, all the tumors and organs (liver, spleen, lung, kidney, heart, and brain) were resected for further imaging and histopathological analysis.
It was found that tumors in the non-sonicated and sonicated control groups 1 and 2 started to grow gradually after the 5th day of observation, whereas the Cy7–Ab-treated tumors in groups 5 and 6 showed modest growth for 13 days and then began to grow rapidly (Figure 7). By the 45th day, the tumors of control groups 1 and 2 reached 821 ± 48 mm3 and 766 ± 48 mm3, respectively, demonstrating an ~19-fold increase in size compared with the initial volume (~42 mm3), whereas the tumors of groups 5 and 6 exhibited an ~12-fold increase (the final tumor volumes were 522 ± 24 mm3 and 490 ± 19 mm3), which corresponded to moderate ~1.6-fold tumor growth suppression compared with that of group 1. This finding indicated that sonication without the conjugate (group 2) had no detectable effect on the tumors, whereas sonication of Cy7–Ab-injected mice slightly suppressed tumor growth, which could be attributed to the therapeutic effect caused by the antibody. A similar moderate effect (~1.4-fold tumor suppression) was recently observed for Cy7–Ab-injected light-irradiated and non-irradiated (kept in the dark) mice [41]. Thus, the photo- and sonodynamic effects of Cy7–Ab on tumor growth were negligible.
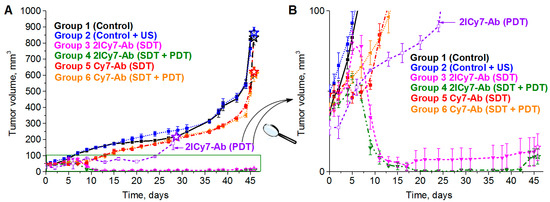
Figure 7.
Tumor growth curves ((A), full range and (B), zoom out) of BT-474 tumor-bearing mice: control (group 1), monitored after ultrasound (group 2), monitored after intravenous (IV) administration of 100 µg (0.5 mg/mL) of 2ICy7–Ab or Cy7–Ab, when irradiated with US (SDT, f = 1 MHz, 0.7 W/cm2, 1 min irradiation/1 min rest, 5 times) (groups 3 and 5), and 2ICy7–Ab or Cy7–Ab, when irradiated with US (SDT, the same conditions) and subsequently exposed to light (PDT, 730 nm LED, 63 J/cm2) for 30 min (groups 4 and 6). For comparison, the tumor volumes for the mice irradiated with light alone (PDT, 730 nm LED, 63 J/cm2) [41] are also shown (violet curve 2ICy7–Ab (PDT)). The tumor volumes were measured in vivo using the caliper method. The volumes of the resected tumors (see Figure 6) are shown by stars. The tumor volume at each time point is represented by the mean ± SEM for five mice in each group. PDT is photodynamic therapy; SDT is sonodynamic therapy.
In contrast, the tumors of the US-irradiated (group 3) and US + light-irradiated (group 4) mice that were administered 2Cy7–Ab initially showed the same trend of growth as those in groups 5 and 6, but one week after irradiation, they started to rapidly decrease. During the next week, the sonicated tumors (group 3) exhibited an ~3.5-fold decrease (to 12 ± 9 mm3) in size compared with the initial tumor size (~42 mm2), which corresponded to ~68-fold tumor growth suppression compared with that of control group 1 and ~42-fold suppression compared with that of Cy7–Ab-treated groups 5 and 6.
Moreover, tumors in the US + light-irradiated group 4 exhibited an ~4.7-fold reduction to 9 ± 5 mm3, which corresponded to ~91-fold tumor growth suppression compared with that in control group 1 and ~56-fold suppression compared with that in Cy7–Ab-treated groups 5 and 6. These results provide evidence that SDT + PDT with 2ICy7–Ab (group 4) could be an even more efficient treatment approach, causing almost complete tumor elimination, than sonication alone (group 3), even though the contribution of PDT to SDT + PDT is moderate. The use of PDT in the clinic is, however, limited by its light penetration depth. However, 2ICy7–Ab-mediated PDT (without SDT) causes only 5.4-fold tumor growth suppression [41], while SDT results in 68-fold tumor suppression (~13 times in our experimental setup) but also in noticeable tumor reduction.
Importantly, the sensitizers can decompose upon US and/or light exposure, as shown in Figure 1. At the applied US dose (210 J/cm2, blue stars in Figure 1), the concentration of the 2ICy7–Ab conjugate decreased to ~47% of the initial value, which means that an even higher US dose can be applied to achieve a better treatment effect after a single IV injection. At the same time, upon light irradiation (a light dose of 63 J/cm2, red star in Figure 1), this conjugate almost completely decomposes, and further photodynamic treatment requires an additional injection of 2ICy7–Ab. Thus, taking into account stronger tumor growth suppression, deeper penetration into the body, and much higher sonostability (D1/2,US~382 J/cm2 by absorption and 349 J/cm2 by emission, Table 1) than photostability (D1/2,Light~9.3 J/cm2 by absorption and 7.8 J/cm2 by emission), SDT with the 2ICy7–Ab conjugate is considered a substantially more efficient treatment modality than PDT.
The tumor volumes measured by the caliper method in vivo on the 45th day were consistent with those measured after tumor resection (the volumes of the resected tumors are shown as stars in Figure 7). The resected tumors in the US-irradiated group 2 (864 ± 34 mm3) were similar in size to those in control group 1 (834 ± 45 mm3). The tumors of groups 5 and 6 were 621 ± 27 mm3 and 606 ± 46 mm3, respectively, which was approximately 1.4-fold lower than that of group 1, whereas the tumors of groups 3 and 4 were significantly smaller, 15 ± 8 mm3 and 9.5 ± 5 mm3, respectively. The significant reduction in tumor size for the 2ICy7–Ab- vs. the Cy7–Ab-treated mice upon PDT [41] and, even greater, upon SDT and SDT + PDT (Figure 6 and Figure 8) agrees well with the φΔ,US and ΦΔ,Light of these conjugates (Table 1).
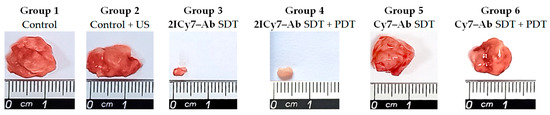
Figure 8.
Representative photographs of tumors resected on the 45th day after intravenous (IV) injection of the conjugates. Non-administered sonicated (group 1) and non-sonicated (group 2) mice were used as controls. PDT is photodynamic therapy; SDT is sonodynamic therapy.
The body weight of the 2ICy7–Ab-treated mice was unchanged during the 45 days of investigation, whereas the control and Cy7–Ab-treated mice exhibited weight loss (Figure S6).
The resected tumors, livers, spleens, lungs, kidneys, hearts, and brains were captured under white light and then histopathologically analyzed. Notably, a Cy7–trastuzumab conjugate was previously evaluated in a breast cancer xenograft for specific accumulation in HER2+ (BT-474) versus HER2–(MDA-MB-231) cell lines [66].
2.7. Histopathological Study
The tumor development and side effects of the sonodynamically treated (group 3) and untreated (group 1) mice were analyzed via histopathological analysis of the tumors and organs (liver, spleen, lung, kidney, heart, and brain). The obtained results are presented in Table 3. Histopathological analysis revealed that all five control mice (group 1) had subcutaneous tumors with extensive necrosis. Moreover, four control animals had metastases: two mice had metastases in the liver, and the other two mice had metastases in the lung, spleen, kidney, and peritoneum. Previously, it was reported that BT-474 can metastasize in vivo [67,68]. Interestingly, the liver and kidney metastases created solid masses, whereas the lung metastases showed an interstitial spread of multiple microscopic foci. Peritoneal metastases were observed in tumor cells surrounding and attached to the spleen capsule without penetrating it. Importantly, lymphovascular invasion (LVI) was identified within one of the spleen blood vessels, indicating that this is the mechanism of tumor spread (Figure 9).

Table 3.
Summary of histopathological results.
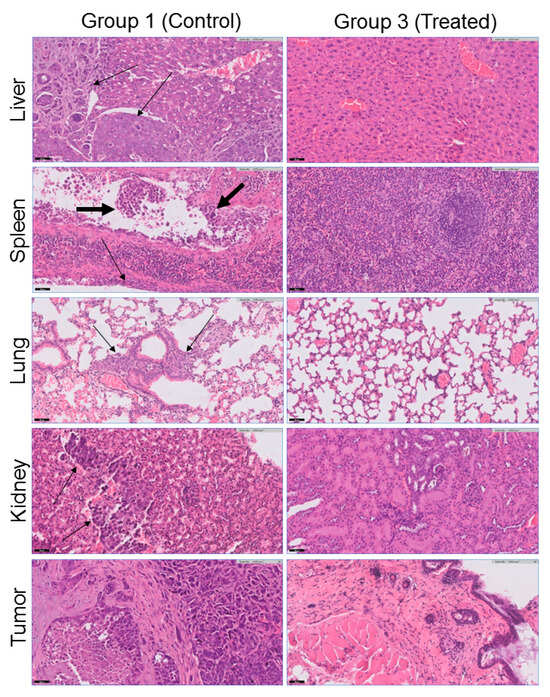
Figure 9.
Representative histopathological findings (hematoxylin/eosin (H&E) staining, magnification ×20) showing differences between the control (group 1) and SDT-treated (group 3) mice. Metastases (marked by thin arrows) were identified in four of five control animals in the liver (image for mouse #1 from group 1 is shown, see Table 3), spleen, peritoneum, lung, and kidney (image for mouse #3 is shown). The liver and kidney metastases created solid masses, while the lung metastases showed an interstitial spread of multiple microscopic foci. LVI was identified within one of the spleen blood vessels, indicating that this is the mechanism of tumor spread, marked by a thick arrow. All control animals (group 1) had a subcutaneous tumor, whereas two of the treated animals (group 3) had no evidence of a tumor at the site of implantation.
In sharp contrast, none of the sonodynamically treated animals (group 3) had metastases. Moreover, two of the treated animals did not have a subcutaneous tumor at the site of tumor implantation (Figure 9). The heart and brain were free of metastases in all the mice in both the control and treated groups (Figure S8). In all the examined tissues (liver, spleen, lung, kidney, heart, and brain), there was no morphological evidence of treatment-related injury, such as nuclear atypia, degenerative changes, inflammation, fibrosis, or vascular alteration. Hence, sonodynamic therapy with an antibody-guided 2ICy7–Ab sensitizer has no morphologically detectable side effects on the main nontargeted, nontumoral tissues.
3. Materials and Methods
3.1. Materials
The Cy7 and 2ICy7 dyes and dye–trastuzumab conjugates 2ICy7–Ab (dye-to-antibody ratio, DAR~1.8) and Cy7–Ab (DAR~1.7) were the same as those used in our previous work [41]. The structure confirmation data are presented in Figures S9–S13. The dyes were >95% pure according to HPLC. All other chemicals were obtained from Alfa Aesar (Petach Tikva, Israel) and Sigma-Aldrich (Jerusalem, Israel). Solvents were purchased from Bio-Lab (Jerusalem, Israel) and used as is.
3.2. Photostability and Sonostability
Solutions of the dyes and conjugates (c~1 μM) in 0.1 M PBS, pH 7.4, were prepared. For the photostability measurements, these solutions were irradiated from a distance of 26.5 cm in a standard 1 cm quartz cell by using a 730 nm, 30 W LED equipped with a 60° lens; the light irradiance was 8 mW/cm2.
Sonication in all experiments was carried out using an ultrasound therapy unit (Dr. Equipment, New Delhi, India). For the sonostability measurements, the sample solutions were sonicated (f = 1 MHz, 0.7 W/cm2) by a US transducer (diameter 30 mm) immersed at a depth of 1 mm on the upper surface of the solutions (5 mL) placed in 55 mm glass Petri dishes. The absorption and emission spectra of the solution were recorded over time in a standard 1 cm quartz cell, and plots of the normalized absorbance and fluorescence intensity vs. time were drawn. Then, the photo- and sonostabilities of the dyes were quantified through the half-lives (τ1/2) calculated from the corresponding monoexponential absorption and fluorescence decay functions (Equation (1)).
where I is the time-dependent absorbance or fluorescence intensity, I0 is the offset of intensity, A is the amplitude, t is the decay constant, and τ is time.
I = I0 + A × exp(–τ/t),
3.3. Quantum Yields of Photodynamic and Sonodynamic Singlet Oxygen Generation
The quantum yields of the photodynamic singlet oxygen generation (ΦΔ,Light) were measured in 0.1 M PBS, pH 7.4, according to a previous procedure [42,69] using singlet oxygen sensor green (SOSG, c = 6 µM) (Thermo Fisher Scientific Inc., Waltham, MA, USA) as the singlet oxygen indicator.
The relative quantum yields of singlet oxygen generation under US exposure (φΔ,US) were measured in 0.1 M PBS, pH 7.4, similar to the reported procedure [69]. Solutions of SOSG (c = 6 µM) [70] and the dye under investigation or the reference dye (Cy7) (c = 1.7–2.3 µM) in PBS (5 mL) were prepared and sonicated at f = 1 MHz, and the applied US intensity was 2 W/cm2. The emission spectra of the solutions were recorded over time in standard 1 cm quartz cells (λ* = 488 nm). The total exposure time reached 8 min. During this time, the emission of SOSG at 530 nm gradually increased. The corresponding plots representing the emission intensity of SOSG versus time were plotted and fitted by a zero-order reaction rate function [69], the rates of singlet oxygen scavenger degradation (r) were calculated (Figure S7), and the relative φΔ,US values of the dyes and conjugates were quantified via Equation (2) [69], with Cy7 in PBS (φΔ,US,Ref ≡ 1 a.u.) used as the reference.
where φΔ,US,Ref is the relative sonodynamic quantum yield of the singlet oxygen generation for the reference dye (1 a.u.), rRef and r are the singlet oxygen scavenger degradation rates obtained from the corresponding fitting curves of the reference dye and the dye under examination, respectively, and ARef and A are the absorbances at λ = 750 nm of the reference dye (Cy7) and the dye or conjugate under examination, respectively.
φΔ = φΔ,US,Ref × (r/rRef) × (ARef/A),
Each φΔ,US was measured three times, and the average value was taken; the reproducibility was within 5%.
3.4. Detection of Free Radicals by EPR
EPR measurements were carried out at room temperature using a Bruker ELEXYS E500 spectrometer operating at X-band frequencies (9.5 GHz) and a Bruker ER4119HS resonator. Solutions of 20 μM Cy7 and 2ICy7 with a 25 mM BMPO spin trap in double-distilled water were prepared. The samples were dissolved in 1 mL of spin trap solution and loaded into a Vitrocom quartz capillary (CV1012-Q-100) with a 1 mm inner diameter.
To measure US-generated radicals, sonication was carried out for 15 min using an MRC ACP-120H bath sonicator (40 kHz, 50 W) (MRC Lab, Holon, Israel), and then the EPR spectra were recorded.
For determination of photogenerated radicals, the samples were measured for 30 min upon light irradiation (730 nm, 30 W LED, light power density of 8 mW/cm2, light dose of 14.4 J/cm2).
The experimental conditions for the EPR measurements were as follows: a microwave power of 20 mW, a 1 Gauss modulation amplitude, and a 100 kHz modulation frequency; the sweep range was 150 Gauss; and the spectra consisted of 300 points. The data were plotted using Microcal Origin software, version 8.6 (OriginLab Corp., Northampton, MA, USA).
For the quantification of radicals, the signal intensity was compared against a linear-fit calibration curve obtained with known concentrations (10 μM, 20 μM, 40 μM, and 80 μM) of 3-carboxy-PROXYL. The intensities of total BMPO-bound radicals, BMPO-OH radicals, and BMPO-OOH were quantified by double integration of the spectra, and the concentrations were calculated using a calibration curve.
3.5. Photothermal Measurements
Solutions (8 mL) of Cy7 and 2ICy7 in DMSO (20 µM) and DMSO (as a control) were placed in non-covered 60 × 12 mm glass Petri dishes and light irradiated (730 nm, 30 W LED, 40 mW/cm2) from the top, perpendicular to the surface of the liquid, from a distance of 10 cm. The initial temperature for all the solutions before irradiation was the same at 25 °C, and the temperature increase was simultaneously recorded upon irradiation via a thermal imager FLUKE TiS20 (Fluke Corp, Everett, WA, USA) positioned at the top of the solutions at an angle of 45°. The graphs for the temperature increase (Δt vs. time) were subsequently plotted.
3.6. Sonothermal Measurements
The measurements were carried out similarly to those of the photothermal experiment, but the irradiation was performed using an ultrasound therapy unit (Dr. Equipment, New Delhi, India) at 0.7 W/cm2, 1.2 W/cm2, and 2.0 W/cm2 (f = 1 MHz). The solutions (3 mL) were placed directly on top of an ultrasound transducer (diameter 3.5 cm) equipped with a plastic border, and the temperature was measured from the top of the solutions.
Each photothermal and sonothermal experiment was performed in triplicate, and the average values were taken.
3.7. Mouse Preparation
All experiments with mice were carried out in compliance with the Israel Council on Animal Care regulations and were approved by the Animal Care Committee of Ariel University (authorization number IL-179-06-19, 21 May 2023).
Thirty-six-week-old athymic BALB/c female nude mice (Harlan Labs, Nes Ziona, Israel) were subcutaneously inoculated on the dorsal right side with the human breast cancer cell line BT-474 (1 × 106 cells in PBS into nu/nu mice, 100 µL per mouse), and tumors were allowed to establish until the tumor sizes reached approximately 42 mm3 (16 days). The tumor-bearing mice were then randomly separated into six groups (5 mice per group). Group 1 was used as the control for tumor growth and background autofluorescence in imaging experiments. Group 2 was used to evaluate the effect of ultrasound alone. Groups 3 and 4 were intravenously (IV) administered (tail) the 2ICy7–Ab conjugate (100 μg in 200 μL of PBS), and groups 5 and 6 were IV administered Cy7–Ab at the same dosage to evaluate the sonodynamic (SDT) and combined sono- (SDT) and photodynamic (PDT) effects.
3.8. Anesthesia
Before imaging and/or photo/sonodynamic treatment, the mice were anesthetized via an intraperitoneal injection of a combination of ketamine and xylazine. Dose: 0.5 mL of ketamine + 0.25 mL of xylazine + 4.25 mL of water for injection. The dose rate was 0.1 mL/10 g of body weight.
3.9. Animal Imaging
Imaging was carried out using an IVIS Spectrum multispectral fluorescence in vivo imaging system (PerkinElmer, Waltham, MA, USA). For imaging, the mice were anesthetized, and imaging was performed after conjugate administration at 6 h and 24 h post-injection. The images were obtained in white light mode with the following fluorescence channel: 710/30 nm excitation bandpass filter and 780/20 nm emission bandpass filter. The data were processed with IVIS Living Image software, version 4.8.2.
3.10. PDT and SDT Experiments
The BT-474 tumor-bearing mice were established as described above until the tumor volume reached approximately 42 mm3. The mice were randomly divided into six groups. Group 1 was used as the control for tumor growth and background autofluorescence in imaging experiments. Group 2 was used to evaluate the effect of ultrasound alone. Groups 3 and 4 were administered 2ICy7–Ab via the tail vein (IV), and groups 5 and 6 were administered Cy7–Ab (100 µg in 200 μL of PBS). The accumulation of the conjugates was monitored by fluorescence imaging at 6 h and 24 h. The accumulation of this conjugate is known to be achieved after 24 h [41]. At this time point, the mice in groups 2–6 were anesthetized and subjected to ultrasound using the ultrasonic device described above. The irradiation conditions were as follows: transducer diameter, 30 mm; gel, f = 1 MHz; applied ultrasound intensity, 0.7 W/cm2; 1 min irradiation/1 min rest, 5 times; and overall US dose, 210 J/cm2. This US dose and sonication regime was selected on the basis of the behavior of the mice. Increasing power and/or dose resulted in anxiety and distress in the mice.
Additionally, groups 4 and 6 were subjected to NIR light irradiation (730 nm, 30 W LED, light power density 70 mW/cm2, 15 min, light dose 63 J/cm2). Then, the tumor sizes were measured in vivo over time using the hands-on digital caliper method, and the tumor volumes were calculated according to Equation (3) [65] and are presented on the plot as the mean ± standard error of the mean (SEM).
Tumor volume (mm3) = 0.5 × Tumor Length × Tumor Width2
3.11. Histological Protocol
The tumor-bearing mice were sacrificed, and the tumors and organs (liver, spleen, lung, kidney, heart, and brain) were harvested and fixed in formalin to prepare paraffin sections. Hematoxylin/eosin (H&E) staining was used for histological analysis.
3.12. Pathological Evaluation of H&E-Stained Sections
The study pathologist was blinded to the experimental protocol used for each animal. H&E-stained sections of the tumor and organs were examined for any morphological abnormalities. The amount of necrosis in the tumor tissues was estimated as a percentage of the total tumor area.
4. Conclusions
In this study, we explored the efficacy of a trastuzumab-guided diiodinated heptamethine cyanine-based sensitizer, 2ICy7–Ab, for sonodynamic therapy (SDT) in an HER2+ xenograft model. The sonosensitizing effect was compared to that of the noniodinated counterpart, Cy7–Ab. Investigations have also been conducted on the combined sonodynamic and photodynamic (PDT) effects. The sonodynamic impact of 2ICy7–Ab in tumor treatment (68-fold tumor growth suppression vs. control) was much more pronounced (~43 times) than that of Cy7–Ab (1.6-fold suppression). Upon combined SDT and PDT, the sonodynamic outcome noticeably surpassed that of the photodynamic treatment alone. After a single intravenous 2ICy7–Ab injection, SDT resulted in a 3.5-fold reduction in tumor volume within 45 days and exhibited 13-fold greater tumor suppression than PDT. Moreover, during this period, SDT with 2ICy7–Ab did not cause any observable (weight, physical conditions, or complete survival) toxic effects in the animals, or morphologically detectable side effects on the non-targeted tissues or organs. The evaluation of the potential long-term side effects of the treatment will be addressed in our future studies.
The iodine atoms in the 2ICy7 dye were identified as pivotal contributors to the enhanced photo- and sonotoxicity of the 2ICy7–Ab conjugate, as the non-iodinated Cy7–Ab analog exhibited no statistically significant cytotoxicity toward tumors. Upon light and ultrasound irradiation, the iodinated 2ICy7 dye and the 2ICy7–Ab conjugate demonstrated noticeably increased quantum yields of singlet oxygen generation compared with their non-iodinated counterparts. Additionally, the higher sonotoxicity of the iodinated versus the non-iodinated dyes is driven by the increase in hydroxyl radical and superoxide generation.
Crucially, 2ICy7–Ab provides a sufficiently strong fluorescent signal, facilitating real-time tracking of the distribution of the sensitizer throughout the body and its accumulation in the tumor. This capability is essential for identifying the optimal time for sonication, thereby enhancing the precision and safety of the treatment. Given that SDT can be applied to the whole body and multiple organs [71] and that it is a deeper treatment approach than PDT, we assume that SDT with the 2ICy7–Ab conjugate is, in general, a more promising therapeutic approach than PDT. However, determining the localization of the conjugate in host organs via fluorescence imaging prior to treatment is important. Our future study will include an investigation of the impact of the number of iodines, their positioning over the dye scaffold on RS generation, and the efficacy of sensitizer-antibody conjugates on various HER2 overexpressed and HER2 low-expressed tumors in vivo. The limitations associated with the DAR range, aggregativity, solubility, and bystander effect of our conjugates will also be explored. Another limitation relates to the heterogeneity of spontaneously arising tumors [72]. The further development of the proposed approach to address this issue is challenging.
5. Patents
A patent on iodinated cyanine–antibody conjugates for sonodynamic applications is pending.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms251810137/s1.
Author Contributions
Conceptualization, T.M., G.G., and L.P.; methodology, L.P. and G.G.; validation, D.K., O.S., S.A.-R., R.C., L.P. and G.G.; investigation, D.K., O.S., S.A.-R. and R.C.; resources, G.G. and L.P.; data curation, D.K., O.K., S.A.-R., R.C., G.G. and L.P.; writing—original draft preparation, D.K., S.A.-R., R.C., G.G. and L.P.; writing—review and editing, T.M., G.G. and L.P.; visualization, S.A.-R., R.C. and L.P.; supervision, G.G. and L.P.; project administration, T.M., G.G. and L.P.; funding acquisition, T.M., G.G. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States–Israel Binational Science Foundation, grant number 2021122, and the Center for Absorption in Science of the Ministry of Immigrant Absorption of Israel under the KAMEA program.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee of Ariel University (protocol code IL-179-06-19, 21 May 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors thank Helena Tuchinsky (Ariel University) for mouse preparation, Arkadi Hesin (Ariel University) for assisting with the mouse imaging experiments, Vered Marks (Ariel University) for the NMR measurements, and Itay Pitussi (Ariel University) for the HRMS measurements.
Conflicts of Interest
Tajib Mirzabekov was employed by Biomirex, Inc. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Bellew, J.W. Therapeutic modalities past, present and future: Their role in the patient care management model. In Modalities for Therapeutic Intervention, 6th ed.; Bellew, J.W., Michlovitz, S.L., Nolan, T.P., Jr., Eds.; McGraw Hill: New York, NY, USA, 2016. [Google Scholar]
- Nowak, K.M.; Schwartz, M.R.; Breza, V.R.; Price, R.J. Sonodynamic therapy: Rapid progress and new opportunities for non-invasive tumor cell killing with sound. Cancer Lett. 2022, 532, 215592. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Liu, L.; Wang, P. Sonodynamic therapy (SDT) for cancer treatment: Advanced sensitizers by ultrasound activation to injury tumor. ACS Appl. Bio Mater. 2020, 3, 3456–3475. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Idris, M.A.; Bilyaminu, I.B.; Liu, D. Sonodynamic antimicrobial chemotherapy: An emerging alternative strategy for microbial inactivation. Ultrason. Sonochem. 2021, 75, 105591. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic excitation of Rose Bengal for eradication of gram-positive and gram-negative bacteria. Biomed. Res. Int. 2013, 2013, 684930. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Pavarina, A.C.; Mima, E.G.O.; McHale, A.P.; Callan, J.F. Antimicrobial sonodynamic and photodynamic therapies against Candida albicans. Biofouling 2018, 34, 357–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043. [Google Scholar] [CrossRef]
- Pan, X.; Wang, W.; Huang, Z.; Liu, S.; Guo, J.; Zhang, F.; Yuan, H.; Li, X.; Liu, F.; Liu, H. MOF-derived double-layer hollow nanoparticles with oxygen generation ability for multimodal imaging-guided sonodynamic therapy. Angew. Chem. 2020, 132, 13659–13663. [Google Scholar] [CrossRef]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, R.; Liu, D.; Li, B.; Lin, J.; Zhang, W. The tumor affinity of chlorin e6 and its sonodynamic effects on non-small cell lung cancer. Ultrason. Sonochem. 2013, 20, 667–673. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Z.; Zheng, Y.; Song, J.; Li, J.; Zeng, Y.; Liu, X. Ultrasound-driven biomimetic nanosystem suppresses tumor growth and metastasis through sonodynamic therapy, CO therapy, and indoleamine 2,3-dioxygenase inhibition. ACS Nano 2020, 14, 8985–8999. [Google Scholar] [CrossRef]
- Xie, L.; Feng, X.; Huang, M.; Zhang, K.; Liu, Q. Sonodynamic therapy combined to 2-deoxyglucose potentiate cell metastasis inhibition of breast cancer. Ultrasound Med. Biol. 2019, 45, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tu, J.; Yang, D.; Raymond, J.L.; Roy, R.A.; Zhang, D. Photo- and sono-dynamic therapy: A review of mechanisms and considerations for pharmacological agents used in therapy incorporating light and sound. Curr. Pharmaceut. Design 2019, 25, 401–412. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology (ESR). ESR statement on portable ultrasound devices. Insights Imaging 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, M. Sonodynamic therapy: Another “light” in tumor treatment by exogenous stimulus. Smart Mater. Med. 2021, 2, 145–149. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light technology for efficient and effective photodynamic therapy: A critical review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Inglut, C.T.; Gaitan, B.; Najafali, D.; Lopez, I.A.; Connolly, N.P.; Orsila, S.; Perttilä, R.; Woodworth, G.F.; Chen, Y.; Huang, H.C. Predictors and limitations of the penetration depth of photodynamic effects in the rodent brain. Photochem. Photobiol. 2020, 96, 301–309. [Google Scholar] [CrossRef]
- Trendowski, M. Using the promise of sonodynamic therapy in the clinical setting against disseminated cancers. Chemother. Res. Pract. 2015, 2015, 316015. [Google Scholar] [CrossRef]
- Sazgarnia, A.; Shanei, A.; Eshghi, H.; Hassanzadeh-Khayyat, M.; Esmaily, H.; Shanei, M.M. Detection of sonoluminescence signals in a gel phantom in the presence of protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics 2013, 53, 29–35. [Google Scholar] [CrossRef]
- Guo, S.; Sun, X.; Cheng, J.; Xu, H.; Dan, J.; Shen, J.; Zhou, Q.; Zhang, Y.; Meng, L.; Cao, W.; et al. Apoptosis of THP-1 macrophages induced by protoporphyrin IX-mediated sonodynamic therapy. Int. J. Nanomed. 2013, 8, 2239–2246. [Google Scholar] [CrossRef][Green Version]
- Hsieh, Y.; Wu, C.; Chang, C.; Yu, J. Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: When plasma membranes are the main targets. J. Cell. Physiol. 2003, 194, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yan, M.; Zhang, H.; Xue, J.; Chen, J. A phthalocyanine-based photosensitizer for effectively combating triple negative breast cancer with enhanced photodynamic anticancer activity and immune response. Eur. J. Med. Chem. 2022, 241, 114644. [Google Scholar] [CrossRef] [PubMed]
- Martins, Y.A.; Fonseca, M.J.V.; Pavan, T.Z.; Lopez, R.F.V. Bifunctional therapeutic application of low-frequency ultrasound associated with zinc phthalocyanine-loaded micelles. Int. J. Nanomed. 2020, 15, 8075–8095. [Google Scholar] [CrossRef] [PubMed]
- Balçik-Erçin, P.; Çetin, M.; Göksel, M.; Durmuş, M. Improved targeting for photodynamic therapy via a biotin–phthalocyanine conjugate: Synthesis, photophysical and photochemical measurements, and in vitro cytotoxicity assay. New J. Chem. 2020, 44, 3392–3401. [Google Scholar] [CrossRef]
- Gong, Z.; Dai, Z. Design and challenges of sonodynamic therapy system for cancer theranostics: From equipment to sensitizers. Adv. Sci. 2021, 8, 2002178. [Google Scholar] [CrossRef]
- Prada, F.; Sheybani, N.D.; Franzini, A.; Moore, D.; Cordeiro, D.; Sheehan, J.; Timbie, K.; Xu, Z. Fluorescein-mediated sonodynamic therapy in a rat glioma model. J. Neurooncol. 2020, 148, 445–454. [Google Scholar] [CrossRef]
- Hou, R.; Liang, X.; Li, X.; Zhang, X.; Ma, X.; Wang, F. In situ conversion of Rose Bengal microbubbles into nanoparticles for ultrasound imaging guided sonodynamic therapy with enhanced antitumor efficacy. Biomater. Sci. 2020, 8, 2526–2536. [Google Scholar] [CrossRef]
- Vanerio, N.; Stijnen, M.; de Mol, B.A.; Kock, L.M. Biomedical applications of photo- and sono-activated Rose Bengal: A review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef]
- Lin, H.; Li, S.; Wang, J.; Chu, C.; Zhang, Y.; Pang, X.; Lv, P.; Wang, X.; Zhao, Q.; Chen, J.; et al. A single-step multi-level supramolecular system for cancer sonotheranostics. Nanoscale Horiz. 2019, 4, 190–195. [Google Scholar] [CrossRef]
- Tang, Q.; Chang, S.; Tian, Z.; Sun, J.; Hao, L.; Wang, Z.; Zhu, S. Efficacy of indocyanine green-mediated sonodynamic therapy on rheumatoid arthritis fibroblast-like synoviocytes. Ultrasound Med. Biol. 2017, 43, 2690–2698. [Google Scholar] [CrossRef]
- Wang, R.; Song, C.; Gao, A.; Liu, Q.; Guan, W.; Mei, J.; Ma, L.; Cui, D. Antibody-conjugated liposomes loaded with indocyanine green for oral targeted photoacoustic imaging-guided sonodynamic therapy of Helicobacter pylori infection. Acta Biomater. 2022, 143, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.G.; Lima-Sousa, R.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int. J. Pharm. 2018, 542, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Q.; Deng, Z. IR-780 dye as a sonosensitizer for sonodynamic therapy of breast tumor. Sci. Rep. 2016, 6, 25968. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.Y.; Liu, Y.; Chen, B.W.; Liu, Y.Y.; Wang, Y.S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Zhang, S.; Yang, X.; Shi, W.; Li, P.; Pan, D.; Shen, L. Cu2-xO@TiO2-y Z-scheme heterojunctions for sonodynamic-chemodynamic combined tumor eradication. Chem. Eng. J. 2022, 435, 1347777. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Wang, Z.; Ivanov, M.; Gao, Y.; Bussotti, L.; Foggi, P.; Zhang, H.; Russo, N.; Dick, B.; Zhao, J.; Di Donato, M.; et al. Spin–orbit charge-transfer intersystem crossing (ISC) in compact electron donor–acceptor dyads: ISC mechanism and application as novel and potent photodynamic therapy reagents. Chem. Eur. J. 2020, 26, 1091–1102. [Google Scholar] [CrossRef]
- Prakash, A.V.; Yazabak, F.; Hovor, I.; Nakonechny, F.; Kulyk, O.; Semenova, O.; Bazylevich, A.; Gellerman, G.; Patsenker, L. Highly efficient near-IR cyclohexene cyanine photosensitizers for antibacterial photodynamic therapy. Dyes Pigm. 2023, 211, 111053. [Google Scholar] [CrossRef]
- Kobzev, D.; Semenova, O.; Tatarets, A.; Bazylevich, A.; Gellerman, G.; Patsenker, L. Antibody-guided iodinated cyanine for near-IR photoimmunotherapy. Dyes Pigm. 2023, 212, 111101. [Google Scholar] [CrossRef]
- Atchison, J.; Kamila, S.; Nesbitt, H.; Logan, K.A.; Nicholas, D.M.; Fowley, C.; Davis, J.; Callan, B.; McHalea, A.P.; Callan, J.F. Iodinated cyanine dyes: A new class of sensitisers for use in NIR activated photodynamic therapy (PDT). Chem. Commun. 2017, 53, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chi, J.; Xia, J.; Zhang, Y.; Han, S.; Sun, Y. Iodinated cyanine dyes for fast near-infrared-guided deep tissue synergistic phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 25720–25729. [Google Scholar] [CrossRef] [PubMed]
- Semenova, O.; Kobzev, D.; Hovor, I.; Atrash, M.; Nakonechny, F.; Kulyk, O.; Bazylevich, A.; Gellerman, G.; Patsenker, L. Effect of solubilizing group on the antibacterial activity of heptamethine cyanine photosensitizers. Pharmaceutics 2023, 15, 247. [Google Scholar] [CrossRef] [PubMed]
- Bokan, M.; Nakonechny, F.; Talalai, E.; Kobzev, D.; Gellerman, G.; Patsenker, L. Photodynamic effect of novel hexa-iodinated quinono-cyanine dye on Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2020, 31, 101866. [Google Scholar] [CrossRef] [PubMed]
- Semenova, O.; Kobzev, D.; Yazbak, F.; Nakonechny, F.; Kolosova, O.; Tatarets, A.; Gellerman, G.; Patsenker, L. Unexpected effect of iodine atoms in heptamethine cyanine dyes on the photodynamic eradication of Gram-positive and Gram-negative pathogens. Dyes Pigm. 2021, 195, 109745. [Google Scholar] [CrossRef]
- Ebaston, T.M.; Nakonechny, F.; Talalai, E.; Gellerman, G.; Patsenker, L. Iodinated xanthene-cyanine NIR dyes as potential photosensitizers for antimicrobial photodynamic therapy. Dyes Pigm. 2021, 184, 108854. [Google Scholar] [CrossRef]
- Lee, G.P.; Willis, A.; Pernal, S.; Phakatkar, A.; Shokuhfar, T.; Blot, V.; Engelhard, H.H. Targeted sonodynamic destruction of glioblastoma cells using antibody-titanium dioxide nanoparticle conjugates. Nanomedicine 2021, 16, 523–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Yang, H.; Yu, L.; Xu, Y.; Sharma, A.; Yin, P.; Li, X.; Kim, J.S.; Sun, Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021, 50, 11227–11248. [Google Scholar] [CrossRef]
- Lin, X.; Huang, R.; Huang, Y.; Wang, K.; Li, H.; Bao, Y.; Wu, C.; Zhang, Y.; Tian, X.; Wang, X. Nanosonosensitizer-augmented sonodynamic therapy combined with checkpoint blockade for cancer immunotherapy. Int. J. Nanomed. 2021, 16, 1889–1899. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of cyanine derived dyes in photodynamic therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Della Pelle, G.; Delgado López, A.; Salord Fiol, M.; Kostevšek, N. Cyanine dyes for photo-thermal therapy: A comparison of synthetic liposomes and natural erythrocyte-based carriers. Int. J. Mol. Sci. 2021, 22, 6914. [Google Scholar] [CrossRef] [PubMed]
- Eren, Z. Ultrasound as a basic and auxiliary process for dye remediation: A review. J. Environ. Manag. 2012, 104, 127–141. [Google Scholar] [CrossRef]
- León, G.; Miguel, B.; Manzanares, L.; Saavedra, M.I.; Guzmán, M.A. Kinetic study of the ultrasound effect on Acid Brown 83 dye degradation by hydrogen peroxide oxidation processes. Chem. Eng. 2021, 5, 52. [Google Scholar] [CrossRef]
- Huang, B.; Wang, L.; Tang, K.; Chen, S.; Xu, Y.; Liao, H.; Niu, C. IR780 Based sonotherapeutic nanoparticles to combat multidrug-resistant bacterial infections. Front Chem. 2022, 10, 840598. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Wysocki, M.; Michalak, M.; Dlugaszewska, J.; Güzel, E.; Sobotta, L. The dual synergy of photodynamic and sonodynamic therapy in the eradication of methicillin-resistant Staphylococcus aureus. Appl. Sci. 2023, 13, 3810. [Google Scholar] [CrossRef]
- Zang, L.-Y.; Misras, H.P. EPR Kinetic studies of superoxide radicals generated during the autoxidation of l-methyl-4-phenyl-2,3-dihydropyridiniuma, a bioactivated intermediate of Parkinsonian-inducing neurotoxin l-methyl-4-phenyl-l,2,3,6-tetrahydropyridine. J. Bio. Chem. 1992, 267, 23601–23608. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, M.; Cheng, Z.; Yang, Z.; Yang, L.; Liu, B.; Bian, Y.; Al Kheraif, A.A.; Ma, P.; Lin, M. A mild and efficient sonothermal tumor therapy enhanced by sonodynamic effect with biodegradable red phosphorus nanoparticles. Chem. Eng. J. 2024, 482, 148711. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, J.; Hu, J.; Sun, K.; Lu, W.; Zeng, F.; Chen, J.; Liu, M.; Cai, Z.; He, X.; et al. Tumor-targeting near-infrared dimeric heptamethine cyanine photosensitizers with an aromatic diphenol linker for imaging-guided cancer phototherapy. Adv. Heal. Mater. 2023, 12, e2203080. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light sources and dosimetry techniques for photodynamic therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Faustino-Rocha, A.; Oliveira, P.A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Soares-Maia, R.; Da Costa, R.G.; Colaço, B.; Pires, M.J.; Colaço, J.; Ferreira, R.; et al. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab. Anim. 2013, 42, 217–224. [Google Scholar] [CrossRef]
- Kobzev, D.; Prasad, C.; Walunj, D.; Gotman, H.; Semenova, O.; Bazylevich, A.; Patsenker, L.; Gellerman, G. Synthesis and biological evaluation of theranostic Trastuzumab-SN38 conjugate for near-IR fluorescence imaging and targeted therapy of HER2+ breast cancer. Eur. J. Med. Chem. 2023, 252, 115298. [Google Scholar] [CrossRef]
- Iorns, E.; Drews-Elger, K.; Ward, T.M.; Dean, S.; Clarke, J.; Berry, D.; El Ashry, D.; Lippman, M. A new mouse model for the study of human breast cancer metastasis. PLoS ONE 2012, 7, e47995. [Google Scholar] [CrossRef]
- Nanni, P.; Nicoletti, G.; Palladini, A.; Croci, S.; Murgo, A.; Ianzano, M.L.; Grosso, V.; Stivani, V.; Antognoli, A.; Lamolinara, A.; et al. Multiorgan metastasis of human HER-2+ breast cancer in Rag2-/-;Il2rg-/- mice and treatment with PI3K inhibitor. PLoS ONE 2012, 7, e39626. [Google Scholar] [CrossRef]
- Lin, H.; Shen, Y.; Chen, D.; Lin, L.; Wilson, B.C.; Li, B.; Xie, S. Feasibility study on quantitative measurements of singlet oxygen generation using Singlet Oxygen Sensor Green. J. Fluoresc. 2013, 23, 41–47. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. Singlet Oxygen Sensor Green Reagent|Thermo Fisher Scientific-DK 2019, 3–5. Available online: https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp36002.pdf (accessed on 31 August 2024).
- Saisei Immunotherapy Clinic. Introduction to Sonodynamic Therapy (SDT). Available online: https://saisei-mirai.or.jp/en/sonophotodynamic_therapy/ (accessed on 2 September 2024).
- Heppner, G.H.; Dexter, D.L.; DeNucci, T.; Miller, F.R.; Calabresi, P. Heterogeneity in drug sensitivity among tumor cell subpopulations of a single mammary tumor. Cancer Res. 1978, 38, 3758–3763. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).