NMDARs in Alzheimer’s Disease: Between Synaptic and Extrasynaptic Membranes
Abstract
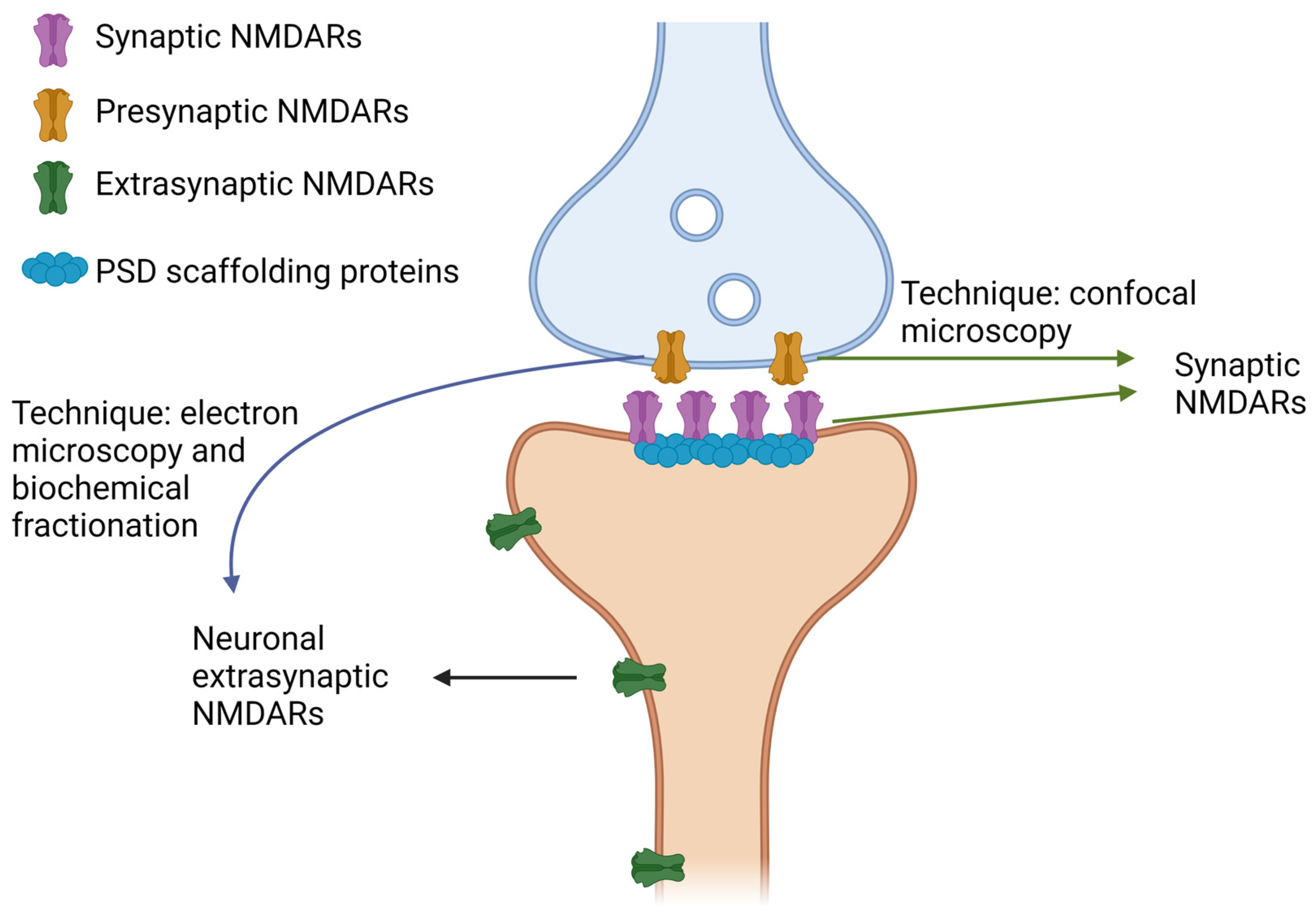
:1. Structure, Function, and Subcellular Localization of NMDARs
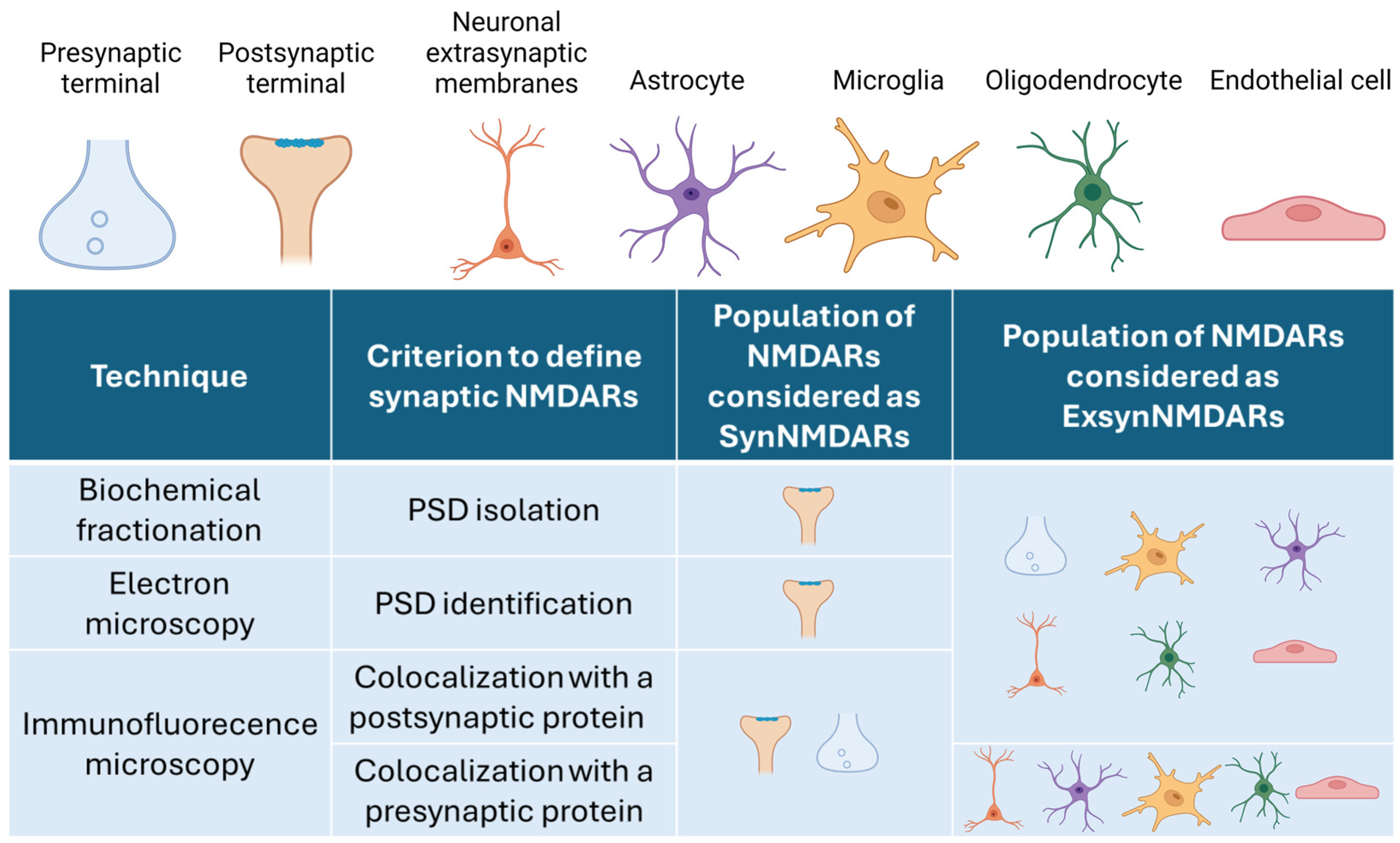
2. How to Distinguish SynNMDARs and ExsynNMDARs
The Conception of ExsynNMDARs
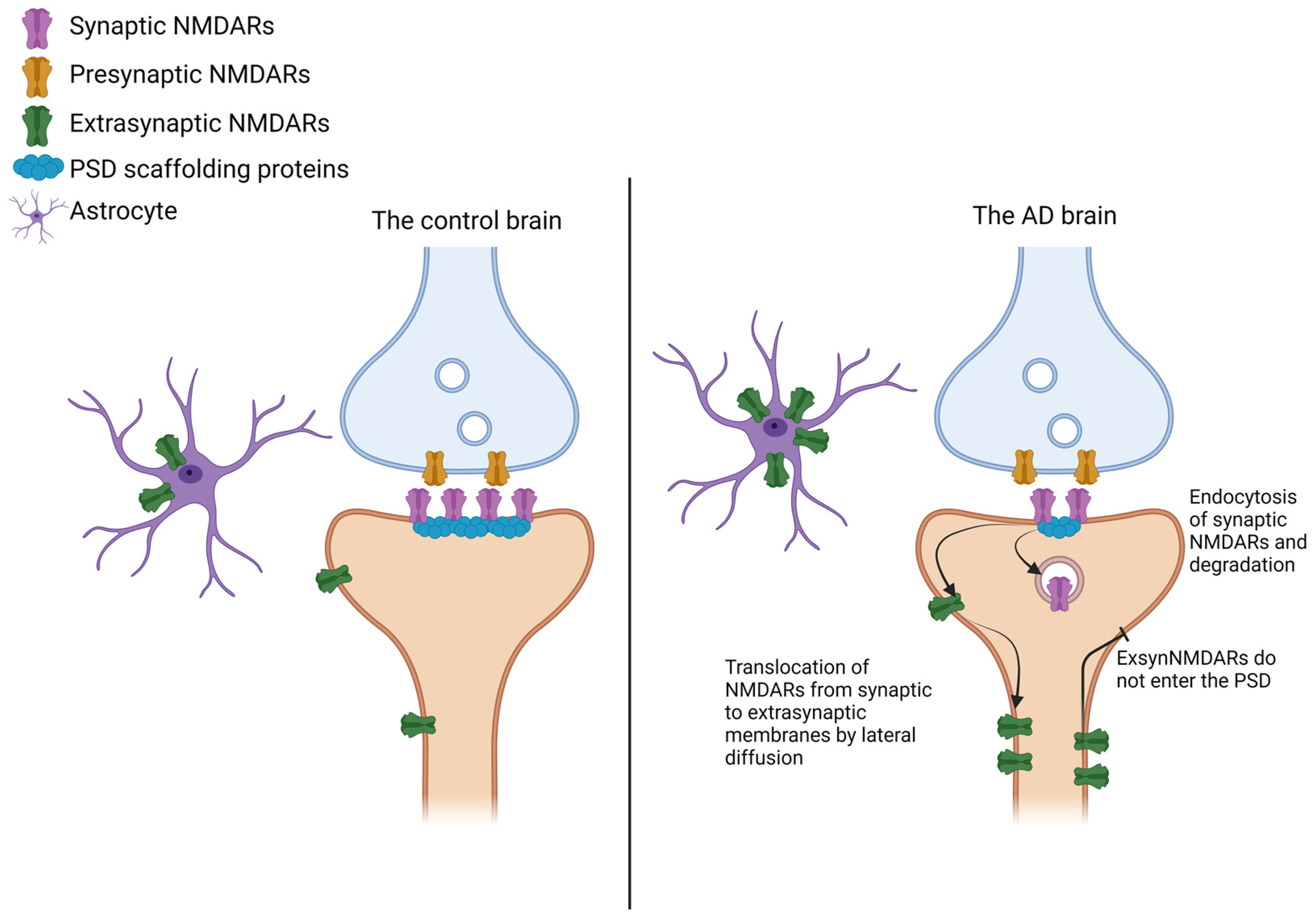
3. NMDAR Distribution in Alzheimer’s Disease
3.1. Distribution of SynNMDARs and ExsynNMDARs in Animal Models of AD
3.1.1. Distribution of SynNMDARs and ExsynNMDARs in Tauopathy Mice Models
3.1.2. Distribution of SynNMDARs and ExsynNMDARs in Aβ-Treated Cultures and Mice Models
3.2. NMDAR Subunit Levels in the Brain of Individuals with AD
3.2.1. Regional NMDAR Transcript Levels in the Brain of Individuals with AD
3.2.2. Total Protein Levels of NMDAR Subunits in the Brain of Patients with AD
3.2.3. NMDAR Subunits Protein Levels in Synaptic and Extrasynaptic Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morris, R.G. NMDA receptors and memory encoding. Neuropharmacology 2013, 74, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tsien, J.Z.; Huerta, P.T.; Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996, 87, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tsien, J.Z. Memory and the NMDA receptors. N. Engl. J. Med. 2009, 361, 302–303. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Sanz-Clemente, A.; Nicoll, R.A.; Roche, K.W. Diversity in NMDA receptor composition: Many regulators, many consequences. Neuroscientist 2013, 19, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Otaño, I.; Larsen, R.S.; Wesseling, J.F. Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat. Rev. Neurosci. 2016, 17, 623–635. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J. Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat. Rev. Neurosci. 2020, 21, 169–178. [Google Scholar] [CrossRef]
- Froudist-Walsh, S.; Xu, T.; Niu, M.; Rapan, L.; Zhao, L.; Margulies, D.S.; Zilles, K.; Wang, X.J.; Palomero-Gallagher, N. Gradients of neurotransmitter receptor expression in the macaque cortex. Nat. Neurosci. 2023, 26, 1281–1294. [Google Scholar] [CrossRef]
- Gladding, C.M.; Raymond, L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol. Cell Neurosci. 2011, 48, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Oliet, S.H. Organization, control and function of extrasynaptic NMDA receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130601. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Wang, Y.X.; Hua, F.; Yi, Z.; Zhou, A.; Ge, L.; Stephenson, F.A.; Wenthold, R.J. Organization of NMDA receptors at extrasynaptic locations. Neuroscience 2010, 167, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Larsen, R.S.; Philpot, B.D.; Paulsen, O. Roles of Presynaptic NMDA Receptors in Neurotransmission and Plasticity. Trends Neurosci. 2016, 39, 26–39. [Google Scholar] [CrossRef]
- Franchini, L.; Stanic, J.; Ponzoni, L.; Mellone, M.; Carrano, N.; Musardo, S.; Zianni, E.; Olivero, G.; Marcello, E.; Pittaluga, A.; et al. Linking NMDA Receptor Synaptic Retention to Synaptic Plasticity and Cognition. iScience 2019, 19, 927–939. [Google Scholar] [CrossRef]
- Ivanov, A.; Pellegrino, C.; Rama, S.; Dumalska, I.; Salyha, Y.; Ben-Ari, Y.; Medina, I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiol. 2006, 572, 789–798. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Massey, P.V.; Johnson, B.E.; Moult, P.R.; Auberson, Y.P.; Brown, M.W.; Molnar, E.; Collingridge, G.L.; Bashir, Z.I. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J. Neurosci. 2004, 24, 7821–7828. [Google Scholar] [CrossRef]
- Lu, W.; Man, H.; Ju, W.; Trimble, W.S.; MacDonald, J.F.; Wang, Y.T. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 2001, 29, 243–254. [Google Scholar] [CrossRef]
- Karpova, A.; Mikhaylova, M.; Bera, S.; Bär, J.; Reddy, P.P.; Behnisch, T.; Rankovic, V.; Spilker, C.; Bethge, P.; Sahin, J.; et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell 2013, 152, 1119–1133. [Google Scholar] [CrossRef]
- Rönicke, R.; Mikhaylova, M.; Rönicke, S.; Meinhardt, J.; Schröder, U.H.; Fändrich, M.; Reiser, G.; Kreutz, M.R.; Reymann, K.G. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol. Aging 2011, 32, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Bading, H. Synaptic activity-mediated suppression of p53 and induction of nuclear calcium-regulated neuroprotective genes promote survival through inhibition of mitochondrial permeability transition. J. Neurosci. 2009, 29, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, F.; Papadia, S.; Fricker, M.; Bell, K.F.; Soriano, F.X.; Martel, M.A.; Puddifoot, C.; Habel, M.; Wyllie, D.J.; Ikonomidou, C.; et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J. Neurosci. 2010, 30, 2623–2635. [Google Scholar] [CrossRef]
- Dick, O.; Bading, H. Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors. J. Biol. Chem. 2010, 285, 19354–19361. [Google Scholar] [CrossRef]
- Vanhoutte, P.; Bading, H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr. Opin. Neurobiol. 2003, 13, 366–371. [Google Scholar] [CrossRef]
- Pegasiou, C.M.; Zolnourian, A.; Gomez-Nicola, D.; Deinhardt, K.; Nicoll, J.A.R.; Ahmed, A.I.; Vajramani, G.; Grundy, P.; Verhoog, M.B.; Mansvelder, H.D.; et al. Age-Dependent Changes in Synaptic NMDA Receptor Composition in Adult Human Cortical Neurons. Cereb. Cortex 2020, 30, 4246–4256. [Google Scholar] [CrossRef]
- González-González, I.M.; Gray, J.A.; Ferreira, J.; Conde-Dusman, M.J.; Bouchet, D.; Perez-Otaño, I.; Groc, L. GluN3A subunit tunes NMDA receptor synaptic trafficking and content during postnatal brain development. Cell Rep. 2023, 42, 112477. [Google Scholar] [CrossRef] [PubMed]
- Erreger, K.; Dravid, S.M.; Banke, T.G.; Wyllie, D.J.; Traynelis, S.F. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J. Physiol. 2005, 563, 345–358. [Google Scholar] [CrossRef]
- Gray, J.A.; Shi, Y.; Usui, H.; During, M.J.; Sakimura, K.; Nicoll, R.A. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: Single-cell NMDA receptor subunit deletion in vivo. Neuron 2011, 71, 1085–1101. [Google Scholar] [CrossRef]
- Gardoni, F.; Di Luca, M. Protein-protein interactions at the NMDA receptor complex: From synaptic retention to synaptonuclear protein messengers. Neuropharmacology 2021, 190, 108551. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Heine, M.; Cousins, S.L.; Stephenson, F.A.; Lounis, B.; Cognet, L.; Choquet, D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc. Natl. Acad. Sci. USA 2006, 103, 18769–18774. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.G.; Miller, A.J.; Westbrook, G.L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006, 95, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S. Distribution of extrasynaptic NMDA receptors on neurons. Sci. World J. 2012, 2012, 267120. [Google Scholar] [CrossRef]
- Yan, J.; Bengtson, C.P.; Buchthal, B.; Hagenston, A.M.; Bading, H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 2020, 370, eaay3302. [Google Scholar] [CrossRef]
- Yu, S.P.; Jiang, M.Q.; Shim, S.S.; Pourkhodadad, S.; Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: Implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.A.; Wyllie, D.J.; Hardingham, G.E. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience 2009, 158, 334–343. [Google Scholar] [CrossRef]
- Papadia, S.; Soriano, F.X.; Léveillé, F.; Martel, M.A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008, 11, 476–487. [Google Scholar] [CrossRef]
- von Engelhardt, J.; Coserea, I.; Pawlak, V.; Fuchs, E.C.; Köhr, G.; Seeburg, P.H.; Monyer, H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology 2007, 53, 10–17. [Google Scholar] [CrossRef]
- Crawley, O.; Conde-Dusman, M.J.; Pérez-Otaño, I. GluN3A NMDA receptor subunits: More enigmatic than ever? J. Physiol. 2022, 600, 261–276. [Google Scholar] [CrossRef]
- Marshall, C.A.; McBride, J.D.; Changolkar, L.; Riddle, D.M.; Trojanowski, J.Q.; Lee, V.M. Inhibition of CK2 mitigates Alzheimer’s tau pathology by preventing NR2B synaptic mislocalization. Acta Neuropathol. Commun. 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Nong, Y.; Almeida, C.G.; Paul, S.; Moran, T.; Choi, E.Y.; Nairn, A.C.; Salter, M.W.; Lombroso, P.J.; Gouras, G.K.; et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005, 8, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Koeglsperger, T.; Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011, 31, 6627–6638. [Google Scholar] [CrossRef] [PubMed]
- McKay, S.; Bengtson, C.P.; Bading, H.; Wyllie, D.J.; Hardingham, G.E. Recovery of NMDA receptor currents from MK-801 blockade is accelerated by Mg2+ and memantine under conditions of agonist exposure. Neuropharmacology 2013, 74, 119–125. [Google Scholar] [CrossRef]
- Xia, P.; Chen, H.S.; Zhang, D.; Lipton, S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef]
- Wu, Y.N.; Johnson, S.W. Memantine selectively blocks extrasynaptic NMDA receptors in rat substantia nigra dopamine neurons. Brain Res. 2015, 1603, 1–7. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef]
- Boeckers, T.M. The postsynaptic density. Cell Tissue Res. 2006, 326, 409–422. [Google Scholar] [CrossRef]
- Dosemeci, A.; Tao-Cheng, J.H.; Vinade, L.; Jaffe, H. Preparation of postsynaptic density fraction from hippocampal slices and proteomic analysis. Biochem. Biophys. Res. Commun. 2006, 339, 687–694. [Google Scholar] [CrossRef]
- Matas, E.; John Francis William, D.; Toro, C.T. Abnormal expression of post-synaptic proteins in prefrontal cortex of patients with schizophrenia. Neurosci. Lett. 2021, 745, 135629. [Google Scholar] [CrossRef] [PubMed]
- He, R.B.; Li, L.; Liu, L.Z.; Ma, Y.J.; Fan, S.J.; Liu, L.R.; Li, W.B.; Xian, X.H. Ceftriaxone improves impairments in synaptic plasticity and cognitive behavior in APP/PS1 mouse model of Alzheimer’s disease by inhibiting extrasynaptic NMDAR-STEP. J. Neurochem. 2023, 166, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, I.; Filipkowski, R.K.; Priller, C.; Ris, L.; Neyton, J.; Croes, S.; Terwel, D.; Gysemans, M.; Devijver, H.; Borghgraef, P.; et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol. Aging 2009, 30, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Knox, R.; Pathipati, P.; Ferriero, D. Developmental localization of NMDA receptors, Src and MAP kinases in mouse brain. Neurosci. Lett. 2011, 503, 215–219. [Google Scholar] [CrossRef]
- Pérez-Otaño, I.; Luján, R.; Tavalin, S.J.; Plomann, M.; Modregger, J.; Liu, X.B.; Jones, E.G.; Heinemann, S.F.; Lo, D.C.; Ehlers, M.D. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat. Neurosci. 2006, 9, 611–621. [Google Scholar] [CrossRef]
- De Marco García, N.V.; Karayannis, T.; Fishell, G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 2011, 472, 351–355. [Google Scholar] [CrossRef]
- Moreau, A.W.; Kullmann, D.M. NMDA receptor-dependent function and plasticity in inhibitory circuits. Neuropharmacology 2013, 74, 23–31. [Google Scholar] [CrossRef]
- Booker, S.A.; Wyllie, D.J.A. NMDA receptor function in inhibitory neurons. Neuropharmacology 2021, 196, 108609. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Z.; Sharmin, F.; Lin, W.; Ricke, K.M.; Zasloff, M.A.; Stewart, A.F.R.; Chen, H.H. Tyrosine phosphatase PTP1B impairs presynaptic NMDA receptor-mediated plasticity in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 156, 105402. [Google Scholar] [CrossRef]
- Nyíri, G.; Stephenson, F.A.; Freund, T.F.; Somogyi, P. Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience 2003, 119, 347–363. [Google Scholar] [CrossRef]
- Corlew, R.; Brasier, D.J.; Feldman, D.E.; Philpot, B.D. Presynaptic NMDA receptors: Newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist 2008, 14, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Bard, L.; Choquet, D. Surface trafficking of N-methyl-D-aspartate receptors: Physiological and pathological perspectives. Neuroscience 2009, 158, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Bard, L.; Groc, L. Glutamate receptor dynamics and protein interaction: Lessons from the NMDA receptor. Mol. Cell Neurosci. 2011, 48, 298–307. [Google Scholar] [CrossRef]
- Kharazia, V.N.; Weinberg, R.J. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J. Comp. Neurol. 1999, 412, 292–302. [Google Scholar] [CrossRef]
- Lee, M.C.; Ting, K.K.; Adams, S.; Brew, B.J.; Chung, R.; Guillemin, G.J. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS ONE 2010, 5, e14123. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, F. Analysis of Functional NMDA Receptors in Astrocytes. Methods Mol. Biol. 2017, 1677, 241–251. [Google Scholar] [CrossRef]
- Skowrońska, K.; Obara-Michlewska, M.; Zielińska, M.; Albrecht, J. NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis. Int. J. Mol. Sci. 2019, 20, 309. [Google Scholar] [CrossRef]
- Thomas, D.M.; Kuhn, D.M. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005, 1050, 190–198. [Google Scholar] [CrossRef]
- Wu, C.C.; Tzeng, C.Y.; Chang, C.Y.; Wang, J.D.; Chen, Y.F.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Wang, W.Y.; Chen, C.J. NMDA receptor inhibitor MK801 alleviated pro-inflammatory polarization of BV-2 microglia cells. Eur. J. Pharmacol. 2023, 955, 175927. [Google Scholar] [CrossRef]
- Raghunatha, P.; Vosoughi, A.; Kauppinen, T.M.; Jackson, M.F. Microglial NMDA receptors drive pro-inflammatory responses via PARP-1/TRMP2 signaling. Glia 2020, 68, 1421–1434. [Google Scholar] [CrossRef]
- Káradóttir, R.; Cavelier, P.; Bergersen, L.H.; Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005, 438, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Krizbai, I.A.; Deli, M.A.; Pestenácz, A.; Siklós, L.; Szabó, C.A.; András, I.; Joó, F. Expression of glutamate receptors on cultured cerebral endothelial cells. J. Neurosci. Res. 1998, 54, 814–819. [Google Scholar] [CrossRef]
- Kim, K.S.; Jeon, M.T.; Kim, E.S.; Lee, C.H.; Kim, D.G. Activation of NMDA receptors in brain endothelial cells increases transcellular permeability. Fluids Barriers CNS 2022, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.M.; Wang, J.; Fiske, M.P.; Hubalkova, P.; Barse, L.; Gray, J.A.; Sanz-Clemente, A. NMDAR-Activated PP1 Dephosphorylates GluN2B to Modulate NMDAR Synaptic Content. Cell Rep. 2019, 28, 332–341.E5. [Google Scholar] [CrossRef]
- Sanz-Clemente, A.; Matta, J.A.; Isaac, J.T.; Roche, K.W. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 2010, 67, 984–996. [Google Scholar] [CrossRef]
- Jamet, Z.; Mergaux, C.; Meras, M.; Bouchet, D.; Villega, F.; Kreye, J.; Prüss, H.; Groc, L. NMDA receptor autoantibodies primarily impair the extrasynaptic compartment. Brain 2024, 147, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.P.; Ladépêche, L.; Seth, H.; Bard, L.; Varela, J.; Mikasova, L.; Bouchet, D.; Rogemond, V.; Honnorat, J.; Hanse, E.; et al. Surface dynamics of GluN2B-NMDA receptors controls plasticity of maturing glutamate synapses. EMBO J. 2014, 33, 842–861. [Google Scholar] [CrossRef]
- Ferreira, J.S.; Papouin, T.; Ladépêche, L.; Yao, A.; Langlais, V.C.; Bouchet, D.; Dulong, J.; Mothet, J.P.; Sacchi, S.; Pollegioni, L.; et al. Co-agonists differentially tune GluN2B-NMDA receptor trafficking at hippocampal synapses. Elife 2017, 6, e25492. [Google Scholar] [CrossRef]
- Mikasova, L.; Xiong, H.; Kerkhofs, A.; Bouchet, D.; Krugers, H.J.; Groc, L. Stress hormone rapidly tunes synaptic NMDA receptor through membrane dynamics and mineralocorticoid signalling. Sci. Rep. 2017, 7, 8053. [Google Scholar] [CrossRef]
- Sanz-Clemente, A.; Gray, J.A.; Ogilvie, K.A.; Nicoll, R.A.; Roche, K.W. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013, 3, 607–614. [Google Scholar] [CrossRef]
- Yi, Z.; Petralia, R.S.; Fu, Z.; Swanwick, C.C.; Wang, Y.X.; Prybylowski, K.; Sans, N.; Vicini, S.; Wenthold, R.J. The role of the PDZ protein GIPC in regulating NMDA receptor trafficking. J. Neurosci. 2007, 27, 11663–11675. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.W.; Gelfand, V.I.; Spector, I.; Craig, A.M. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: Differential attachment of NMDA versus AMPA receptors. J. Neurosci. 1998, 18, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Specht, C.G.; Waites, C.L.; Butler-Munro, C.; Leal-Ortiz, S.; Foote, J.W.; Genoux, D.; Garner, C.C.; Montgomery, J.M. SAP97 directs NMDA receptor spine targeting and synaptic plasticity. J. Physiol. 2011, 589, 4491–4510. [Google Scholar] [CrossRef]
- Escamilla, S.; Badillos, R.; Comella, J.X.; Solé, M.; Pérez-Otaño, I.; Sánchez-Mut, J.S.; Sáez-Valero, J.; Cuchillo-Ibáñez, I. Synaptic and extrasynaptic distribution of NMDA receptors in cortex of Alzheimer’s disease patients. Alzheimer’s Dement. 2024, in press. [Google Scholar] [CrossRef]
- Torrez, V.R.; Zimmer, E.R.; Kalinine, E.; Haas, C.B.; Zenki, K.C.; Muller, A.P.; Souza, D.O.; Portela, L.V. Memantine mediates astrocytic activity in response to excitotoxicity induced by PP2A inhibition. Neurosci. Lett. 2019, 696, 179–183. [Google Scholar] [CrossRef]
- Wu, H.M.; Tzeng, N.S.; Qian, L.; Wei, S.J.; Hu, X.; Chen, S.H.; Rawls, S.M.; Flood, P.; Hong, J.S.; Lu, R.B. Novel neuroprotective mechanisms of memantine: Increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology 2009, 34, 2344–2357. [Google Scholar] [CrossRef]
- Murakawa-Hirachi, T.; Mizoguchi, Y.; Ohgidani, M.; Haraguchi, Y.; Monji, A. Effect of memantine, an anti-Alzheimer’s drug, on rodent microglial cells in vitro. Sci. Rep. 2021, 11, 6151. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef]
- Bading, H. Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J. Exp. Med. 2017, 214, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Carles, A.; Freyssin, A.; Perin-Dureau, F.; Rubinstenn, G.; Maurice, T. Targeting. Int. J. Mol. Sci. 2024, 25, 3733. [Google Scholar] [CrossRef]
- Masliah, E.; Alford, M.; DeTeresa, R.; Mallory, M.; Hansen, L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1996, 40, 759–766. [Google Scholar] [CrossRef]
- Jacob, C.P.; Koutsilieri, E.; Bartl, J.; Neuen-Jacob, E.; Arzberger, T.; Zander, N.; Ravid, R.; Roggendorf, W.; Riederer, P.; Grünblatt, E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J. Alzheimer’s Dis. 2007, 11, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Gebhardt, F.M.; Mitrovic, A.D.; Vandenberg, R.J.; Dodd, P.R. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 553.e1–553.e11. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, F.; El Gaamouch, F.; Gouix, E.; Lecocq, M.; Lobner, D.; Nicole, O.; Buisson, A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008, 22, 4258–4271. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on its Current and Future Applications. J. Alzheimer’s Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D. A review of the effects of memantine on clinical progression in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2012, 27, 769–776. [Google Scholar] [CrossRef]
- Karimi Tari, P.; Parsons, C.G.; Collingridge, G.L.; Rammes, G. Memantine: Updating a rare success story in pro-cognitive therapeutics. Neuropharmacology 2024, 244, 109737. [Google Scholar] [CrossRef]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Zhao, T.; Gong, M.; Wang, X.; Zhang, Y.; Xu, L.; Li, W.; Jia, J. The role of N-methyl-D-aspartate glutamate receptors in Alzheimer’s disease: From pathophysiology to therapeutic approaches. Prog. Neurobiol. 2023, 231, 102534. [Google Scholar] [CrossRef]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef] [PubMed]
- Bordji, K.; Becerril-Ortega, J.; Nicole, O.; Buisson, A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ß production. J. Neurosci. 2010, 30, 15927–15942. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Tuo, Q.Z.; Liuyang, Z.Y.; Xie, A.J.; Feng, X.L.; Yan, X.; Qiu, M.; Li, S.; Wang, X.L.; Cao, F.Y.; et al. Extrasynaptic NMDA receptor-induced tau overexpression mediates neuronal death through suppressing survival signaling ERK phosphorylation. Cell Death Dis. 2016, 7, e2449. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.S.; Liu, A.C.; Chen, J.; Pan, Z.Y.; Wan, Q.; Li, Z.Q.; Wang, Z.F. Overactivation of NR2B-containing NMDA receptors through entorhinal-hippocampal connection initiates accumulation of hyperphosphorylated tau in rat hippocampus after transient middle cerebral artery occlusion. J. Neurochem. 2015, 134, 566–577. [Google Scholar] [CrossRef]
- Amadoro, G.; Ciotti, M.T.; Costanzi, M.; Cestari, V.; Calissano, P.; Canu, N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc. Natl. Acad. Sci. USA 2006, 103, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Hoey, S.E.; Williams, R.J.; Perkinton, M.S. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J. Neurosci. 2009, 29, 4442–4460. [Google Scholar] [CrossRef]
- Varshavskaya, K.B.; Mitkevich, V.A.; Makarov, A.A.; Barykin, E.P. Synthetic, Cell-Derived, Brain-Derived, and Recombinant β-Amyloid: Modelling Alzheimer’s Disease for Research and Drug Development. Int. J. Mol. Sci. 2022, 23, 15036. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Zhang, Y. Animal models of Alzheimer’s disease: Applications, evaluation, and perspectives. Zool. Res. 2022, 43, 1026–1040. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, C.; Qiu, Y.; Zhou, Z.; Bao, J.; Qian, W. Dendritic/Post-synaptic Tau and Early Pathology of Alzheimer’s Disease. Front. Mol. Neurosci. 2021, 14, 671779. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Ruiz, R.; Aguado, C.; Martín-Belmonte, A.; Moreno-Martínez, A.E.; Merchán-Rubira, J.; Hernández, F.; Ávila, J.; Fukazawa, Y.; Luján, R. Different modes of synaptic and extrasynaptic NMDA receptor alteration in the hippocampus of P301S tau transgenic mice. Brain Pathol. 2023, 33, e13115. [Google Scholar] [CrossRef]
- Ma, D.L.; Luo, Y.; Huang, R.; Zhao, Z.R.; Zhang, L.; Li, Y.L.; Wang, Q.; Li, L. Cornel Iridoid Glycoside Suppresses Hyperactivity Phenotype in rTg4510 Mice through Reducing Tau Pathology and Improving Synaptic Dysfunction. Curr. Med. Sci. 2020, 40, 1031–1039. [Google Scholar] [CrossRef]
- Hering, H.; Sheng, M. Dendritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Regan, P.; Mitchell, S.J.; Kim, S.C.; Lee, Y.; Yi, J.H.; Barbati, S.A.; Shaw, C.; Cho, K. Regulation of Synapse Weakening through Interactions of the Microtubule Associated Protein Tau with PACSIN1. J. Neurosci. 2021, 41, 7162–7170. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Huang, S.M.; Peng, H.H.; Lin, S.W.; Lin, S.R.; Chin, T.Y. Imbalance of synaptic and extrasynaptic NMDA receptors induced by the deletion of CRMP1 accelerates age-related cognitive decline in mice. Neurobiol. Aging 2024, 135, 48–59. [Google Scholar] [CrossRef]
- Nakamura, F.; Kumeta, K.; Hida, T.; Isono, T.; Nakayama, Y.; Kuramata-Matsuoka, E.; Yamashita, N.; Uchida, Y.; Ogura, K.; Gengyo-Ando, K.; et al. Amino- and carboxyl-terminal domains of Filamin-A interact with CRMP1 to mediate Sema3A signalling. Nat. Commun. 2014, 5, 5325. [Google Scholar] [CrossRef] [PubMed]
- Quach, T.T.; Moutal, A.; Khanna, R.; Deems, N.P.; Duchemin, A.M.; Barrientos, R.M. Collapsin Response Mediator Proteins: Novel Targets for Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 949–960. [Google Scholar] [CrossRef]
- Pallas-Bazarra, N.; Draffin, J.; Cuadros, R.; Antonio Esteban, J.; Avila, J. Tau is required for the function of extrasynaptic NMDA receptors. Sci. Rep. 2019, 9, 9116. [Google Scholar] [CrossRef]
- Goebel-Goody, S.M.; Davies, K.D.; Alvestad Linger, R.M.; Freund, R.K.; Browning, M.D. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 2009, 158, 1446–1459. [Google Scholar] [CrossRef]
- Whiteman, I.T.; Minamide, L.S.; Goh, d.L.; Bamburg, J.R.; Goldsbury, C. Rapid changes in phospho-MAP/tau epitopes during neuronal stress: Cofilin-actin rods primarily recruit microtubule binding domain epitopes. PLoS ONE 2011, 6, e20878. [Google Scholar] [CrossRef]
- Rosenberger, A.F.; Morrema, T.H.; Gerritsen, W.H.; van Haastert, E.S.; Snkhchyan, H.; Hilhorst, R.; Rozemuller, A.J.; Scheltens, P.; van der Vies, S.M.; Hoozemans, J.J. Increased occurrence of protein kinase CK2 in astrocytes in Alzheimer’s disease pathology. J. Neuroinflamm. 2016, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.J.; Sabatini, B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Niidome, T.; Akaike, A.; Kihara, T.; Sugimoto, H. Amyloid beta-peptide preconditioning reduces glutamate-induced neurotoxicity by promoting endocytosis of NMDA receptor. Biochem. Biophys. Res. Commun. 2006, 351, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kurup, P.; Zhang, Y.; Xu, J.; Venkitaramani, D.V.; Haroutunian, V.; Greengard, P.; Nairn, A.C.; Lombroso, P.J. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J. Neurosci. 2010, 30, 5948–5957. [Google Scholar] [CrossRef]
- Dinamarca, M.C.; Colombres, M.; Cerpa, W.; Bonansco, C.; Inestrosa, N.C. Beta-amyloid oligomers affect the structure and function of the postsynaptic region: Role of the Wnt signaling pathway. Neurodegener. Dis. 2008, 5, 149–152. [Google Scholar] [CrossRef]
- Olajide, O.J.; Chapman, C.A. Amyloid-β (1-42) peptide induces rapid NMDA receptor-dependent alterations at glutamatergic synapses in the entorhinal cortex. Neurobiol. Aging 2021, 105, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.H.; Wenthold, R.J. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J. Biol. Chem. 1999, 274, 151–157. [Google Scholar] [CrossRef]
- Rammes, G.; Mattusch, C.; Wulff, M.; Seeser, F.; Kreuzer, M.; Zhu, K.; Deussing, J.M.; Herms, J.; Parsons, C.G. Involvement of GluN2B subunit containing N-methyl-d-aspartate (NMDA) receptors in mediating the acute and chronic synaptotoxic effects of oligomeric amyloid-beta (Aβ) in murine models of Alzheimer’s disease (AD). Neuropharmacology 2017, 123, 100–115. [Google Scholar] [CrossRef]
- Dodd, P.R.; Hardy, J.A.; Baig, E.B.; Kidd, A.M.; Bird, E.D.; Watson, W.E.; Johnston, G.A. Optimization of freezing, storage, and thawing conditions for the preparation of metabolically active synaptosomes from frozen rat and human brain. Neurochem. Pathol. 1986, 4, 177–198. [Google Scholar] [CrossRef]
- Mishizen-Eberz, A.J.; Rissman, R.A.; Carter, T.L.; Ikonomovic, M.D.; Wolfe, B.B.; Armstrong, D.M. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiol. Dis. 2004, 15, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; TesFaye, E.; Yasuda, R.P.; Mash, D.C.; Armstrong, D.M.; Wolfe, B.B. Effects of post-mortem delay on subunits of ionotropic glutamate receptors in human brain. Brain Res. Mol. Brain Res. 2000, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.F.; Cao, H.; Fu, A.K.Y.; Ip, N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 25800–25809. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Dodd, P.R.; Oakley, A.E.; Perry, R.H.; Edwardson, J.A.; Kidd, A.M. Metabolically active synaptosomes can be prepared from frozen rat and human brain. J. Neurochem. 1983, 40, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bayés, À.; Collins, M.O.; Galtrey, C.M.; Simonnet, C.; Roy, M.; Croning, M.D.; Gou, G.; van de Lagemaat, L.N.; Milward, D.; Whittle, I.R.; et al. Human post-mortem synapse proteome integrity screening for proteomic studies of postsynaptic complexes. Mol. Brain 2014, 7, 88. [Google Scholar] [CrossRef]
- Bi, H.; Sze, C.I. N-methyl-D-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer’s disease. J. Neurol. Sci. 2002, 200, 11–18. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Differential expression of N-methyl-D-aspartate receptor NR2 isoforms in Alzheimer’s disease. J. Neurochem. 2004, 90, 913–919. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate(NMDA) receptor NR1 subunit mRNA expression in Alzheimer’s disease. J. Neurochem. 2001, 78, 175–182. [Google Scholar] [CrossRef]
- Das, S.; Li, Z.; Wachter, A.; Alla, S.; Noori, A.; Abdourahman, A.; Tamm, J.A.; Woodbury, M.E.; Talanian, R.V.; Biber, K.; et al. Distinct transcriptomic responses to Aβ plaques, neurofibrillary tangles, and APOE in Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 74–90. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Bossers, K.; Wirz, K.T.; Meerhoff, G.F.; Essing, A.H.; van Dongen, J.W.; Houba, P.; Kruse, C.G.; Verhaagen, J.; Swaab, D.F. Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer’s disease. Brain 2010, 133, 3699–3723. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Qin, J.; Lai, Y.; Zhang, C.; Zhang, X. Large-Scale Integration of Single-Cell RNA-Seq Data Reveals Astrocyte Diversity and Transcriptomic Modules across Six Central Nervous System Disorders. Biomolecules 2023, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.; Bi, H.; Kleinschmidt-DeMasters, B.K.; Filley, C.M.; Martin, L.J. N-Methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J. Neurol. Sci. 2001, 182, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Sanz, C.; Balantzategi, U.; Quintela-López, T.; Ruiz, A.; Luchena, C.; Zuazo-Ibarra, J.; Capetillo-Zarate, E.; Matute, C.; Zugaza, J.L.; Alberdi, E. Amyloid β/PKC-dependent alterations in NMDA receptor composition are detected in early stages of Alzheimer’s disease. Cell Death Dis. 2022, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, E.; Gaisler-Salomon, I.; Biegon, A. Hippocampal glutamate NMDA receptor loss tracks progression in Alzheimer’s disease: Quantitative autoradiography in postmortem human brain. PLoS ONE 2013, 8, e81244. [Google Scholar] [CrossRef]
- Yeung, J.H.Y.; Walby, J.L.; Palpagama, T.H.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Glutamatergic receptor expression changes in the Alzheimer’s disease hippocampus and entorhinal cortex. Brain Pathol. 2021, 31, e13005. [Google Scholar] [CrossRef]
- Lee, H.G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef]
- Krasnow, A.M.; Attwell, D. NMDA Receptors: Power Switches for Oligodendrocytes. Neuron 2016, 91, 3–5. [Google Scholar] [CrossRef]
- Cao, N.; Yao, Z.X. Oligodendrocyte N-methyl-D-aspartate receptor signaling: Insights into its functions. Mol. Neurobiol. 2013, 47, 845–856. [Google Scholar] [CrossRef]
- Liu, H.; Leak, R.K.; Hu, X. Neurotransmitter receptors on microglia. Stroke Vasc. Neurol. 2016, 1, 52–58. [Google Scholar] [CrossRef]
- Hogan-Cann, A.D.; Anderson, C.M. Physiological Roles of Non-Neuronal NMDA Receptors. Trends Pharmacol. Sci. 2016, 37, 750–767. [Google Scholar] [CrossRef]
- Tovar, K.R.; Westbrook, G.L. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999, 19, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Shyu, W.C.; Wang, Y.T. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol. Med. 2011, 17, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ding, Q.; Chen, Z.; Yun, H.; Wang, H. Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J. Biol. Chem. 2013, 288, 24151–24159. [Google Scholar] [CrossRef]
- Jhou, J.F.; Tai, H.C. The Study of Postmortem Human Synaptosomes for Understanding Alzheimer’s Disease and Other Neurological Disorders: A Review. Neurol. Ther. 2017, 6, 57–68. [Google Scholar] [CrossRef]
- Höhn, L.; Hußler, W.; Richter, A.; Smalla, K.H.; Birkl-Toeglhofer, A.M.; Birkl, C.; Vielhaber, S.; Leber, S.L.; Gundelfinger, E.D.; Haybaeck, J.; et al. Extracellular Matrix Changes in Subcellular Brain Fractions and Cerebrospinal Fluid of Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2023, 24, 5532. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.M.; Kim, P.; Meador-Woodruff, J.H. Fractionation of Subcellular Compartments from Human Brain Tissue. Methods Mol. Biol. 2019, 1941, 201–223. [Google Scholar] [CrossRef]
- Luabeya, M.K.; Vanisberg, M.A.; Jeanjean, A.P.; Baudhuin, P.; Laduron, P.M.; Maloteaux, J.M. Fractionation of human brain by differential and isopycnic equilibration techniques. Brain Res. Brain Res. Protoc. 1997, 1, 83–90. [Google Scholar] [CrossRef]
- Maloteaux, J.M.; Luabeya, M.K.; Vanisberg, M.A.; Jeanjean, A.P.; Baudhuin, P.; Scherman, D.; Laduron, P.M. Subcellular distribution of receptor sites in human brain: Differentiation between heavy and light structures of high and low density. Brain Res. 1995, 687, 155–166. [Google Scholar] [CrossRef]
- Zhang, W.; Ross, P.J.; Ellis, J.; Salter, M.W. Targeting NMDA receptors in neuropsychiatric disorders by drug screening on human neurons derived from pluripotent stem cells. Transl. Psychiatry 2022, 12, 243. [Google Scholar] [CrossRef]
- Lao, K.; Ji, N.; Zhang, X.; Qiao, W.; Tang, Z.; Gou, X. Drug development for Alzheimer’s disease: Review. J. Drug Target. 2019, 27, 164–173. [Google Scholar] [CrossRef] [PubMed]
| mRNA Levels | ||||||||
| Reference | Year | Technique | Brain Area | Sample Size (AD Braak Stage) | Levels with Respect to Control | Cell Type (When Specified) | ||
| GRIN1 | GRIN2A | GRIN2B | ||||||
| [139] | 2001 | qPCR | Temporal and cingulate cortex | 10 (no Braak specified) | Down | N/A | N/A | |
| [138] | 2004 | qPCR | Hippocampus, anterior cingulate gyrus, and superior temporal cortex | 10 (no Braak specified) | Down | Down | Down | |
| [132] | 2004 | qPCR | Hippocampus | 10 (I–II); 10 (III–IV); 10 (V–VI) | Down | No change | Down | |
| [137] | 2002 | qPCR | Hippocampus | 10 (no Braak specified) | Down | Down | ||
| [142] | 2010 | Microarray | Prefrontal cortex | 14 (I–II); 14 (III–IV); 14 (V–VI) | Down | |||
| [141] | 2019 | snRNAseq | Prefrontal cortex | 10 (I–II); 21 (III–IV); 17 (V–VI) | Up at early stages but down at late stages | Down | No change | Excitatory neurons |
| [140] | 2024 | RNAseq | Superior temporal gyrus | 10 (V–VI) | No change | No change | No change | |
| [134] | 2020 | RNAseq | Prefrontal cortex | 12 (IV–VI) | Up | Up | Endothelial cells | |
| Down | Down | Oligodendrocytes | ||||||
| Total Protein Levels | ||||||||
| Reference | Year | Technique | Brain Area | Sample Size (AD Braak Stage) | Levels with Respect to Control | Cell Type (When Specified) | ||
| GluN1 | GluN2A | GluN2B | ||||||
| [138] | 2004 | WB | Hippocampus, anterior cingulate gyrus, and superior temporal cortex | 10 (no Braak specified) | Down | Down | ||
| [132] | 2004 | WB | Hippocampus | Down | Up (in early stage) | Down | ||
| [146] | 2013 | Quantitative autoradiography | Hippocampus | 23 (IV–VI) | General NMDAR reduction | General NMDAR reduction | General NMDAR reduction | |
| [144] | 2001 | WB | Entorhinal cx | 6 (III–VI) | No change | Down | Down | |
| Hippocampus | Down | No change | Down | |||||
| Caudate | No change | No change | No change | |||||
| Occipital cortex | No change | No change | No change | |||||
| [147] | 2021 | Quantitative confocal microscopy | Hippocampus | 8 (IV–VI) | Up | Up | General and specifically in astrocytes | |
| [133] | 2000 | WB | Hippocampus | 6 (no Braak specified) | Down | No change | Down | |
| Frontal cx | Down | Down | Down | |||||
| Entorhinal cx | No change | No change | No change | |||||
| Tauopathy Mice Models | ||||||||||
| Reference | Year | Technique | Criterion SynNMDAR | Criterion ExsynNMDAR | Model/Cell Culture Treatment | NMDARs Levels Respect to WT or Control | Observations | Other Findings | ||
| SynNMDAR | ExsynNMDAR | Total NMDAR | ||||||||
| [120] | 2019 | Microscopy | Y1472-GluN3B | Y1336-GluN3B | tau KO mice | No change | No change | No change | Hippocampus | tau KO lacks ExsynNMDAR currents |
| [112] | 2010 | Biochemical | Solubility in SDS | Solubility in pH 8 | tau KO mice | Down | Up | No change | Hippocampus | |
| [113] | 2023 | SDS-FRL | (Self-developed semi-automatic software) Dendritic spines were considered as such if (1) they emerged from a dendritic shaft or (2) they opposed an axon terminal recognized by the presence of synaptic vesicles on their cross-fractured portions | Non-specific background labeling was measured on E-face structures surrounding the measured P-faces (specific staining surrounding spines) | Tg P301S mice | No change * | Up ** | * In excitatory neurons, decreased SynNMDARs but unaltered ExsynGluN1 | ** Specifically in interneuron dendrites of the stratum oriens | |
| [41] | 2022 | Microscopy | Colocalization with PSD95 | The rest | Neurons treated with tau from AD brain tau for 7 days | Down | Up | Down | Mouse cultured hippocampal neurons | |
| Amyloidosis Mice Models | ||||||||||
| Reference | Year | Technique | Criterion Syn NMDAR | Criterion ExsynNMDAR | Treatment/Model | NMDARs Level Respect to WT or Control | Observations | Other Findings | ||
| SynNMDAR | ExsynNMDAR | Total NMDAR | ||||||||
| [43] | 2005 | Microscopy | Colocalization with synapsin | No colocalization with synapsin | Cultured cortical neurons treated with Aβ 1 h | Down GluN1 | Suggests redistribution to extrasynaptic membranes | Detect reduced GluN1 in surface levels but no changes in total levels. Suggests redistribution to extrasynaptic membranes. | ||
| Biotinylation | No change | Reduced surface expression of GluN2B and GluN1, no change in total levels | ||||||||
| [44] | 2011 | Biochemical | Triton soluble fraction | Triton insoluble fraction | Mice slices treated with Aβ -> fractionation | Down GluN2B | No change | |||
| Microscopy | Colocalization with synapsin | No colocalization with synapsin | Cultured hippocampal neurons + Aβ | Down GluN2B | No change | |||||
| [52] | 2023 | Biochemical | Triton insolubility | Triton solubility | APP/PS1 mouse | Down GluN2B | Up GluN2B | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla, S.; Sáez-Valero, J.; Cuchillo-Ibáñez, I. NMDARs in Alzheimer’s Disease: Between Synaptic and Extrasynaptic Membranes. Int. J. Mol. Sci. 2024, 25, 10220. https://doi.org/10.3390/ijms251810220
Escamilla S, Sáez-Valero J, Cuchillo-Ibáñez I. NMDARs in Alzheimer’s Disease: Between Synaptic and Extrasynaptic Membranes. International Journal of Molecular Sciences. 2024; 25(18):10220. https://doi.org/10.3390/ijms251810220
Chicago/Turabian StyleEscamilla, Sergio, Javier Sáez-Valero, and Inmaculada Cuchillo-Ibáñez. 2024. "NMDARs in Alzheimer’s Disease: Between Synaptic and Extrasynaptic Membranes" International Journal of Molecular Sciences 25, no. 18: 10220. https://doi.org/10.3390/ijms251810220
APA StyleEscamilla, S., Sáez-Valero, J., & Cuchillo-Ibáñez, I. (2024). NMDARs in Alzheimer’s Disease: Between Synaptic and Extrasynaptic Membranes. International Journal of Molecular Sciences, 25(18), 10220. https://doi.org/10.3390/ijms251810220





