Ascochlorin Attenuates the Early Stage of Adipogenesis via the Wnt/β-Catenin Pathway and Inhibits High-Fat-Diet-Induced Obesity in Mice
Abstract
1. Introduction
2. Results
2.1. ASC Inhibits Lipid Accumulation in 3T3-L1 Preadipocytes
2.2. ASC Affects the Expression of Key Transcriptional Regulators of Adipogenesis in 3T3-L1 Cells
2.3. ASC Affects the Cell Cycle and Transcriptional Regulation during the Differentiation of 3T3-L1 Cells
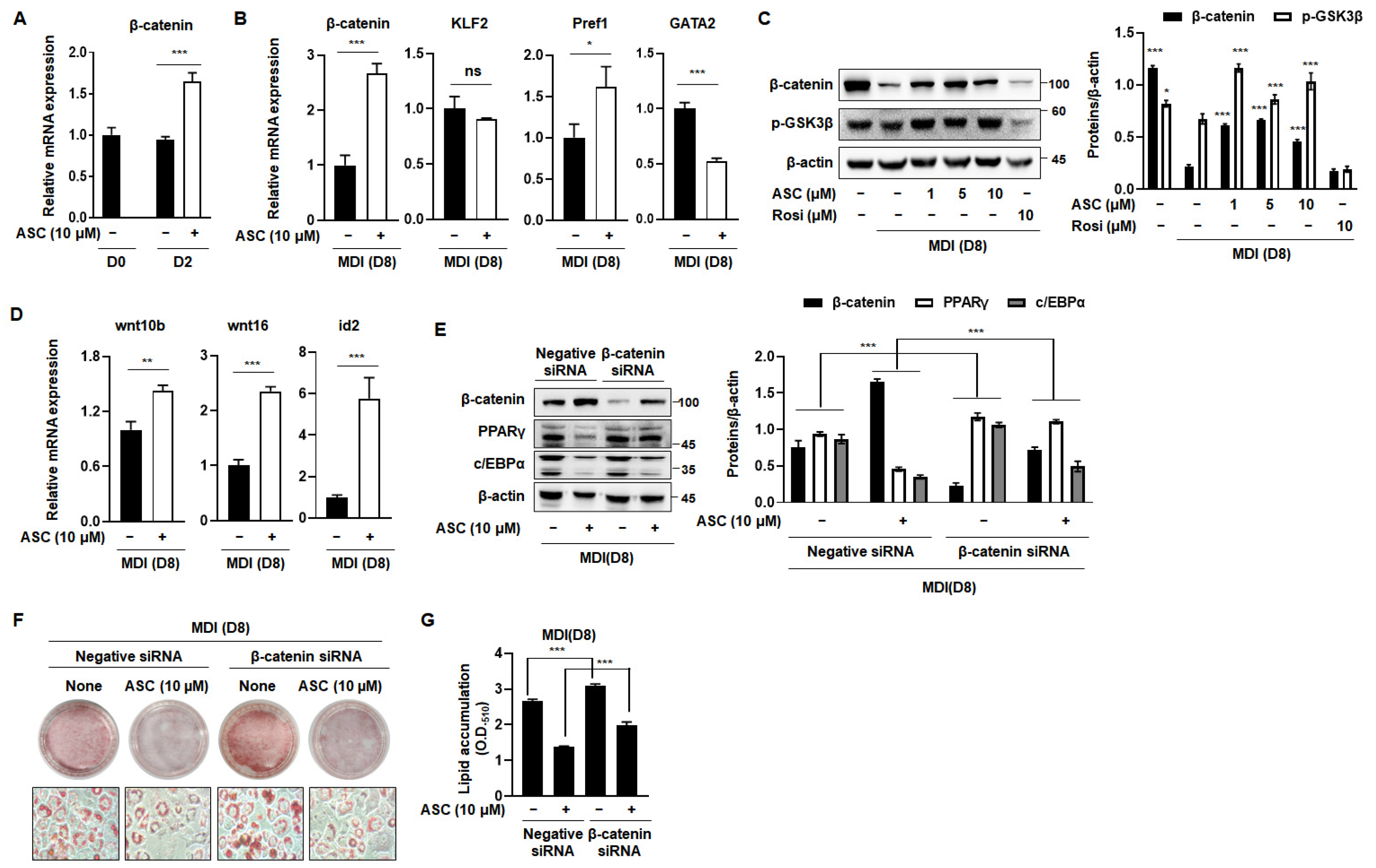
2.4. ASC Regulates Β-Catenin Expression and Inhibits Adipogenesis in MDI-Treated 3T3-L1 Cells
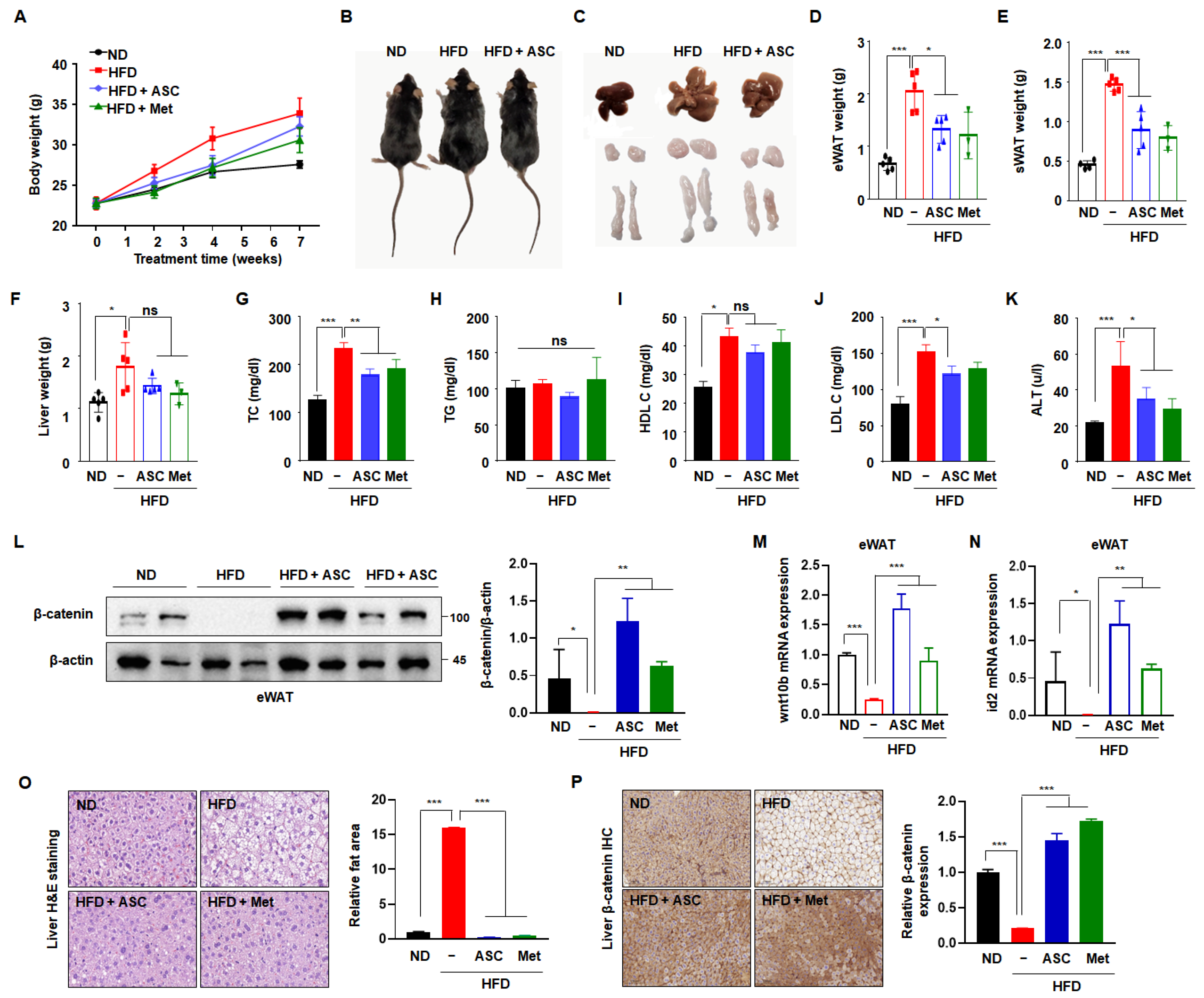
2.5. ASC Ameliorates HFD-Induced Obesity
3. Discussion
4. Materials and Methods
4.1. Cells and Materials
4.2. Cell Viability Assay
4.3. Oil Red O Staining Analysis of Adipocytes
4.4. Triglyceride Assay
4.5. BODIPY Staining Assay
4.6. Real-Time Polymerase Chain Reaction (RT-PCR) Assay
4.7. Immunoblotting Assay
4.8. Cell Cycle Analysis
4.9. siRNA Transfection
4.10. Animals
4.11. Immunohistochemistry (IHC)
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2020, 10, 615375. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Mammi, C.; Marzolla, V.; Calanchini, M.; Antelmi, A.; Rosano, G.M.; Fabbri, A.; Caprio, M. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. J. Cell. Biochem. 2010, 110, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Fernyhough, M.E.; Okine, E.; Hausman, G.; Vierck, J.L.; Dodson, M.V. PPARγ and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest. Anim. Endocrinol. 2007, 33, 367–378. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–224. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef]
- De Winter, T.J.; Nusse, R. Running against the Wnt: How Wnt/β-catenin suppresses adipogenesis. Front. Cell Dev. Biol. 2021, 9, 627429. [Google Scholar] [CrossRef]
- Chen, T.-X.; Cheng, X.-Y.; Wang, Y.; Yin, W. Toosendanin inhibits adipogenesis by activating Wnt/β-catenin signaling. Sci. Rep. 2018, 8, 4626. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Liang, F.; Zhu, Q.; Cai, S.; Tong, X.; He, Z.; Liu, X.; Chen, Y.; Mo, D. HMGB2 orchestrates mitotic clonal expansion by binding to the promoter of C/EBPβ to facilitate adipogenesis. Cell Death Dis. 2021, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Yamamoto, A.; Yoneda, T.; Hori, S.; Okochi, N.; Kagotani, K.; Okumura, K.; Takebayashi, S.-I. Reorganization of the DNA replication landscape during adipogenesis is closely linked with adipogenic gene expression. J. Cell Sci. 2023, 136, jcs260778. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, H.-D.; Lee, S.-H.; Kwak, C.-H.; Chang, Y.-C.; Lee, Y.-C.; Chung, T.-W.; Magae, J.; Kim, C.-H. Ascochlorin induces caspase-independent necroptosis in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2019, 239, 111898. [Google Scholar] [CrossRef] [PubMed]
- Min-Wen, J.C.; Yan-Jiang, B.C.; Mishra, S.; Dai, X.; Magae, J.; Shyh-Chang, N.; Kumar, A.P.; Sethi, G. Molecular targets of ascochlorin and its derivatives for cancer therapy. Adv. Protein Chem. Struct. Biol. 2017, 108, 199–225. [Google Scholar] [PubMed]
- Kang, J.H.; Kim, J.K.; Park, W.H.; Park, K.K.; Lee, T.S.; Magae, J.; Nakajima, H.; Kim, C.H.; Chang, Y.C. Ascochlorin suppresses oxLDL-induced MMP-9 expression by inhibiting the MEK/ERK signaling pathway in human THP-1 macrophages. J. Cell. Biochem. 2007, 102, 506–514. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–833. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, C.H.; Lee, S.K.; Ha, S.H.; Park, J.; Chung, T.W.; Ha, K.T.; Suh, S.J.; Chang, Y.C.; Chang, H.W. Anti-inflammatory effect of ascochlorin in LPS-stimulated RAW 264.7 macrophage cells is accompanied with the down-regulation of iNOS, COX-2 and proinflammatory cytokines through NF-κB, ERK1/2, and p38 signaling pathway. J. Cell. Biochem. 2016, 117, 978–987. [Google Scholar] [CrossRef]
- Choi, Y.R.; Na, H.-J.; Lee, J.; Kim, Y.-S.; Kim, M.J. Isoeugenol Inhibits Adipogenesis in 3T3-L1 Preadipocytes with Impaired Mitotic Clonal Expansion. Nutrients 2024, 16, 1262. [Google Scholar] [CrossRef]
- Raciti, G.A.; Fiory, F.; Campitelli, M.; Desiderio, A.; Spinelli, R.; Longo, M.; Nigro, C.; Pepe, G.; Sommella, E.; Campiglia, P. Citrus aurantium L. dry extracts promote C/ebpβ expression and improve adipocyte differentiation in 3T3-L1 cells. PLoS ONE 2018, 13, e0193704. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Y.-Y.; Li, B.-Y.; Peng, W.-Q.; Tang, Q.-Q. Histone demethylase KDM5A is transactivated by the transcription factor C/EBPβ and promotes preadipocyte differentiation by inhibiting Wnt/β-catenin signaling. J. Biol. Chem. 2019, 294, 9642–9654. [Google Scholar] [CrossRef]
- Feng, S.; Reuss, L.; Wang, Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Shaik Mohamed Sayed, U.F.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef] [PubMed]
- Kurek, J.M.; Zielińska-Wasielica, J.; Kowalska, K.; Krejpcio, Z.; Olejnik, A. Modulating effects of steviol and steviol glycosides on adipogenesis, lipogenesis, glucose uptake and insulin resistance in 3T3-L1 adipocyte model. J. Funct. Foods 2022, 94, 105141. [Google Scholar] [CrossRef]
- Kim, M.; Cho, H.J.; Jeong, Y.J.; Chung, I.K.; Magae, J.; Chang, Y.C. 4-O-methylascochlorin suppresses differentiation of 3T3-L1 preadipocytes by inhibiting PPARgamma expression through regulation of AMPK/mTOR signaling pathways. Arch. Biochem. Biophys. 2015, 583, 79–86. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Vasileva, L.V.; Savova, M.S. Antiobesity molecules of natural origin: Call for lead finding acceleration. Food Front. 2021, 2, 23–24. [Google Scholar] [CrossRef]
- Goshu, B.T. Molecular modulators of adipogenesis as treatment options for obesity. Preprints 2021, 2021060620. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Feinberg, M.W.; Watanabe, M.; Gray, S.; Haspel, R.L.; Denkinger, D.J.; Kawahara, R.; Hauner, H.; Jain, M.K. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J. Biol. Chem. 2003, 278, 2581–2584. [Google Scholar] [CrossRef]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, B.-Y.; Choi, S.-H.; Jung, T.-D.; Choi, S.-I.; Lim, J.-H.; Lee, O.-H. Sulforaphane attenuates bisphenol A-induced 3T3-L1 adipocyte differentiation through cell cycle arrest. J. Funct. Foods 2018, 44, 17–23. [Google Scholar] [CrossRef]
- Nusse, R.; Fuerer, C.; Ching, W.; Harnish, K.; Logan, C.; Zeng, A.; ten Berge, D.; Kalani, Y. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef] [PubMed]



| Genes | Sense Primer (5′–3′) | Antisense Primer (5′–3′) |
|---|---|---|
| PPAR-γ | ATCAAAGTAGAACCTGCATC | ACCCTTGCATCCTTCACAAG |
| C/EBP-α | GAACAGCAACGAGTACCGGGT | CCCATGGCCTTGACCAAGGAG |
| pref-1 | TGGCTGTGTCAATGGAGTCTGC | CCACGCAAGTTCCATTGTTGGC |
| GATA2 | CTTCAACCATCTCGACTCGCAG | GCAACAAGTGTGGTCGGCACAT |
| β-catenin | GTTCGCCTTCATTATGGACTGCC | ATAGCACCCTGTTCCCGCAAAG |
| Wnt10b | ACCACGACATGGACTTCGGAGA | CCGCTTCAGGTTTTCCGTTACC |
| Wnt16 | CCCTCTTTGGCTATGAGCTGAG | GGTGGTTTCACAGGAACATTCGG |
| Tcf7l2 | CGCTGACAGTCAACGCATCTATG | GGAGGATTCCTGCTTGACTGTC |
| Axin2 | ATGGAGTCCCTCCTTACCGCAT | GTTCCACAGGCGTCATCTCCTT |
| Nkd1 | GCCTGAGAAGATTGACAGCCTAG | CTCCACAGAGACATCACACTGC |
| β-actin | AGGCCCAGAGCAAGAGAGGTA | CCATGTCGTCCCAGTTGGTAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.-H.; Jeong, Y.-J.; Son, S.W.; Kwon, S.Y.; Song, K.-H.; Son, H.-S.; Jeon, E.-J.; Chang, Y.-C. Ascochlorin Attenuates the Early Stage of Adipogenesis via the Wnt/β-Catenin Pathway and Inhibits High-Fat-Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2024, 25, 10226. https://doi.org/10.3390/ijms251810226
Yu M-H, Jeong Y-J, Son SW, Kwon SY, Song K-H, Son H-S, Jeon E-J, Chang Y-C. Ascochlorin Attenuates the Early Stage of Adipogenesis via the Wnt/β-Catenin Pathway and Inhibits High-Fat-Diet-Induced Obesity in Mice. International Journal of Molecular Sciences. 2024; 25(18):10226. https://doi.org/10.3390/ijms251810226
Chicago/Turabian StyleYu, Mi-Hee, Yun-Jeong Jeong, Sung Wook Son, So Yoon Kwon, Kwon-Ho Song, Ho-Sang Son, Eon-Ju Jeon, and Young-Chae Chang. 2024. "Ascochlorin Attenuates the Early Stage of Adipogenesis via the Wnt/β-Catenin Pathway and Inhibits High-Fat-Diet-Induced Obesity in Mice" International Journal of Molecular Sciences 25, no. 18: 10226. https://doi.org/10.3390/ijms251810226
APA StyleYu, M.-H., Jeong, Y.-J., Son, S. W., Kwon, S. Y., Song, K.-H., Son, H.-S., Jeon, E.-J., & Chang, Y.-C. (2024). Ascochlorin Attenuates the Early Stage of Adipogenesis via the Wnt/β-Catenin Pathway and Inhibits High-Fat-Diet-Induced Obesity in Mice. International Journal of Molecular Sciences, 25(18), 10226. https://doi.org/10.3390/ijms251810226






