Bioinformatics Identification and Expression Analysis of Acetyl-CoA Carboxylase Reveal Its Role in Isoflavone Accumulation during Soybean Seed Development
Abstract
1. Introduction
2. Results
2.1. Identification of ACC Genes from Soybean Genome
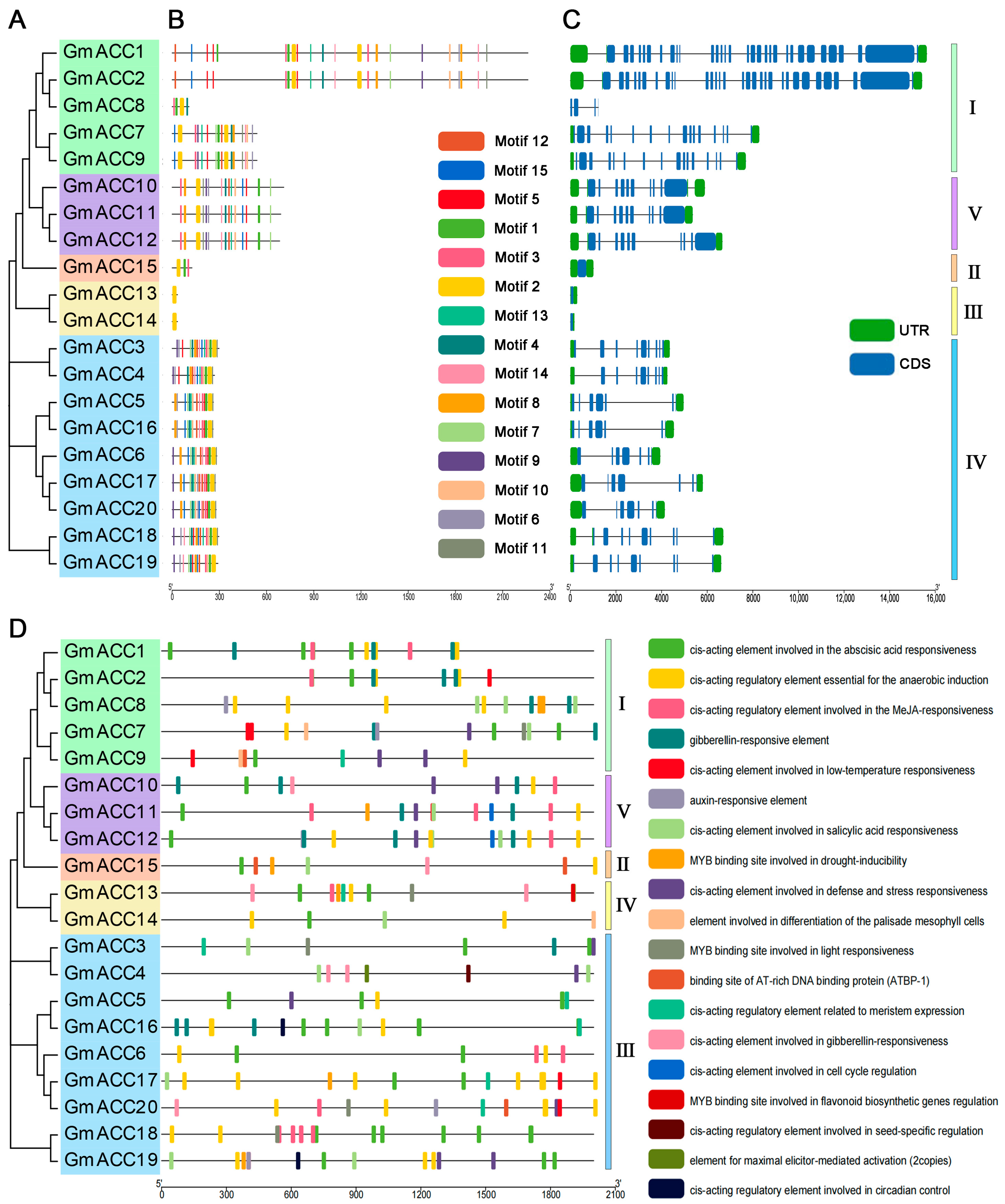
2.2. Phylogenetic Analysis of GmACCs
2.3. Conserved Motifs and Gene Structure Analysis of GmACCs
2.4. Cis-Acting Element Analysis of GmACC Gene Promoters
2.5. Collinearity and Amino Acid Substitution Selection Pressure Analyses of GmACCs
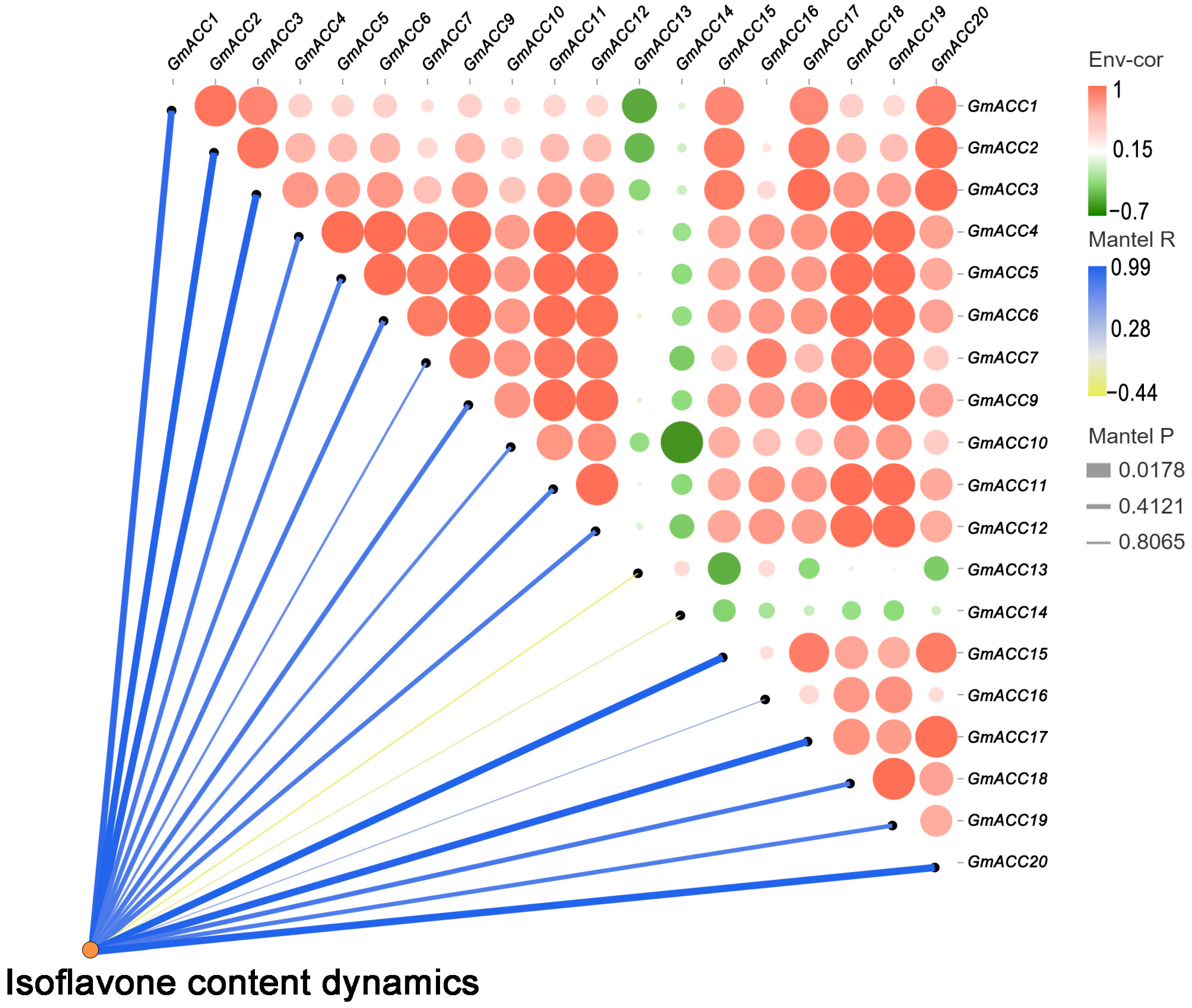
2.6. Expression Pattern Analysis of GmACCs
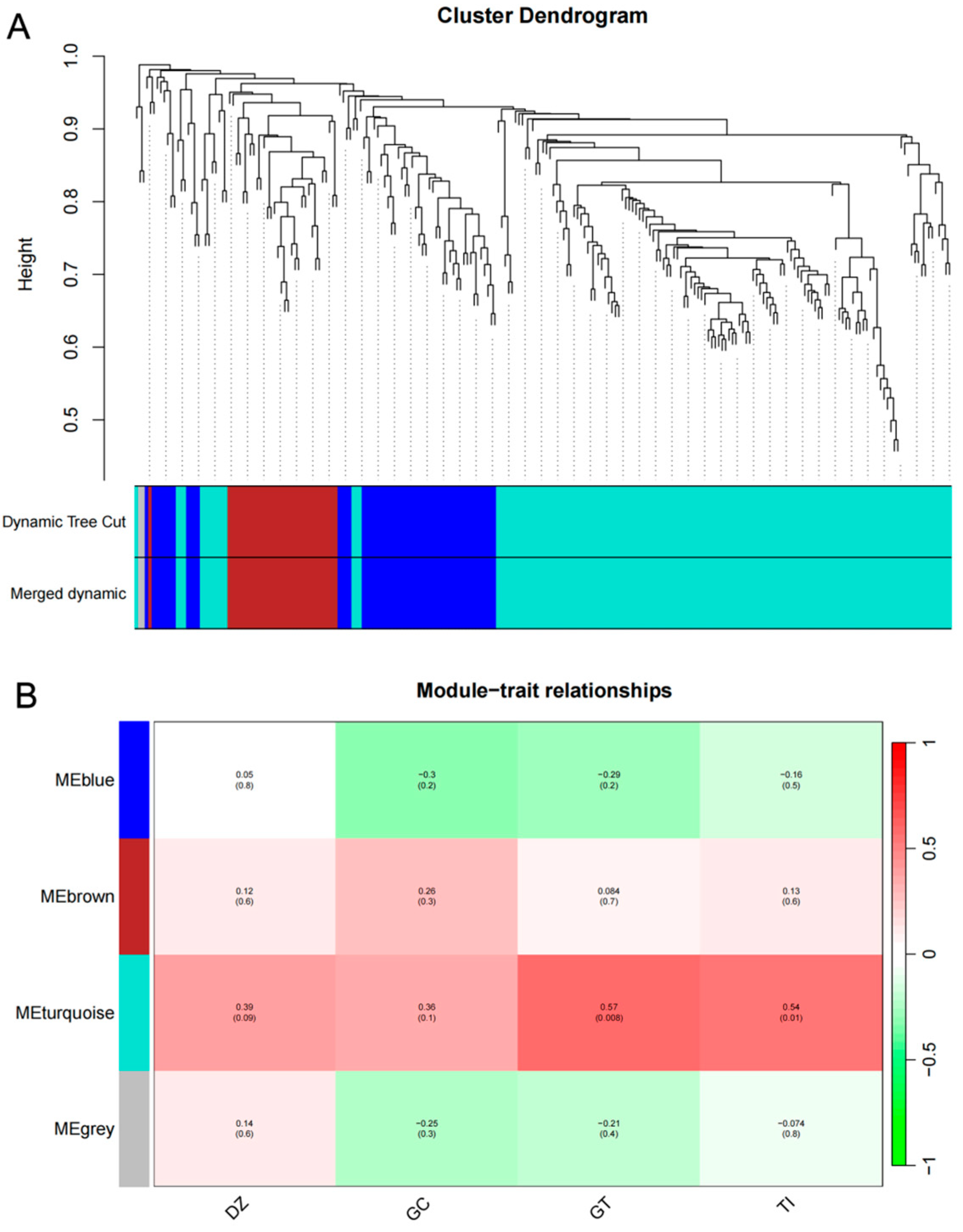
2.7. Construction and Analysis of Weighted Gene Co-Expression Networks
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of ACC Genes in Soybean
4.2. Prediction of Physicochemical Properties of GmACC Protein
4.3. Construction of a Phylogenetic Tree
4.4. Gene Structure, Conserved Motif Analysis, and Promoter Cis-Regulatory Element Characterization of the GmACC Genes
4.5. Collinearity Analysis and Selective Pressure for Duplicated Genes
4.6. Plant Materials and Gene Expression Analysis
4.7. Total RNA Isolation and RT-qPCR Expression Analysis
4.8. Transcriptome Sequencing Analysis and Correlation Analysis
4.9. Analysis of Weighted Gene Co-Expression Networks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mateos-Aparicio, I.; Redondo Cuenca, A.; Villanueva-Suárez, M.J.; Zapata-Revilla, M.A. Soybean, a Promising Health Source. Nutr. Hosp. 2008, 23, 305–312. [Google Scholar] [PubMed]
- Azam, M.; Zhang, S.; Abdelghany, A.M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Seed Isoflavone Profiling of 1168 Soybean Accessions from Major Growing Ecoregions in China. Food Res. Int. 2020, 130, 108957. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Wei, X.; Sun, Y.; Dong, S. Molecular Mechanism of Drought Resistance in Soybean Roots Revealed Using Physiological and Multi-Omics Analyses. Plant Physiol. Biochem. 2024, 208, 108451. [Google Scholar] [CrossRef] [PubMed]
- Horitani, M.; Yamada, R.; Taroura, K.; Maeda, A.; Anai, T.; Watanabe, S. Identification of Genes Responsible for the Synthesis of Glycitein Isoflavones in Soybean Seeds. Plants 2024, 13, 156. [Google Scholar] [CrossRef]
- Zeng, G.; Li, D.; Han, Y.; Teng, W.; Wang, J.; Qiu, L.; Li, W. Identification of QTL Underlying Isoflavone Contents in Soybean Seeds among Multiple Environments. Theor. Appl. Genet. 2009, 118, 1455–1463. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Zhao, X.; Li, Y.; Teng, W.; Li, D.; Zhan, Y.; Li, W. Mapping Isoflavone QTL with Main, Epistatic and QTL × Environment Effects in Recombinant Inbred Lines of Soybean. PLoS ONE 2015, 10, e0118447. [Google Scholar] [CrossRef] [PubMed]
- Yoshiara, L.Y.; Madeira, T.B.; Delaroza, F.; da Silva, J.B.; Ida, E.I. Optimization of Soy Isoflavone Extraction with Different Solvents Using the Simplex-Centroid Mixture Design. Int. J. Food Sci. Nutr. 2012, 63, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Anguraj Vadivel, A.K.; McDowell, T.; Renaud, J.B.; Dhaubhadel, S. A Combinatorial Action of GmMYB176 and GmbZIP5 Controls Isoflavonoid Biosynthesis in Soybean (Glycine Max). Commun. Biol. 2021, 4, 356. [Google Scholar] [CrossRef]
- Graham, T.L. Flavonoid and Isoflavonoid Distribution in Developing Soybean Seedling Tissues and in Seed and Root Exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef]
- Dwivedi, S.; Singh, V.; Sharma, K.; Sliti, A.; Baunthiyal, M.; Shin, J.-H. Significance of Soy-Based Fermented Food and Their Bioactive Compounds Against Obesity, Diabetes, and Cardiovascular Diseases. Plant Foods Hum. Nutr. 2024, 79, 1–11. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Wu, X.; Gillman, J.D.; Lee, J.-D.; Zhong, R.; Yu, O.; Shannon, G.; Ellersieck, M.; Nguyen, H.T.; Sleper, D.A. Intricate Environment-Modulated Genetic Networks Control Isoflavone Accumulation in Soybean Seeds. BMC Plant Biol. 2010, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.; Wang, R.-F.; Hou, C.; Yu, T.; Miao, K.; Cao, F.-Y.; Fang, R.-J.; Liu, L. Functional Characterization of Two Chalcone Isomerase (CHI) Revealing Their Responsibility for Anthocyanins Accumulation in Mulberry. Plant Physiol. Biochem. 2021, 161, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Sun, T.; Zhang, C.; Liu, X.; Li, Y. Genome-Wide Classification and Evolutionary Analysis Reveal Diverged Patterns of Chalcone Isomerase in Plants. Biomolecules 2022, 12, 961. [Google Scholar] [CrossRef]
- Anguraj Vadivel, A.K.; Krysiak, K.; Tian, G.; Dhaubhadel, S. Genome-Wide Identification and Localization of Chalcone Synthase Family in Soybean (Glycine Max [L.] Merr) . BMC Plant Biol. 2018, 18, 325. [Google Scholar] [CrossRef]
- Sepiol, C.J.; Yu, J.; Dhaubhadel, S. Genome-Wide Identification of Chalcone Reductase Gene Family in Soybean: Insight into Root-Specific GmCHRs and Phytophthora Sojae Resistance. Front. Plant Sci. 2017, 8, 2073. [Google Scholar] [CrossRef]
- Post-Beittenmiller, D.; Jaworski, J.G.; Ohlrogge, J.B. In Vivo Pools of Free and Acylated Acyl Carrier Proteins in Spinach. Evidence for Sites of Regulation of Fatty Acid Biosynthesis. J. Biol. Chem. 1991, 266, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Shinohara, K.; Yamada, K.; Sasaki, Y. Acetyl-CoA Carboxylase in Higher Plants: Most Plants Other than Gramineae Have Both the Prokaryotic and the Eukaryotic Forms of This Enzyme. Plant Cell Physiol. 1996, 37, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Nagano, Y. Plant Acetyl-CoA Carboxylase: Structure, Biosynthesis, Regulation, and Gene Manipulation for Plant Breeding. Biosci. Biotechnol. Biochem. 2004, 68, 1175–1184. [Google Scholar] [CrossRef]
- Salie, M.J.; Thelen, J.J. Regulation and Structure of the Heteromeric Acetyl-CoA Carboxylase. Biochim. Biophys. Acta 2016, 1861, 1207–1213. [Google Scholar] [CrossRef]
- Kozaki, A.; Mayumi, K.; Sasaki, Y. Thiol-Disulfide Exchange between Nuclear-Encoded and Chloroplast-Encoded Subunits of Pea Acetyl-CoA Carboxylase. J. Biol. Chem. 2001, 276, 39919–39925. [Google Scholar] [CrossRef]
- Nikolau, B.J.; Ohlrogge, J.B.; Wurtele, E.S. Plant Biotin-Containing Carboxylases. Arch. Biochem. Biophys. 2003, 414, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ilarslan, H.; Brachova, L.; Qian, H.-R.; Li, L.; Che, P.; Wurtele, E.S.; Nikolau, B.J. Reverse-Genetic Analysis of the Two Biotin-Containing Subunit Genes of the Heteromeric Acetyl-Coenzyme A Carboxylase in Arabidopsis Indicates a Unidirectional Functional Redundancy. Plant Physiol. 2011, 155, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dunwell, J.M.; Zhang, Y.-M. An Integrated Omics Analysis Reveals Molecular Mechanisms That Are Associated with Differences in Seed Oil Content between Glycine Max and Brassica Napus. BMC Plant Biol. 2018, 18, 328. [Google Scholar] [CrossRef] [PubMed]
- Elborough, K.M.; Winz, R.; Deka, R.K.; Markham, J.E.; White, A.J.; Rawsthorne, S.; Slabas, A.R. Biotin Carboxyl Carrier Protein and Carboxyltransferase Subunits of the Multi-Subunit Form of Acetyl-CoA Carboxylase from Brassica Napus: Cloning and Analysis of Expression during Oilseed Rape Embryogenesis. Biochem. J. 1996, 315, 103–112. [Google Scholar] [CrossRef]
- Yanai, Y.; Kawasaki, T.; Shimada, H.; Wurtele, E.S.; Nikolau, B.J.; Ichikawa, N. Genomic Organization of 251 kDa Acetyl-CoA Carboxylase Genes in Arabidopsis: Tandem Gene Duplication Has Made Two Differentially Expressed Isozymes. Plant Cell Physiol. 1995, 36, 779–787. [Google Scholar] [CrossRef]
- Incledon, B.J.; Hall, J.C. Acetyl-Coenzyme A Carboxylase: Quaternary Structure and Inhibition by Graminicidal Herbicides. Pestic. Biochem. Physiol. 1997, 57, 255–271. [Google Scholar] [CrossRef]
- Chen, Y.; Elizondo-Noriega, A.; Cantu, D.C.; Reilly, P.J. Structural Classification of Biotin Carboxyl Carrier Proteins. Biotechnol. Lett. 2012, 34, 1869–1875. [Google Scholar] [CrossRef]
- Megha, S.; Wang, Z.; Kav, N.N.V.; Rahman, H. Genome-Wide Identification of Biotin Carboxyl Carrier Subunits of Acetyl-CoA Carboxylase in Brassica and Their Role in Stress Tolerance in Oilseed Brassica Napus. BMC Genom. 2022, 23, 707. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Wang, Y.; Liu, Z.; Ijaz, B.; Huang, Y.; Hua, J. Genome-Wide Identification and Expression Analysis of the Biotin Carboxyl Carrier Subunits of Heteromeric Acetyl-CoA Carboxylase in Gossypium. Front. Plant Sci. 2017, 8, 624. [Google Scholar] [CrossRef]
- Alban, C.; Job, D.; Douce, R. Biotin Metabolism in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 17–47. [Google Scholar] [CrossRef]
- Shui, L.; Huo, K.; Chen, Y.; Zhang, Z.; Li, Y.; Niu, J. Integrated Metabolome and Transcriptome Revealed the Flavonoid Biosynthetic Pathway in Developing Vernonia Amygdalina Leaves. PeerJ 2021, 9, e11239. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Mooney, B.P.; Hajduch, M.; Joshi, T.; Zhou, M.; Xu, D.; Thelen, J.J. System Analysis of an Arabidopsis Mutant Altered in de Novo Fatty Acid Synthesis Reveals Diverse Changes in Seed Composition and Metabolism. Plant Physiol. 2009, 150, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The Flavonoid Biosynthetic Pathway in Arabidopsis: Structural and Genetic Diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.; Lim, K.-H.; Saw, P.-N.; Koffas, M.A.G. Engineering Central Metabolic Pathways for High-Level Flavonoid Production in Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 3877–3886. [Google Scholar] [CrossRef]
- Zong, Y.; Li, S.; Xi, X.; Cao, D.; Wang, Z.; Wang, R.; Liu, B. Comprehensive Influences of Overexpression of a MYB Transcriptor Regulating Anthocyanin Biosynthesis on Transcriptome and Metabolome of Tobacco Leaves. Int. J. Mol. Sci. 2019, 20, 5123. [Google Scholar] [CrossRef]
- Yan, F.; Githiri, S.M.; Liu, Y.; Sang, Y.; Wang, Q.; Takahashi, R. Loss-of-Function Mutation of Soybean R2R3 MYB Transcription Factor Dilutes Tawny Pubescence Color. Front. Plant Sci. 2019, 10, 1809. [Google Scholar] [CrossRef]
- Amato, A.; Cardone, M.F.; Ocarez, N.; Alagna, F.; Ruperti, B.; Fattorini, C.; Velasco, R.; Mejía, N.; Zenoni, S.; Bergamini, C. VviAGL11 Self-Regulates and Targets Hormone- and Secondary Metabolism-Related Genes during Seed Development. Hortic. Res. 2022, 9, uhac133. [Google Scholar] [CrossRef]
- Cooper, B.; Campbell, K.B.; Garrett, W.M. Salicylic Acid and Phytoalexin Induction by a Bacterium That Causes Halo Blight in Beans. Phytopathology® 2022, 112, 1766–1775. [Google Scholar] [CrossRef]
- Sreedasyam, A.; Plott, C.; Hossain, M.S.; Lovell, J.T.; Grimwood, J.; Jenkins, J.W.; Daum, C.; Barry, K.; Carlson, J.; Shu, S.; et al. JGI Plant Gene Atlas: An Updateable Transcriptome Resource to Improve Functional Gene Descriptions across the Plant Kingdom. Nucleic Acids Res. 2023, 51, 8383–8401. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Holub, E.B. The Arms Race Is Ancient History in Arabidopsis, the Wildflower. Nat. Rev. Genet. 2001, 2, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Clarke, J.D.; Zhang, Y.; Dong, X. Activation of an EDS1-Mediated R-Gene Pathway in the Snc1 Mutant Leads to Constitutive, NPR1-Independent Pathogen Resistance. Mol. Plant Microbe Interact. 2001, 14, 1131–1139. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, G.; Jiang, X.; Cao, S.; Chen, Z.J.; Song, Q. Altered Chromatin Architecture and Gene Expression during Polyploidization and Domestication of Soybean. Plant Cell 2021, 33, 1430–1446. [Google Scholar] [CrossRef]
- Sun, S.; Wang, B.; Jiang, Q.; Li, Z.; Jia, S.; Wang, Y.; Guo, H. Genome-Wide Analysis of BpDof Genes and the Tolerance to Drought Stress in Birch (Betula Platyphylla). PeerJ 2021, 9, e11938. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, C.; Pei, X.; Wang, S.; Huang, Y.; Li, J.; Liu, B.; Kong, F.; Yang, Q.-Y.; Fang, C. SoyMD: A Platform Combining Multi-Omics Data with Various Tools for Soybean Research and Breeding. Nucleic Acids Res. 2024, 52, D1639–D1650. [Google Scholar] [CrossRef]
- Wakil, S.J. Enzymatic Synthesis of Fatty Acids. Comp. Biochem. Physiol. 1962, 4, 123–158. [Google Scholar] [CrossRef]
- Katagiri, T.; Amao, Y. Recent Advances in Light-Driven C–H Bond Activation and Building C–C Bonds with CO2 as a Feedstock for Carbon Capture and Utilization Technology. Green Chem. 2020, 22, 6682–6713. [Google Scholar] [CrossRef]
- Li, S.J.; Cronan, J.E. The Genes Encoding the Two Carboxyltransferase Subunits of Escherichia Coli Acetyl-CoA Carboxylase. J. Biol. Chem. 1992, 267, 16841–16847. [Google Scholar] [CrossRef]
- Belkebir, A.; Paepe, R.D.; Tremolieres, A.; Aid, F.; Benhassaine-Kesri, G. Sethoxydim Affects Lipid Synthesis and Acetyl-CoA Carboxylase Activity in Soybean. J. Exp. Bot. 2006, 57, 3553–3562. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Pu, X.; Gao, R.; Demurtas, O.C.; Fleck, S.J.; Richter, M.; He, C.; Ji, A.; Sun, W.; Kong, J.; et al. Tandem Gene Duplications Drive Divergent Evolution of Caffeine and Crocin Biosynthetic Pathways in Plants. BMC Biol. 2020, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Barakat, A.; Guyot, R.; Cooke, R.; Delseny, M. Extensive Duplication and Reshuffling in the Arabidopsis Genome. Plant Cell 2000, 12, 1093–1102. [Google Scholar] [CrossRef]
- Lu, L.; Hou, Q.; Wang, L.; Zhang, T.; Zhao, W.; Yan, T.; Zhao, L.; Li, J.; Wan, X. Genome-Wide Identification and Characterization of Polygalacturonase Gene Family in Maize (Zea Mays L.). Int. J. Mol. Sci. 2021, 22, 10722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, R.; Jiang, K.-W.; Qi, J.; Hu, Y.; Guo, J.; Zhu, R.; Zhang, T.; Egan, A.N.; Yi, T.-S.; et al. Nuclear Phylotranscriptomics and Phylogenomics Support Numerous Polyploidization Events and Hypotheses for the Evolution of Rhizobial Nitrogen-Fixing Symbiosis in Fabaceae. Mol. Plant 2021, 14, 748–773. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Allen, F.L.; Johnson, R.D.; Pantalone, V.R.; Sams, C.E. Correlations of Oil and Protein with Isoflavone Concentration in Soybean [Glycine Max (L.) Merr.]. J. Agric. Food Chem. 2005, 53, 7128–7135. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Li, D.; Wu, J.; Li, X.; Yang, Y. Overexpression of FAD2 Promotes Seed Germination and Hypocotyl Elongation in Brassica Napus. Plant Cell Tiss. Organ Cult. 2010, 102, 205–211. [Google Scholar] [CrossRef]
- Shivaiah, K.-K.; Ding, G.; Upton, B.; Nikolau, B.J. Non-Catalytic Subunits Facilitate Quaternary Organization of Plastidic Acetyl-CoA Carboxylase1[OPEN]. Plant Physiol. 2020, 182, 756–775. [Google Scholar] [CrossRef]
- Wang, M.; Garneau, M.G.; Poudel, A.N.; Lamm, D.; Koo, A.J.; Bates, P.D.; Thelen, J.J. Overexpression of Pea α-Carboxyltransferase in Arabidopsis and Camelina Increases Fatty Acid Synthesis Leading to Improved Seed Oil Content. Plant J. 2022, 110, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, Z.; Zhao, Y. Overexpression of Heteromeric GhACCase Subunits Enhanced Oil Accumulation in Upland Cotton | Plant Molecular Biology Reporter. Plant Mol. Biol. Rep. 2017, 35, 287–297. [Google Scholar] [CrossRef]
- Salie, M.J.; Zhang, N.; Lancikova, V.; Xu, D.; Thelen, J.J. A Family of Negative Regulators Targets the Committed Step of de Novo Fatty Acid Biosynthesis. Plant Cell 2016, 28, 2312–2325. [Google Scholar] [CrossRef]
- Madoka, Y.; Tomizawa, K.-I.; Mizoi, J.; Nishida, I.; Nagano, Y.; Sasaki, Y. Chloroplast Transformation with Modified accD Operon Increases Acetyl-CoA Carboxylase and Causes Extension of Leaf Longevity and Increase in Seed Yield in Tobacco. Plant Cell Physiol. 2002, 43, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Misra, P.; Khan, M.P.; Swarnkar, G.; Tewari, M.C.; Bhambhani, S.; Trivedi, R.; Chattopadhyay, N.; Trivedi, P.K. Co-Expression of Arabidopsis Transcription Factor, AtMYB12, and Soybean Isoflavone Synthase, GmIFS1, Genes in Tobacco Leads to Enhanced Biosynthesis of Isoflavones and Flavonols Resulting in Osteoprotective Activity. Plant Biotechnol. J. 2014, 12, 69–80. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Li, Y.; Wang, X.; Pan, X.; Wang, F.; Zhang, Q. Developmental Dynamic Transcriptome and Systematic Analysis Reveal the Major Genes Underlying Isoflavone Accumulation in Soybean. Front. Plant Sci. 2023, 14, 1014349. [Google Scholar] [CrossRef] [PubMed]
- D’Agostina, A.; Boschin, G.; Resta, D.; Annicchiarico, P.; Arnoldi, A. Changes of Isoflavones during the Growth Cycle of Lupinus Albus. J. Agric. Food Chem. 2008, 56, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Dhaubhadel, S.; Gijzen, M.; Moy, P.; Farhangkhoee, M. Transcriptome Analysis Reveals a Critical Role of CHS7 and CHS8 Genes for Isoflavonoid Synthesis in Soybean Seeds. Plant Physiol. 2007, 143, 326–338. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Li, J.; Ahsan, M.; Agyenim-Boateng, K.G.; Qi, J.; Feng, Y.; Liu, Y.; Li, B.; Qiu, L.; et al. Identification of Hub Genes Regulating Isoflavone Accumulation in Soybean Seeds via GWAS and WGCNA Approaches. Front. Plant Sci. 2023, 14, 1120498. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Huai, Y.; Abdelghany, A.M.; Shaibu, A.S.; Qi, J.; Feng, Y.; Liu, Y.; Li, J.; Qiu, L.; et al. Identification of Genes for Seed Isoflavones Based on Bulk Segregant Analysis Sequencing in Soybean Natural Population. Theor. Appl. Genet. 2023, 136, 13. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, S.; Li, J.; Pei, R.; Tian, L.; Qi, J.; Azam, M.; Agyenim-Boateng, K.G.; Shaibu, A.S.; Liu, Y.; et al. Dual-Function C2H2-Type Zinc-Finger Transcription Factor GmZFP7 Contributes to Isoflavone Accumulation in Soybean. New Phytol. 2023, 237, 1794–1809. [Google Scholar] [CrossRef]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. In Posidonia Oceanica Cadmium Induces Changes in DNA Methylation and Chromatin Patterning. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef]
- Kusano, M.; Tohge, T.; Fukushima, A.; Kobayashi, M.; Hayashi, N.; Otsuki, H.; Kondou, Y.; Goto, H.; Kawashima, M.; Matsuda, F.; et al. Metabolomics Reveals Comprehensive Reprogramming Involving Two Independent Metabolic Responses of Arabidopsis to UV-B Light. Plant J. 2011, 67, 354–369. [Google Scholar] [CrossRef]
- Thelen, J.J.; Ohlrogge, J.B. Both Antisense and Sense Expression of Biotin Carboxyl Carrier Protein Isoform 2 Inactivates the Plastid Acetyl-Coenzyme A Carboxylase in Arabidopsis Thaliana. Plant J. 2002, 32, 419–431. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, S.; Yao, W.; Cheng, Z.; Zhou, B.; Jiang, T. Genome-Wide Analysis and Expression Profile of the bZIP Gene Family in Poplar. BMC Plant Biol. 2021, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Mor, B.; Garhwal, S.; Kumar, A. A Systematic Review of Hidden Markov Models and Their Applications. Arch. Computat. Methods Eng. 2021, 28, 1429–1448. [Google Scholar] [CrossRef]
- Ye, Y.; Nikovics, K.; To, A.; Lepiniec, L.; Fedosejevs, E.T.; Van Doren, S.R.; Baud, S.; Thelen, J.J. Docking of Acetyl-CoA Carboxylase to the Plastid Envelope Membrane Attenuates Fatty Acid Production in Plants. Nat. Commun. 2020, 11, 6191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Gao, F.; Guan, P.; Gao, J.; Guo, Z.; Guo, J.; Cui, H.; Li, Y.; Zhang, G.; Li, Z.; et al. Identification and Analysis of Differentially Expressed Trihelix Genes in Maize (Zea Mays) under Abiotic Stresses. PeerJ 2023, 11, e15312. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-C.; Shen, H.-B. Cell-PLoc: A Package of Web Servers for Predicting Subcellular Localization of Proteins in Various Organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T.; Jia, Z.-H.; Xuan, J.-P.; Pan, D.-L.; Guo, Z.-R.; Zhang, J.-Y. Genome-Wide Bioinformatics Analysis of MAPK Gene Family in Kiwifruit (Actinidia Chinensis). Int. J. Mol. Sci. 2018, 19, 2510. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.-H. Evolview v3: A Webserver for Visualization, Annotation, and Management of Phylogenetic Trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Rombauts, S.; Dehais, P.; Van Montagu, M.; Rouze, P. PlantCARE, a Plant Cis-Acting Regulatory Element Database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Xu, F.; He, L.; Gao, S.; Su, Y.; Li, F.; Xu, L. Comparative Analysis of Two Sugarcane Ancestors Saccharum Officinarum and S. Spontaneum Based on Complete Chloroplast Genome Sequences and Photosynthetic Ability in Cold Stress. IJMS 2019, 20, 3828. [Google Scholar] [CrossRef] [PubMed]
- Breitler, J.-C.; Campa, C.; Georget, F.; Bertrand, B.; Etienne, H. A Single-Step Method for RNA Isolation from Tropical Crops in the Field. Sci. Rep. 2016, 6, 38368. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, H.; Yan, Y.; Zhu, G.; Chen, Z. Development and Application of a Multiplex Fluorescent PCR for Shigella Detection and Species Identification. J. Fluoresc. 2022, 32, 707–713. [Google Scholar] [CrossRef]
- Wu, D.; Li, D.; Zhao, X.; Zhan, Y.; Teng, W.; Qiu, L.; Zheng, H.; Li, W.; Han, Y. Identification of a Candidate Gene Associated with Isoflavone Content in Soybean Seeds Using Genome-Wide Association and Linkage Mapping. Plant J. 2020, 104, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Peng, S.; Lai, Z.; Mai, Y. Seasonal Variation of Temperature Affects HMW-PAH Accumulation in Fishery Species by Bacterially Mediated LMW-PAH Degradation. Sci. Total Environ. 2022, 853, 158617. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Cai, Z.; Cheng, Y.; Ma, Z.; Liu, X.; Ma, Q.; Xia, Q.; Zhang, G.; Mu, Y.; Nian, H. Fine-Mapping of QTLs for Individual and Total Isoflavone Content in Soybean (Glycine Max L.) Using a High-Density Genetic Map. Theor. Appl. Genet. 2018, 131, 555–568. [Google Scholar] [CrossRef]
- Kim, J.M.; Seo, J.S.; Lee, J.W.; Lyu, J.I.; Ryu, J.; Eom, S.H.; Ha, B.-K.; Kwon, S.-J. QTL Mapping Reveals Key Factors Related to the Isoflavone Contents and Agronomic Traits of Soybean (Glycine Max). BMC Plant Biol. 2023, 23, 517. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, W.; Ao, C.; Huang, J. Integrative Analysis of the Transcriptome and Metabolome Reveals Bacillus Atrophaeus WZYH01-Mediated Salt Stress Mechanism in Maize (Zea Mays L.). J. Biotechnol. 2024, 383, 39–54. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, B.; Du, H.; Hao, Q.; Zhang, L.; Chen, Z.; Mao, J.; Zhu, C.; Yan, M.; Qin, H.; et al. Co-Expression of Metabolites and Sensory Attributes through Weighted Correlation Network Analysis to Explore Flavor-Contributing Factors in Various Pyrus Spp. Cultivars. Food Chem. X 2024, 21, 101189. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Gene ID | Chromosome | Length (aa) | MW | pI | Subcellular Localization Prediction | Group |
|---|---|---|---|---|---|---|---|
| GmACC1 | Glyma.04G104900. | 4 | 2260 | 252,370.45 | 6.00 | Cytoplasmic | I |
| GmACC2 | Glyma.06G105900 | 6 | 2260 | 252,169.99 | 5.92 | Cytoplasmic | I |
| GmACC3 | Glyma.13G363500 | 13 | 298 | 32,437.37 | 8.49 | Plasma Membrane, Chloroplast | III |
| GmACC4 | Glyma.15G010300 | 15 | 269 | 29,161.57 | 8.81 | Nuclear, Chloroplast, Mitochondrial | III |
| GmACC5 | Glyma.18G243500 | 18 | 262 | 27,657.07 | 9.47 | Chloroplast | III |
| GmACC6 | Glyma.18G265300 | 18 | 284 | 29,764.27 | 8.45 | Chloroplast | III |
| GmACC7 | Glyma.05G221100 | 5 | 539 | 58,888.72 | 7.22 | Mitochondrial | I |
| GmACC8 | Glyma.07G137400 | 7 | 107 | 11,530.56 | 9.03 | Mitochondrial | I |
| GmACC9 | Glyma.08G027600 | 8 | 539 | 58,807.60 | 7.22 | Mitochondrial | I |
| GmACC10 | Glyma.18G195700 | 18 | 709 | 78,666.60 | 7.64 | Mitochondrial, Cytoplasmic | V |
| GmACC11 | Glyma.18G195900 | 18 | 690 | 76,918.92 | 8.63 | Mitochondrial, Cytoplasmic | V |
| GmACC12 | Glyma.18G196000 | 18 | 683 | 75,989.91 | 8.88 | Cytoplasmic, Mitochondrial | V |
| GmACC13 | Glyma.10G208900 | 10 | 34 | 3865.83 | 10.30 | Mitochondrial, Chloroplast, Nuclear | IV |
| GmACC14 | Glyma.15G003800 | 15 | 34 | 3847.76 | 10.00 | Mitochondrial, Chloroplast | IV |
| GmACC15 | Glyma.15G248500 | 15 | 126 | 14,897.90 | 4.51 | Nuclear | II |
| GmACC16 | Glyma.09G248900 | 9 | 261 | 27,536.94 | 9.37 | Chloroplast | III |
| GmACC17 | Glyma.13G057400 | 13 | 276 | 28,870.48 | 8.69 | Chloroplast | III |
| GmACC18 | Glyma.11G233700 | 11 | 297 | 32,064.75 | 9.01 | Mitochondrial | III |
| GmACC19 | Glyma.18G023300 | 18 | 291 | 31,540.09 | 9.10 | Mitochondrial | III |
| GmACC20 | Glyma.19G028800 | 19 | 280 | 29,326.88 | 8.16 | Chloroplast | III |
| No. | Sequence | Duplication Type | Ka | Ks | Ka/Ks | Divergence Time (MYA) |
|---|---|---|---|---|---|---|
| 1 | GmACC1 and GmACC2 | Segmental | 0.013 | 0.093 | 0.139 | 3.116 |
| 2 | GmACC3 and GmACC4 | Segmental | 0.034 | 0.064 | 0.528 | 2.134 |
| 3 | GmACC6 and GmACC20 | Segmental | 0.200 | 0.543 | 0.368 | 18.085 |
| 4 | GmACC7 and GmACC9 | Segmental | 0.008 | 0.124 | 0.066 | 4.141 |
| 5 | GmACC16 and GmACC5 | Segmental | 0.017 | 0.090 | 0.193 | 3.002 |
| 6 | GmACC17 and GmACC6 | Segmental | 0.183 | 0.552 | 0.331 | 18.416 |
| 7 | GmACC18 and GmACC19 | Segmental | 0.049 | 0.104 | 0.466 | 3.476 |
| 8 | GmACC17 and GmACC20 | Segmental | 0.046 | 0.077 | 0.601 | 2.569 |
| 9 | GmACC10 and GmACC11 | Tandem | 0.037 | 0.072 | 0.517 | 2.393 |
| 10 | GmACC11 and GmACC12 | Tandem | 0.014 | 0.054 | 0.259 | 1.798 |
| 11 | GmACC10 and GmACC12 | Tandem | 0.035 | 0.075 | 0.473 | 2.501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, Z.; Zhu, Y.; Zhan, Y.; Li, Y.; Teng, W.; Han, Y.; Zhao, X. Bioinformatics Identification and Expression Analysis of Acetyl-CoA Carboxylase Reveal Its Role in Isoflavone Accumulation during Soybean Seed Development. Int. J. Mol. Sci. 2024, 25, 10221. https://doi.org/10.3390/ijms251810221
Wu X, Yang Z, Zhu Y, Zhan Y, Li Y, Teng W, Han Y, Zhao X. Bioinformatics Identification and Expression Analysis of Acetyl-CoA Carboxylase Reveal Its Role in Isoflavone Accumulation during Soybean Seed Development. International Journal of Molecular Sciences. 2024; 25(18):10221. https://doi.org/10.3390/ijms251810221
Chicago/Turabian StyleWu, Xu, Zhenhong Yang, Yina Zhu, Yuhang Zhan, Yongguang Li, Weili Teng, Yingpeng Han, and Xue Zhao. 2024. "Bioinformatics Identification and Expression Analysis of Acetyl-CoA Carboxylase Reveal Its Role in Isoflavone Accumulation during Soybean Seed Development" International Journal of Molecular Sciences 25, no. 18: 10221. https://doi.org/10.3390/ijms251810221
APA StyleWu, X., Yang, Z., Zhu, Y., Zhan, Y., Li, Y., Teng, W., Han, Y., & Zhao, X. (2024). Bioinformatics Identification and Expression Analysis of Acetyl-CoA Carboxylase Reveal Its Role in Isoflavone Accumulation during Soybean Seed Development. International Journal of Molecular Sciences, 25(18), 10221. https://doi.org/10.3390/ijms251810221






