Phylogenetic Lineages and Diseases Associated with Moraxella catarrhalis Isolates Recovered from Bulgarian Patients
Abstract
:1. Background
2. Results
2.1. Studied Population
2.2. Serotyping
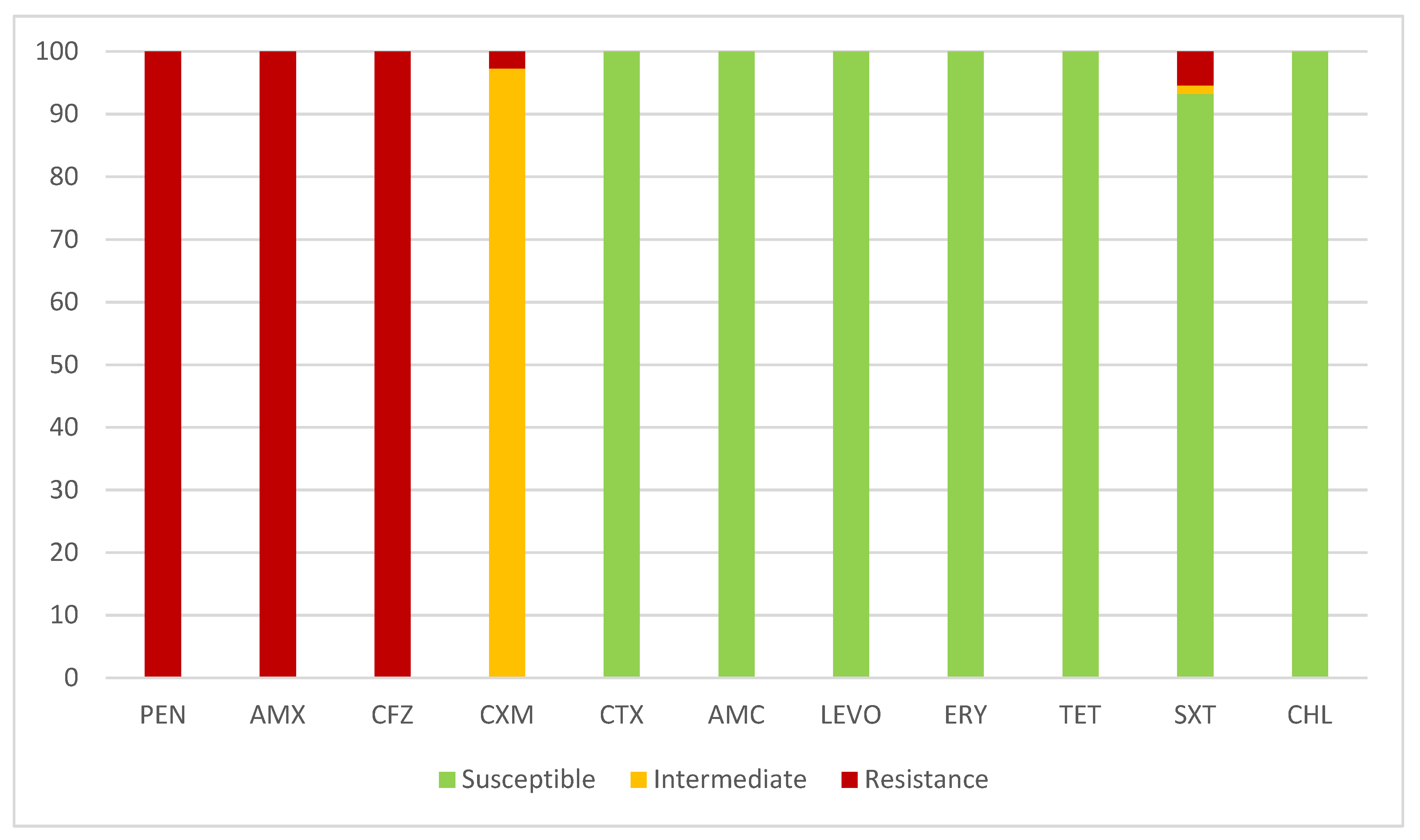
2.3. Antimicrobial Susceptibility
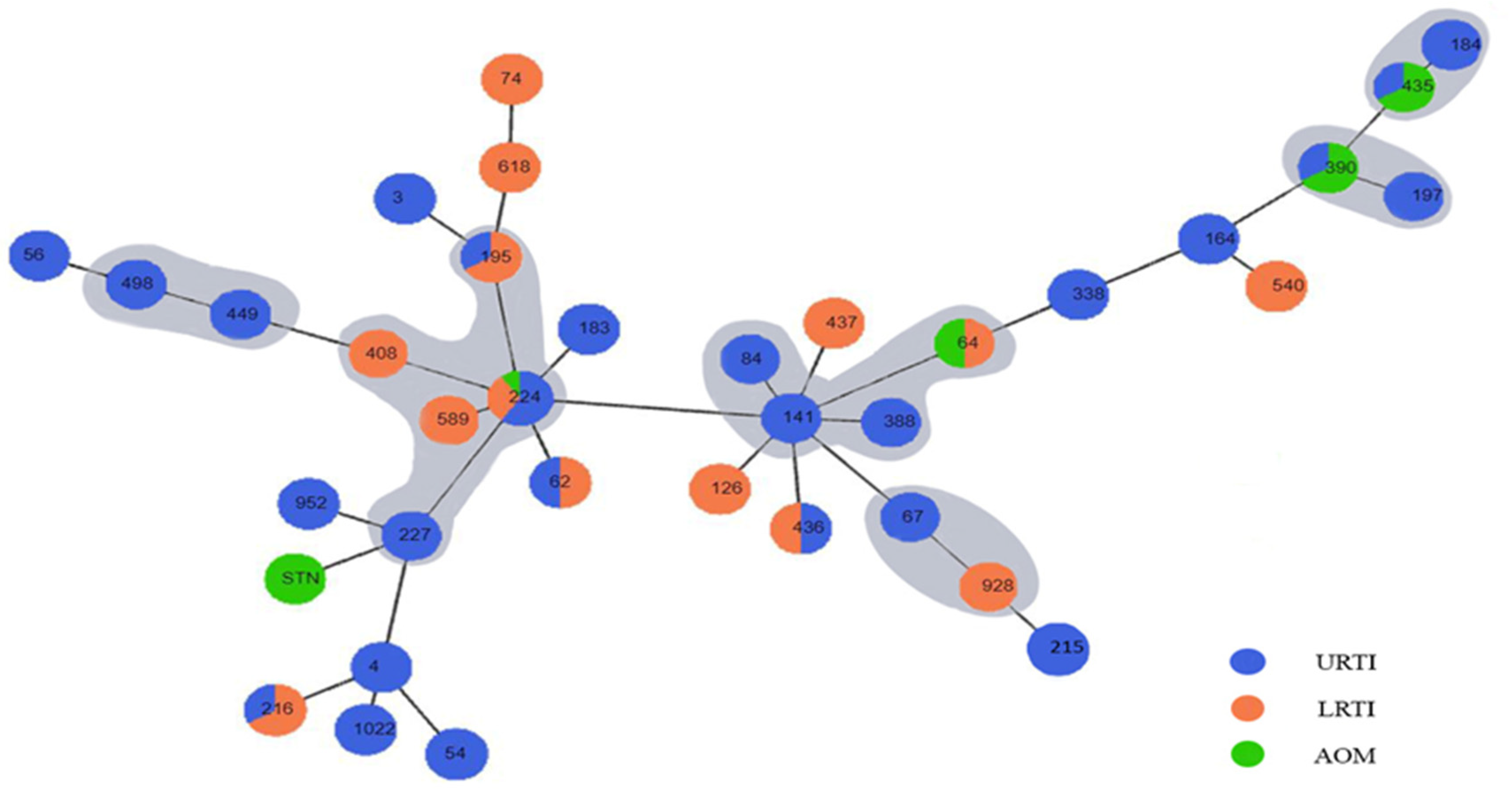
2.4. Genotyping
3. Discussion
4. Materials and Methods
4.1. Specimen Collection
4.2. Antimicrobial Susceptibility Testing
4.3. Serotyping
4.4. DNA Extraction and PCR Amplifications
4.5. Multilocus Sequence Typing (MLST) of M. catarrhalis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karalus, R.; Campagnari, A. Moraxella catarrhalis: A review of an important human mucosal pathogen. Microbes Infect. 2000, 2, 547–559. [Google Scholar] [CrossRef]
- Deng, W.J.; Zhang, J.F.; Li, P.Y.; Zhou, J.L.; Yao, Z.J.; Ye, X.H. Co-carriage of Streptococcus pneumoniae and Moraxella catarrhalis among preschool children and its influencing factors. Zhongguo Dang Dai Er Ke Za Zhi 2022, 24, 874–880. [Google Scholar] [CrossRef]
- Verduin, C.M.; Hol, C.; Fleer, A.; van Dijk, H.; van Belkum, A. Moraxella catarrhalis: From emerging to established pathogen. Clin. Microbiol. Rev. 2002, 15, 125–144. [Google Scholar] [CrossRef]
- Morris, D.E.; Osman, K.L.; Cleary, D.W.; Clarke, S.C. The characterization of Moraxella catarrhalis carried in the general population. Microb. Genom. 2022, 8, 000820. [Google Scholar] [CrossRef]
- Aebi, C. Moraxella catarrhalis—Pathogen or commensal? In Hot Topics in Infection and Immunity in Children VII; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2011; Volume 697, pp. 107–116. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Murphy, T.F.; Parameswaran, G.I. Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis. 2009, 49, 124–131. [Google Scholar] [CrossRef]
- Gupta, N.; Arora, S.; Kundra, S. Moraxella catarrhalis as a respiratory pathogen. Indian J. Pathol. Microbiol. 2011, 54, 769–771. [Google Scholar] [CrossRef]
- Van Hare, G.F.; Shurin, P.A. The increasing importance of Branhamella catarrhalis in respiratory infections. Pediatr. Infect. Dis. J. 1987, 6, 92–94. [Google Scholar] [CrossRef]
- Mbaki, N.; Rikitomi, N.; Nagatake, T.; Matsumoto, K.; Tohoku, J. Correlation between Branhamella catarrhalis adherence to oropharyngeal cells and seasonal incidence of lower respiratory tract infections. Tohoku J. Exp. Med. 1987, 153, 111–121. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Aris, E.; Bourne, S.; Clarke, S.C.; Peeters, M.; Pascal, T.G.; Schoonbroodt, S.; Tuck, A.C.; Kim, V.; Ostridge, K.; et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 2017, 72, 919–927. [Google Scholar] [CrossRef]
- Marchisio, P.; Gironi, S.; Esposito, S.; Schito, G.C.; Mannelli, S.; Principi, N. Seasonal variations in nasopharyngeal carriage of respiratory pathogens in healthy Italian children attending day-care centres or schools. J. Med. Microbiol. 2001, 50, 1095–1099. [Google Scholar] [CrossRef]
- Venekamp, R.P.; Damoiseaux, R.A.; Schilder, A.G. Acute Otitis Media in Children. Am. Fam. Physician 2017, 95, 109–110. [Google Scholar]
- El Feghaly, R.E.; Nedved, A.; Katz, S.E.; Frost, H.M. New insights into the treatment of acute otitis media. Expert Rev. Anti-Infect. Ther. 2023, 21, 523–534. [Google Scholar] [CrossRef]
- Zhang, X.B.; Wu, X.; Nong, G.M. Update on protracted bacterial bronchitis in children. Ital. J. Pediatr. 2020, 46, 38. [Google Scholar] [CrossRef]
- Shaikh, N.; Hoberman, A.; Shope, T.R.; Jeong, J.H.; Kurs-Lasky, M.; Martin, J.M.; Bhatnagar, S.; Muniz, G.B.; Block, S.L.; Andrasko, M.; et al. Identifying Children Likely to Benefit from Antibiotics for Acute Sinusitis: A Randomized Clinical Trial. JAMA 2023, 330, 349–358. [Google Scholar] [CrossRef]
- Nawa, M.; Mwansa, J.; Mwaba, J.; Kaonga, P.; Mukubesa, A.N.; Simuyandi, M.; Chisenga, C.C.; Alabi, P.; Mwananyanda, L.; Thea, D.M.; et al. Microbiologic and virulence characteristics of Moraxella catarrhalis isolates from Zambian children presenting with acute pneumonia. Pediatr. Pulmonol. 2022, 57, 3084–3093. [Google Scholar] [CrossRef]
- Siegel, H.; Lang, S.; Maier, P.; Reinhard, T. Bacterial Conjunctivitis: Current Aspects of Diagnosis and Therapy. Klin. Monbl. Augenheilkd. 2024, 241, 231–246. [Google Scholar] [CrossRef]
- Perez, A.C.; Murphy, T.F. Potential impact of a Moraxella catarrhalis vaccine in COPD. Vaccine 2019, 37, 5551–5558. [Google Scholar] [CrossRef]
- Wiegers, H.M.G.; van Nijen, L.; van Woensel, J.B.M.; Bem, R.A.; de Jong, M.D.; Calis, J.C.J. Bacterial co-infection of the respiratory tract in ventilated children with bronchiolitis; a retrospective cohort study. BMC Infect. Dis. 2019, 19, 938. [Google Scholar] [CrossRef]
- Hirai, J.; Kinjo, T.; Koga, T.; Haranaga, S.; Motonaga, E.; Fujita, J. Clinical characteristics of community-acquired pneumonia due to Moraxella catarrhalis in adults: A retrospective single-centre study. BMC Infect. Dis. 2020, 20, 821. [Google Scholar] [CrossRef]
- Mitov, I.G.; Gergova, R.T.; Ouzounova-Raykova, V.V. Distribution of genes encoding virulence factors ompB2, ompCD, ompE, β-lactamase and serotype in pathogenic and colonizing strains of Moraxella catarrhalis. Arch. Med. Res. 2010, 41, 530–535. [Google Scholar] [CrossRef]
- Harb, H.; Al-Obaidi, H.; Irannejad, K.; Bagheri, F.A. Unique Case of Moraxella catarrhalis Meningitis Following Neurosurgical Intervention. Cureus 2024, 16, e59689. [Google Scholar] [CrossRef]
- Ioannou, P.; Alexakis, K.; Baliou, S.; Kofteridis, D.P. Infective Endocarditis by Moraxella Species: A Systematic Review. J. Clin. Med. 2022, 11, 1854. [Google Scholar] [CrossRef]
- Siwakoti, S.; Bajracharya, S.; Adhikaree, N.; Sah, R.; Rajbhandari, R.S.; Khanal, B. Early-Onset Neonatal Meningitis Caused by an Unusual Pathogen-Moraxella catarrhalis. Case Rep. Pediatr. 2019, 2019, 4740504. [Google Scholar] [CrossRef]
- Daoud, A.; Abuekteish, F.; Masaadeh, H. Neonatal meningitis due to Moraxella catarrhalis and review of the literature. Ann. Trop. Paediatr. 1996, 16, 199–201. [Google Scholar] [CrossRef]
- Kobayashi, Y. Bacteremic Moraxella catarrhalis pneumonia. J. Infect. Chemother. 2000, 6, 68. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Mundy, L.M. Etiology of community-acquired pneumonia. Clin. Chest Med. 2005, 26, 47–55. [Google Scholar] [CrossRef]
- Gergova, R.T.; Iankov, I.D.; Haralambieva, I.H.; Mitov, I.G. Bactericidal monoclonal antibody against Moraxella catarrhalis lipooligosaccharide cross-reacts with Haemophilus spp. Curr. Microbiol. 2007, 54, 85–90. [Google Scholar] [CrossRef]
- de Vries, S.P.; Bootsma, H.J.; Hays, J.P.; Hermans, P.W. Molecular aspects of Moraxella catarrhalis pathogenesis. Microbiol. Mol. Biol. Rev. 2009, 73, 389–406. [Google Scholar] [CrossRef]
- Rikitomi, N.; Ahmed, K.; Nagatake, T. Moraxella (Branhamella) catarrhalis adherence to human bronchial and oropharyngeal cells: The role of adherence in lower respiratory tract infections. Microbiol. Immunol. 1997, 41, 487–494. [Google Scholar] [CrossRef]
- Reddy, M.S.; Murphy, T.F.; Faden, H.S.; Bernstein, J.M. Middle ear mucin glycoprotein: Purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol.—Head Neck Surg. 1997, 116, 175–180. [Google Scholar] [CrossRef]
- Luke, N.R.; Howlett, A.J.; Shao, J.; Campagnari, A.A. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect. Immun. 2004, 72, 6262–6270. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Autenrieth, I.B.; Frick, J.S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Sankaranarayanan, K.; Khosla, C. Biosynthesis and structure-activity relationships of the lipid a family of glycolipids. Curr. Opin. Chem. Biol. 2017, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lee, J.; Widmalm, G.; Im, W. Preferred conformations of lipooligosaccharides and oligosaccharides of Moraxella catarrhalis. Glycobiology 2020, 30, 86–94. [Google Scholar] [CrossRef] [PubMed]
- McGregor, K.; Chang, B.J.; Mee, B.J.; Riley, T.V. Moraxella catarrhalis: Clinical significance, antimicrobial susceptibility and BRO beta-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 219–234. [Google Scholar] [CrossRef]
- Khan, M.A.; Northwood, J.B.; Levy, F.; Verhaegh, S.J.; Farrell, D.J.; Van Belkum, A.; Hays, J.P. bro β-lactamase and antibiotic resistances in a global cross-sectional study of Moraxella catarrhalis from children and adults. J. Antimicrob. Chemother. 2010, 65, 91–97. [Google Scholar] [CrossRef]
- Bootsma, H.J.; van Dijk, H.; Vauterin, P.; Verhoef, J.; Mooi, F.R. Genesis of BRO β-lactamase-producing Moraxella catarrhalis: Evidence for transformation-mediated horizontal transfer. Mol. Microbiol. 2000, 36, 93–104. [Google Scholar] [CrossRef]
- Bristy, S.A.; Hossain, M.A.; Hasan, M.I.; Mahmud, S.M.H.; Moni, M.A.; Rahman, M.H. An integrated complete-genome sequencing and systems biology approach to predict antimicrobial resistance genes in the virulent bacterial strains of Moraxella catarrhalis. Brief. Funct. Genom. 2023, 22, 375–391. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Xiang, X.; Liao, P.; Niu, C. Mutation of TonB-Dependent Receptor Encoding Gene MCR_0492 Potentially Associates with Macrolides Resistance in Moraxella catarrhalis Isolates. Infect. Drug Resist. 2022, 15, 2419–2426. [Google Scholar] [CrossRef]
- Sánchez Arlegui, A.; Del Arco Rodríguez, J.; De Velasco Vázquez, X.; Gallego Rodrigo, M.; Gangoiti, I.; Mintegi, S. Bacterial pathogens and antimicrobial resistance in acute otitis media. An. Pediatr. Engl. Ed. 2024, 100, 173–179. [Google Scholar] [CrossRef]
- Ngo, C.C.; Massa, H.M.; Thornton, R.B.; Cripps, A.W. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PLoS ONE 2016, 11, e0150949. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.M.; Johnson, B.C.; McCormack, J.G. Moraxella catarrhalis: Pathogenic significance in respiratory tract infections treated by community practitioners. Clin. Infect. Dis. 1996, 22, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Gergova, R.; Markovska, R. Antimicrobial resistance of Bulgarian isolates Moraxella catarrhalis during the period 1999–2018. J. IMAB 2020, 26, 3208–3212. [Google Scholar] [CrossRef]
- Hare, K.M.; Seib, K.L.; Chang, A.B.; Harris, T.M.; Spargo, J.C.; Smith-Vaughan, H.C. Antimicrobial susceptibility and impact of macrolide antibiotics on Moraxella catarrhalis in the upper and lower airways of children with chronic endobronchial suppuration. J. Med. Microbiol. 2019, 68, 1140–1147. [Google Scholar] [CrossRef]
- Król-Turmińska, K.; Olender, A.; Bogut, A. Tetracycline resistance in Moraxella catarrhalis clinical strains isolated in Poland. New Microbiol. 2020, 43, 103–106. [Google Scholar]
- Kovács, E.; Sahin-Tóth, J.; Tóthpál, A.; van der Linden, M.; Tirczka, T.; Dobay, O. Co-carriage of Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis among three different age categories of children in Hungary. PLoS ONE 2020, 15, e0229021. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef]
- Bandet, T.; Whitehead, S.; Blondel-Hill, E.; Wagner, K.; Cheeptham, N. Susceptibility of clinical Moraxella catarrhalis isolates in British Columbia to six empirically prescribed antibiotic agents. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 155–158. [Google Scholar] [CrossRef]
- Zhao, N.; Ren, H.; Deng, J.; Du, Y.; Li, Q.; Zhou, P.; Zhou, H.; Jiang, X.; Qin, T. Genotypic and Phenotypic Characteristics of Moraxella catarrhalis from Patients and Healthy Asymptomatic Participants among Preschool Children. Pathogens 2022, 11, 984. [Google Scholar] [CrossRef]
- Flamm, R.K.; Sader, H.S.; Farrell, D.J.; Jones, R.N. Macrolide and tetracycline resistance among Moraxella catarrhalis isolates from 2009 to 2011. Diagn. Microbiol. Infect. Dis. 2012, 74, 198–200. [Google Scholar] [CrossRef]
- Raveendran, S.; Kumar, G.; Sivanandan, R.N.; Dias, M. Moraxella catarrhalis: A Cause of Concern with Emerging Resistance and Presence of BRO Beta-Lactamase Gene-Report from a Tertiary Care Hospital in South India. Int. J. Microbiol. 2020, 2020, 7316257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ding, R.; Jia, X.M.; Huang, J.J.; Yu, S.; Chan, H.T.; Li, W.; Mao, L.L.; Zhang, L.; Zhang, X.Y.; et al. Correlation of Moraxella catarrhalis macrolide susceptibility with the ability to adhere and invade human respiratory epithelial cells. Emerg. Microbes Infect. 2022, 11, 2055–2068. [Google Scholar] [CrossRef]
- Liu, Y.L.; Xiao, M.; Cheng, J.W.; Xu, H.P.; Xu, Z.P.; Ye, S.; Zhang, W.J.; Kudinha, T.; Kong, F.; Xu, Y.C. Moraxella catarrhalis Macrolide-Resistant isolates Are highly concentrated in Two MLST clonal complexes -CCN10 and CC363. Front. Microbiol. 2017, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, H.; Wang, F.; Liang, S.; Cheng, L.; Du, X.; Pang, F.; Tian, J.; Kan, B.; Xu, J.; et al. Multilocus sequence typing-based analysis of Moraxella catarrhalis population structure reveals clonal spreading of drug-resistant strains isolated from childhood pneumonia. Infect. Genet. Evol. 2017, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Masaki, H.; Gotoh, K.; Furumoto, A.; Terada, M.; Watanabe, K.; Watanabe, H. Molecular epidemiological study of Moraxella catarrhalis isolated from nosocomial respiratory infection patients in a community hospital in Japan. Intern. Med. 2009, 48, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; YambaYamba, L.; Wasserstrom, L.; Rünow, E.; Göransson, T.; Nilsson, A.; Ahl, J.; Riesbeck, K. Exploring the microbial landscape: Uncovering the pathogens associated with community-acquired pneumonia in hospitalized patients. Front. Public Health 2023, 11, 1258981. [Google Scholar] [CrossRef]
- Blakeway, L.V.; Tan, A.; Lappan, R.; Ariff, A.; Pickering, J.L.; Peacock, C.S.; Blyth, C.C.; Kahler, C.M.; Chang, B.J.; Lehmann, D.; et al. Moraxella catarrhalis Restriction-Modification Systems Are Associated with Phylogenetic Lineage and Disease. Genome Biol. Evol. 2018, 10, 2932–2946. [Google Scholar] [CrossRef]
- Earl, J.P.; de Vries, S.P.; Ahmed, A.; Powell, E.; Schultz, M.P.; Hermans, P.W.; Hill, D.J.; Zhou, Z.; Constantinidou, C.I.; Hu, F.Z.; et al. Comparative Genomic Analyses of the Moraxella catarrhalis Serosensitive and Seroresistant Lineages Demonstrate Their Independent Evolution. Genome Biol. Evol. 2016, 8, 955–974. [Google Scholar] [CrossRef]
| Age 1 | Diagnosis 2 | Serotype | |||||
|---|---|---|---|---|---|---|---|
| Children | 0–11 y n = 51 (69.9%) | URTI | AOM | LRTI | A | B | C |
| 43 (58.9%) | 8 (11.0%) | - | 46 (63.0%) | 4 (5.5%) | 1 (1.4%) | ||
| Adults | 50–74 y n = 22 (30.1%) | 3 (4.1%) | - | 19 (26.0%) | 20(27.4%) | 1 (1.4%) | 1 (1.4%) |
| Total n (100%) | 73 (100%) | 46 (63.0%) | 8 (11.0%) | 19 (26.0%) | 66 (90.4%) | 5 (6.8%) | 2 (2.8%) |
| CC 1 | Strain | Age | Gender 2 | Diagnosis 3 | Sample 4 | Serotype | abcZ | adk | efp | fumC | glyRS | mutY | ppa | trpE | ST 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC141 | 57/1379 | 50 | f | Bronchitis chr | Sputum | A | 2 | 17 | 12 | 2 | 3 | 3 | 3 | 2 | 64 |
| 557 | 2 | f | Rhinophar | Nph | A | 2 | 6 | 2 | 2 | 20 | 3 | 3 | 2 | 141 | |
| 8080 | 2 | f | Rhinophar | Nph | A | 2 | 6 | 2 | 2 | 20 | 3 | 3 | 2 | 141 | |
| 1353 | 4 | f | Adenoiditis | Nph | A | 2 | 6 | 2 | 2 | 20 | 3 | 3 | 2 | 141 | |
| 20/1321 | 3 | m | Adenoiditis | Nph | A | 2 | 9 | 2 | 2 | 20 | 3 | 3 | 2 | 84 | |
| 58/1558 | 1 | m | Rhinophar | Nph | A | 8 | 6 | 3 | 2 | 20 | 26 | 3 | 2 | 388 | |
| CC224 | 77 | 1 | f | Rhinophar | Nph | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 |
| 17/179 | 5 | m | Adenoiditis | Nph | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 61 | 3 | m | Adenoiditis | Nph | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 54/847 | 5 | m | Rhinophar | Nph | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 18 | 6 | f | Rhinophar | Nph | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 711 | 6 | f | Rhinophar | Nph | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 555 | 71 | m | COPD | Sputum | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 608 | 61 | m | Bronchitis chr | Sputum | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 26/53K | 71 | m | COPD | Sputum | A | 53 | 3 | 2 | 3 | 57 | 3 | 3 | 2 | 224 | |
| 1843 | 63 | m | COPD | Sputum | A | 53 | 3 | 59 | 3 | 57 | 3 | 3 | 2 | 589 | |
| 109,316 | 68 | m | COPD | Sputum | A | 3 | 3 | 2 | 3 | 57 | 67 | 3 | 2 | 408 | |
| K978 | 5 | m | Rhinophar | Nph | A | 3 | 3 | 2 | 9 | 57 | 3 | 3 | 2 | 227 | |
| 166 | 4 | m | Adenoiditis | Nph | A | 3 | 3 | 2 | 9 | 57 | 3 | 3 | 2 | 227 | |
| 3270 | 2 | m | Rhinophar | Nph | A | 28 | 3 | 2 | 3 | 31 | 31 | 3 | 2 | 195 | |
| 60 | 67 | m | COPD | Sputum | A | 28 | 3 | 2 | 3 | 31 | 31 | 3 | 2 | 195 | |
| 3270 | 2 | m | Rhinophar | Nph | A | 28 | 3 | 2 | 3 | 31 | 31 | 3 | 2 | 195 | |
| CC184 | 5102 | 10 | m | Rhinosinuitis | Nph | A | 8 | 25 | 6 | 4 | 57 | 22 | 2 | 2 | 184 |
| 55/980 | 2 | f | Rhinophar | Nph | A | 8 | 25 | 6 | 4 | 57 | 22 | 2 | 2 | 184 | |
| 22/6215 | 46 | f | Rhinosinuitis | Nph | A | 8 | 25 | 6 | 4 | 57 | 22 | 2 | 2 | 184 | |
| 426 | 5 | f | AOM | MEF | A | 8 | 25 | 6 | 4 | 57 | 22 | 23 | 2 | 435 | |
| 184/241 | 5 | m | Rhinophar | Nph | A | 8 | 25 | 6 | 4 | 57 | 22 | 23 | 2 | 435 | |
| 24/814 | 4 | m | AOM | MEF | B | 8 | 25 | 6 | 4 | 57 | 22 | 23 | 2 | 435 | |
| CC449 | 36/3468 | 6 | m | Rhinophar | Nph | A | 3 | 3 | 2 | 2 | 17 | 15 | 8 | 2 | 449 |
| 37/8657 | 2 | f | Rhinophar | Nph | A | 3 | 3 | 2 | 2 | 17 | 15 | 8 | 2 | 449 | |
| 31/161 | 1 | m | Rhinophar | Nph | B | 3 | 3 | 2 | 2 | 17 | 15 | 8 | 2 | 449 | |
| 2826 | 1 | m | Rhinophar | Nph | A | 3 | 18 | 55 | 2 | 15 | 15 | 8 | 2 | 498 | |
| 11 | 2 | m | Rhinophar | Nph | A | 3 | 18 | 55 | 2 | 15 | 15 | 8 | 2 | 498 | |
| CC390 | 42/785 | 6 | f | AOM | MEF | A | 8 | 25 | 6 | 2 | 37 | 9 | 3 | 2 | 390 |
| 785/42 | 6 | f | AOM | MEF | B | 8 | 25 | 6 | 2 | 37 | 9 | 3 | 2 | 390 | |
| 42/785 | 4 | f | Rhinophar | Nph | A | 8 | 25 | 6 | 2 | 37 | 9 | 3 | 2 | 390 | |
| 3522 | 3 | m | Adenoiditis | Nph | A | 8 | 3 | 6 | 2 | 17 | 9 | 17 | 2 | 197 | |
| CC67 | 184 | 11 | m | Rhinosinuitis | Nph | A | 2 | 17 | 2 | 3 | 20 | 2 | 2 | 2 | 67 |
| 571 | 60 | m | COPD | Sputum | A | 2 | 2 | 111 | 3 | 2 | 2 | 2 | 2 | 928 | |
| ST3 | 75 | 3 | f | Rhinophar | Nph | A | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 3 |
| 1205 | 3 | m | Rhinophar | Nph | A | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | |
| ST54 | 592 | 2 | f | Rhinophar | Nph | A | 8 | 24 | 3 | 7 | 30 | 3 | 23 | 2 | 54 |
| 353 | 59 | m | Rhinosinuitis | Nph | A | 8 | 24 | 3 | 7 | 30 | 3 | 23 | 2 | 54 | |
| 343 | 1 | f | Rhinosinuitis | Nph | A | 8 | 24 | 3 | 7 | 30 | 3 | 23 | 2 | 54 | |
| ST62 | K83 | 3 | m | AOM | MEF | C | 8 | 26 | 2 | 3 | 2 | 22 | 25 | 2 | 62 |
| 1096 | 4 | m | AOM | Nph | A | 8 | 26 | 2 | 3 | 2 | 22 | 25 | 2 | 62 | |
| ST183 | 43/1680 | 2 | m | AOM | Nph | A | 9 | 3 | 2 | 3 | 37 | 19 | 9 | 2 | 183 |
| 141 | 10 | m | Rhinosinuitis | Nph | A | 9 | 3 | 2 | 3 | 37 | 19 | 9 | 2 | 183 | |
| ST215 | K1092 | 1 | f | Rhinophar | Nph | A | 2 | 2 | 2 | 9 | 2 | 18 | 17 | 2 | 215 |
| K1097 | 3 | m | AOM | Nph | A | 2 | 2 | 2 | 9 | 2 | 18 | 17 | 2 | 215 | |
| ST216 | 10,838 | 74 | m | COPD | Sputum | A | 25 | 18 | 3 | 4 | 6 | 9 | 3 | 2 | 216 |
| 56/1497 | 2 | f | Rhinophar | Nph | A | 25 | 18 | 3 | 4 | 6 | 9 | 3 | 2 | 216 | |
| 39/4638 | 51 | f | Bronchitis chr | Sputum | A | 25 | 18 | 3 | 4 | 6 | 9 | 3 | 2 | 216 | |
| ST436 | 8083 | 3 | m | AOM | Nph | B | 27 | 18 | 2 | 2 | 10 | 9 | 9 | 2 | 436 |
| 32/160 | 65 | m | COPD | Sputum | A | 27 | 18 | 2 | 2 | 10 | 9 | 9 | 2 | 436 | |
| ST540 | 22/471 | 53 | m | COPD | Sputum | B | 8 | 17 | 20 | 3 | 128 | 3 | 17 | 2 | 540 |
| 28/3220 | 64 | m | Bronchitis chr | Sputum | A | 8 | 17 | 20 | 3 | 128 | 3 | 17 | 2 | 540 | |
| ST952 | 40/348 | 4 | f | Rhinopharyn | Nph | A | 157 | 2 | 2 | 9 | 21 | 9 | 3 | 2 | 952 |
| 22/5205M | 4 | f | Rhinophar | Nph | A | 157 | 2 | 2 | 9 | 21 | 9 | 3 | 2 | 952 | |
| ST1022 | 81 | 6 | f | AOM | Nph | A | 3 | 3 | 3 | 4 | 3 | 28 | 3 | 2 | 1022 |
| 14 | 2 | m | Rhinophar | Nph | A | 3 | 3 | 3 | 4 | 3 | 28 | 3 | 2 | 1022 | |
| STN | K511 | 4 | f | Adenoiditis | Nph | A | 3 | 9 | 2 | 9 | 129 | 8 | 9 | 5 | STN |
| 512 | 3 | m | Otitis media | MEF | A | 3 | 9 | 2 | 9 | 129 | 8 | 9 | 2 | STN | |
| Singletons | 69,215 | 2 | m | Rhinophar | Nph | A | 3 | 3 | 3 | 4 | 3 | 3 | 3 | 2 | 4 |
| 3034 | 4 | m | Adenoiditis | Nph | A | 3 | 18 | 6 | 3 | 31 | 15 | 17 | 2 | 56 | |
| 64 | 57 | m | COPD | Sputum | A | 8 | 26 | 2 | 3 | 2 | 22 | 25 | 2 | 62 | |
| 30/867 | 4 | f | Otitis media | MEF | A | 2 | 17 | 12 | 2 | 3 | 3 | 3 | 2 | 64 | |
| 34/3907 | 54 | f | Bronchitis chr | Sputum | A | 29 | 2 | 2 | 3 | 34 | 29 | 21 | 2 | 74 | |
| 72AI | 65 | m | Bronchitis chr | Sputum | C | 36 | 6 | 12 | 2 | 50 | 41 | 3 | 2 | 126 | |
| 17 | 5 | f | Adenoiditis | Nph | A | 8 | 17 | 6 | 3 | 27 | 3 | 3 | 2 | 164 | |
| 3168 | 4 | m | Rhinophar | Nph | A | 2 | 17 | 12 | 3 | 3 | 3 | 3 | 2 | 338 | |
| 80 | 55 | m | COPD | Sputum | A | 2 | 17 | 2 | 4 | 107 | 89 | 3 | 2 | 437 | |
| 25/91K | 74 | m | Bronchitis chr | Sputum | A | 108 | 3 | 2 | 3 | 31 | 31 | 21 | 2 | 618 |
| Gene | Primer Set | Base Pairs |
|---|---|---|
| glyRS | F 5′-GCACCGAAGAGTTGCCACCA-3′ R 5′-ACGCAACGGGCAAATCCACC-3′ | 762 bp |
| ppa | F 5′-AATAAAATTCTAGATGCTGGC-3′ R 5′-ACTTATTGCTCTGTCCAGCG-3′ | 523 bp |
| efp | F 5′-CTCTGATTGACAACTGGCAGG-3′ R 5′-GATATTCGCCAGTACGCG-3′ | 582 bp |
| fumC | F 5′-GGGCGGTACAGCAGTCGGCAC-3′ R 5′-CTCATCAAATTCAGCTTCAG-3′ | 675 bp |
| trpE | F 5′-TTATCCCGCATCGAAAATGG-3′ R 5′-GGTTTCATCCCATTCAGCC-3′ | 545 bp |
| mutY | F 5′-GGCAATACCATCATCAGCCG-3′ R 5′-GGTAACTGACTTTGAACGCC-3′ | 609 bp |
| adk | F 5′-GGCATTCCTCAAATCTCAAC-3′ R 5′-GATGGGCTTTATTGTCAAATG-3′ | 631 bp |
| abcZ | F 5′-ACATGCTGATGATGGTGAG-3′ R 5′-CACTGGCAAGTTCAAGCGC-3′ | 610 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrova, A.S.; Boyanov, V.S.; Mihova, K.Y.; Gergova, R.T. Phylogenetic Lineages and Diseases Associated with Moraxella catarrhalis Isolates Recovered from Bulgarian Patients. Int. J. Mol. Sci. 2024, 25, 9769. https://doi.org/10.3390/ijms25189769
Alexandrova AS, Boyanov VS, Mihova KY, Gergova RT. Phylogenetic Lineages and Diseases Associated with Moraxella catarrhalis Isolates Recovered from Bulgarian Patients. International Journal of Molecular Sciences. 2024; 25(18):9769. https://doi.org/10.3390/ijms25189769
Chicago/Turabian StyleAlexandrova, Alexandra S., Vasil S. Boyanov, Kalina Y. Mihova, and Raina T. Gergova. 2024. "Phylogenetic Lineages and Diseases Associated with Moraxella catarrhalis Isolates Recovered from Bulgarian Patients" International Journal of Molecular Sciences 25, no. 18: 9769. https://doi.org/10.3390/ijms25189769





