A Visual Distance-Based Capillary Immunoassay Using Biomimetic Polymer Nanoparticles for Highly Sensitive and Specific C-Reactive Protein Quantification
Abstract
1. Introduction
2. Results
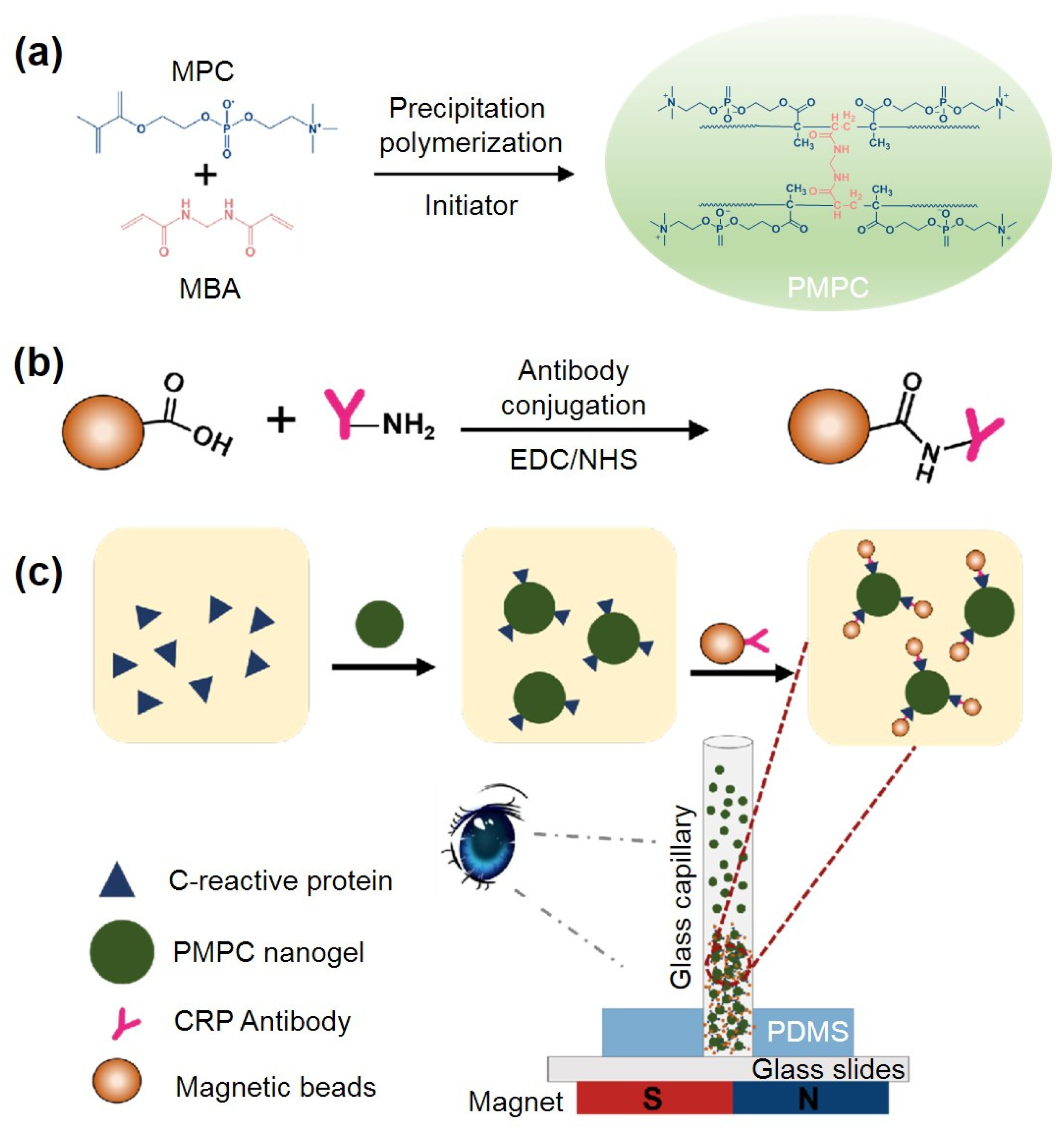
2.1. Principle of PMPC NPs-Based Immunoassay for Visual CRP Quantification
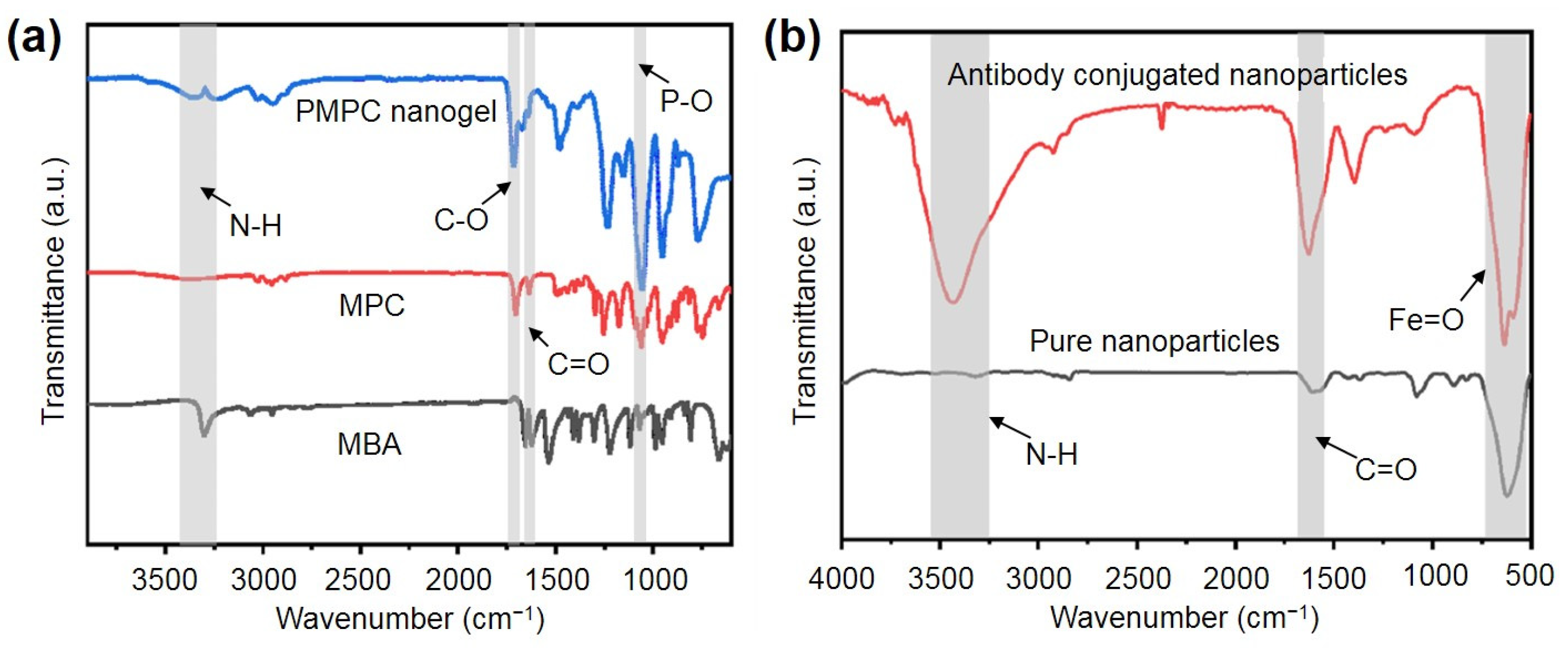
2.2. Synthesis and Characterization of the PMPC NPs and Antibody-Functionalized NPs
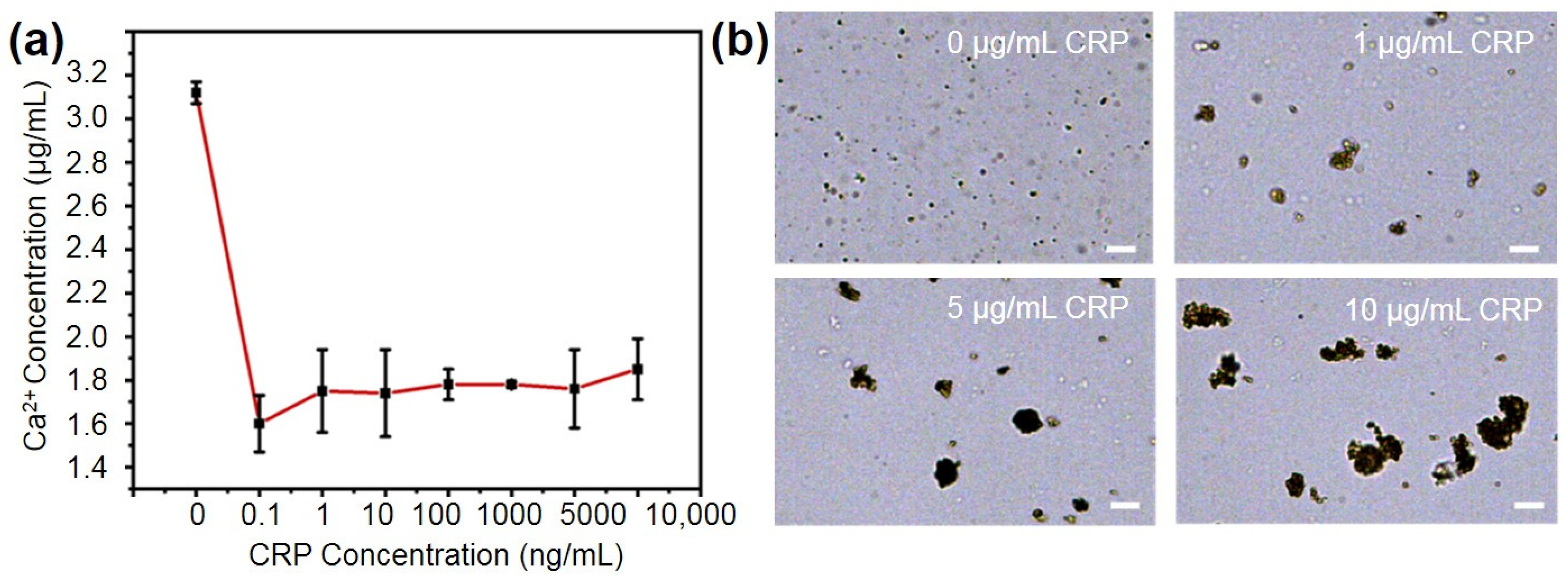
2.3. Performance of the PMPC NPs for Binding CRP
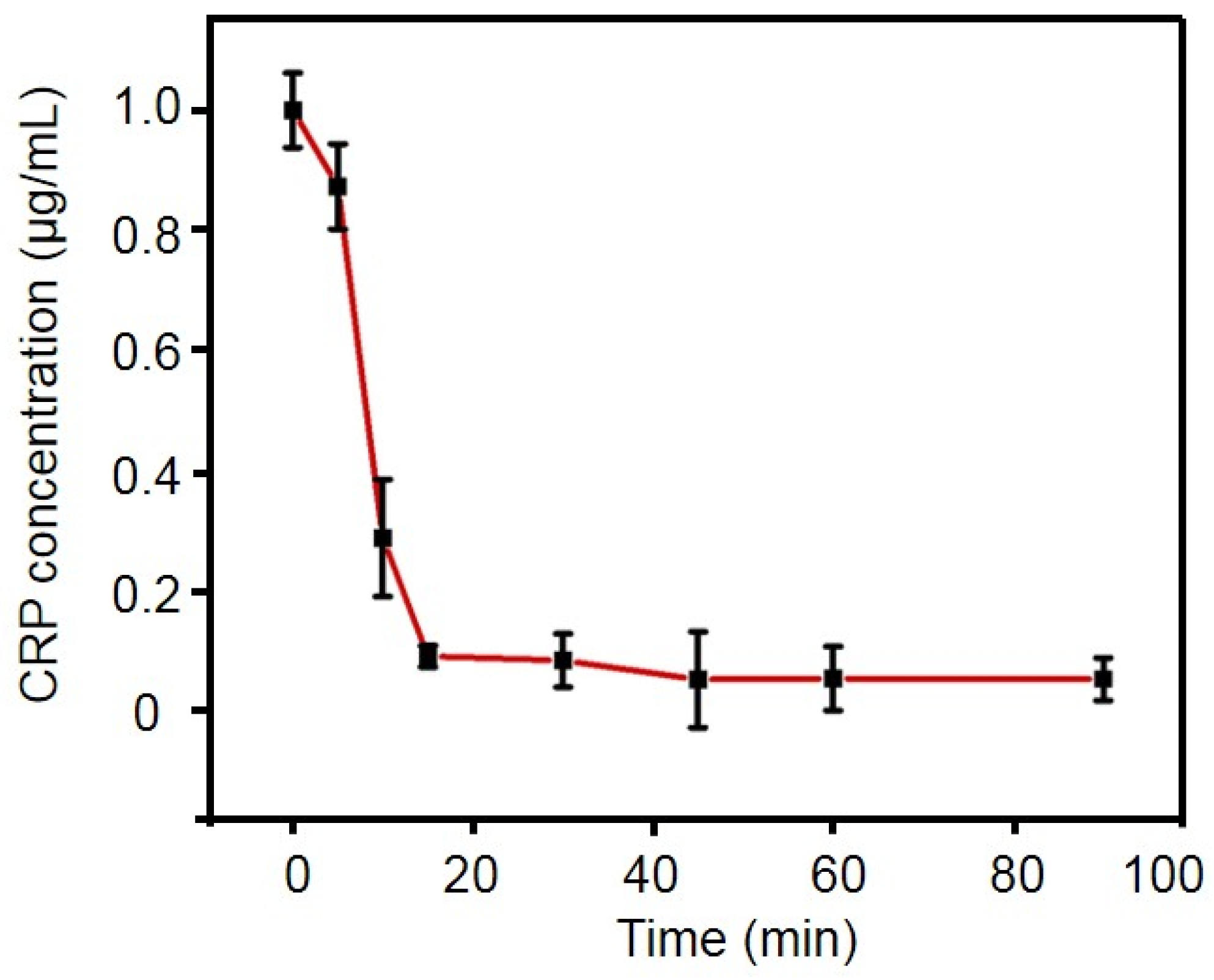
2.4. Optimization of Experimental Conditions
2.5. Linearity Range and Limit of Detection
2.6. Specificity Experiments
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Instrumentation
4.3. Synthesis and Characterization of PMPC Nanoparticles
4.4. Synthesis and Characterization of Antibody-Conjugated Magnetic Nanoparticles
4.5. Optimization of Incubation Time
4.6. Microscope Imaging of Nanoparticle Complex
4.7. Detection of CRP Concentrations Using PMPC NPs
4.8. Specificity Experiment
4.9. Statisticsusing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goda, T.; Kjall, P.; Ishihara, K.; Richter-Dahlfors, A.; Miyahara, Y. Biomimetic Interfaces Reveal Activation Dynamics of C-Reactive Protein in Local Microenvironments. Adv. Healthc. Mater. 2014, 3, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Karol, A.B.; Reford, E.; Joshi, H.; Doroshow, D.B.; Galsky, M.D. C-reactive protein (CRP) as a prognostic biomarker in patients with bladder cancer: A meta-analysis. J. Clin. Oncol. 2023, 41, e16502. [Google Scholar] [CrossRef]
- Olson, M.E.; Hornick, M.G.; Stefanski, A.; Albanna, H.R.; Gjoni, A.; Hall, G.D.; Hart, P.C.; Rajab, I.M.; Potempa, L.A. A biofunctional review of C-reactive protein (CRP) as a mediator of inflammatory and immune responses: Differentiating pentameric and modified CRP isoform effects. Front. Immunol. 2023, 14, 1264383. [Google Scholar] [CrossRef]
- Kolberg, H.-C.; Edimiris, A.; Hoffmann, O.; Wetzig, S.; Shaheen, M.; Stephanou, M.; Kolberg-Liedtke, C. The role of C-reactive protein (CRP) as a prognostic biomarker in patients with early breast cancer (EBC) treated with neoadjuvant chemotherapy (NACT). J. Clin. Oncol. 2021, 39, e12545. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice—A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. Dual elevated remnant cholesterol and C-reactive protein in myocardial infarction, atherosclerotic cardiovascular disease, and mortality. Atherosclerosis 2023, 379, 117141. [Google Scholar] [CrossRef]
- Escadafal, C.; Incardona, S.; Fernandez-Carballo, B.L.; Dittrich, S. The good and the bad: Using C reactive protein to distinguish bacterial from non-bacterial infection among febrile patients in low-resource settings. BMJ Global Health 2020, 5, e002396. [Google Scholar] [CrossRef] [PubMed]
- Van den Bruel, A.; Jones, C.; Thompson, M.; Mant, D. C-reactive protein point-of-care testing in acutely ill children: A mixed methods study in primary care. Arch. Dis. Child. 2016, 101, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023, 61, 1540–1545. [Google Scholar] [CrossRef]
- Li, Y.S.; Men, X.J.; Gao, G.W.; Tian, Y.; Wen, Y.Q.; Zhang, X.J. A distance-based capillary biosensor using wettability alteration. Lab A Chip 2021, 21, 719–724. [Google Scholar] [CrossRef]
- Tian, T.; An, Y.; Wu, Y.P.; Song, Y.L.; Zhu, Z.; Yang, C.Y. Integrated Distance-Based Origami Paper Analytical Device for One-Step Visualized Analysis. Acs Appl. Mater. Interfaces 2017, 9, 30480–30487. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.Y.; Heo, N.S.; Kang, J.W.; Lee, J.B.; Kim, H.J.; Kim, M.I. Nanoceria-based lateral flow immunoassay for hydrogen peroxide-free colorimetric biosensing for C-reactive protein. Anal. Bioanal. Chem. 2022, 414, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.S.; Housaindokht, M.R.; Firouzi, M. Designing and prototyping a novel biosensor based on a volumetric bar-chart chip for urea detection. Lab A Chip 2024, 24, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Ban, M.J.; Jin, X.X.; Li, A.; Tao, J.; Pan, L.G. An integrated distance-based microfluidic aptasensor for visual quantitative detection of with sample-in-answer-out capability. Sens. Actuators B-Chem. 2023, 381, 133480. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Jiao, X.; Li, T.; Lv, Z.; Yang, C.J.; Zhang, X.; Wen, Y. Control of capillary behavior through target-responsive hydrogel permeability alteration for sensitive visual quantitative detection. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.X.; Liu, S.S.; Li, B.X. A label-free visual aptasensor for zearalenone detection based on target-responsive aptamer-cross-linked hydrogel and color change of gold nanoparticles. Food Chem. 2022, 389, 133078. [Google Scholar] [CrossRef]
- Khajouei, S.; Ravan, H.; Ebrahimi, A. DNA hydrogel-empowered biosensing. Adv. Colloid Interface Sci. 2020, 275, 102060. [Google Scholar] [CrossRef]
- Ji, T.; Liu, D.; Liu, F.; Li, J.; Ruan, Q.; Song, Y.; Tian, T.; Zhu, Z.; Zhou, L.; Lin, H.; et al. A pressure-based bioassay for the rapid, portable and quantitative detection of C-reactive protein. Chem. Commun. 2016, 52, 8452–8454. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Zhou, J.; Liu, S.; Tian, T.; Song, Y.; Zhu, Z.; Zhou, L.; Ji, T.; Yang, C. A fully integrated distance readout ELISA-Chip for point-of-care testing with sample-in-answer-out capability. Biosens. Bioelectron. 2017, 96, 332–338. [Google Scholar] [CrossRef]
- Chuang, C.S.; Deng, C.Z.; Fang, Y.F.; Jiang, H.R.; Tseng, P.W.; Sheen, H.J.; Fan, Y.J. A smartphone-based diffusometric immunoassay for detecting C-reactive protein. Sci. Rep. 2019, 9, 17131. [Google Scholar] [CrossRef]
- Huang, R.; Quan, J.; Su, B.; Cai, C.; Cai, S.; Chen, Y.; Mou, Z.; Zhou, P.; Ma, D.; Cui, X. A two-step competition assay for visual, sensitive and quantitative C-reactive protein detection in low-cost microfluidic particle accumulators. Sens. Actuators B Chem. 2022, 359, 131583. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Liu, Q.; Huang, H.; Wang, X.; Shi, Z.; Li, Y.; Liu, S.; Xue, L.; Lei, Y. A smart hydrogel system for visual detection of glucose. Biosens. Bioelectron. 2019, 142, 111547. [Google Scholar] [CrossRef] [PubMed]
- Lucío, M.I.; Cubells-Gómez, A.; Maquieira, A.; Bañuls, M.J. Hydrogel-based holographic sensors and biosensors: Past, present, and future. Anal. Bioanal. Chem. 2022, 414, 993–1014. [Google Scholar] [CrossRef]
- Volanakis, J.E.; Wirtz, K.W.A. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers. Nature 1979, 281, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Abe, N.; Santsuka, H.; Shida, T.; Kishida, K.; Kuwajima, S.; Yamada, M.; Morimatsu, M.; Naiki, M. Efficient preparation of monospecific anti-canine C-reactive protein serum and purification of canine C-reactive protein by affinity chromatography. Veter- Immunol. Immunopathol. 1993, 36, 293–301. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef]
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushita, T.; Chung, U.I.; Nakamura, K.; Kawaguchi, H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef]
- Ishihara, K.; Ziats, N.P.; Tierney, B.P.; Nakabayashi, N.; Anderson, J.M. Protein adsorption from human plasma is reduced on phospholipid polymers. J. Biomed. Mater. Res. 1991, 25, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Pinyorospathum, C.; Chaiyo, S.; Sae-Ung, P.; Hoven, V.P.; Damsongsang, P.; Siangproh, W.; Chailapakul, O. Disposable paper-based electrochemical sensor using thiol-terminated poly(2-methacryloyloxyethyl phosphorylcholine) for the label-free detection of C-reactive protein. Microchim. Acta 2019, 186, 472. [Google Scholar] [CrossRef]
- Matsuura, R.; Tawa, K.; Kitayama, Y.; Takeuchi, T. A plasmonic chip-based bio/chemical hybrid sensing system for the highly sensitive detection of C-reactive protein. Chem. Commun. 2016, 52, 3883–3886. [Google Scholar] [CrossRef]
- Kitayama, Y.; Takeuchi, T. Localized Surface Plasmon Resonance Nanosensing of C-Reactive Protein with Poly(2-methacryloyloxyethyl phosphorylcholine)-Grafted Gold Nanoparticles Prepared by Surface-Initiated Atom Transfer Radical Polymerization. Anal. Chem. 2014, 86, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Lucío, M.I.; Montoto, A.H.; Fernández, E.; Alamri, S.; Kunze, T.; Bañuls, M.-J.; Maquieira, Á. Label-free detection of C-Reactive protein using bioresponsive hydrogel-based surface relief diffraction gratings. Biosens. Bioelectron. 2021, 193, 113561. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.G.; Kim, H.-C.; Lee, S.J.; Baek, C.S.; Jeong, S.W. Protein-Directed Immobilization of Phosphocholine Ligands on a Gold Surface for Multivalent C-Reactive Protein Binding. Curr. Top. Med. Chem. 2013, 13, 519–524. [Google Scholar] [CrossRef]
- Christopeit, T.; Gossas, T.; Danielson, H. Characterization of Ca2+ and phosphocholine interactions with C-reactive protein using a surface plasmon resonance biosensor. Anal. Biochem. 2009, 391, 39–44. [Google Scholar] [CrossRef]
- Krylov, N.A.; Pentkovsky, V.M.; Efremov, R.G. Nontrivial Behavior of Water in the Vicinity and Inside Lipid Bilayers As Probed by Molecular Dynamics Simulations. ACS Nano 2013, 7, 9428–9442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.J.; Wang, J.; Zhai, G.X. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, S. Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 2010, 1, 5730. [Google Scholar] [CrossRef]
- Vishnu, S.K.D.; Ranganathan, P.; Rwei, S.P.; Pattamaprom, C.; Kavitha, T.; Sarojini, P. New reductant-free synthesis of gold nanoparticles-doped chitosan-based semi-IPN nanogel: A robust nanoreactor for exclusively sensitive 5-fluorouracil sensor. Int. J. Biol. Macromol. 2020, 148, 79–88. [Google Scholar] [CrossRef]
- Sánchez-deAlcázar, D.; Rodriguez-Abetxuko, A.; Beloqui, A. Metal-Organic Enzyme Nanogels as Nanointegrated Self-Reporting Chemobiosensors. Acs Appl. Mater. Interfaces 2022, 14, 27589–27598. [Google Scholar] [CrossRef]
- Lee, S.K.; Yim, B.; Park, J.; Kim, N.G.; Kim, B.S.; Park, Y.; Yoon, Y.K.; Kim, J. Method for the Rapid Detection of SARS-CoV-2-Neutralizing Antibodies Using a Nanogel-Based Surface Plasmon Resonance Biosensor. ACS Appl. Polym. Mater. 2023, 5, 2195–2202. [Google Scholar] [CrossRef]
- Díaz-Betancor, Z.; Bañuls, M.J.; Sanza, F.J.; Casquel, R.; Laguna, M.F.; Holgado, M.; Puchades, R.; Maquieira, Á. Phosphorylcholine-based hydrogel for immobilization of biomolecules. Application to fluorometric microarrays for use in hybridization assays and immunoassays, and nanophotonic biosensing. Microchim. Acta 2019, 186, 570. [Google Scholar] [CrossRef] [PubMed]
- Shagymgereyeva, S.; Sarsenbekuly, B.; Kang, W.; Yang, H.; Turtabayev, S. Advances of polymer microspheres and its applications for enhanced oil recovery. Colloids Surf. B Biointerfaces 2023, 233, 113622. [Google Scholar] [CrossRef] [PubMed]
- Farqarazi, S.; Khorasani, M. The effects of synthetic and physical factors on the properties of poly (acrylic acid)-based hydrogels synthesized by precipitation polymerization technique: A review. Rev. Chem. Eng. 2024, 40, 5. [Google Scholar] [CrossRef]
- Zhou, H.-H.; Tang, Y.-L.; Xu, T.-H.; Cheng, B. C-reactive protein: Structure, function, regulation, and role in clinical diseases. Front. Immunol. 2024, 15, 1425168. [Google Scholar] [CrossRef] [PubMed]
- Molinero-Fernandez, A.; Moreno-Guzman, M.; Arruza, L.; López, M.Á.; Escarpa, A. Toward early diagnosis of late-onset sepsis in preterm neonates: Dual magnetoimmunosensor for simultaneous procalcitonin and C-reactive protein determination in diagnosed clinical samples. ACS Sens. 2019, 4, 2117–2123. [Google Scholar] [CrossRef]
- Fukuyama, M.; Maeki, M.; Ishida, A.; Tani, H.; Hibara, A.; Tokeshi, M. One-step non-competitive fluorescence polarization immunoassay based on a Fab fragment for C-reactive protein quantification. Sens. Actuators B Chem. 2021, 326, 128982. [Google Scholar]
- Fu, Q.; Wu, Z.; Li, J.; Wu, Z.; Zhong, H.; Yang, Q.; Liu, Q.; Liu, Z.; Sheng, L.; Xu, M.; et al. Quantitative assessment of disease markers using the naked eye: Point-of-care testing with gas generation-based biosensor immunochromatographic strips. J. Nanobiotechnol. 2019, 17, 67. [Google Scholar] [CrossRef]
- Panraksa, Y.; Apilux, A.; Jampasa, S.; Puthong, S.; Henry, C.S.; Rengpipat, S.; Chailapakul, O. A facile one-step gold nanoparticles enhancement based on sequential patterned lateral flow immunoassay device for C-reactive protein detection. Sens. Actuators B Chem. 2021, 329, 129241. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, S.; Qiang, Z.; Guo, M.; Tan, X.; Li, H.; Yu, Y. Improved Sensitivity and Wide Range Detection of Small Analytes Using a Two-Antigen-Combined Competitive Immunoassay. ACS Omega 2022, 7, 48121–48129. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Liu, Z.; Jiang, X.; Huang, J.; Zhou, P.; Mou, Z.; Ma, D.; Cui, X. A Visual Distance-Based Capillary Immunoassay Using Biomimetic Polymer Nanoparticles for Highly Sensitive and Specific C-Reactive Protein Quantification. Int. J. Mol. Sci. 2024, 25, 9771. https://doi.org/10.3390/ijms25189771
Huang R, Liu Z, Jiang X, Huang J, Zhou P, Mou Z, Ma D, Cui X. A Visual Distance-Based Capillary Immunoassay Using Biomimetic Polymer Nanoparticles for Highly Sensitive and Specific C-Reactive Protein Quantification. International Journal of Molecular Sciences. 2024; 25(18):9771. https://doi.org/10.3390/ijms25189771
Chicago/Turabian StyleHuang, Ruodong, Zhenbo Liu, Xinlin Jiang, Junqi Huang, Ping Zhou, Zongxia Mou, Dong Ma, and Xin Cui. 2024. "A Visual Distance-Based Capillary Immunoassay Using Biomimetic Polymer Nanoparticles for Highly Sensitive and Specific C-Reactive Protein Quantification" International Journal of Molecular Sciences 25, no. 18: 9771. https://doi.org/10.3390/ijms25189771
APA StyleHuang, R., Liu, Z., Jiang, X., Huang, J., Zhou, P., Mou, Z., Ma, D., & Cui, X. (2024). A Visual Distance-Based Capillary Immunoassay Using Biomimetic Polymer Nanoparticles for Highly Sensitive and Specific C-Reactive Protein Quantification. International Journal of Molecular Sciences, 25(18), 9771. https://doi.org/10.3390/ijms25189771




