Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks
Abstract
:1. Introduction
2. Microbiota Host: From Passive Spectator to Essential Modulator in Health and Disease
2.1. Structural Components of Microbiota Host
2.2. Immunological Components of Microbiota Host
3. Maternal Microbiota Disturbance: Implications for Offspring Immune System and Beyond
3.1. Maternal DIET and Microbiota
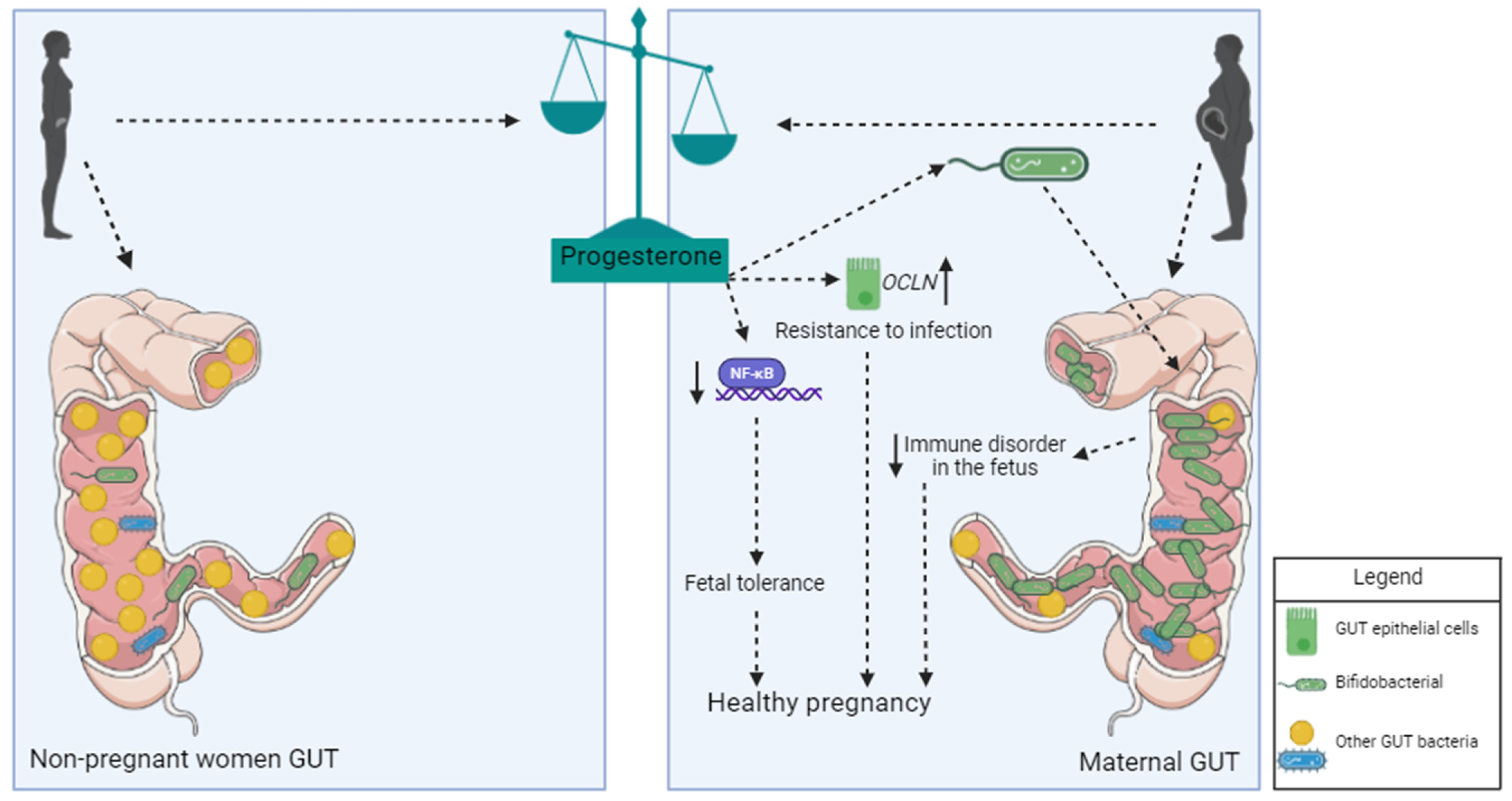
3.2. Influence of Progesterone on Microbiota during Pregnancy
3.3. Maternal Microbiota and Immunity: Implications for Fetal and Neonatal Health
4. Exploring the Interplay between Prenatal Maternal Stress, Microbiota, and Depression
4.1. Stress, Gut Microbiota, and Depression
4.2. The Impact of Prenatal Maternal Stress on Maternal Offspring Microbiome Dynamics
4.3. The Impact of Maternal Microbiota and Prenatal Stress on Offspring Immune System
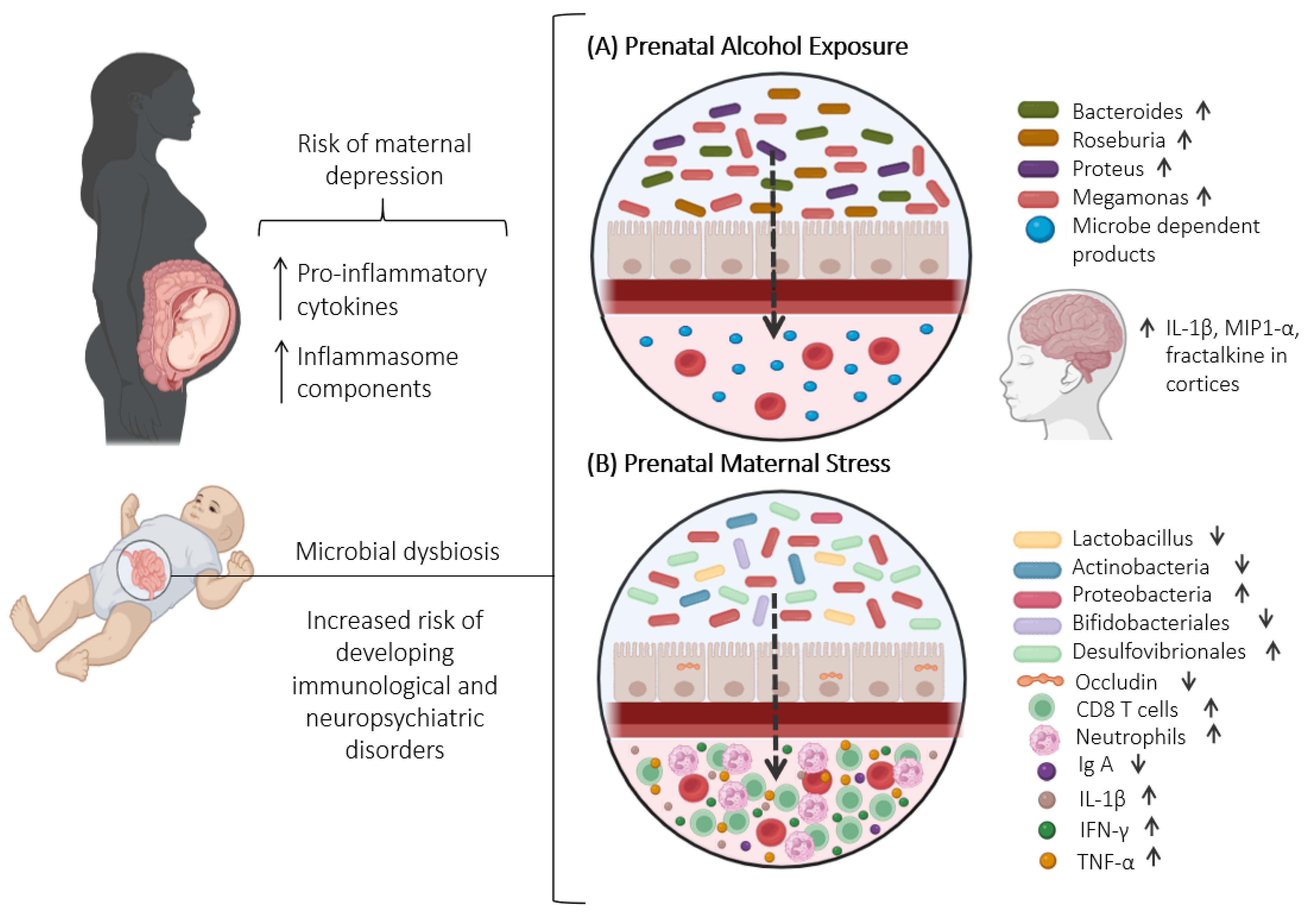
5. Ethanol-Induced Changes in Gut Microbiota: Understanding Consequences and Investigating Prenatal Exposure Effects
5.1. Consequences of Ethanol Consumption in Intestinal Dysbiosis
5.2. Effects of Prenatal Ethanol Exposure on Maternal and Offspring Microbiota
6. Connecting the Dots: Exploring the Link between Microbiota Dysbiosis, Maternal Immunological Changes, and Depression
7. Conclusions
Implications and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Tanelian, A.; Nankova, B.; Miari, M.; Sabban, E.L. Microbial composition, functionality, and stress resilience or susceptibility: Unraveling sex-specific patterns. Biol. Sex Differ. 2024, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers 2023, 9, 11. [Google Scholar] [CrossRef]
- Butts, M.; Sundaram, V.L.; Murughiyan, U.; Borthakur, A.; Singh, S. The Influence of Alcohol Consumption on Intestinal Nutrient Absorption: A Comprehensive Review. Nutrients 2023, 15, 1571. [Google Scholar] [CrossRef]
- Esper, L.H.; Furtado, E.F. Stressful life events and alcohol consumption in pregnant women: A cross-sectional survey. Midwifery 2019, 71, 27–32. [Google Scholar] [CrossRef]
- Lovallo, W.R. Cortisol secretion patterns in addiction and addiction risk. Int. J. Psychophysiol. 2006, 59, 195–202. [Google Scholar] [CrossRef]
- Anderson, A.E.; Hure, A.J.; Kay-Lambkin, F.J.; Loxton, D.J. Women’s perceptions of information about alcohol use during pregnancy: A qualitative study. BMC Public Health 2014, 14, 1048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Branco, E.I.; Kaskutas, L.A. “If it burns going down…”: How focus groups can shape fetal alcohol syndrome (FAS) prevention. Subst. Use Misuse 2001, 36, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Qiao, X.; Yi, L.; Siren, D.; He, J.; Hai, L.; Guo, F.; Xiao, Y.; Ji, R. Camel milk modulates ethanol-induced changes in the gut microbiome and transcriptome in a mouse model of acute alcoholic liver disease. J. Dairy Sci. 2020, 103, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, J.; Du, K.; Liu, S.; Wang, J.; Wang, Q.; Zhao, H.; Li, M.; Yan, D.; Zhang, R.; et al. Intestinal microbiome dysbiosis in alcohol-dependent patients and its effect on rat behaviors. mBio 2023, 14, e0239223. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Partrick, K.A.; Chassaing, B.; Beach, L.Q.; McCann, K.E.; Gewirtz, A.T.; Huhman, K.L. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav. Brain Res. 2018, 345, 39–48. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, T.; Wu, Y.; Liu, Y.; Zou, Z.; Bai, J. Impacts of Maternal Diet and Alcohol Consumption during Pregnancy on Maternal and Infant Gut Microbiota. Biomolecules 2021, 11, 369. [Google Scholar] [CrossRef]
- Yeramilli, V.; Cheddadi, R.; Shah, J.; Brawner, K.; Martin, C. A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites. Metabolites 2023, 13, 535. [Google Scholar] [CrossRef]
- Bodnar, T.S.; Ainsworth-Cruickshank, G.; Billy, V.; Wegener Parfrey, L.; Weinberg, J.; Raineki, C. Alcohol consumption during pregnancy differentially affects the fecal microbiota of dams and offspring. Sci. Rep. 2024, 14, 16121. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes Drive Evolution of Animals and Plants: The Hologenome Concept. mBio 2016, 7, e01395. [Google Scholar] [CrossRef]
- Cohen, Y.; Borenstein, E. The microbiome’s fiber degradation profile and its relationship with the host diet. BMC Biol. 2022, 20, 266. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Barber, A.F.; Noya, S.B.; Williams, J.A.; Li, F.; Daniel, S.G.; Bittinger, K.; Fang, J.; Sehgal, A. The microbiome stabilizes circadian rhythms in the gut. Proc. Natl. Acad. Sci. USA 2023, 120, e2217532120. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef]
- Cheney, A.M.; Costello, S.M.; Pinkham, N.V.; Waldum, A.; Broadaway, S.C.; Cotrina-Vidal, M.; Mergy, M.; Tripet, B.; Kominsky, D.J.; Grifka-Walk, H.M.; et al. Gut microbiome dysbiosis drives metabolic dysfunction in Familial dysautonomia. Nat. Commun. 2023, 14, 218. [Google Scholar] [CrossRef]
- Roy Sarkar, S.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Afroz, K.F.; Reyes, N.; Young, K.; Parikh, K.; Misra, V.; Alviña, K. Altered gut microbiome and autism like behavior are associated with parental high salt diet in male mice. Sci. Rep. 2021, 11, 8364, Erratum in Sci. Rep. 2022, 12, 5686. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Corfield, A.P. The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human. Microorganisms 2018, 6, 78. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Pringault, E.; Kraehenbuhl, J.P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 1996, 14, 275–300. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 2001, 1, 59–67. [Google Scholar] [CrossRef]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef]
- Masopust, D.; Vezys, V.; Wherry, E.J.; Barber, D.L.; Ahmed, R. Cutting edge: Gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 2006, 176, 2079–2083. [Google Scholar] [CrossRef]
- van Wijk, F.; Cheroutre, H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev. Clin. Immunol. 2010, 6, 559–566. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006, 212, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Ishigame, H.; Kakuta, S.; Nagai, T.; Kadoki, M.; Nambu, A.; Komiyama, Y.; Fujikado, N.; Tanahashi, Y.; Akitsu, A.; Kotaki, H.; et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009, 30, 108–119. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Bousbaine, D.; Fisch, L.I.; London, M.; Bhagchandani, P.; Rezende de Castro, T.B.; Mimee, M.; Olesen, S.; Reis, B.S.; VanInsberghe, D.; Bortolatto, J.; et al. A conserved Bacteroidetes antigen induces anti-inflammatory intestinal T lymphocytes. Science 2022, 377, 660–666. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Siracusa, F.; Schaltenberg, N.; Kumar, Y.; Lesker, T.R.; Steglich, B.; Liwinski, T.; Cortesi, F.; Frommann, L.; Diercks, B.P.; Bönisch, F.; et al. Short-term dietary changes can result in mucosal and systemic immune depression. Nat. Immunol. 2023, 24, 1473–1486. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Gut Microbiome and Female Health. Biology 2022, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Mungroo, M.R.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Use of Gut Microbial Modulation Strategies as Interventional Strategies for Ageing. Microorganisms 2022, 10, 1869. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.M.; Guerri, C.; Ureña, J.; Pascual, M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Wolfe, R.R.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am. J. Obstet. Gynecol. 1992, 167, 913–919. [Google Scholar] [CrossRef]
- Connor, K.L.; Chehoud, C.; Altrichter, A.; Chan, L.; DeSantis, T.Z.; Lye, S.J. Maternal metabolic, immune, and microbial systems in late pregnancy vary with malnutrition in mice. Biol. Reprod. 2018, 98, 579–592. [Google Scholar] [CrossRef]
- Mirpuri, J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: Implications for public health initiatives. Pediatr. Res. 2021, 89, 301–306. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 2013, 191, 3200–3209. [Google Scholar] [CrossRef]
- Zhou, Z.; Bian, C.; Luo, Z.; Guille, C.; Ogunrinde, E.; Wu, J.; Zhao, M.; Fitting, S.; Kamen, D.L.; Oates, J.C.; et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci. Rep. 2019, 9, 8367. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. The Role of NFκB in Healthy and Preeclamptic Placenta: Trophoblasts in the Spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Cohen, C.J.; Kerenyi, T.D.; Gusberg, S.B. A blocking factor in amniotic fluid causing leukocyte migration enhancement. Am. J. Obstet. Gynecol. 1979, 133, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Shohat, B.; Faktor, J.M. Immunosuppressive activity of human amniotic fluid of normal and abnormal pregnancies. Int. J. Fertil. 1988, 33, 273–277. [Google Scholar]
- Lang, A.K.; Searle, R.F. The immunomodulatory activity of human amniotic fluid can be correlated with transforming growth factor-beta 1 (TGF-beta 1) and beta 2 activity. Clin. Exp. Immunol. 1994, 97, 158–163. [Google Scholar] [CrossRef]
- Winters, A.D.; Romero, R.; Greenberg, J.M.; Galaz, J.; Shaffer, Z.D.; Garcia-Flores, V.; Kracht, D.J.; Gomez-Lopez, N.; Theis, K.R. Does the Amniotic Fluid of Mice Contain a Viable Microbiota? Front. Immunol. 2022, 13, 820366. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Rackaityte, E.; Halkias, J.; Fukui, E.M.; Mendoza, V.F.; Hayzelden, C.; Crawford, E.D.; Fujimura, K.E.; Burt, T.D.; Lynch, S.V. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020, 26, 599–607. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nõlvak, H.; Preem, J.K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Muylaert, D.; Kubota, H.; Sakai, T.; Oishi, K.; Martin, R.; Ben Amor, K.; Oozeer, R.; et al. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl. Environ. Microbiol. 2011, 77, 6788–6793. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, Y.; Kato, N.; Watanabe, K.; Kato, H. Molecular epidemiological study of vertical transmission of vaginal Lactobacillus species from mothers to newborn infants in Japanese, by arbitrarily primed polymerase chain reaction. J. Infect. Chemother. 2002, 8, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Tanaka, R.; Fukuda, M.; Oyaizu, H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 1999, 65, 4506–4512. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Brushett, S.; Prins, J.; Zhernakova, A. The maternal gut microbiome during pregnancy and its role in maternal and infant health. Curr. Opin. Microbiol. 2023, 74, 102309. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef]
- Savino, F.; Cresi, F.; Pautasso, S.; Palumeri, E.; Tullio, V.; Roana, J.; Silvestro, L.; Oggero, R. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr. 2004, 93, 825–829. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736.e3. [Google Scholar] [CrossRef]
- Haro, C.; Garcia-Carpintero, S.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Delgado-Lista, J.; Perez-Martinez, P.; Rangel Zuñiga, O.A.; Quintana-Navarro, G.M.; Landa, B.B.; Clemente, J.C.; et al. The gut microbial community in metabolic syndrome patients is modified by diet. J. Nutr. Biochem. 2016, 27, 27–31. [Google Scholar] [CrossRef]
- Blackburn, D.G. Reconstructing the Evolution of Viviparity and Placentation. J. Theor. Biol. 1998, 192, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Renfree, M.B.; Hore, T.A.; Shaw, G.; Graves, J.A.; Pask, A.J. Evolution of genomic imprinting: Insights from marsupials and monotremes. Annu. Rev. Genom. Hum. Genet. 2009, 10, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.J.; Dobrinskikh, E.; Soderborg, T.K.; Janssen, R.C.; Takahashi, D.L.; Dean, T.A.; Varlamov, O.; Hennebold, J.D.; Gannon, M.; Aagaard, K.M.; et al. Maternal diet alters long-term innate immune cell memory in fetal and juvenile hematopoietic stem and progenitor cells in nonhuman primate offspring. Cell Rep. 2023, 42, 112393. [Google Scholar] [CrossRef]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef]
- Telemo, E.; Hanson, L.A. Antibodies in milk. J. Mammary Gland Biol. Neoplasia 1996, 1, 243–249. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243, Erratum in Gut 2023, 72, e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Zhang, H.; Shi, G.; Zheng, X.; Chang, J.; Lin, Q.; Tian, Z.; Yang, H. The impact of gut microbial signals on hematopoietic stem cells and the bone marrow microenvironment. Front. Immunol. 2024, 15, 1338178. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Gorky, J.; Schwaber, J. The role of the gut-brain axis in alcohol use disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Yoshioka, T.; Ohashi, M.; Matsumoto, K.; Omata, T.; Hamano, T.; Yamazaki, M.; Kimiki, S.; Okano, K.; Kobayashi, R.; Yamada, D.; et al. Repeated psychological stress, chronic vicarious social defeat stress, evokes irritable bowel syndrome-like symptoms in mice. Front. Neurosci. 2022, 16, 993132. [Google Scholar] [CrossRef]
- Drossman, D.A. Do psychosocial factors define symptom severity and patient status in irritable bowel syndrome? Am. J. Med. 1999, 107, 41S–50S. [Google Scholar] [CrossRef]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef]
- Bailey, M.T.; Lubach, G.R.; Coe, C.L. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 414–421. [Google Scholar] [CrossRef]
- Pandey, G.N.; Zhang, H.; Sharma, A.; Ren, X. Innate immunity receptors in depression and suicide: Upregulated NOD-like receptors containing pyrin (NLRPs) and hyperactive inflammasomes in the postmortem brains of people who were depressed and died by suicide. J. Psychiatry Neurosci. 2021, 46, E538–E547. [Google Scholar] [CrossRef]
- Kimura, L.F.; Novaes, L.S.; Picolo, G.; Munhoz, C.D.; Cheung, C.W.; Camarini, R. How environmental enrichment balances out neuroinflammation in chronic pain and comorbid depression and anxiety disorders. Br. J. Pharmacol. 2022, 179, 1640–1660. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, J.D.; Perdue, M.H. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G7–G13. [Google Scholar] [CrossRef] [PubMed]
- Paton, S.E.J.; Solano, J.L.; Coulombe-Rozon, F.; Lebel, M.; Menard, C. Barrier-environment interactions along the gut-brain axis and their influence on cognition and behaviour throughout the lifespan. J. Psychiatry Neurosci. 2023, 48, E190–E208. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Shityakov, S.; Förster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Collins, S.M.; Bercik, P. The Relationship between Intestinal Microbiota and the Central Nervous System in Normal Gastrointestinal Function and Disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M.; Geffard, M.; Bosmans, E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr. Scand. 2013, 127, 344–354. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Cima, I.; Corazza, N.; Dick, B.; Fuhrer, A.; Herren, S.; Jakob, S.; Ayuni, E.; Mueller, C.; Brunner, T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 2004, 200, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Amasi-Hartoonian, N.; Sforzini, L.; Cattaneo, A.; Pariante, C.M. Cause or consequence? Understanding the role of cortisol in the increased inflammation observed in depression. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100356. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 121, 114–121. [Google Scholar] [CrossRef]
- Ligumsky, M.; Simon, P.L.; Karmeli, F.; Rachmilewitz, D. Role of interleukin 1 in inflammatory bowel disease—Enhanced production during active disease. Gut 1990, 31, 686–689. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, C.; Xing, Y.; Xue, G.; Zhang, Q.; Pan, F.; Wu, G.; Hu, Y.; Guo, Q.; Lu, A.; et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 2017, 8, 1896. [Google Scholar] [CrossRef]
- Seo, S.U.; Kamada, N.; Muñoz-Planillo, R.; Kim, Y.G.; Kim, D.; Koizumi, Y.; Hasegawa, M.; Himpsl, S.D.; Browne, H.P.; Lawley, T.D.; et al. Distinct Commensals Induce Interleukin-1β via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity 2015, 42, 744–755. [Google Scholar] [CrossRef]
- Huang, L.S.; Anas, M.; Xu, J.; Zhou, B.; Toth, P.T.; Krishnan, Y.; Di, A.; Malik, A.B. Endosomal trafficking of two-pore K+ efflux channel TWIK2 to plasmalemma mediates NLRP3 inflammasome activation and inflammatory injury. eLife 2023, 12, e83842. [Google Scholar] [CrossRef]
- Wong, M.L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Cho, Y.; Ambade, A.; Szabo, G. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J. Neuroinflamm. 2018, 15, 298. [Google Scholar] [CrossRef]
- Ding, L.; Liu, J.; Yang, Y.; Cui, Z.; Du, G. Chronically socially isolated mice exhibit depressive-like behavior regulated by the gut microbiota. Heliyon 2024, 10, e29791. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Moloney, R.D.; Golubeva, A.V.; McVey Neufeld, K.A.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016, 339, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Liu, P.; Gao, M.; Liu, Z.; Zhang, Y.; Tu, H.; Lei, L.; Wu, P.; Zhang, A.; Yang, C.; Li, G.; et al. Gut Microbiome Composition Linked to Inflammatory Factors and Cognitive Functions in First-Episode, Drug-Naive Major Depressive Disorder Patients. Front. Neurosci. 2022, 15, 800764. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486, Erratum in Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 564. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Q.; Hou, Y.; Zhang, X.; Yin, Z.; Cai, X.; Wei, W.; Wang, J.; He, D.; Wang, G.; et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav. Immun. 2022, 102, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Ivković, S.; Mitić, M.; Adžić, M. Tryptophan metabolites in depression: Modulation by gut microbiota. Front. Behav. Neurosci. 2022, 16, 987697. [Google Scholar] [CrossRef]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020, 83, 239–247. [Google Scholar] [CrossRef]
- Yin, X.; Sun, N.; Jiang, N.; Xu, X.; Gan, Y.; Zhang, J.; Qiu, L.; Yang, C.; Shi, X.; Chang, J.; et al. Prevalence and associated factors of antenatal depression: Systematic reviews and meta-analyses. Clin. Psychol. Rev. 2021, 83, 101932. [Google Scholar] [CrossRef]
- Staneva, A.; Bogossian, F.; Pritchard, M.; Wittkowski, A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth 2015, 28, 179–193. [Google Scholar] [CrossRef]
- Horrigan, T.J.; Schroeder, A.V.; Schaffer, R.M. The triad of substance abuse, violence, and depression are interrelated in pregnancy. J. Subst. Abus. Treat. 2000, 18, 55–58. [Google Scholar] [CrossRef]
- Grote, N.K.; Bridge, J.A.; Gavin, A.R.; Melville, J.L.; Iyengar, S.; Katon, W.J. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 2010, 67, 1012–1024. [Google Scholar] [CrossRef]
- Halligan, S.L.; Murray, L.; Martins, C.; Cooper, P.J. Maternal depression and psychiatric outcomes in adolescent offspring: A 13-year longitudinal study. J. Affect. Disord. 2007, 97, 145–154. [Google Scholar] [CrossRef]
- Kingsbury, M.; Weeks, M.; MacKinnon, N.; Evans, J.; Mahedy, L.; Dykxhoorn, J.; Colman, I. Stressful Life Events During Pregnancy and Offspring Depression: Evidence From a Prospective Cohort Study. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 709–716.e2. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587, Erratum in Microorganisms 2020, 8, E2046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; Lu, B.; Wang, G. The role of gut microbiota in the pathogenesis and treatment of postpartum depression. Ann. Gen. Psychiatry 2023, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.; Cantrell, K.; Huang, S.; McDonald, D.; Haiminen, N.; Carrieri, A.P.; Zhu, Q.; Gonzalez, A.; McGrath, I.; Beck, K.L.; et al. Efficient computation of Faith’s phylogenetic diversity with applications in characterizing microbiomes. Genome Res. 2021, 31, 2131–2137. [Google Scholar] [CrossRef]
- Galley, J.D.; Mashburn-Warren, L.; Blalock, L.C.; Lauber, C.L.; Carroll, J.E.; Ross, K.M.; Hobel, C.; Coussons-Read, M.; Dunkel Schetter, C.; Gur, T.L. Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain Behav. Immun. 2023, 107, 253–264. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- O’Donnell, K.; O’Connor, T.G.; Glover, V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev. Neurosci. 2009, 31, 285–292. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Bugge Jensen, A.; Freeman, L.; Khalife, N.; O’Connor, T.G.; Glover, V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology 2012, 37, 818–826. [Google Scholar] [CrossRef]
- Hantsoo, L.; Kornfield, S.; Anguera, M.C.; Epperson, C.N. Inflammation: A Proposed Intermediary Between Maternal Stress and Offspring Neuropsychiatric Risk. Biol. Psychiatry 2019, 85, 97–106. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; Claassen-Weitz, S.; Gardner-Lubbe, S.; Botha, G.; Kaba, M.; Zar, H.J.; Nicol, M.P.; Stein, D.J. Association of maternal prenatal psychological stressors and distress with maternal and early infant faecal bacterial profile. Acta Neuropsychiatr. 2020, 32, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Hamidi, M. Maternal stress during the third trimester of pregnancy and the neonatal microbiome. J. Matern. Fetal Neonatal Med. 2023, 36, 2214835. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Crampton, S.; Desbonnet, L.; Edge, D.; O’Sullivan, O.; Lomasney, K.W.; Zhdanov, A.V.; Crispie, F.; Moloney, R.D.; Borre, Y.E.; et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015, 60, 58–74. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Nuccitelli, S.; Carnevali, P.; Brigidi, P.; Vitali, B.; Nobili, F.; Rami, R.; Garaguso, I.; Mengheri, E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel Dis. 2009, 15, 1526–1536. [Google Scholar] [CrossRef]
- Gur, T.L.; Palkar, A.V.; Rajasekera, T.; Allen, J.; Niraula, A.; Godbout, J.; Bailey, M.T. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav. Brain Res. 2019, 359, 886–894. [Google Scholar] [CrossRef]
- Coretti, L.; Cristiano, C.; Florio, E.; Scala, G.; Lama, A.; Keller, S.; Cuomo, M.; Russo, R.; Pero, R.; Paciello, O.; et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep. 2017, 7, 45356. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, R.; Li, L.; Jin, G.; Zhou, B.; Huang, H.; Li, M.; Yang, Y.; Liu, X.; Cao, X.; et al. Prenatal Maternal Stress Exacerbates Experimental Colitis of Offspring in Adulthood. Front. Immunol. 2021, 12, 700995. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Rosin, S.; Xia, K.; Azcarate-Peril, M.A.; Carlson, A.L.; Propper, C.B.; Thompson, A.L.; Grewen, K.; Knickmeyer, R.C. A preliminary study of gut microbiome variation and HPA axis reactivity in healthy infants. Psychoneuroendocrinology 2021, 124, 105046. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019, 55, 100797. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef]
- Maes, M.; Vasupanrajit, A.; Jirakran, K.; Klomkliew, P.; Chanchaem, P.; Tunvirachaisakul, C.; Plaimas, K.; Suratanee, A.; Payungporn, S. Adverse childhood experiences and reoccurrence of illness impact the gut microbiome, which affects suicidal behaviours and the phenome of major depression: Towards enterotypic phenotypes. Acta Neuropsychiatr. 2023, 35, 328–345. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef]
- Chen, H.J.; Bischoff, A.; Galley, J.D.; Peck, L.; Bailey, M.T.; Gur, T.L. Discrete role for maternal stress and gut microbes in shaping maternal and offspring immunity. Neurobiol. Stress 2022, 21, 100480. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Maccioni, L.; Fu, Y.; Horsmans, Y.; Leclercq, I.; Stärkel, P.; Kunos, G.; Gao, B. Alcohol-associated bowel disease: New insights into pathogenesis. eGastroenterology 2023, 1, e100013. [Google Scholar] [CrossRef] [PubMed]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Toh, E.; Ross, R.A.; Heathers, L.E.; Chandler, K.; Oshodi, A.; McGee, B.; Modlik, E.; Linton, T.; Mangiacarne, D.; et al. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci. Rep. 2017, 7, 4462. [Google Scholar] [CrossRef]
- Peterson, V.L.; Jury, N.J.; Cabrera-Rubio, R.; Draper, L.A.; Crispie, F.; Cotter, P.D.; Dinan, T.G.; Holmes, A.; Cryan, J.F. Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav. Brain Res. 2017, 323, 172–176. [Google Scholar] [CrossRef]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef]
- Carbia, C.; Bastiaanssen, T.F.S.; Iannone, L.F.; García-Cabrerizo, R.; Boscaini, S.; Berding, K.; Strain, C.R.; Clarke, G.; Stanton, C.; Dinan, T.G.; et al. The Microbiome-Gut-Brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine 2023, 89, 104442. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, L.; Gong, C.; Li, T.; Xia, Y. The diversity of the intestinal microbiota in patients with alcohol use disorder and its relationship to alcohol consumption and cognition. Front. Psychiatry 2022, 13, 1054685. [Google Scholar] [CrossRef] [PubMed]
- Baltazar-Díaz, T.A.; González-Hernández, L.A.; Aldana-Ledesma, J.M.; Peña-Rodríguez, M.; Vega-Magaña, A.N.; Zepeda-Morales, A.S.M.; López-Roa, R.I.; Del Toro-Arreola, S.; Martínez-López, E.; Salazar-Montes, A.M.; et al. Escherichia/Shigella, SCFAs, and Metabolic Pathways-The Triad That Orchestrates Intestinal Dysbiosis in Patients with Decompensated Alcoholic Cirrhosis from Western Mexico. Microorganisms 2022, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Keshavarzian, A.; Engen, P.; Forsyth, C.B.; Sikaroodi, M.; Gillevet, P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol. Clin. Exp. Res. 2009, 33, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wei, J.; Shen, M.; Ding, Y.; Lu, Y.; Ishaq, H.M.; Li, D.; Yan, D.; Wang, Q.; Zhang, R. Integrated Analyses of the Gut Microbiota, Intestinal Permeability, and Serum Metabolome Phenotype in Rats with Alcohol Withdrawal Syndrome. Appl. Environ. Microbiol. 2021, 87, e0083421. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Q.; Guo, L.; Zeng, H.; Ding, C.; Zhang, W.; Xu, D.; Wang, X.; Qiu, J.; Dong, Q.; et al. Gut Microbiota and Relevant Metabolites Analysis in Alcohol Dependent Mice. Front. Microbiol. 2018, 9, 1874. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Dong, X.; Hu, T.; Wang, L.; Zhao, W.; Zhu, S.; Li, G.; Hu, Y.; Gao, Q.; et al. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors 2019, 4, 187–199. [Google Scholar] [CrossRef]
- Xiao, H.W.; Ge, C.; Feng, G.X.; Li, Y.; Luo, D.; Dong, J.L.; Li, H.; Wang, H.; Cui, M.; Fan, S.J. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 2018, 287, 23–30. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Cuesta, C.M.; Pascual, M.; Pérez-Moraga, R.; Rodríguez-Navarro, I.; García-García, F.; Ureña-Peralta, J.R.; Guerri, C. TLR4 Deficiency Affects the Microbiome and Reduces Intestinal Dysfunctions and Inflammation in Chronic Alcohol-Fed Mice. Int. J. Mol. Sci. 2021, 22, 12830. [Google Scholar] [CrossRef]
- Bailey, S.M.; Pietsch, E.C.; Cunningham, C.C. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic. Biol. Med. 1999, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Tsuruya, A.; Kuwahara, A.; Saito, Y.; Yamaguchi, H.; Tsubo, T.; Suga, S.; Inai, M.; Aoki, Y.; Takahashi, S.; Tsutsumi, E.; et al. Ecophysiological consequences of alcoholism on human gut microbiota: Implications for ethanol-related pathogenesis of colon cancer. Sci. Rep. 2016, 6, 27923. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Wei, H.; Yu, C.; Zhang, C.; Ren, Y.; Guo, L.; Wang, T.; Chen, F.; Li, Y.; Zhang, X.; Wang, H.; et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 160, 114308. [Google Scholar] [CrossRef]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.-J.; Jonkers, D.M. Short-chain fatty acids activate AMP-Activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Singhal, R.; Donde, H.; Ghare, S.; Stocke, K.; Zhang, J.; Vadhanam, M.; Reddy, S.; Gobejishvili, L.; Chilton, P.; Joshi-Barve, S.; et al. Decrease in acetyl-CoA pathway utilizing butyrate-producing bacteria is a key pathogenic feature of alcohol-induced functional gut microbial dysbiosis and development of liver disease in mice. Gut Microbes 2021, 13, 1946367. [Google Scholar] [CrossRef]
- Leclercq, S.; Le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G.; et al. Gut Microbiota-Induced Changes in β-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep. 2020, 33, 108238. [Google Scholar] [CrossRef] [PubMed]
- Bjørkhaug, S.T.; Aanes, H.; Neupane, S.P.; Bramness, J.G.; Malvik, S.; Henriksen, C.; Skar, V.; Medhus, A.W.; Valeur, J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 2019, 10, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Hillemacher, T.; Bachmann, O.; Kahl, K.G.; Frieling, H. Alcohol, microbiome, and their effect on psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 85, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Day, A.W.; Kumamoto, C.A. Gut Microbiome Dysbiosis in Alcoholism: Consequences for Health and Recovery. Front. Cell Infect. Microbiol. 2022, 12, 840164. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Al-Atiyat, R.M.; Albatshan, H.A.; Aljassim, R.; Aljumaah, M.R.; Alkhulaifi, M.M.; Stanley, D.M. Effects of concentration of corn distillers dried grains with solubles and enzyme supplementation on cecal microbiota and performance in broiler chickens. Appl. Microbiol. Biotechnol. 2017, 101, 7017–7026. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Hensler, J.G.; Ladenheim, E.E.; Lyons, W.E. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J. Neurochem. 2003, 85, 1139–1147. [Google Scholar] [CrossRef]
- Charlton, M.E.; Sweetnam, P.M.; Fitzgerald, L.W.; Terwilliger, R.Z.; Nestler, E.J.; Duman, R.S. Chronic ethanol administration regulates the expression of GABAA receptor alpha 1 and alpha 5 subunits in the ventral tegmental area and hippocampus. J. Neurochem. 1997, 68, 121–127. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, D.; Yu, H.; Shen, H.; Lan, X.; Liu, H.; Chen, X.; Wu, X.; Zhang, G.; Wang, X. AMPAkine CX516 alleviated chronic ethanol exposure-induced neurodegeneration and depressive-like behavior in mice. Toxicol. Appl. Pharmacol. 2022, 439, 115924. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, D.; Yu, H.; Yuan, H.; Shen, H.; Lan, X.; Liu, H.; Chen, X.; Meng, F.; Wu, X.; et al. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation. Mol. Psychiatry 2023, 28, 919–930. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, M.; Zheng, L.; Cen, Q.; Wang, F.; Zhu, L.; Pang, R.; Zhang, A. Bifidobacterium: A probiotic for the prevention and treatment of depression. Front. Microbiol. 2023, 14, 1174800. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Gómez, P.; Pérez-Hernández, M.; O’Shea, E.; Caso, J.R.; Martín-Hernandez, D.; Cervera, L.A.; Centelles, M.L.G.; Gutiérrez-Lopez, M.D.; Colado, M.I. Changes in brain kynurenine levels via gut microbiota and gut-barrier disruption induced by chronic ethanol exposure in mice. FASEB J. 2019, 33, 12900–12914. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.M.; Kumar, G.; Zhang, S.J.; Zhong, Q.H.; Zhang, H.Y.; Gui, G.; Wu, L.L.; Fan, H.Z.; Sheng, J.W. Therapeutic Interventions of Gut-Brain Axis as Novel Strategies for Treatment of Alcohol Use Disorder Associated Cognitive and Mood Dysfunction. Front. Neurosci. 2022, 16, 820106. [Google Scholar] [CrossRef] [PubMed]
- Lippai, D.; Bala, S.; Petrasek, J.; Csak, T.; Levin, I.; Kurt-Jones, E.A.; Szabo, G. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 2013, 94, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Bhaumik, R.; Dwivedi, Y. Toll-like receptors in the depressed and suicide brain. J. Psychiatr. Res. 2014, 53, 62–68. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol. Res. 2017, 38, 163–171. [Google Scholar]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut. Dig. Dis. 2015, 33, 338–345. [Google Scholar] [CrossRef]
- Gabriel, K.; Hofmann, C.; Glavas, M.; Weinberg, J. The hormonal effects of alcohol use on the mother and fetus. Alcohol. Health Res. World 1998, 22, 170–177. [Google Scholar]
- Qiu, X.; Sun, X.; Li, H.O.; Wang, D.H.; Zhang, S.M. Maternal alcohol consumption and risk of postpartum depression: A meta-analysis of cohort studies. Public Health 2022, 213, 163–170. [Google Scholar] [CrossRef]
- Tan, C.H.; Denny, C.H.; Cheal, N.E.; Sniezek, J.E.; Kanny, D. Alcohol use and binge drinking among women of childbearing age—United States, 2011–2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Charness, M.E.; Riley, E.P.; Sowell, E.R. Drinking During Pregnancy and the Developing Brain: Is Any Amount Safe? Trends Cogn. Sci. 2016, 20, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.J.; Goodlett, C.R.; Hannigan, J.H. Animal models of fetal alcohol spectrum disorders: Impact of the social environment. Dev. Disabil. Res. Rev. 2009, 15, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.L.; Zhou, F.C.; Goodlett, C.R. Effects of one- and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol 2014, 48, 99–111. [Google Scholar] [CrossRef]
- Cantacorps, L.; Alfonso-Loeches, S.; Moscoso-Castro, M.; Cuitavi, J.; Gracia-Rubio, I.; López-Arnau, R.; Escubedo, E.; Guerri, C.; Valverde, O. Maternal alcohol binge drinking induces persistent neuroinflammation associated with myelin damage and behavioural dysfunctions in offspring mice. Neuropharmacology 2017, 123, 368–384. [Google Scholar] [CrossRef]
- Brocardo, P.S.; Boehme, F.; Patten, A.; Cox, A.; Gil-Mohapel, J.; Christie, B.R. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology 2012, 62, 1607–1618. [Google Scholar] [CrossRef]
- O’Connor, M.J.; Kasari, C. Prenatal alcohol exposure and depressive features in children. Alcohol. Clin. Exp. Res. 2000, 24, 1084–1092. [Google Scholar] [CrossRef]
- Bodnar, T.S.; Lee, C.; Wong, A.; Rubin, I.; Wegener Parfrey, L.; Weinberg, J. Evidence for long-lasting alterations in the fecal microbiota following prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2022, 46, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Parkes, G.C.; Rayment, N.B.; Hudspith, B.N.; Petrovska, L.; Lomer, M.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 31–39. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiong, L.; Li, L.; Li, M.; Chen, M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2017, 32, 28–38. [Google Scholar] [CrossRef]
- Li, Z.; Hu, G.; Zhu, L.; Sun, Z.; Jiang, Y.; Gao, M.J.; Zhan, X. Study of growth, metabolism, and morphology of Akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.J.; Xie, J.; Chen, J.J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Virdee, M.S.; Saini, N.; Kay, C.D.; Neilson, A.P.; Kwan, S.T.C.; Helfrich, K.K.; Mooney, S.M.; Smith, S.M. An enriched biosignature of gut microbiota-dependent metabolites characterizes maternal plasma in a mouse model of fetal alcohol spectrum disorder. Sci. Rep. 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; de Timary, P.; Delzenne, N.M.; Stärkel, P. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl. Psychiatry 2017, 7, e1048. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Montagud-Romero, S.; Forteza, J.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J. Neuroinflamm. 2017, 14, 145. [Google Scholar] [CrossRef]
- Bake, S.; Pinson, M.R.; Pandey, S.; Chambers, J.P.; Mota, R.; Fairchild, A.E.; Miranda, R.C.; Sohrabji, F. Prenatal alcohol-induced sex differences in immune, metabolic and neurobehavioral outcomes in adult rats. Brain Behav. Immun. 2021, 98, 86–100. [Google Scholar] [CrossRef]
- Zaretsky, M.V.; Alexander, J.M.; Byrd, W.; Bawdon, R.E. Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol. 2004, 103, 546–550. [Google Scholar] [CrossRef]
- Gleditsch, D.D.; Shornick, L.P.; Van Steenwinckel, J.; Gressens, P.; Weisert, R.P.; Koenig, J.M. Maternal inflammation modulates infant immune response patterns to viral lung challenge in a murine model. Pediatr. Res. 2014, 76, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. The concept of depression as a dysfunction of the immune system. Curr. Immunol. Rev. 2010, 6, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Buka, S.L.; Tsuang, M.T.; Torrey, E.F.; Klebanoff, M.A.; Bernstein, D.; Yolken, R.H. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry 2001, 58, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Solati, J.; Hosseini, M.H.; Shahi, H.R.; Saki, G.; Salari, A.A. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res. Bull. 2012, 87, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Henricks, A.M.; Sullivan, E.D.K.; Dwiel, L.L.; Li, J.Y.; Wallin, D.J.; Khokhar, J.Y.; Doucette, W.T. Maternal immune activation and adolescent alcohol exposure increase alcohol drinking and disrupt cortical-striatal-hippocampal oscillations in adult offspring. Transl. Psychiatry 2022, 12, 288. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Bienenstock, J.; Kunze, W.; Forsythe, P. Microbiota and the Gut–Brain Axis. Nutr. Rev. 2015, 73, 28–31. [Google Scholar] [CrossRef]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef]
| Prenatal Maternal Stress and Microbiota | ||||
|---|---|---|---|---|
| Author | Prenatal Stress Exposure | Specie | Microbiota (Offspring) | Outcomes |
| Zijlmans et al. (2015) doi: 10.1016/j.psyneuen.2015.01.006 [147] | Maternal prenatal stress (self-reports and/or salivary cortisol levels) | Humans | ↓ Lactic acid bacteria (Lactobacillus, Lactoccus, Aerococcus); ↓ Actinobacteria; ↑ Proteobacteria (Escherichia, Serratia and Enterobacter). | Specific colonization patterns were correlated with maternal-reported gastrointestinal symptoms and allergic reactions in the infant. |
| Galley et al. (2023) doi: 10.1016/j.bbi.2022.10.005 [145] | Maternal anxiety, depression, or perceived stress | Humans | ↓ Bifidobacterium dentium in the offspring of mothers with higher anxiety, perceived stress, and depression. | B. dentium was positively associated with elevated IL-6 and IL-8 during pregnancy |
| Naudé et al. (2020) doi: 10.1017/neu.2019.43 [151] | Prenatal distinct psychological profiles, including exposure to lifelong intimate partner violence (IPV) | Humans | Infants born to mothers that were exposed to high levels of IPV: ↑ Citrobacter and three unclassified genera (Enterobacteriaceae family) detected at birth. | - |
| Weiss, Hamidi (2023) doi: 10.1080/14767058.2023.2214835 [152] | Stress during the third trimester of pregnancy | Humans | Lower stress levels: ↑ Bacteroides, Eggerthella, and Enterobacteriacea. Higher stress levels: ↑ Lactobacillus and Bifidobacterium. | - |
| Golubeva et al. (2015) doi: 10.1016/j.psyneuen.2015.06.002 [155] | Restraint stress (gestational day 14–20) | Rats | ↑ Oscillibacter, Anaerotruncus, and Peptococcus; ↓ Lactobacillus (tendency). male offspring | - |
| Gur et al. (2019) doi: 10.1016/j.bbr.2018.06.025 [158] | Restraint stress (gestational day 10–16) | Mice | ↓ Bacteroides and Parabacteroides. male offspring | - |
| Sun et al. (2021) doi: 10.3389/fimmu.2021.700995 [160] | Variable stress beginning at gestational day 10 | Mice | ↓ anti-inflammatory probiotics: Bifidobacteriales, Bifidobacteriaceae, and Bifidobacterium. ↑ pro-inflammatory bacteria: Desulfovibrionales, Desulfovibrionaceae, and Desulfovibrio. | Prenatal maternal stress caused persistent overgrowth of Desulfovibrio resulting in exacerbated experimental colitis in adulthood. |
| Bailey, Lubach, Coe (2004) doi: 10.1097/00005176-200404000-00009 [99] | Stressed during pregnancy using an acoustical startle paradigm | Monkeys | ↓ Bifidobacteria and Lactobacilli. | - |
| Alcohol and Microbiota | ||||
|---|---|---|---|---|
| Author | Alcohol Exposure | Specie | Microbiota | Outcomes/Main Results |
| Mutlu et al. (2009) doi: 10.1111/j.1530-0277.2009.01022.x [185] | Received alcohol or dextrose intragastrically by gavage twice daily for up to 10 weeks | Rats | Alterations in the composition of mucosa-associated microbiota in the colon | Little or no dysbiosis after 4–6 weeks of alcohol feeding, but dysbiosis after 10 weeks of alcohol exposure |
| Bull-Otterson et al. (2013) doi: 10.1371/journal. pone.0053028 [175] | Mice were fed liquid Lieber–DeCarli diet without or with alcohol (5% v/v) for 6 weeks | Mice | ↑ Proteobacteria, Actinobacteria ↓ Bacteriodetes, Firmicutes | ↑ In plasma endotoxin ↑ Fecal pH ↑ Hepatic inflammation |
| Leclercq et al. (2014) doi: 10.1073/pnas. 1415174111 [181] | Individuals with AUD | Humans | ↑ Lachnospiraceae (Dorea) and Blautia ↓ Ruminococcaceae (Ruminococcus, Faecalibacterium, Subdoligranulum, Oscillibacter and Anaerofilum) | ↑ Intestinal permeability (IP) ↑ Inflammatory markers (TNFα, IL-1β, IL-6, IL-8, and IL-10) AUD individuals with high IP presented higher depression, anxiety, and alcohol-craving scores |
| Tsuruya et al. (2016) doi: 10.1038/srep27923 [193] | Individuals with AUD | Humans | ↑ Streptococcus and Coprobacillus ↓ Bacteroides and Ruminococcus (anaerobes) | Lower production of acetaldehyde from ethanol metabolism |
| Peterson et al. (2017) doi: 10.1016/j.bbr.2017.01.049 [177] | C57BL/6J mice were exposed to 4 weeks of vaporized ethanol | Mice | ↑ Alistipes ↓ Clostridium IV and XIVb, Dorea and Coprococcus | ↓ α-diversity of microbiota |
| Dubinkina et al. (2017) doi: 10.1186/s40168-017-0359-2 [180] | Individuals with AUD | Humans | ↑ Enterobacteriaceae, ↓ Clostridiales—Individuals with AUD without liver cirrhosis. | Strong negative influence of alcohol dependence and associated liver dysfunction on the intestinal microbiota |
| Grander et al. (2018) doi: 10.1136/gutjnl-2016-313432 [182] | Individuals with acute alcoholic steatohepatitis | Humans | ↓ Akkermansia muciniphila | Oral administration of A. muciniphila protected against ethanol-induced hepatic injuries |
| Wang et al. (2018) doi: 10.3389/fmicb. 2018.01874 [187] | Consumption of increasing alcohol concentration 3%, 6%, 10% (v/v) | Mice | ↑ Firmicutes, Clostridiales and Lachnospiraceae, Alistipes, Odoribacter | Groups exposed to alcohol or with alcohol abstinence presented anxiety and depression compared |
| Xiao et al. (2018) doi: 10.1016/j.toxlet. 2018.01.021 [189] | Chronically fed alcohol | Mice | ↑ Erysipelotriquia, Erythrobacter, Allobaculum and Blautia | Increased gut microbiome diversity, with reduced commensal gut taxa. Anxiety behaviors associated with alcohol withdrawal |
| Xu et al. (2019) doi: 10.1002/biof.1469 [188] | Exposure to 2–8% (v/v) ethanol was 21 days | Mice | ↑ Actinobacteria and Cyanobacteria ↑ Adlercreutzia spp., Allobaculum spp., Turicibacter spp. ↓ Helicobacter spp. | Animals exhibited anxiety/depression-like behaviors |
| Leclercq et al. (2020) doi: 10.1016/j.celrep. 2020.108238 [202] | Individuals with alcohol dependence | Humans | ↑ Lachnospiraceae ↓ Faecalibacterium praustnizii | ↑ Intestinal Permeability ↑ Introversion and social anxiety |
| Microbial transfer from human donors with alcohol dependence to mice | Mice | ↑ Firmicutes and Akkermansia muciniphila ↓ Blautia, Faecalibacterium, and Bacteroidetes | ↑ Depressive-like behavior ↑ Corticosterone level ↓ Social behavior | |
| Cuesta et al. (2021) doi: 10.3390/ijms222312830 [191] | TLR4 knockout mice treated with 10% (v/v) alcohol for 3 months | Mice | ↓ Firmicutes | TLR4 is a key factor in determining the intestinal microbiota as TLR4 KO mice did not show the usual signs of ethanol-induced inflammation |
| Singhal et al. (2021) doi: 10.1080/19490976.2021.1946367 [201] | C57BL/6 mice were fed a Lieber-DeCarli liquid diet containing ethanol 5%(v/v) for 7 weeks | Mice | ↓ Firmicutes, Lachnospiraceace, Clostridiaceae, Ruminococccaceae | Loss of butyrate-producing bacteria and butyrate genes |
| Yang et al. (2021) doi: 10.1128/AEM.00834-21 [186] | 6% (v/v) alcohol was added to drinking water for 5 weeks | Rats | ↑ Firmicutes and Bacteroidetes ↓ Prevotellaceae, Ruminococcus-1, Lachnospiraceae, Roseburia, Prevotellaceae and Lachnoclostridium | ↓ Amino acids and lipids (serum metabolome disorders) ↓ Concentration of serotonin in the hippocampus ↓ Lipopolysaccharide ↑ Intestinal permeability biomarkers |
| Du et al. (2022) doi: 10.3389/fpsyt. 2022.1054685 [183] | Individuals with alcohol dependence | Humans | ↑ Faecalibacterium, Gemmiger and Lachnospiracea incertae sedis ↓ Megamonas and Escherichia | The cognitive capacity of the participants was assessed using the Montreal Cognitive Assessment (MoCA) and the Mini-Mental State Examination (MMSE), revealing a negative correlation between the MoCA and MMSE scores with years of alcohol consumption and dependence on alcohol. |
| Baltazar-Díaz et al. (2022) doi: 10.3390/microorganisms10061231 [184] | Individuals with alcoholic cirrhosis | Humans | ↑ Escherichia/Shigella e Prevotella ↓ Blautia, Faecalibacterium | ↓ α—diversity of microbiota ↓ Acetyl-CoA fermentation to butyrate |
| Wang et al. (2023) doi: 10.1128/mbio.02392-23 [12] | Individuals with alcohol dependence | Humans | ↑ Saccharimonadaceae, Lachnospiraceae, and Fusobacterium ↓ Ruminococcaceae, Erysipelotrichaceae, and Roseburia | ↑ Anxiety and depression behaviors ↑ Preference for alcohol |
| Microbial transfer from human donors with alcohol dependence to rats | Rats | |||
| Wei et al. (2023) doi: 10.1016/j.biopha. 2023.114308 [198] | C57BL/6 J mice were fed Lieber-DeCarli liquid diets (28% ethanol) for 6 weeks | Mice | ↑ Streptococcus, Parasutterella, Dubosiella, Muribaculum, Bifidobacterium, Bacteroides, Odoribacter, Alistipes, Lactobacillus and Parabacteroides ↓ Ileibacterium, Lachnoclostridium, and Coriobacteriaceae | Neuronal necrosis in the hippocampus and prefrontal cortex Exacerbate neurodegeneration and cognitive dysfunction ↑ TNF-α, IL-6, IL-1β and IL-18 in the hippocampus |
| Yao et al. (2023) doi: 10.1038/s41380-022-01841-y [211] | C57BL/6N mice were exposed to ethanol-free drinking model for 90 days (Fecal microbiota transplantation model) | Mice | ↑ Firmicutes, Actinobacteria, Erysipelotrichi, Erysipelotrichales, Bacillales, Erysipelotrichaceae, Staphylococcaceae, Allobaculum ↓ Bacteroidetes, Bacteroidia, Verrucomicrobiae | ↑ Intestinal permeability ↑ Serum inflammatory factor levels Activation of NLRP3 inflammasome in the hippocampus, leading to neuroinflammation and depressive-like behavior |
| Carbia et al. (2023) doi: 10.1016/j.ebiom.2023.104442 [179] | Young people aged 18–25 years reported their alcohol use (at least 60 g or more of pure alcohol on at least one occasion in the last 30 days) | Humans | ↑ Veillonella dispar ↓ Alistipes | Difficulties in emotional recognition |
| Prenatal Alcohol Exposure and Microbiota | |||
|---|---|---|---|
| Author | Alcohol Exposure | Specie | Microbiota/Microbe-Dependent Products |
| Virdee et al. (2021) doi: 10.1038/s41598-020-80093-8 [235] | Pregnant C57BL/6 mice were gavaged with 3 g/kg alcohol during GD8.5-17.5 | Mice | Specific microbe-dependent products (MDP) in the microbiome might serve as a biosignature for identifying PAE Mothers: High levels of MDPs in plasma, most of them phenolic acids and indole derivatives Offspring: High levels of MDPs implicated in neuroinflammation, depression, and anxiety (4-ethylphenylsulfate, oxindole, indolepropionate, p-cresol sulfate, and salicylate) |
| Wang et al. (2021) doi: 10.3390/biom11030369 [15] | Maternal alcohol consumption during pregnancy | Humans | Mothers: ↑ Phascolarctobacterium and Blautia ↓ Faecalibacterium Offspring: ↑ Megamonas |
| Bodnar et al. (2022) doi: 10.1111/acer.14784 [229] | Pregnant Sprague-Dawley rats received an ethanol diet during GD17-GD21 | Rats | Offspring: ↑ Bacteroides, Roseburia (at the phylum Firmicutes), and Proteus PAE generated sex-specific changes in the microbiota. In males, α-diversity was higher in PAE, and in females, this difference was not detected. Significant alterations in Bacteroides, Bifidobacterium, Akkermansia, and Ruminiclostridium were found between PAE and control males. In females, Bacteroides, Proteobacteria, Faecalitalea, Proteus, Roseburia, and Gastranaerophilales were identified to be altered by PAE. |
| Bodnar et al. (2024) doi: 10.1038/s41598-024-64313-z [17] | Pregnant Sprague-Dawley rats with ad libitum access to an alcohol-containing liquid diet with 36% of total calories derived from ethanol during GD1-GD21 | Rats | Mothers: ↑ Desulfovibrionaceae, Akkermansia Offspring: ↑ Firmicutes, Parabacterteroides, Alistipes ↓ Ruminococcus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarini, R.; Marianno, P.; Hanampa-Maquera, M.; Oliveira, S.d.S.; Câmara, N.O.S. Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks. Int. J. Mol. Sci. 2024, 25, 9776. https://doi.org/10.3390/ijms25189776
Camarini R, Marianno P, Hanampa-Maquera M, Oliveira SdS, Câmara NOS. Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks. International Journal of Molecular Sciences. 2024; 25(18):9776. https://doi.org/10.3390/ijms25189776
Chicago/Turabian StyleCamarini, Rosana, Priscila Marianno, Maylin Hanampa-Maquera, Samuel dos Santos Oliveira, and Niels Olsen Saraiva Câmara. 2024. "Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks" International Journal of Molecular Sciences 25, no. 18: 9776. https://doi.org/10.3390/ijms25189776





