Increased Motility in Campylobacter jejuni and Changes in Its Virulence, Fitness, and Morphology Following Protein Expression on Ribosomes with Altered RsmA Methylation
Abstract
:1. Introduction
2. Results
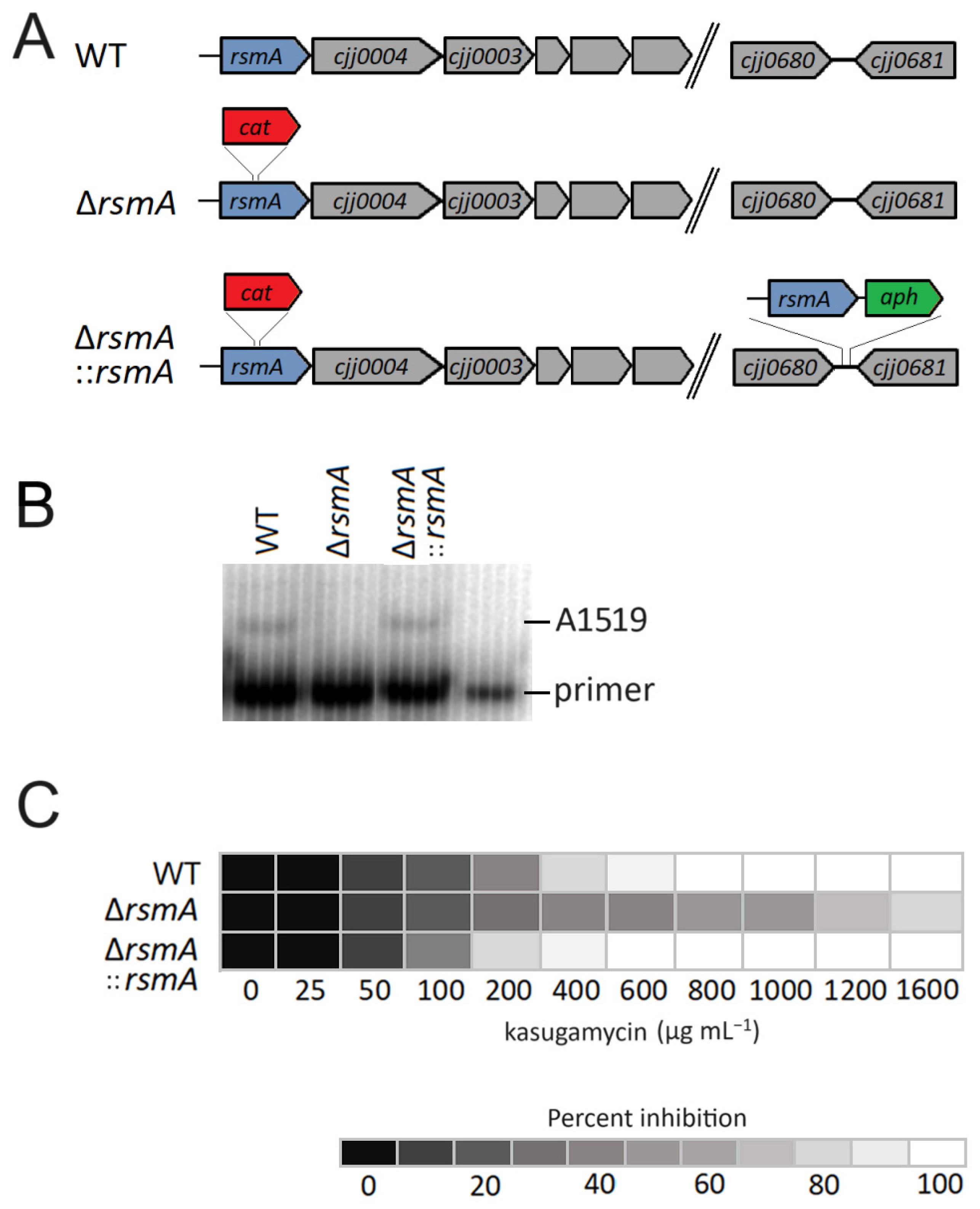
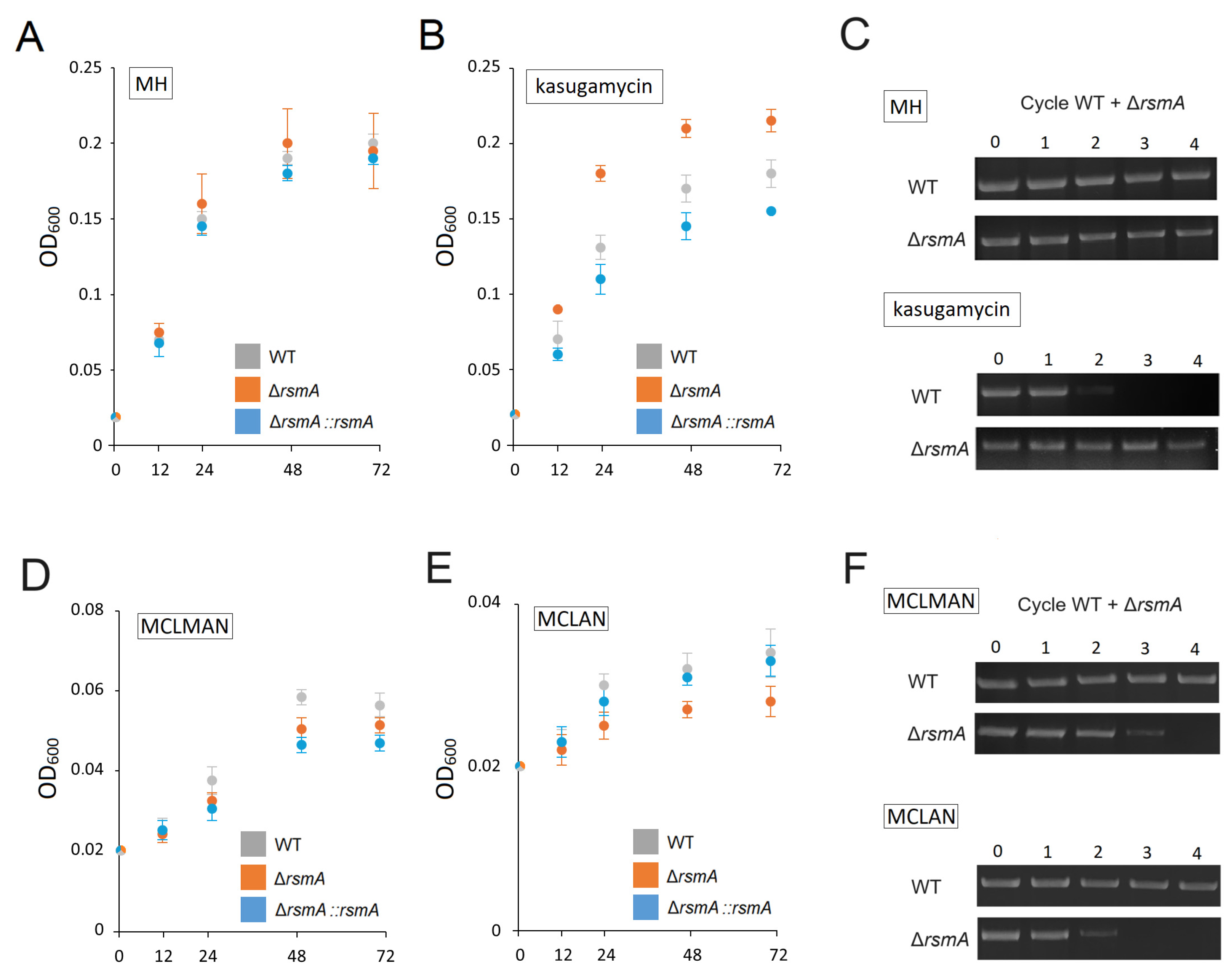
2.1. Changes in the C. jejuni Growth Phenotype after Loss of RsmA Methylation
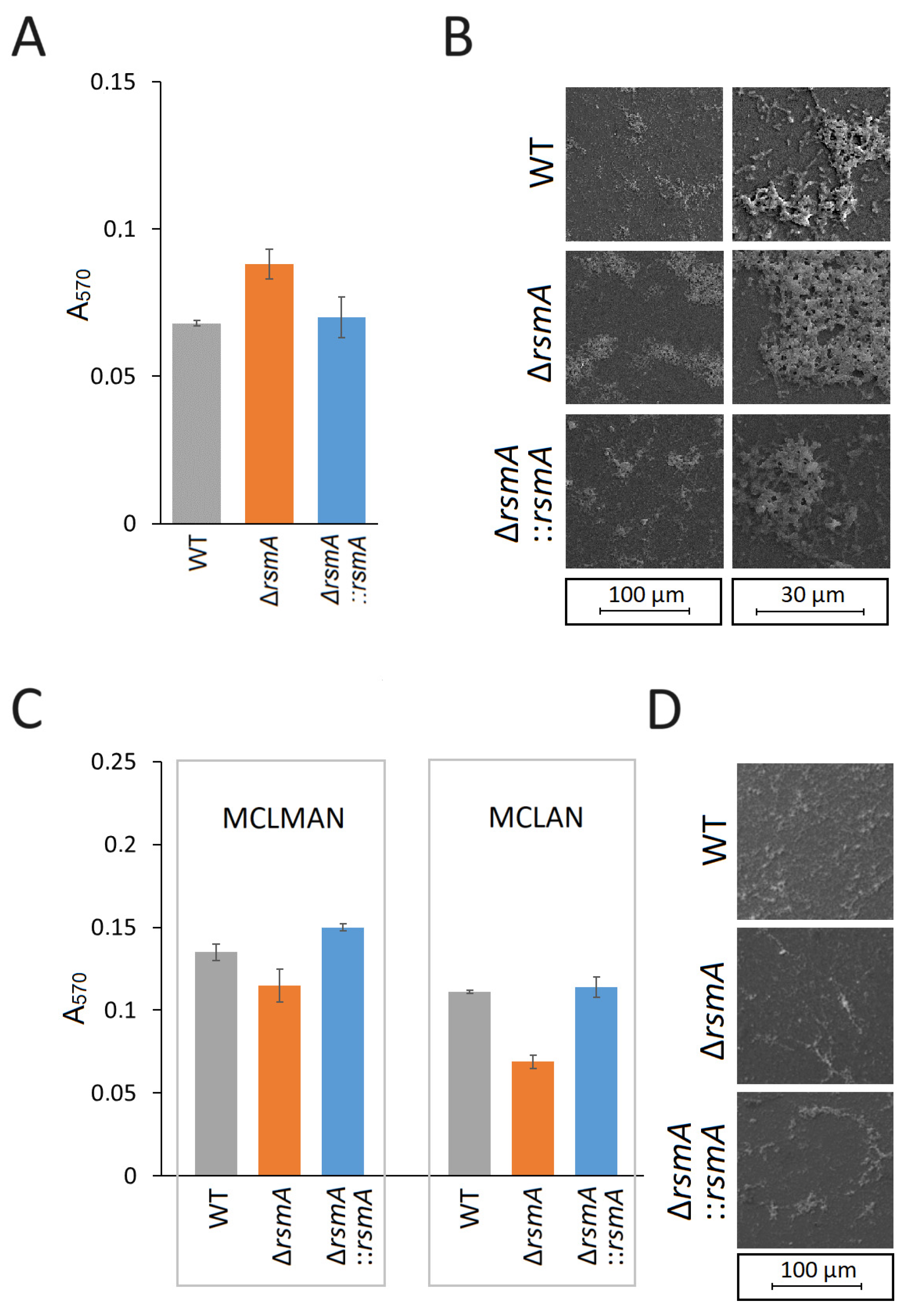
2.2. Changes in C. jejuni Biofilm Formation after Loss of RsmA Methylation
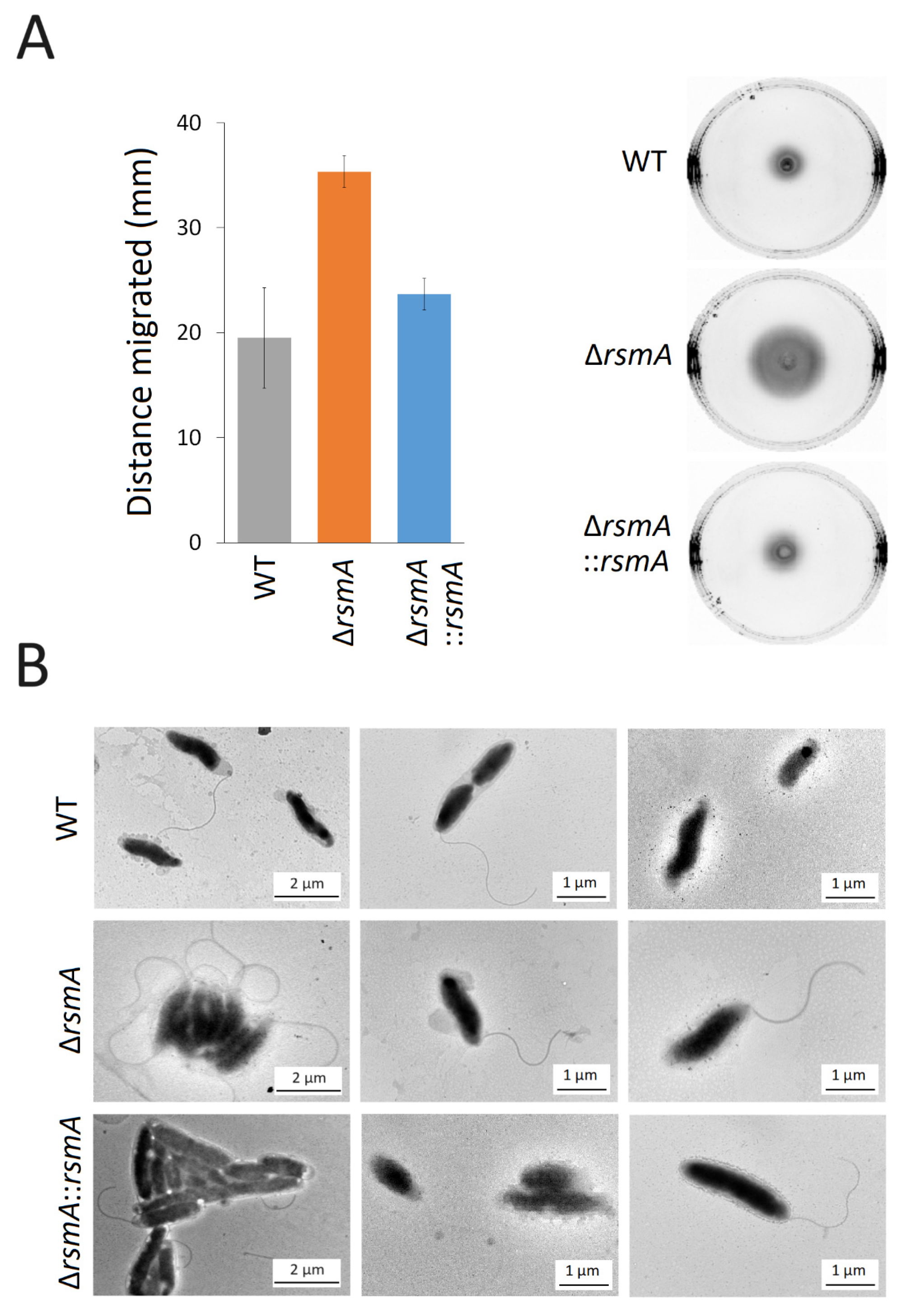
2.3. Changes in C. jejuni Virulence after Loss of RsmA Methylation
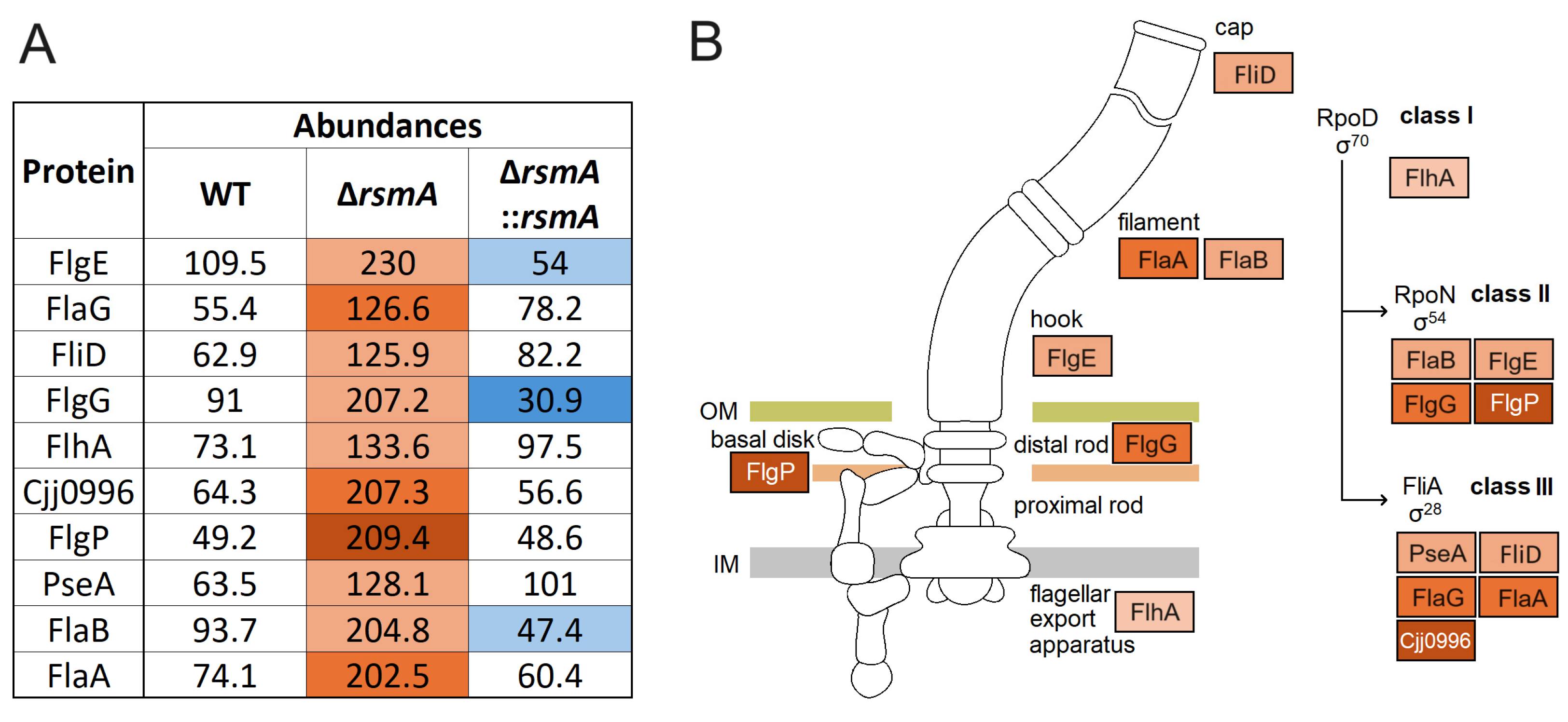
2.4. Upregulated Expression of Proteins Associated with Motility in the C. jejuni rsmA Mutant
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Construction of rsmA Knockout Mutant
| Strains | Relevant Characteristics | Source/Reference |
|---|---|---|
| C. jejuni 81-176 | Wild-type | [53] |
| C. jejuni 81-176 ΔrsmA | Cmr, rsmA deletion mutant | This study |
| C. jejuni 81-176 ΔrsmA::rsmA | Cmr, Kmr, rsmA deletion mutant complemented with C. jejuni rsmA | This study |
4.3. Motility
4.4. Virulence Phenotypes
4.5. MIC Determination
4.6. Primer Extension
4.7. Competition Growth Assay and Florescence PCR
4.8. Transmission Electron Microscopy (TEM)
4.9. Scanning Electron Microscopy (SEM)
4.10. Protein Extraction and Peptide Labeling
4.11. LC-MS3 Tandem Mass Spectrometry
4.12. Protein Identification and Quantification
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barre syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sałamaszyńska-Guz, A.; Rose, S.; Lykkebo, C.A.; Taciak, B.; Bącal, P.; Uśpieński, T.; Douthwaite, S. Biofilm formation and motility are promoted by Cj0588-directed methylation of rRNA in Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2018, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Sałamaszyńska-Guz, A.; Serafińska, I.; Bącal, P.; Douthwaite, S. Virulence properties of Campylobacter jejuni are enhanced by displaying a mycobacterial TlyA methylation pattern in its rRNA. Cell. Microbiol. 2020, 22, e13199. [Google Scholar] [CrossRef] [PubMed]
- Sałamaszyńska-Guz, A.; Rasmussen, P.K.; Murawska, M.; Douthwaite, S. Campylobacter jejuni virulence factors identified by modulating their synthesis on ribosomes with altered rRNA methylation. Front. Cell. Infect. Microbiol. 2022, 11, 803730. [Google Scholar] [CrossRef] [PubMed]
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; Purta, E.; Kurkowska, M.; Shirvanizadeh, N.; Destefanis, E.; Groza, P.; et al. MODOMICS: A database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022, 50, D231–D235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purta, E.; O’Connor, M.; Bujnicki, J.M.; Douthwaite, S. YgdE is the 2′-O-ribose methyltransferase RlmM specific for nucleotide C2498 in bacterial 23S rRNA. Mol. Microbiol. 2009, 72, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Sergiev, P.V.; Aleksashin, N.A.; Chugunova, A.A.; Polikanov, Y.S.; Dontsova, O.A. Structural and evolutionary insights into ribosomal RNA methylation. Nat. Chem. Biol. 2018, 14, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.K.; Kelley, S.T.; Spiegelman, G.B.; Pace, N.R. The genetic core of the universal ancestor. Genome Res. 2003, 13, 407–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Buul, C.P.; van Knippenberg, P.H. Nucleotide sequence of the ksgA gene of Escherichia coli: Comparison of methyltransferases effecting dimethylation of adenosine in ribosomal RNA. Gene 1985, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Helser, T.L.; Davies, J.E.; Dahlberg, J.E. Mechanism of kasugamycin resistance in Escherichia coli. Nat. New Biol. 1972, 235, 6–9. [Google Scholar] [CrossRef]
- Connolly, K.; Rife, J.P.; Culver, G. Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA. Mol. Microbiol. 2008, 70, 1062–1075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Connor, M.; Thomas, C.L.; Zimmermann, R.A.; Dahlberg, A.E. Decoding fidelity at the ribosomal A and P sites: Influence of mutations in three different regions of the decoding domain in 16S rRNA. Nucleic Acids Res. 1997, 25, 1185–1193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergman, M.A.; Loomis, W.P.; Mecsas, J.; Starnbach, M.N.; Isberg, R.R. CD8(+) T cells restrict Yersinia pseudotuberculosis infection: Bypass of anti-phagocytosis by targeting antigen-presenting cells. PLoS Pathog. 2009, 5, e1000573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mecsas, J.; Bilis, I.; Falkow, S. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infect. Immun. 2001, 69, 2779–2787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, D.H.; Zhou, X.; Kim, H.Y.; Call, D.R.; Guard, J. Transposon mutagenesis of Salmonella enterica serovar Enteritidis identifies genes that contribute to invasiveness in human and chicken cells and survival in egg albumen. Infect. Immun. 2012, 80, 4203–4215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kyuma, T.; Kizaki, H.; Ryuno, H.; Sekimizu, K.; Kaito, C. 16S rRNA methyltransferase KsgA contributes to oxidative stress resistance and virulence in Staphylococcus aureus. Biochimie 2015, 119, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Douthwaite, S.; Fourmy, D.; Yoshizawa, S. Nucleotide methylations in rRNA that confer resistance to ribosome-targeting antibiotics. In Fine-Tuning of RNA Functions by Modification and Editing; Grosjean, H., Ed.; Topics in Current Genetics; Springer: New York, NY, USA, 2005; Volume 12, pp. 287–309. [Google Scholar]
- Laperyre, B. Conserved ribosomal RNA modifications and their putative roles in ribosomal biogenesis and translation. In Fine-Tuning of RNA Functions by Modification and Editing; Grosjean, H., Ed.; Topics in Current Genetics; Springer: New York, NY, USA, 2005; Volume 12, pp. 263–284. [Google Scholar]
- Bahena-Ceron, R.; Teixeira, C.; Ponce, J.R.J.; Wolff, P.; Couzon, F.; Francois, P.; Klaholz, B.P.; Vandenesch, F.; Romby, P.; Moreau, K.; et al. RlmQ: A newly discovered rRNA modification enzyme bridging RNA modification and virulence traits in Staphylococcus aureus. RNA 2024, 30, 200–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helser, T.L.; Davies, J.E.; Dahlberg, J.E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat. New Biol. 1971, 233, 12–14. [Google Scholar] [CrossRef] [PubMed]
- van Buul, C.P.; Hamersma, M.; Visser, W.; van Knippenberg, P.H. Partial methylation of two adjacent adenosines in ribosomes from Euglena gracilis chloroplasts suggests evolutionary loss of an intermediate stage in the methyl-transfer reaction. Nucleic Acids Res. 1984, 12, 9205–9208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seistrup, K.H.; Rose, S.; Birkedal, U.; Nielsen, H.; Huber, H.; Douthwaite, S. Bypassing rRNA methylation by RsmA/Dim1during ribosome maturation in the hyperthermophilic archaeon Nanoarchaeum equitans. Nucleic Acids Res. 2017, 45, 2007–2015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vila-Sanjurjo, A.; Squires, C.L.; Dahlberg, A.E. Isolation of kasugamycin resistant mutants in the 16S ribosomal RNA of Escherichia coli. J. Mol. Biol. 1999, 293, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.L.; Lee, A. Motility of Campylobacter jejuni in a viscous environment: Comparison with conventional rod-shaped bacteria. J. Gen. Microbiol. 1988, 134, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lis, L.; Connerton, I.F. The minor flagellin of Campylobacter jejuni (FlaB) confers defensive properties against bacteriophage infection. Front. Microbiol. 2016, 7, 1908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, T.; Barker, C.S.; Matsunami, H.; Aizawa, S.I.; Samatey, F.A. The FlaG regulator is involved in length control of the polar flagella of Campylobacter jejuni. Microbiology 2018, 164, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Sommerlad, S.M.; Hendrixson, D.R. Analysis of the roles of FlgP and FlgQ in flagellar motility of Campylobacter jejuni. J. Bacteriol. 2007, 189, 179–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Roujeinikova, A. The structure, composition, and role of periplasmic stator scaffolds in polar bacterial flagellar motors. Front. Microbiol. 2021, 12, 639490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frirdich, E.; Biboy, J.; Huynh, S.; Parker, C.T.; Vollmer, W.; Gaynor, E.C. Morphology heterogeneity within a Campylobacter jejuni helical population: The use of calcofluor white to generate rod-shaped C. jejuni 81-176 clones and the genetic determinants responsible for differences in morphology within 11168 strains. Mol. Microbiol. 2017, 104, 948–971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frirdich, E.; Vermeulen, J.; Biboy, J.; Soares, F.; Taveirne, M.E.; Johnson, J.G.; DiRita, V.J.; Girardin, S.E.; Vollmer, W.; Gaynor, E.C. Peptidoglycan LD-carboxypeptidase Pgp2 influences Campylobacter jejuni helical cell shape and pathogenic properties and provides the substrate for the DL-carboxypeptidase Pgp1. J. Biol. Chem. 2014, 289, 8007–8018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frirdich, E.; Vermeulen, J.; Biboy, J.; Vollmer, W.; Gaynor, E.C. Multiple Campylobacter jejuni proteins affecting the peptidoglycan structure and the degree of helical cell curvature. Front. Microbiol. 2023, 14, 1162806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lertsethtakarn, P.; Ottemann, K.M.; Hendrixson, D.R. Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 2011, 65, 389–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrillo, C.D.; Taboada, E.; Nash, J.H.; Lanthier, P.; Kelly, J.; Lau, P.C.; Verhulp, R.; Mykytczuk, O.; Sy, J.; Findlay, W.A.; et al. Genome-wide expression analyses of Campylobacter jejuni NCTC11168 reveals coordinate regulation of motility and virulence by flhA. J. Biol. Chem. 2004, 279, 20327–20338. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Celandroni, F.; Salvett, S.; Beecher, D.J.; Gominet, M.; Lereclus, D.; Wong, A.C.; Senesi, S. Requirement of flhA for swarming differentiation, flagellin export, and secretion of virulence-associated proteins in Bacillus thuringiensis. J. Bacteriol. 2002, 184, 6424–6433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGee, D.J.; Coker, C.; Testerman, T.L.; Harro, J.M.; Gibson, S.V.; Mobley, H.L.T. The Helicobacter pylori flbA flagellar biosynthesis and regulatory gene is required for motility and virulence and modulates urease of H. pylori and Proteus mirabilis. J. Med. Microbiol. 2002, 51, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Fleiszig, S.M.; Arora, S.K.; Van, R.; Ramphal, R. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun. 2001, 69, 4931–4937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grant, C.C.; Konkel, M.E.; Cieplak, W., Jr.; Tompkins, L.S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect. Immun. 1993, 61, 1764–1771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wassenaar, T.M.; van der Zeijst, B.A.; Ayling, R.; Newell, D.G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 1993, 139, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reeser, R.J.; Medler, R.T.; Billington, S.J.; Jost, B.H.; Joens, L.A. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 2007, 73, 1908–1913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Svensson, S.L.; Pryjma, M.; Gaynor, E.C. Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS ONE 2014, 9, e106063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taniguchi, T.; Ohki, M.; Urata, A.; Ohshiro, S.; Tarigan, E.; Kiatsomphob, S.; Vetchapitak, T.; Sato, H.; Misawa, N. Detection and identification of adhesins involved in adhesion of Campylobacter jejuni to chicken skin. Int. J. Food Microbiol. 2021, 337, 108929. [Google Scholar] [CrossRef] [PubMed]
- Goon, S.; Ewing, C.P.; Lorenzo, M.; Pattarini, D.; Majam, G.; Guerry, P. A sigma28-regulated nonflagella gene contributes to virulence of Campylobacter jejuni 81-176. Infect. Immun. 2006, 74, 769–772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crofts, A.A.; Poly, F.M.; Ewing, C.P.; Kuroiwa, J.M.; Rimmer, J.E.; Harro, C.; Sack, D.; Talaat, K.R.; Porter, C.K.; Gutierrez, R.L.; et al. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat. Microbiol. 2018, 3, 494–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chekabab, S.M.; Harel, J.; Dozois, C.M. Interplay between genetic regulation of phosphate homeostasis and bacterial virulence. Virulence 2014, 5, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, J.; Bloom, B.R.; Rubin, E.J. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. USA 2005, 102, 8327–8332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tischler, A.D.; Leistikow, R.L.; Kirksey, M.A.; Voskuil, M.I.; McKinney, J.D. Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect. Immun. 2013, 81, 317–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Campo, M.; Kaya, Y.; Ofengand, J. Identification and site of action of the remaining four putative pseudouridine synthases in Escherichia coli. RNA 2001, 7, 1603–1615. [Google Scholar] [PubMed]
- Zhang, Y.; Aleksashin, N.A.; Klepacki, D.; Anderson, C.; Vazquez-Laslop, N.; Gross, C.A.; Mankin, A.S. The context of the ribosome binding site in mRNAs defines specificity of action of kasugamycin, an inhibitor of translation initiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2118553119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuwirth, B.S.; Day, J.M.; Hau, C.W.; Janssen, G.R.; Dahlberg, A.E.; Cate, J.H.; Vila-Sanjurjo, A. Structural analysis of kasugamycin inhibition of translation. Nat. Struct. Mol. Biol. 2006, 13, 879–886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alazzam, B.; Bonnassie-Rouxin, S.; Dufour, V.; Ermel, G. MCLMAN, a new minimal medium for Campylobacter jejuni NCTC 11168. Res. Microbiol. 2011, 162, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Alm, R.A.; Trust, T.J.; Guerry, P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 1993, 130, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Korlath, J.A.; Osterholm, M.T.; Judy, L.A.; Forfang, J.C.; Robinson, R.A. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 1985, 152, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Douthwaite, S.; Powers, T.; Lee, J.Y.; Noller, H.F. Defining the structural requirements for a helix in 23S ribosomal RNA that confers erythromycin resistance. J. Mol. Biol. 1989, 209, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Monshupanee, T.; Johansen, S.K.; Dahlberg, A.E.; Douthwaite, S. Capreomycin susceptibility is increased by TlyA-directed 2′-O-methylation on both ribosomal subunits. Mol. Microbiol. 2012, 85, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Heissel, S.; Bunkenborg, J.; Kristiansen, M.P.; Holmbjerg, A.F.; Grimstrup, M.; Mørtz, E.; Kofoed, T.; Højrup, P. Evaluation of spectral libraries and sample preparation for DIA-LC-MS analysis of host cell proteins: A case study of a bacterially expressed recombinant biopharmaceutical protein. Protein Expr. Purif. 2018, 147, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Description | Protein | Gene ID | Abundance Ratio | |
|---|---|---|---|---|
| ΔrsmA/ WT | ΔrsmA/ ΔrsmA::rsmA | |||
| Flagellar hook protein | FlgE | CJJ81176_0025 | 2.1 | 4.2 |
| Flagellar protein FlaG | FlaG | CJJ81176_0572 | 2.2 | 1.6 |
| Flagellar hook-associated protein | FliD | CJJ81176_0573 | 2.0 | 1.5 |
| Flagellar basal-body rod protein | FlgG | CJJ81176_0721 | 2.2 | 6.6 |
| Flagellar biosynthesis protein | FlhA | CJJ81176_0890 | 1.8 | 1.3 |
| PaaI family thioesterase | Cjj0996 | CJJ81176_0996 | 3.2 | 3.6 |
| Putative lipoprotein. | FlgP | CJJ81176_1045 | 4.2 | 4.3 |
| Flagellin modification protein | PseA | CJJ81176_1333 | 2.0 | 1.2 |
| Flagellin | FlaB | CJJ81176_1338 | 2.1 | 4.3 |
| Flagellin | FlaA | CJJ81176_1339 | 2.7 | 3.3 |
| 30S ribosomal protein S12 | RpsL | CJJ81176_0511 | 2.2 | 1.5 |
| Putative imidazole glycerol phosphate synthase subunit | HisF2 | CJJ81176_1331 | 2.1 | 1.4 |
| Imidazole glycerol phosphate synthase subunit | HisH1 | CJJ81176_1332 | 2.9 | 1.4 |
| Amino acid-binding protein | GlnH | CJJ81176_0836 | 0.5 | 0.4 |
| Phosphate ABC transporter. periplasmic phosphate-binding protein | PstS | CJJ81176_0642 | 3.0 | 1.3 |
| Thioredoxin family protein | Cjj1656 | CJJ81176_1656 | 1.9 | 1.4 |
| Peptidase. M24 family | PepP | CJJ81176_0681 | 1.9 | 1.9 |
| Peptidase. M23/M37 family | Cjj1105 | CJJ81176_1105 | 1.8 | 1.3 |
| Pseudouridine synthase | RluB | CJJ81176_0003 | 0.4 | 0.7 |
| pTet and pVir proteins | ||||
| Uncharacterized protein | Cpp33 | CJJ81176_pTet0030 | 2.6 | 1.4 |
| Single-stranded DNA-binding protein | Ssb | CJJ81176_pTet0031 | 2.1 | 1.3 |
| Uncharacterized protein | Cpp35 | CJJ81176_pTet0032 | 2.1 | 1.3 |
| VirB8 | VirB8 | CJJ81176_pVir0001 | 0.5 | 0.6 |
| VirB9 | VirB9 | CJJ81176_pVir0002 | 0.5 | 0.6 |
| Uncharacterized protein | Cjp07 | CJJ81176_pVir0008 | 0.5 | 0.5 |
| Uncharacterized protein | Cjp08 | CJJ81176_pVir0009 | 0.4 | 0.6 |
| VirB4 | VirB4 | CJJ81176_pVir0053 | 0.5 | 0.6 |
| Uncharacterized protein | Cpp20 | CJJ81176_pTet0015 | 0.4 | 0.6 |
| Uncharacterized protein | Cpp47 | CJJ81176_pTet0044 | 0.4 | 0.5 |
| Ribosomal RNA small subunit methyltransferase A | RsmA | CJJ81176_0005 | 0.2 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sałamaszyńska-Guz, A.; Murawska, M.; Bącal, P.; Ostrowska, A.; Kwiecień, E.; Stefańska, I.; Douthwaite, S. Increased Motility in Campylobacter jejuni and Changes in Its Virulence, Fitness, and Morphology Following Protein Expression on Ribosomes with Altered RsmA Methylation. Int. J. Mol. Sci. 2024, 25, 9797. https://doi.org/10.3390/ijms25189797
Sałamaszyńska-Guz A, Murawska M, Bącal P, Ostrowska A, Kwiecień E, Stefańska I, Douthwaite S. Increased Motility in Campylobacter jejuni and Changes in Its Virulence, Fitness, and Morphology Following Protein Expression on Ribosomes with Altered RsmA Methylation. International Journal of Molecular Sciences. 2024; 25(18):9797. https://doi.org/10.3390/ijms25189797
Chicago/Turabian StyleSałamaszyńska-Guz, Agnieszka, Małgorzata Murawska, Paweł Bącal, Agnieszka Ostrowska, Ewelina Kwiecień, Ilona Stefańska, and Stephen Douthwaite. 2024. "Increased Motility in Campylobacter jejuni and Changes in Its Virulence, Fitness, and Morphology Following Protein Expression on Ribosomes with Altered RsmA Methylation" International Journal of Molecular Sciences 25, no. 18: 9797. https://doi.org/10.3390/ijms25189797






