Simultaneous Electrochemical Detection of Catechol and Hydroquinone Based on a Carbon Nanotube Paste Electrode Modified with Electro-Reduced Graphene Oxide
Abstract
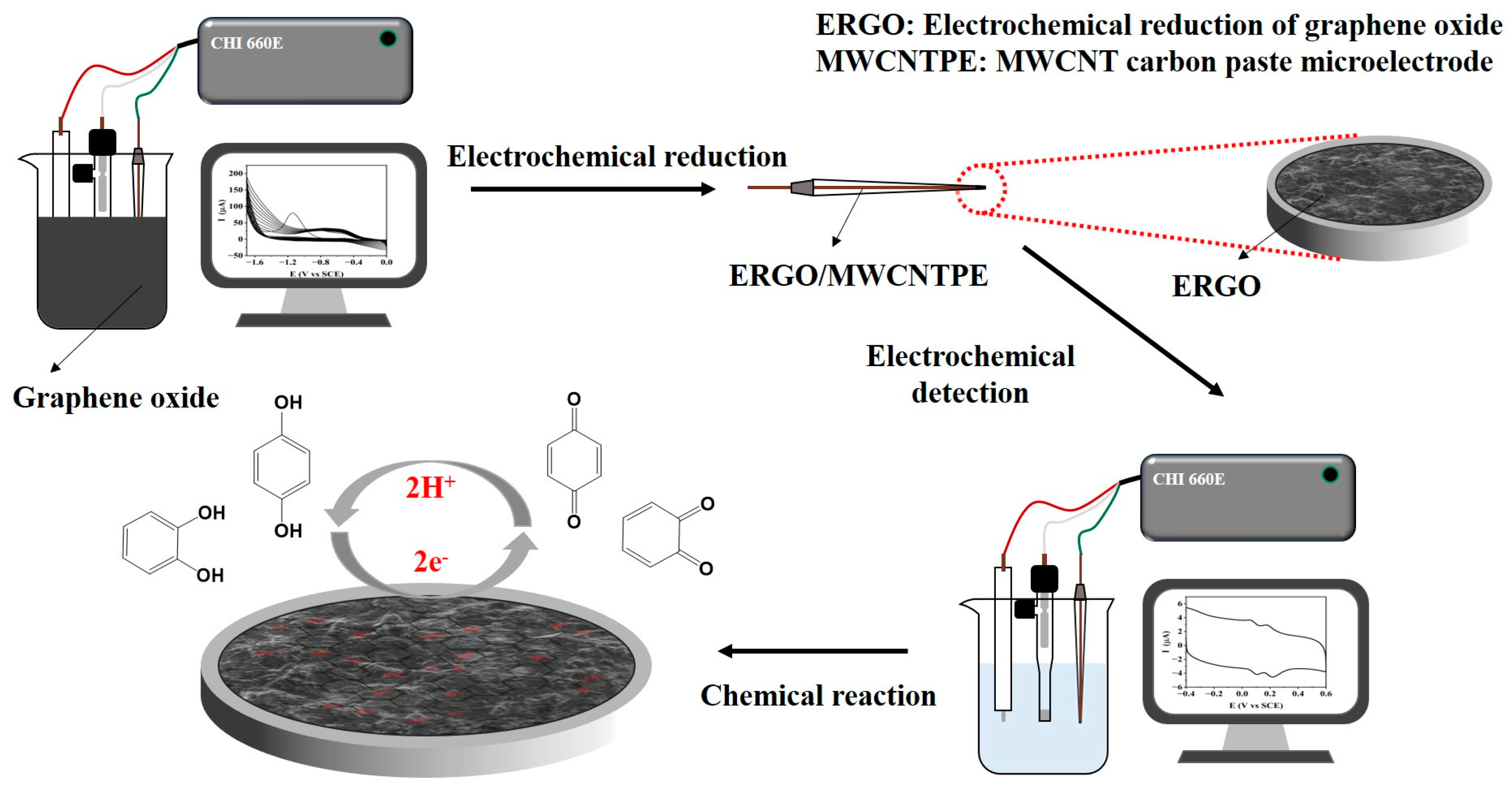
1. Introduction
2. Results and Discussion
2.1. Electrochemical Reduction of Graphene Oxide
2.2. Morphological and Structural Characterization of Different Electrodes
2.3. Electrochemical Behavior of the Modified Electrode
2.4. Optimization of Experimental Conditions
2.5. Influence of the pH Value of the Electrolyte
2.6. Influence of the Scan Rate
2.7. Adsorption Quantity of CC on Different Electrodes
2.8. Individual and Simultaneous Determination of CC and HQ
2.9. Reproducibility, Repeatability, and Selectivity
2.10. Real Sample Analysis
3. Materials and Methods
3.1. Reagents and Samples
3.2. Apparatus
3.3. Preparation of Carbon Paste Microelectrodes
3.3.1. Fabrication of Bare MWCNTPEs
3.3.2. Fabrication of the ERGO/MWCNTPE
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindsay, R.H.; Hill, J.B.; Gaitan, E.; Cooksey, R.C.; Jolley, R.L. Antithyroid effects of coal-derived pollutants. J. Toxicol. Environ. Health 1992, 37, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Ong, H.Y.; Shi, C.Y.; Ong, C.N. Simultaneous determination of hydroquinone, catechol and phenol in urine using high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B 1993, 619, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, M.; Levshakova, A.; Tumkin, I.; Fatkullin, M.; Gurevich, E.; Manshina, A.; Rodriguez, R.D.; Khairullina, E. Simultaneous electrochemical detection of hydroquinone and catechol using flexible laser-induced metal-polymer composite electrodes. Microchem. J. 2024, 204, 111106. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Chen, Z.; Chen, J.; Hu, Y.; Zhu, J.-J. Electrochemical sensor based on Ce-MOF/carbon nanotube composite for the simultaneous discrimination of hydroquinone and catechol. J. Hazard. Mater. 2021, 416, 125895. [Google Scholar] [CrossRef]
- Ni, Y.; Xia, Z.; Kokot, S. A kinetic spectrophotometric method for simultaneous determination of phenol and its three derivatives with the aid of artificial neural network. J. Hazard. Mater. 2011, 192, 722–729. [Google Scholar] [CrossRef]
- Patil, S.K.; Patil, S.A.; Vadiyar, M.M.; Awale, D.V.; Sartape, A.S.; Walekar, L.S.; Kolekar, G.B.; Ghorpade, U.V.; Kim, J.H.; Kolekar, S.S. Tailor-made dicationic ionic liquid as a fluorescent sensor for detection of hydroquinone and catechol. J. Mol. Liq. 2017, 244, 39–45. [Google Scholar] [CrossRef]
- Chen, T.-S.; Liou, S.-Y.; Kuo, W.-W.; Wu, H.-C.; Jong, G.-P.; Wang, H.-F.; Shen, C.-Y.; Padma, V.V.; Huang, C.-Y.; Chang, Y.-L. Rapid method for the quantification of hydroquinone concentration: Chemiluminescent analysis. Luminescence 2015, 30, 947–949. [Google Scholar] [CrossRef]
- Marrubini, G.; Calleri, E.; Coccini, T.; Castoldi, A.F.; Manzo, L. Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column hplc with fluorimetric detection. Chromatographia 2005, 62, 25–31. [Google Scholar] [CrossRef]
- Han, B.; Chen, Y.; Wang, H.; Yan, J.; Liu, G.; Huang, Z.; Zhou, C. A Biosensor for Simultaneous Detection of Epinephrine and Ascorbic Acid Based on Fe(III)–Polyhistidine-Functionalized Multi-Wall Carbon Nanotube Composites. Int. J. Mol. Sci. 2024, 25, 7883. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Motakef-Kazami, N.; Faridbod, F.; Khoee, S.; Norouzi, P. Determination of Pb2+ ions by a modified carbon paste electrode based on multi-walled carbon nanotubes (MWCNTs) and nanosilica. J. Hazard. Mater. 2010, 173, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Niranjana, J.S.; Koyakutty, H.; Thomas, A.A.; Bushiri, M.J. Novel synthesis of multi-layered rGO/Fe3O4 nanocomposite in a single step and its efficient electrochemical sensing of vitamin C. Mater. Sci. Eng. B 2023, 290, 116283. [Google Scholar] [CrossRef]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A novel sandwich-type SERS immunosensor for selective and sensitive carcinoembryonic antigen (CEA) detection. Anal. Chim. Acta 2020, 1139, 100–110. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, S.; Zhu, C.; Liu, C.; Liu, X.; Hu, S.; Zheng, D.; Zhang, J. Application of surfactants in the electrochemical sensing and biosensing of biomolecules and drug molecules. Anal. Methods 2024, 16, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, M.; Wang, X.; Qu, H.; Liu, Y.; Chen, S. Simultaneous electrochemical determination of catechol and hydroquinone in seawater using Co3O4/MWCNTs/GCE. Mater. Chem. Phys. 2019, 234, 217–223. [Google Scholar] [CrossRef]
- Sun, L.; Guo, H.; Pan, Z.; Liu, B.; Zhang, T.; Yang, M.; Wu, N.; Zhang, J.; Yang, F.; Yang, W. In-situ reducing platinum nanoparticles on covalent organic framework as a sensitive electrochemical sensor for simultaneous detection of catechol, hydroquinone and resorcinol. Colloids Surf. A 2022, 635, 128114. [Google Scholar] [CrossRef]
- Yue, Y.; Hu, G.; Zheng, M.; Guo, Y.; Cao, J.; Shao, S. A mesoporous carbon nanofiber-modified pyrolytic graphite electrode used for the simultaneous determination of dopamine, uric acid, and ascorbic acid. Carbon 2012, 50, 107–114. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Cardenas-Riojas, A.A.; Baena-Moncada, A.M.; Sotomayor, M.D.P.T. Voltammetric sensor based on glassy carbon electrode modified with hierarchical porous carbon, silver sulfide nanoparticles and fullerene for electrochemical monitoring of nitrite in food samples. Food Chem. 2022, 383, 132384. [Google Scholar] [CrossRef]
- Anagawadi, R.B.; Mahanthesha, K.R. Glycine functionalized multiwalled carbon nanotube modified carbon paste electrode for the selective determination of Norepinephrine: A Voltammetric study. Results Chem. 2024, 7, 101479. [Google Scholar] [CrossRef]
- Mǎgeruşan, L.; Pogǎcean, F.; Cozar, B.-I.; Tripon, S.-C.; Pruneanu, S. Harnessing graphene-modified electrode sensitivity for enhanced ciprofloxacin detection. Int. J. Mol. Sci. 2024, 25, 3691. [Google Scholar] [CrossRef]
- Sun, C.-L.; Lai, S.-Y.; Tsai, K.-J.; Wang, J.; Zhou, J.; Chen, H.-Y. Application of nanoporous core–shell structured multi-walled carbon nanotube–graphene oxide nanoribbons in electrochemical biosensors. Microchem. J. 2022, 179, 107586. [Google Scholar] [CrossRef]
- Kumara Swamy, N.; Narasimha Shetty Mohana, K.; Madhusudana, A.M.; Manjunatha, J.G.; Manukumar, H.M. Graphene nanoribbon derived from multi-walled carbon nanotube: An efficient viral gene hosting and biosensing molecular platform for the electroanalysis of HIV-1 gene. Results Chem. 2024, 7, 101393. [Google Scholar] [CrossRef]
- Settu, K.; Lai, Y.-C.; Liao, C.-T. Carbon nanotube modified laser-induced graphene electrode for hydrogen peroxide sensing. Mater. Lett. 2021, 300, 130106. [Google Scholar] [CrossRef]
- Yi, K.; Xu, S.; Cheng, H.; Chen, S.; Jiang, S.; Tu, J. A label-free sensor based on a carbon nanotube-graphene platform for the detection of non-Hodgkin lymphoma genes. Alex. Eng. J. 2023, 84, 93–99. [Google Scholar] [CrossRef]
- Akkarachanchainon, N.; Rattanawaleedirojn, P.; Chailapakul, O.; Rodthongkum, N. Hydrophilic graphene surface prepared by electrochemically reduced micellar graphene oxide as a platform for electrochemical sensor. Talanta 2017, 165, 692–701. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Khan, M.U.; Shaida, M.A. Reduction mechanism of graphene oxide including various parameters affecting the C/O ratio. Mater. Today Commun. 2023, 36, 106577. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Asadpour-Zeynali, K.; Ozkan, S.A. Preparation of porous Cu metal organic framework/ZnTe nanorods/Au nanoparticles hybrid platform for nonenzymatic determination of catechol. J. Electroanal. Chem. 2020, 856, 113672. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Q.; Chen, J.; Lin, Y.; Yang, Y.; Wang, C.; Xie, Y.; Zhao, P.; Fei, J. Temperature-controlled electrochemical sensor based on environmentally responsive polymer/BiPO4/BiOCl/multi-walled carbon nanotube composite for the detection of catechol in water. Colloids Surf. A 2023, 657, 130543. [Google Scholar] [CrossRef]
- Yu, S.; Liu, S.; Jiang, X.; Yang, N. Recent advances on electrochemistry of diamond related materials. Carbon 2022, 200, 517–542. [Google Scholar] [CrossRef]
- Chen, G. Application of carbon based material for the electrochemical detection of heavy metal ions in water environment. Int. J. Electrochem. Sci. 2020, 15, 4252–4263. [Google Scholar] [CrossRef]
- Michalkiewicz, S.; Skorupa, A.; Jakubczyk, M. Carbon materials in electroanalysis of preservatives: A review. Materials 2021, 14, 7630. [Google Scholar] [CrossRef]
- Famá, L.; Pettarin, V.; Goyanes, S.; Bernal, C. Starch/multi-walled carbon nanotubes composites with improved mechanical properties. Carbohydr. Polym. 2011, 83, 1226–1231. [Google Scholar] [CrossRef]
- Vengurlekar, S.; Chaturvedi, S.C. Chapter 10—Elevating toward a new innovation: Carbon nanotubes (CNTs). In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 271–294. [Google Scholar]
- Kanagaraj, R.; John, S.A. Highly sensitive determination of nitrite using FMWCNTs-conducting polymer composite modified electrode. Sens. Actuators B 2015, 215, 119–124. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G. Determination of Mn(II) ion by a modified carbon paste electrode based on multi-walled carbon nanotubes (MWCNTs) in different water samples. Sens. Actuators B 2014, 202, 699–707. [Google Scholar] [CrossRef]
- Malode, S.J.; Sharma, P.; Hasan, M.R.; Shetti, N.P.; Mascarenhas, R.J. 4—Carbon and carbon paste electrodes. In Electrochemical Sensors; Maruccio, G., Narang, J., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 79–114. [Google Scholar]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Pourmoghaddam, N.; Ayas, N. Synthesis, structure characterization, and electrochemical hydrogen storage of reduced graphene oxide and graphene. Int. J. Hydrog. Energy 2024, 82, 1422–1434. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, W.; Gu, Y.; Hao, S.; Yan, X.; Zhang, Z.; Yang, M. Simultaneous determination of catechol and hydroquinone based on poly(sulfosalicylic acid)/functionalized graphene modified electrode. J. Appl. Electrochem. 2014, 44, 1059–1067. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-wave voltammetry. ChemTexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Li, J.; Qu, J.; Yang, R.; Qu, L.; Harrington, P.d.B. A sensitive and selective electrochemical sensor based on graphene quantum dot/gold nanoparticle nanocomposite modified electrode for the determination of quercetin in biological samples. Electroanalysis 2016, 28, 1322–1330. [Google Scholar] [CrossRef]
- Ahmed, J.; Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Novel SWCNTs-mesoporous silicon nanocomposite as efficient non-enzymatic glucose biosensor. Appl. Surf. Sci. 2021, 552, 149477. [Google Scholar] [CrossRef]
- Yang, M.; Guo, H.; Sun, L.; Wu, N.; Wang, M.; Yang, F.; Zhang, T.; Zhang, J.; Pan, Z.; Yang, W. Simultaneous electrochemical detection of hydroquinone and catechol using MWCNT-COOH/CTF-1 composite modified electrode. Colloids Surf. A 2021, 625, 126917. [Google Scholar] [CrossRef]
- Indrajith Naik, E.; Sunil Kumar Naik, T.S.; Pradeepa, E.; Singh, S.; Naik, H.S.B. Design and fabrication of an innovative electrochemical sensor based on Mg-doped ZnO nanoparticles for the detection of toxic catechol. Mater. Chem. Phys. 2022, 281, 125860. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar diffusion to macro disc electrodes—what electrode size is required for the Cottrell and Randles-Sevcik equations to apply quantitatively? J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]
- Sundfors, F.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Kinetics of electron transfer between Fe(CN)63−/4− and poly(3,4-ethylenedioxythiophene) studied by electrochemical impedance spectroscopy. Electrochim. Acta 2002, 47, 2245–2251. [Google Scholar] [CrossRef]
- Anson, F.C. Application of potentiostatic current integration to the study of the adsorption of cobalt(iii)-(ethylenedinitrilo(tetraacetate) on mercury electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Liu, B.; Guo, H.; Sun, L.; Pan, Z.; Peng, L.; Wang, M.; Wu, N.; Chen, Y.; Wei, X.; Yang, W. Electrochemical sensor based on covalent organic frameworks/MWCNT for simultaneous detection of catechol and hydroquinone. Colloids Surf. A 2022, 639, 128335. [Google Scholar] [CrossRef]
- Erogul, S.; Bas, S.Z.; Ozmen, M.; Yildiz, S. A new electrochemical sensor based on Fe3O4 functionalized graphene oxide-gold nanoparticle composite film for simultaneous determination of catechol and hydroquinone. Electrochim. Acta 2015, 186, 302–313. [Google Scholar] [CrossRef]
- Velmurugan, M.; Karikalan, N.; Chen, S.-M.; Cheng, Y.-H.; Karuppiah, C. Electrochemical preparation of activated graphene oxide for the simultaneous determination of hydroquinone and catechol. J. Colloid Interface Sci. 2017, 500, 54–62. [Google Scholar] [CrossRef]
- Yang, C.; Chai, Y.; Yuan, R.; Xu, W.; Chen, S. Gold nanoparticle–graphene nanohybrid bridged 3-amino-5-mercapto-1,2,4-triazole-functionalized multiwall carbon nanotubes for the simultaneous determination of hydroquinone, catechol, resorcinol and nitrite. Anal. Methods 2013, 5, 666–672. [Google Scholar] [CrossRef]
- Zhu, J.; Fan, R.; Wang, Y.; Lv, Z.; Han, Y.; Peng, J.; Zheng, X.; Lin, R. Determination of catechol and hydroquinone by using perilla frutescens activated carbon modified glassy carbon electrode. J. Braz. Chem. Soc. 2020, 31, 25–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Huai, S.; Jiao, J.; Xu, Q.; Wu, H.; Zhang, H. Fullerene and platinum composite-based electrochemical sensor for the selective determination of catechol and hydroquinone. J. Electroanal. Chem. 2020, 878, 114726. [Google Scholar] [CrossRef]
- Alshahrani, L.A.; Liu, L.; Sathishkumar, P.; Nan, J.; Gu, F.L. Copper oxide and carbon nano-fragments modified glassy carbon electrode as selective electrochemical sensor for simultaneous determination of catechol and hydroquinone in real-life water samples. J. Electroanal. Chem. 2018, 815, 68–75. [Google Scholar] [CrossRef]
- Si, W.; Lei, W.; Zhang, Y.; Xia, M.; Wang, F.; Hao, Q. Electrodeposition of graphene oxide doped poly(3,4-ethylenedioxythiophene) film and its electrochemical sensing of catechol and hydroquinone. Electrochim. Acta 2012, 85, 295–301. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, W.; Gao, Y.; Wang, Q. Simultaneous determination of catechol and hydroquinone using non-enzymatic Co3O4@ carbon core/shell composites based sensor. J. Electrochem. Soc. 2019, 166, B1069. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, X.; Zhu, S.; Cao, H.; Chen, Y.; Hu, S. Sensing nitric oxide with a carbon nanofiber paste electrode modified with a CTAB and nafion composite. Microchim. Acta 2015, 182, 2403–2410. [Google Scholar] [CrossRef]









| Electrodes | Linear Range (μM) | Detection Limit (μM) | Ref. | ||
|---|---|---|---|---|---|
| CC | HQ | CC | HQ | ||
| COFs/MWCNT/GCE | 4–450 | 4–450 | 0.36 | 0.38 | [54] |
| AuNPs/Fe3O4-GO/GCE | 2–145 | 3–137 | 0.8 | 1.1 | [55] |
| aGO/SCPE | 1–350 | 1–312 | 0.182 | 0.27 | [56] |
| CNT-SH@Au-GR/GCE | 11–126 | 55–125 | 1.0 | 4.2 | [57] |
| PFHSAAC/GCE | 1–150 | 1–200 | 0.423 | 0.357 | [58] |
| Ce-MOF/CNTs/GCE | 5–50 | 10–100 | 3.5 | 5.3 | [5] |
| Pt/C60/PGE | 50–1500 | 50–1100 | 2.97 | 2.19 | [59] |
| CuO-CNF/GCE | 0–150 | 3–80 | 2.0 | 1.0 | [60] |
| PEDOT/GO/GCE | 2–400 | 2.5–200 | 1.6 | 1.6 | [61] |
| Co3O4@carbon/GCE | 0.6–116.4 | 0.8–127.1 | 0.03 | 0.03 | [62] |
| ERGO/MWCNTPE | 0.4–400 | 0.4–400 | 0.083 | 0.028 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|
| HQ | CC | HQ | CC | HQ | CC | HQ | CC | |
| Tap water | 50 | 50 | 48.96 | 50.50 | 97.92 | 101.00 | 4.86 | 1.89 |
| 100 | 100 | 99.28 | 100.67 | 99.28 | 100.67 | 1.18 | 0.98 | |
| 150 | 150 | 149.14 | 149.67 | 99.42 | 99.78 | 4.59 | 4.05 | |
| Urine | 50 | 50 | 49.47 | 50.81 | 98.94 | 101.62 | 1.61 | 5.55 |
| 100 | 100 | 100.78 | 99.58 | 100.78 | 99.58 | 2.55 | 3.88 | |
| 150 | 150 | 150.71 | 150.83 | 100.47 | 100.55 | 0.63 | 3.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Liu, C.; Liu, X.; Zhu, C.; Zheng, D. Simultaneous Electrochemical Detection of Catechol and Hydroquinone Based on a Carbon Nanotube Paste Electrode Modified with Electro-Reduced Graphene Oxide. Int. J. Mol. Sci. 2024, 25, 9829. https://doi.org/10.3390/ijms25189829
Chen T, Liu C, Liu X, Zhu C, Zheng D. Simultaneous Electrochemical Detection of Catechol and Hydroquinone Based on a Carbon Nanotube Paste Electrode Modified with Electro-Reduced Graphene Oxide. International Journal of Molecular Sciences. 2024; 25(18):9829. https://doi.org/10.3390/ijms25189829
Chicago/Turabian StyleChen, Tingfei, Chao Liu, Xiaojun Liu, Chunnan Zhu, and Dongyun Zheng. 2024. "Simultaneous Electrochemical Detection of Catechol and Hydroquinone Based on a Carbon Nanotube Paste Electrode Modified with Electro-Reduced Graphene Oxide" International Journal of Molecular Sciences 25, no. 18: 9829. https://doi.org/10.3390/ijms25189829
APA StyleChen, T., Liu, C., Liu, X., Zhu, C., & Zheng, D. (2024). Simultaneous Electrochemical Detection of Catechol and Hydroquinone Based on a Carbon Nanotube Paste Electrode Modified with Electro-Reduced Graphene Oxide. International Journal of Molecular Sciences, 25(18), 9829. https://doi.org/10.3390/ijms25189829





