Diallyl Trisulfide and Cardiovascular Health: Evidence and Potential Molecular Mechanisms
Abstract
:1. Introduction
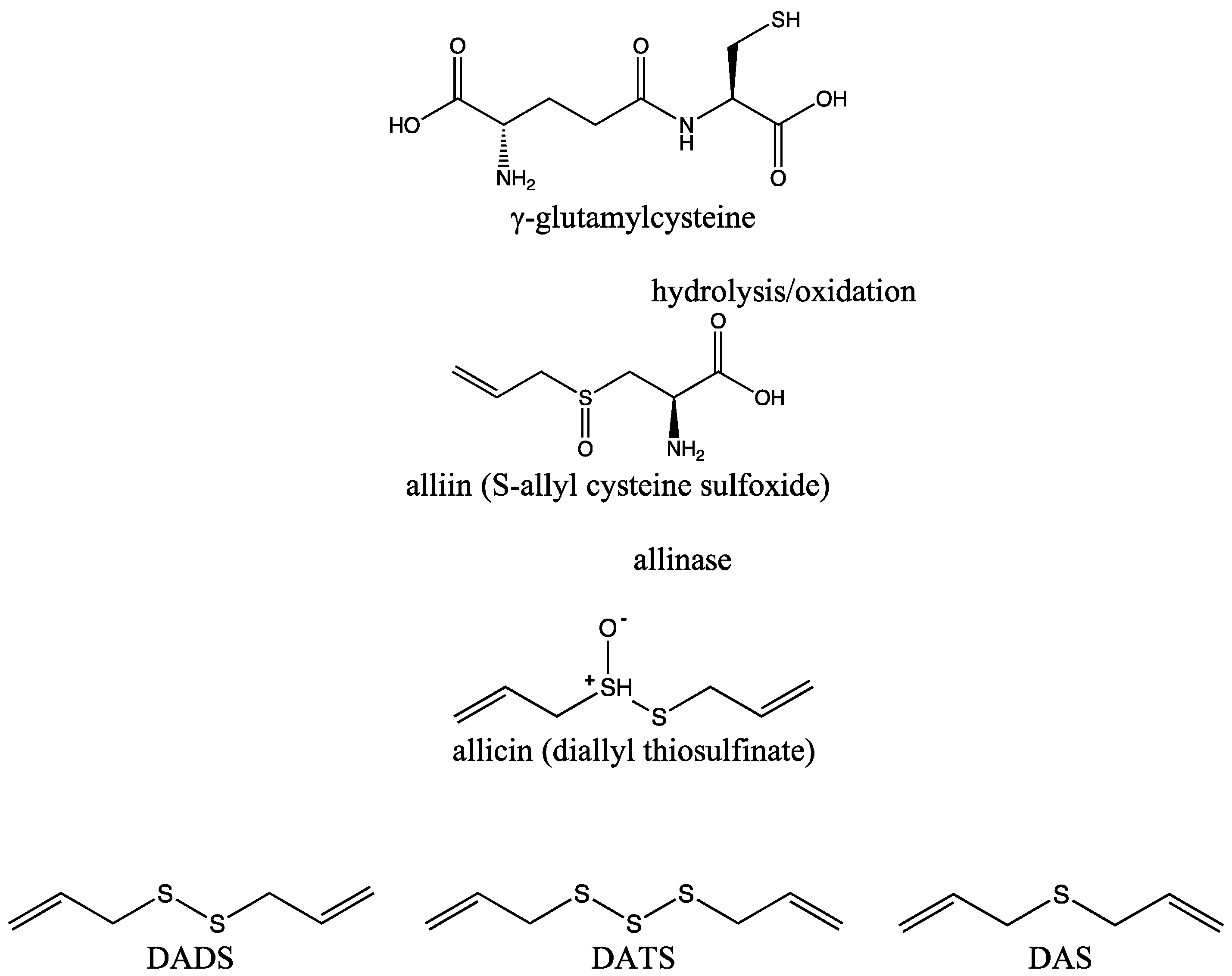
2. DATS and Its Biochemical Synthesis
3. Metabolism, Pharmacokinetics, and Potential Side Effects of DATS
4. Effects of DATS on Cardiac Structure and Function in Experimental Models
5. DATS in Diabetic Cardiomyopathy
6. DATS in Hypertension and Hypertrophic Cardiomyopathy
7. DATS in Myocardial Infarction
8. DATS Dosing for Cardioprotection
9. Potential Molecular Mechanisms Responsible for Cardioprotective Effects of DATS
9.1. Antioxidative Effects of DATS
9.2. Anti-Apoptotic Effects of DATS
9.3. Anti-Inflammatory Effects of DATS
9.4. Effects of DATS on Mitochondria
9.5. Effects of DATS on Cardiac Ion Channels
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kromhout, D. Epidemiology of cardiovascular diseases in Europe. Public Health Nutr. 2001, 4, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.; Abshire, M.; Davidson, P. Assessment of breathlessness: A critical dimension of identifying cardiovascular disease. Aust. Nurs. Midwifery J. 2018, 25, 36–39. [Google Scholar]
- Rauf, A.; Abu-Izneid, T.; Thiruvengadam, M.; Imran, M.; Olatunde, A.; Shariati, M.A.; Bawazeer, S.; Naz, S.; Shirooie, S.; Sanches-Silva, A.; et al. Garlic (Allium sativum L.): Its Chemistry, Nutritional Composition, Toxicity, and Anticancer Properties. Curr. Top Med Chem. 2022, 22, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Ünsal, N.; Koçak, D.P.; Yilmaz, H.; Şahin, F.; Yildirim, C.M. The apoptotic effect of garlic (Allium sativum) derived SEVs on different types of cancer cell lines in vitro. Turk. J. Biol. 2024, 48, 182–191. [Google Scholar] [CrossRef]
- Iwar, K.; Ochar, K.; Seo, Y.A.; Ha, B.K.; Kim, S.H. Alliums as Potential Antioxidants and Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 8079. [Google Scholar] [CrossRef]
- Gao, X.; Xue, Z.; Ma, Q.; Guo, Q.; Xing, L.; Santhanam, R.K.; Zhang, M.; Chen, H. Antioxidant and antihypertensive effects of garlic protein and its hydrolysates and the related mechanism. J. Food Biochem. 2020, 44, e13126. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Raslan, M.T.; Sabala, R.F.; Abd-Elghany, S.M.; Mahros, M.A.; Elshebrawy, H.A. Antimicrobial effect of garlic against foodborne pathogens in ground mutton. Food Microbiol. 2024, 120, 104462. [Google Scholar] [CrossRef]
- Sarangi, A.; Das, B.S.; Panigrahi, L.L.; Arakha, M.; Bhattacharya, D. Formulation of Garlic Essential Oil-assisted Silver Nanoparticles and Mechanistic Evaluation of their Antimicrobial Activity against a Spectrum of Pathogenic Microorganisms. Curr. Top. Med. Chem. 2024, 24, 2000–2012. [Google Scholar] [CrossRef]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. Int. J. Mol. Sci. 2024, 25, 1801. [Google Scholar] [CrossRef]
- Zugaro, S.; Benedetti, E.; Caioni, G. Garlic (Allium sativum L.) as an Ally in the Treatment of Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 685–698. [Google Scholar] [CrossRef]
- Hegazy, E.M.; Sabry, A.; Khalil, W.K.B. Neuroprotective effects of onion and garlic root extracts against Alzheimer’s disease in rats: Antimicrobial, histopathological, and molecular studies. BioTechnologia 2022, 103, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.M.M.B.; Araujo, A.C.; Tofano, R.J.; Barbalho, S.M. Garlic: A systematic review of the effects on cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 6797–6819. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; Hoffmann, G. An umbrella review of garlic intake and risk of cardiovascular disease. Phytomedicine 2016, 23, 1127–1133. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Korma, S.A.; Salem, H.M.; Abd El-Mageed, T.A.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F.A.; Ahmed, A.E.; et al. Garlic bioactive substances and their therapeutic applications for improving human health: A comprehensive review. Front. Immunol. 2024, 15, 1277074. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid.-Based Complement. Altern. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef]
- Bradley, J.M.; Organ, C.L.; Lefer, D.J. Garlic-Derived Organic Polysulfides and Myocardial Protection. J. Nutr. 2016, 146, 403S–409S. [Google Scholar] [CrossRef] [PubMed]
- Londhe, V.P.; Gavasane, A.T.; Nipate, S.S.; Bandawane, D.D.; Chaudhari, P.D. Role of garlic (Allium sativum) in various diseases: An overview. J. Pharm. Res. Opin. 2011, 1, 129–134. [Google Scholar]
- van den Driessche, J.J.; Plat, J.; Mensink, J.P. Effects of superfoods on risk factors of metabolic syndrome: A systematic review of human intervention trials. Food Funct. 2018, 9, 1944–1966. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef]
- Kamel, A.; Saleh, M. Recent studies on the chemistry and biological activities of the organosulfur compounds of garlic (Allium sativum). Stud. Nat. Prod. Chem. 2000, 23, 455–485. [Google Scholar] [CrossRef]
- Iciek, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Kwiecień, I.; Górny, M.; Włodek, L. S-sulfhydration as a cellular redox regulation. Biosci. Rep. 2015, 36, e00304. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Gould, E.; Tinson, R.; Groom, M.; Hamilton, C.J. Think Yellow and Keep Green-Role of Sulfanes from Garlic in Agriculture. Antioxidants 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Lee, J.H.; Kim, J.H.; Choi, G.H.; Cho, N.J.; Park, B.J. Determination of dimethyl disulfide, diallyl disulfide, and diallyl trisulfide in biopesticides containing Allium sativum extract by gas chromatography. Korean J. Environ. Agric. 2014, 33, 381–387. [Google Scholar] [CrossRef]
- Shukla, Y.; Kalra, N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007, 247, 167–181. [Google Scholar] [CrossRef]
- Wen, S.Y.; Tsai, C.Y.; Pai, P.Y.; Chen, Y.W.; Yang, Y.C.; Aneja, R.; Huang, C.Y.; Kuo, W.W. Diallyl trisulfide suppresses doxorubicin-induced cardiomyocyte apoptosis by inhibiting MAPK/NF-κB signaling through attenuation of ROS generation. Environ. Toxicol. 2018, 33, 93–103. [Google Scholar] [CrossRef]
- Tisserand, R.; Balacs, T. Essential oil safety. Int. J. Aromather. 1996, 7, 28–32. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Constituent profiles [Internet]. In Essential Oil Safety, 2nd ed.; Churchill Livingstone: London, UK, 2014; pp. 483–647. [Google Scholar] [CrossRef]
- Sun, X.; Guo, T.; He, J.; Zhao, M.; Yan, M.; Cui, F.; Deng, Y. Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi 2006, 126, 521–527. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.; Lech, J.; Rosen, S.L.; Hartman, T.G. Determination of allicin, S-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J. Nutr. 2001, 131, 968S–971S. [Google Scholar] [CrossRef]
- Suarez, F.; Springfield, J.; Furne, J.; Levitt, M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am. J. Physiol. 1999, 276, G425–G430. [Google Scholar] [CrossRef]
- Egen-Schwind, C.; Eckard, R.; Kemper, F.H. Metabolism of garlic constituents in the isolated perfused rat liver. Planta Medica 1992, 58, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716S–725S. [Google Scholar] [CrossRef]
- De, A.; Roychowdhury, P.; Bhuyan, N.R.; Ko, Y.T.; Singh, S.K.; Dua, K.; Kuppusamy, G. Folic Acid Functionalized Diallyl Trisulfide-Solid Lipid Nanoparticles for Targeting Triple Negative Breast Cancer. Molecules 2023, 28, 1393. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Hosono, T.; Misawa, S.; Seki, T.; Ariga, T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2004, 42, 743–749. [Google Scholar] [CrossRef]
- Munday, R.; Munday, C.M. Relative activities of organosulfur compounds derived from onions and garlic in increasing tissue activities of quinone reductase and glutathione transferase in rat tissues. Nutr. Cancer 2001, 40, 205–210. [Google Scholar] [CrossRef]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Kuo, W.W.; Tsai, S.J.; Lii, C.K. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric. Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef]
- Kennett, E.C.; Kuchel, P.W. Redox reactions and electron transfer across the red cell membrane. IUBMB Life 2003, 55, 375–385. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Et Biophys. Acta 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Et Biophys. Acta 1998, 1379, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Aggarwal, A.; Dey, P.; Chauhan, A.K.; Rashid, S.; Chen, K.T.; Sharma, R. Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: An updated review. Front. Nutr. 2023, 10, 1120377. [Google Scholar] [CrossRef] [PubMed]
- Sunter, W. Warfarin and garlic. Pharm. J. 1991, 246, 72. [Google Scholar]
- Chan, K.; Yin, M.; Chao, W. Effect of diallyl trisulfide-rich garlic oil on blood coagulation and plasma activity of anticoagulation factors in rats. Food Chem. Toxicol. 2007, 45, 502–507. [Google Scholar] [CrossRef]
- Abou-Diwan, C.; Ritchie, J. Drug interactions with garlic and ginger supplements. In Efficacy, Toxicity, Interactions with Western Drugs, and Effects on Clinical Laboratory Tests; John and Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 333–350. [Google Scholar] [CrossRef]
- Persaud, H. A case study: Raw garlic consumption and an increased risk of bleeding. J. Herb. Med. 2022, 32, 100544. [Google Scholar] [CrossRef]
- Hoshino, T.; Kashimoto, N.; Kasuga, S. Effects of garlic preparations on the gastrointestinal mucosa. J. Nutr. 2001, 131, 1109S–1113S. [Google Scholar] [CrossRef]
- Jappe, U.; Bonnekoh, B.; Hausenm, B.M.; Gollnick, H. Garlic-related dermatoses: Case report and review of the literature. Am. J. Contact Dermat. 1999, 10, 37–39. [Google Scholar] [CrossRef]
- Anibarro, B.; Fontela, J.L.; De La Hoz, F. Occupational asthma induced by garlic dust. J. Allergy Clin. Immunol. 1997, 100, 734–738. [Google Scholar] [CrossRef]
- Tattelman, E. Health effects of garlic. Am. Fam. Physician 2005, 72, 103–106. [Google Scholar]
- Kamal, Z.; Al-Amgad, Z.; Zigo, F.; Farkašová, Z.; Zigová, M.; Ahmad, A.M.; Said, A.H.; Rehan, I.F. Heavy consumption of garlic (Allium sativum) exert nephro- and pulmonary toxicity at maternal and embryonic level in the Albino rats. J. Appl. Anim. Res. 2023, 51, 776–788. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; He, C.; Kang, J.; Ye, J.; Xiao, Z.; Zhu, J.; Chen, A.; Feng, S.; Li, X.; et al. Novel H2S Releasing Nanofibrous Coating for In Vivo Dermal Wound Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 27474–27481. [Google Scholar] [CrossRef]
- Citi, V.; Piragine, E.; Testai, L.; Breschi, M.C.; Calderone, V.; Martelli, A. The Role of Hydrogen Sulfide and H2S-donors in Myocardial Protection Against Ischemia/Reperfusion Injury. Curr. Med. Chem. 2018, 25, 4380–4401. [Google Scholar] [CrossRef]
- Chatzianastasiou, A.; Bibli, S.I.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Yao, C.H.; Way, C.L.; Lee, K.W.; Tsai, C.Y.; Ou, H.C.; Kuo, W.W. Diallyl trisulfide and diallyl disulfide ameliorate cardiac dysfunction by suppressing apoptotic and enhancing survival pathways in experimental diabetic rats. J. Appl. Physiol. 2013, 114, 402–410. [Google Scholar] [CrossRef]
- Kuo, W.W.; Wang, W.J.; Tsai, C.Y.; Way, C.L.; Hsu, H.H.; Chen, L.M. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. Int. J. Cardiol. 2013, 168, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Dan, Q.; Zhao, Y.; Wu, Y.J.; Zhu, C.; Liu, L.; Xu, B.; Liu, Y.Q.; Li, Y. Effect of allitridum on remodeling of the transient outward potassium current of ventricular myocytes of spontaneously hypertensive rats. Yao Xue Xue Bao=Acta Pharm. Sin. 2015, 50, 39–44. [Google Scholar]
- Zhang, W.J.; Shi, Y.X.; Wang, B.B.; Cui, Y.J.; Guo, J.Z.; Li, B. Allitridum mimics effect of ischemic preconditioning by activation of protein kinase C. Acta Pharmacol. Sin. 2001, 22, 132–136. [Google Scholar]
- Yu, L.; Di, W.; Dong, X.; Li, Z.; Xue, X.; Zhang, J.; Wang, Q.; Xiao, X.; Han, J.; Yang, Y.; et al. Diallyl trisulfide exerts cardioprotection against myocardial ischemia-reperfusion injury in diabetic state, role of AMPK-mediated AKT/GSK-3β/HIF-1α activation. Oncotarget 2017, 8, 74791–74805. [Google Scholar] [CrossRef]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Bolevich, S.; Nikolic Turnic, T.R.; Mitrovic, S.L.; Jovicic, N.U.; Tyagi, S.C.; et al. The cardioprotective effects of diallyl trisulfide on diabetic rats with ex vivo induced ischemia/reperfusion injury. Mol. Cell. Biochem. 2019, 460, 151–164. [Google Scholar] [CrossRef]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic Derived Diallyl Trisulfide in Experimental Metabolic Syndrome: Metabolic Effects and Cardioprotective Role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Kondo, K.; Bhushan, S.; Zlatopolsky, M.A.; King, A.L.; Aragon, J.P.; Grinsfelder, D.B.; Condit, M.E.; Lefer, D.J. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H2410–H2418. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, H.; Lu, Y.; Wang, X.; Zhang, B.; Cong, S.; Zhao, Y.; Ji, M.; Tao, H.; Wei, L. Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia-reperfusion injury. Int. J. Nanomed. 2019, 14, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.; Kondo, K.; Bhushan, S.; Bir, S.C.; Kevil, C.G.; Murohara, T.; Lefer, D.J.; Calvert, J.W. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ. Heart Fail. 2013, 6, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, W.; Dai, J.; Huang, J.; Shi, M.; Chu, X.; Wang, F.; Guo, C.; Wang, C.; Pang, L.; et al. Donor heart preservation with a novel long-term and slow-releasing hydrogen sulfide system. Nitric Oxide 2018, 81, 1–10. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, J.; Wang, J.; Gao, Y.; Niu, W.; Zhao, M.; Zhu, H.; Guo, L.; Lu, P.; Wang, S. The effects of allitridi and amiodarone on the conduction system and reverse use-dependence in the isolated hearts of rats with myocardial infarction. J. Ethnopharmacol. 2012, 141, 674–684. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Andreeva-Gateva, P.A.; Mihaleva, I.D.; Dimova, I.I. Type 2 diabetes mellitus and cardiovascular risk; what the pharmacotherapy can change through the epigenetics. Postgrad. Med. J. 2020, 132, 109–125. [Google Scholar] [CrossRef]
- Liu, C.T.; Hse, H.; Lii, C.K.; Chen, P.S.; Sheen, L.Y. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur. J. Pharmacol. 2005, 516, 165–173. [Google Scholar] [CrossRef]
- Yu, L.; Li, S.; Tang, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: Role of SIRT1 activation. Apoptosis Int. J. Program. Cell Death 2017, 22, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Slivnick, J.; Lampert, B.C. Hypertension and Heart Failure. Heart Fail. Clin. 2019, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.M.; Coleman, C.I.; Teevan, C.; Vachhani, P.; White, C.M. Effects of garlic on blood pressure in patients with and without systolic hypertension: A meta-analysis. Ann. Pharmacother. 2008, 42, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Brankovic, S.; Radenkovic, M.; Kitic, D.; Veljkovic, S.; Ivetic, V.; Pavlovic, D.; Miladinovic, B. Comparison of the hypotensive and bradycardic activity of ginkgo, garlic, and onion extracts. Clin. Exp. Hypertens. 2011, 33, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Böhm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef]
- Lee, G.J.; Kim, S.K.; Kang, S.W.; Kim, O.K.; Chae, S.J.; Choi, S.; Shin, J.H.; Park, H.K.; Chung, J.H. Real time measurement of myocardial oxygen dynamics during cardiac ischemia-reperfusion of rats. Analyst 2012, 137, 5312–5319. [Google Scholar] [CrossRef]
- Heusch, G. Cardioprotection: Chances and challenges of its translation to the clinic. Lancet 2013, 381, 166–175. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, S.; Li, X.; Zhang, L.; Tan, H.; Xu, L.; Chen, Y.; Geng, Y.; Lin, Y.; Aertker, B.; et al. Pharmacological postconditioning with lactic acid and hydrogen rich saline alleviates myocardial reperfusion injury in rats. Sci. Rep. 2015, 5, 9858. [Google Scholar] [CrossRef]
- Driessen, H.E.; van Veen, T.A.B.; Boink, G.J.J. Emerging molecular therapies targeting myocardial infarction-related arrhythmias. Europace 2017, 19, 518–528. [Google Scholar] [CrossRef]
- Zhu, C.; Su, Y.; Juriasingani, S.; Zheng, H.; Veramkovich, V.; Jiang, J.; Sener, A.; Whiteman, M.; Lacefield, J.; Nagpal, D.; et al. Supplementing preservation solution with mitochondria-targeted H2S donor AP39 protects cardiac grafts from prolonged cold ischemia-reperfusion injury in heart transplantation. Am. J. Transplant. 2019, 19, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Morihara, N.; Sumioka, I.; Moriguchi, T.; Uda, N. Aged garlic extract enhances production of nitric oxide. Life Sci. 2007, 80, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Wang, Y.; Zhang, C.; Hao, L. Development and evaluation of DATS-loaded liposomes in vitro and in vivo. J. Nanosci. Nanotechnol. 2015, 15, 479–485. [Google Scholar]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsugoor, M.H.; Alharbi, K.N.; et al. Lipid-Based Nanoparticle Formulation of Diallyl Trisulfide Chemosensitizes the Growth Inhibitory Activity of Doxorubicin in Colorectal Cancer Model: A Novel In Vitro, In Vivo and In Silico Analysis. Molecules 2022, 27, 192. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Wen, S.Y.; Shibu, M.A.; Yang, Y.C.; Peng, H.; Wang, B.; Wei, Y.M.; Chang, H.Y.; Lee, C.Y.; Huang, C.Y.; et al. Diallyl trisulfide protects against high glucose-induced cardiac apoptosis by stimulating the production of cystathionine gamma-lyase-derived hydrogen sulfide. Int. J. Cardiol. 2015, 195, 300–310. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Wang, C.C.; Lai, T.Y.; Tsu, H.N.; Wang, C.H.; Liang, H.Y.; Kuo, W.W. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef]
- Liu, L.L.; Yan, L.; Chen, Y.H.; Zeng, G.H.; Zhou, Y.; Chen, H.P.; Peng, W.Y.; He, M.; Huang, Q.R. A role for diallyl trisulfide in mitochondrial antioxidative stress contributes to its protective effects against vascular endothelial impairment. Eur. J. Pharmacol. 2014, 725, 23–31. [Google Scholar] [CrossRef]
- Lei, Y.P.; Liu, C.T.; Sheen, L.Y.; Chen, H.W.; Lii, C.K. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol. Nutr. Food Res. 2010, 54, S42–S52. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49 (Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Hayashida, R.; Kondo, K.; Morita, S.; Unno, K.; Shintani, S.; Shimizu, Y.; Calvert, Y.W.; Shibata, R.; Murohara, T. Diallyl Trisulfide Augments Ischemia-Induced Angiogenesis via an Endothelial Nitric Oxide Synthase-Dependent Mechanism. Circ. J. 2017, 81, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Chen, H.W.; Wang, R.Y.; Lei, Y.P.; Sheen, Y.L.; Lii, C.K. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-κB activation in RAW 264.7 macrophages. J. Agric. Food Chem. 2006, 54, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Wang, K.; Zhu, X.; Lin, G.; Zhao, Z.; Li, S.; Cai, J.; Cao, J. Diallyl trisulfide inhibits naphthalene-induced oxidative injury and the production of inflammatory responses in A549 cells and mice. Int. Immunopharmacol. 2015, 29, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Pertynska-Marczewska, M.; Merhi, Z. Relationship of Advanced Glycation End Products With Cardiovascular Disease in Menopausal Women. Reprod. Sci. 2015, 22, 774–782. [Google Scholar] [CrossRef]
- Churchill, E.N.; Mochly-Rosen, D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem. Soc. Trans. 2007, 35 Pt 5, 1040–1042. [Google Scholar] [CrossRef]
- Hsieh, D.J.; Ng, S.C.; Zeng, R.Y.; Padma, V.V.; Huang, C.Y.; Kuo, W.W. Diallyl Trisulfide (DATS) Suppresses AGE-Induced Cardiomyocyte Apoptosis by Targeting ROS-Mediated PKCδ Activation. Int. J. Mol. Sci. 2020, 21, 2608. [Google Scholar] [CrossRef]
- Wen, S.Y.; Ng, S.C.; Ho, W.K.; Huang, H.Z.; Huang, C.Y.; Kuo, W.W. Activation of PI3K/Akt mediates the protective effect of diallyl trisulfide on doxorubicin induced cardiac apoptosis. Curr. Res. Toxicol. 2023, 5, 100136. [Google Scholar] [CrossRef]
- Yu, J.; Liu, F.; Yin, P.; Zhao, H.; Luan, W.; Hou, X.; Zhong, Y.; Jia, D.; Zan, J.; Ma, W.; et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Int. J. Biol. Stress. 2013, 16, 99–113. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sadoshima, J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc. Med. 2011, 21, 27–32. [Google Scholar] [CrossRef]
- Quesada, I.; de Paola, M.; Torres-Palazzolo, C.; Camargo, A.; Ferder, L.; Manucha, W.; Castro, C. Effect of Garlic’s Active Constituents in Inflammation, Obesity and Cardiovascular Disease. Curr. Hypertens. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhuang, X.; Xie, C.; Hu, X.; Dong, X.; Guo, Y.; Li, S.; Liao, X. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell. Physiol. Biochem. 2016, 40, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Han, M.H.; Hwang, H.J.; Kim, G.Y.; Moon, S.K.; Hyun, J.W.; Kim, W.J.; Choi, Y.H. Diallyl trisulfide exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 macrophages by suppressing the Toll-like receptor 4/nuclear factor-κB pathway. Int. J. Mol. Med. 2015, 35, 487–495. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Nakanishi, E.; Kuwata, H.; Chen, J.; Nakasone, Y.; He, X.; He, J.; Liu, X.; Zhang, S.; Zhang, B.; et al. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol. Nutr. Food Res. 2013, 57, 2049–2060. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert. Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Qi, J.; Yang, B.; Cao, H.L.; Wang, R.Y.; Wang, X.; Chi, R.F.; Guo, C.L.; Yang, Z.M.; Liu, H.M.; et al. Diallyl Trisulfide Suppresses Angiotensin II-Induced Vascular Remodeling Via Inhibition of Mitochondrial Fission. Cardiovasc. Drugs Ther. 2020, 34, 605–618. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, H.M.; Wei, X.; Gong, X.; Lu, Z.Y.; Huang, Z.H. Diallyl trisulfide attenuates hyperglycemia-induced endothelial apoptosis by inhibition of Drp1-mediated mitochondrial fission. Acta Diabetol. 2019, 56, 1177–1189. [Google Scholar] [CrossRef]
- Lim, S.; Lee, S.Y.; Seo, H.H.; Ham, O.; Lee, C.; Park, J.H.; Park, J.; Seung, M.; Yun, I.; Han, S.M.; et al. Regulation of mitochondrial morphology by positive feedback interaction between PKCδ and Drp1 in vascular smooth muscle cell. J. Cell. Biochem. 2015, 116, 648–660. [Google Scholar] [CrossRef]
- Sunaga, D.; Tanno, M.; Kuno, A.; Ishikawa, S.; Ogasawara, M.; Yano, T.; Miki, T.; Miura, T. Accelerated recovery of mitochondrial membrane potential by GSK-3β inactivation affords cardiomyocytes protection from oxidant-induced necrosis. PLoS ONE 2014, 9, e112529. [Google Scholar] [CrossRef]
- Nandi, S.; Ravindran, S.; Kurian, G.A. Role of endogenous hydrogen sulfide in cardiac mitochondrial preservation during ischemia reperfusion injury. Biomed. Pharmacother. 2018, 97, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, X.; Li, N.; Sun, M.; Lv, J.; Xu, Z. Herbal drugs against cardiovascular disease: Traditional medicine and modern development. Drug Discov. Today 2015, 20, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Khatua, T.N.; Adela, R.; Banerjee, S.K. Garlic and cardioprotection: Insights into the molecular mechanisms. Can. J. Physiol. Pharmacol. 2013, 91, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.Y.; Rao, F.; Kuang, S.J.; Wu, S.L.; Shan, Z.X.; Li, X.H.; Zhou, Z.L.; Lin, Q.X.; Liu, X.Y.; Yang, M.; et al. Allitridi inhibits transient outward potassium currents in human atrial myocytes. Clin. Exp. Pharmacol. Physiol. 2011, 38, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Hondeghem, L.M.; Snyders, D.J. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation 1990, 81, 686–690. [Google Scholar] [CrossRef]
- Bryant, S.M.; Kong, C.; Cannell, M.B.; Orchard, C.H.; James, A.F. Loss of caveolin-3-dependent regulation of ICa in rat ventricular myocytes in heart failure. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H521–H529. [Google Scholar] [CrossRef]

| Animal Species | Experimental Model | Route/Dose/Duration | Major Cardiac Effects | Potential Use | Ref. |
|---|---|---|---|---|---|
| Male Wistar albino rats | Diabetic cardiomyopathy induced by 65 mg/kg of streptozotocin | Per os by gavage, 40 mg/kg body weight, every other day for 16 days | ↑ HR ↓ LVESD ↑ LVPWd, LVPWs ↑ FS | Alleviation of diabetic cardiomyopathy | [58] |
| Male Wistar albino rats | Diabetes induced by 65 mg/kg of streptozotocin | Per os by gavage, 40 mg/kg body weight, every other day for 16 days | ↑ EF | [59] | |
| Male spontaneously hypertensive rats [SHR] | Isolation of ventricular myocytes | Intraperitoneal injection, 7.5 mg/kg or 15 mg/kg for 8 weeks | ↓ LV hypertrophy Recovery of the transient outward potassium current of ventricular myocytes | Alleviation of LV hypertrophy in hypertension | [60] |
| Male rabbits | Ex vivo induced I/R injury on Langendorff apparatus-30 min ischemia followed by 120 min reperfusion | Acute heart perfusion, 60 μmol/L for 5 min, followed by 10 min drug-free interval before induced I/R injury | ↓ LVsp ↓ infarct size | Preconditioning agent | [61] |
| Male Sprague Dawley rats | Diabetes induced by 40 mg/kg of streptozotocin and myocardial infarction by LCA-30 min ischemia followed by 3 h of reperfusion | Per os by gavage, 20 mg/kg body weight 3 days before myocardial I/R injury and once again after 20 min of ischemia | ↑ LVsp ↑ +dP/dt max, −dP/dt max ↓ infarct size | Preconditioning agent in diabetic condition | [62] |
| Male Wistar albino rats | Diabetes induced by 60 mg/kg of streptozotocin and ex vivo induced I/R injury on Langendorff apparatus-30 min ischemia followed by 60 min of reperfusion | Per os by gavage, 40 mg/kg body weight, every other day for 21 days | ↓ IVSd ↑ FS ↓ LVPWd, LVPWs ↑dp/dt max, dp/dt min ↑ HR ↑ CF ↓ myocardial structure turbulence | [63] | |
| Male Wistar albino rats | Metabolic syndrome induced by one month of high fat diet followed with 25 mg/kg of streptozotocin and ex vivo induced I/R injury on Langendorff apparatus-30 min ischemia followed by 60 min of reperfusion | Per os by gavage, 40 mg/kg body weight, every other day for 21 days | ↑ FS ↑E F ↓ DBP ↑ dp/dt max, dp/dt min ↓ CF ↓ myocardial structure turbulence | Preconditioning agent in condition of metabolic syndrome | [64] |
| Male C57 BL6/J mice | Myocardial infarction by LCA ligation-45 min ischemia followed by 24 h of reperfusion | Intravenous injection, 200 μg/kg 5 min before reperfusion; Intraperitoneal injection, 200 μg/kg 22.5 min before reperfusion | ↓ infarct size ↓ LVEDD, LVESD ↑ EF ↑ FS | Postconditioning agent | [65] |
| Male C57 BL6/J mice | Myocardial infarction by LAD ligation-30 min ischemia followed by 24 h of reperfusion | Intravenous injection of MIONs, 0.71 µg/100 g | ↑ HR ↑ EF ↑ FS | [66] | |
| Male C57 BL6/J mice | Heart failure by TAC | Intraperitoneal injection, 200 μg/kg 24 h after TAC and for the next 12 weeks/day | ↓ LVEDD, LVESD ↑ EF ↓ cardiac hypertrophy ↓ fibrosis | [67] | |
| Male Sprague Dawley rats | Heart transplantation model | Preservation solution, 3 µg/mL in MSN 6 h before transplantation | ↑ LVDP ↑ dP/dt max ↓ arrhythmia score ↓ time of reanimation ↓% myocardial neutrophilic infiltrate ↓ necrosis ↑ survival rates of heterotopic hearts ↑ EF ↑ FS ↓ LVIDs, LVIDd ↓ fibrotic area | Improving the survival of allografts | [68] |
| Male Sprague Dawley rats | LAD | Acute heart perfusion, 7.5 mg/L For 10 min, followed by 15 min drug-free interval | prolonged ERP and MAPD90 ↑ ERP/MAPD90 ratio ↓ His bundle (A-H, H-V) conduction ↓ incidences of arrhythmia | Postconditioning and antiarrhythmic agent | [69] |
| Experimental Model | Dose/Duration/Route | Molecular Mechanisms | Molecular Finding(s) | Molecular Effect(s) | Ref. |
|---|---|---|---|---|---|
| In vitro isolated H9c2 cardiomyocytes exposed to HG | 33 mM | Activation of IGF1R/pAkt survival pathway | ↑ CSE expression ↓ Bak and caspase 3 ↓ pIGF1R and p-AKT proteins ↓ NOX-2, p-47 | ↓ OS ↓ cellular apoptosis | [87] |
| In vitro isolated H9c2 cardiomyoblasts exposed to HG | 10 µM | Activation of PI3K/Akt/Nrf signaling pathway | ↑ Nrf2 protein expression ↑ Antioxidant enzymes (HO-1, SOD-1, SOD-2) ↓ Keap1, GSK3β expression ↓ apoptotic bodies, ↓cleaved-caspase 3 | [88] | |
| In vitro isolated H9c2 cardiomyoblasts and neonatal cardiomyocytes exposed to HG | 1–10 µM | Inhibition of ROS-stimulated JNK/NF-κB signaling pathway | ↓ JNK phosphorylation ↓ c-Jun phosphorylation ↓ apoptotic bodies ↓ caspase-3 | [59] | |
| In vitro H9c2 cardiomyoblasts exposed to HG I/R injury in DM rats | 10 μM 6 h 20 mg/kg 3 days before IRI | Activation of AMPK-mediated AKT/GSK-3β/HIF-1α signaling pathway | ↑ Bcl-2 expression ↓ cleaved caspase-3 and Bax expression | ↓ cellular apoptosis | [62] |
| In vitro DOX-induced H9c2 cardiomyocytes In vivo DOX-induced rats | 1, 5, 10 µM | Inhibition of ROS-dependent JNK/ERK/NFkB signaling pathway | ↓ p22phox and p47phox protein levels ↓ phosphorylated JNK/ERK ↓ Bax, caspase 3 | [25] | |
| In vivo obese DM rats In vitro HUVECs exposed to HG | 5.0 mg/kg/day i.v. 7 days 25–100 µmol/L 24 h | Preserved activity of mitochondrial antioxidant defense system (SOD, GSH-Px) * Suggested further research | ↓ endothelial injury Improved endothelial maximal relaxation percent ↑ cell viability, ↓ LDH activity ↓ ROS, MDA in mitochondria ↑ NO bioavailability ↓ mitochondrial disfunction (↑ ΔΨm, ATP levels, and O2 consumption) | ↓ mitochondrial OS and HG-induced endothelial injury | [89] |
| I/R injury in STZ-induced DM rats | 40 mg/kg/day 3 days | Activation of SIRT-1 signaling pathway | ↓ apoptotic index, ↓ caspase 3, ↓ cleaved caspase-3 ↓ p-PERK/PERK and p-eIF2α/eIF2α ratio ↓ ATF4, CHOP and caspase-12 Activation of Nrf-2/HO-1 signaling ↓ Nox-2 and Nox-4 protein expressions ↓ O2−, ↓ MDA, ↓ SOD | ↓ OS and ER stress-induced cardiac apoptosis | [73] |
| Ex vivo I/R injury in STZ-induced DM rats | Per os by gavage 40 mg/kg body weight, every other day for 21 days | Inhibition of ROS and apoptosis | ↓ O2−, ↑ NO2− ↑ CAT, ↑ SOD ↓ tunel staining ↑ SOD-2 and Bcl-2 expression ↓ Bax and caspase-3 expression | ↓ OS ↓ apoptosis | [63] |
| Ex vivo I/R injury in rats with metabolic syndrome | Per os by gavage 40 mg/kg body weight, every other day for 21 days | Inhibition of ROS, apoptosis, and inflammation | ↓ TBARS, ↓ O2−, ↑ NO2− ↑ CAT, ↑ GSH, ↑ SOD ↓ tunel staining ↓ picrosirius red and vimentin staining ↑ Bcl-2 and caspase-3 expression ↑ HSP70 ↓ Bax and caspase-9 expression ↑ SOD-1, ↑ SOD-2 ↓ NF-kB, TNF-alpha and IL-17A expression | ↓ OS ↓ apoptosis ↓ inflammation | [64] |
| In vitro HUVECs exposed to LDL | 50 µM | Activation of PI3/PKB-dependent eNOS signaling pathway | preserved caveolin-1-associated eNOS ↑ cGMP content ↓ chymotrypsin-like proteasome activity ↑ PKB phosphorylation | Improved eNOS activity and NO production | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novakovic, J.; Muric, M.; Bradic, J.; Ramenskaya, G.; Jakovljevic, V.; Jeremic, N. Diallyl Trisulfide and Cardiovascular Health: Evidence and Potential Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 9831. https://doi.org/10.3390/ijms25189831
Novakovic J, Muric M, Bradic J, Ramenskaya G, Jakovljevic V, Jeremic N. Diallyl Trisulfide and Cardiovascular Health: Evidence and Potential Molecular Mechanisms. International Journal of Molecular Sciences. 2024; 25(18):9831. https://doi.org/10.3390/ijms25189831
Chicago/Turabian StyleNovakovic, Jovana, Maja Muric, Jovana Bradic, Galina Ramenskaya, Vladimir Jakovljevic, and Nevena Jeremic. 2024. "Diallyl Trisulfide and Cardiovascular Health: Evidence and Potential Molecular Mechanisms" International Journal of Molecular Sciences 25, no. 18: 9831. https://doi.org/10.3390/ijms25189831
APA StyleNovakovic, J., Muric, M., Bradic, J., Ramenskaya, G., Jakovljevic, V., & Jeremic, N. (2024). Diallyl Trisulfide and Cardiovascular Health: Evidence and Potential Molecular Mechanisms. International Journal of Molecular Sciences, 25(18), 9831. https://doi.org/10.3390/ijms25189831







