Antibiotic Resistance Hotspot: Comparative Genomics Reveals Multiple Strains of Multidrug-Resistant Citrobacter portucalensis in Edible Snails
Abstract
1. Introduction
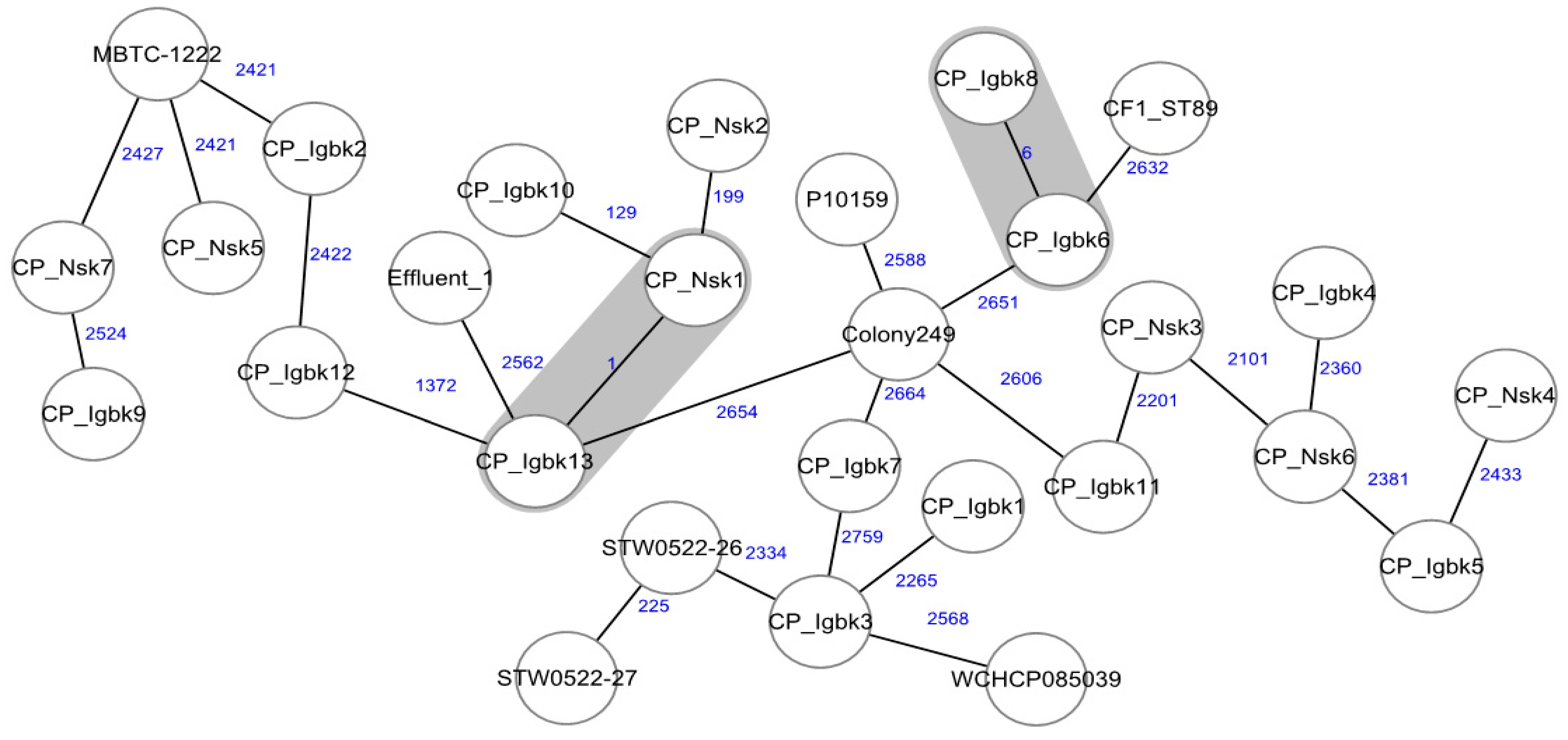
2. Results and Discussion
3. Materials and Methods
3.1. Isolation of Citrobacter
3.2. In Vitro Characterization of Sensitivity to Antimicrobial Agents
3.3. Identification of Bacteria Using MALDI-TOF MS
3.4. Whole Genome Sequencing (WGS) of the Isolates and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission [EC]. Regulation EC 853/2004 on Laying Down Specific Rules for Food of Animal Origin. 2004. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 8 September 2023).
- Cognitive Market Research. Global Edible Snail Market Report, CMR165668. 2023. Available online: https://www.cognitivemarketresearch.com/edible-snail-market-report (accessed on 8 September 2023).
- Okafor, A.C.; Ogbo, F.C. Occurrence and Enumeration of Multiple Bacterial Pathogens in Edible Snails from South East Nigeria. Food Sci. Technol. 2019, 7, 23–30. [Google Scholar] [CrossRef][Green Version]
- Okafor, A.C.; Ogbo, F.C. Virulence Potentials of Bacillus Strains Recovered From Edible Snails and Survival During Culinary Preparation. Food Control. 2020, 108, 106834. [Google Scholar] [CrossRef]
- Okafor, A.C.; Ogbo, F.C. Potentials of Common Desliming Agents in Decontaminating Snail Meat During Culinary Processing (Oral Presentation). In Proceedings of the 43rd Annual Conference of Nigerian Society for Microbiology (NSM), University of Port Harcourt, Port Harcourt, Nigeria, 6–10 September 2021; pp. 58–60. [Google Scholar]
- Oberhettinger, P.; Schüle, L.; Marschal, M.; Bezdan, D.; Ossowski, S.; Dörfel, D.; Vogel, W.; Rossen, J.W.; Willmann, M.; Peter, S. Description of Citrobacter cronae sp. nov., isolated from human rectal swabs and stool samples. Int. J. Syst. Evol. Microbiol. 2020, 70, 2998–3003. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-H.; Wang, N.-Y.; Wu, A.Y.-J.; Lin, C.-C.; Lee, C.-M.; Liu, C.-P. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J. Microbiol. Immunol. Infect. 2018, 51, 565–572. [Google Scholar] [CrossRef]
- Okafor, A.C.; Rosel, A.C.; Ogbo, F.C.; Stöger, A.; Allerberger, F.; Ruppitsch, W. Draft Genome Sequence of Citrobacter cronae Awk (Sequence Type 880), Associated with Edible Snails Commercially Available in Awka, Nigeria. Microbiol. Genome Announc. 2022, 11, e0063422. [Google Scholar] [CrossRef]
- Pawar, K.D.; Banskar, S.; Rane, S.D.; Charan, S.S.; Kulkarni, G.J.; Sawant, S.S.; Ghate, H.V.; Patole, M.S.; Shouche, Y.S. Bacterial diversity in different regions of gastrointestinal tract of Giant African Snail (Achatina fulica). Microbiol. Open 2012, 1, 415–426. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Roman, S.B.; Miller, J.M. Ability of commercial identification systems to identify newly recognized species of Citrobacter. J. Clin. Microbiol. 1995, 33, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Kolínská, R.; Španělová, P.; Dřevínek, M.; Hrabák, J.; Žemličková, H. Species identification of strains belonging to genus Citrobacter using the biochemical method and MALDI-TOF mass spectrometry. Folia Microbiol. 2014, 60, 53–59. [Google Scholar] [CrossRef]
- Quainoo, S.; Coolen, J.P.M.; van Hijum, S.A.F.T.; Huynen, M.A.; Melchers, W.J.G.; van Schaik, W.; Wertheim, H.F.L. Whole-genome sequencing of bacterial pathogens: The future of nosocomial outbreak analysis. Clin. Microbiol. Rev. 2017, 30, 1015–1063. [Google Scholar] [CrossRef]
- Lessenger, J.E. Diseases from Animals, Poultry, and Fish. In Agricultural Medicine; Springer: New York, NY, USA, 2006; pp. 367–382. [Google Scholar]
- Pepperell, C.; Kus, J.V.; Gardam, M.A.; Humar, A.; Burrows, L.L. Low-virulence Citrobacter species encode resistance to multiple antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3555–3560. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Goncalves, B.R.; da Silva, M.S.; Novais, Â.; Machado, E.; Carrico, J.A.; Peixe, L. Citrobacter portucalensis sp. nov., isolated from an aquatic sample. Int. J. Syst. Evol. Microbiol. 2017, 67, 3513–3517. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Rathje, J.; Habermann, D.; Brinks, E.; Cho, G.S.; Franz, C.M. Draft genome sequence of multidrug-resistant strain Citrobacter portucalensis MBTC-1222, isolated from uziza (Piper guineense) leaves in Nigeria. Genome Announc. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xie, H.; Huang, D.; Zhou, W.; Liu, Y.; Shen, H.; Zhou, K. Detection of a clinical carbapenem-resistant Citrobacter portucalensis strain and the dissemination of C. portucalensis in clinical settings. J. Glob. Antimicrob. Resist. 2021, 27, 79–81. [Google Scholar] [CrossRef]
- Chah, J.M.; Inegbedion, G. Characteristics of snail farming in Edo South Agricultural Zone of Edo State, Nigeria. Trop. Anim. Health Prod. 2013, 45, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Leopold, S.R.; Goering, R.V.; Witten, A.; Harmsen, D.; Mellmann, A. Bacterial whole-genome sequencing revisited: Portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J. Clin. Microbiol. 2014, 52, 2365–2370. [Google Scholar] [CrossRef]
- Hasan, S.; Sultana, M.; Hossain, M.A. Complete Genome Arrangement Revealed the Emergence of a Poultry Origin Superbug Citrobacter Portucalensis Strain NR-12. J. Glob. Antimicrob. Resist. 2019, 18, 126–129. [Google Scholar] [CrossRef]
- Sellera, F.P.; Fernandes, M.R.; Fuga, B.; Fontana, H.; Vásquez-Ponce, F.; Goldberg, D.W.; Monte, D.F.; Rodrigues, L.; Cardenas-Arias, A.R.; Lopes, R.; et al. Phylogeographical landscape of citrobacter portucalensis carrying clinically relevant resistomes. Microbiol. Spectr. 2022, 10, e0150621. [Google Scholar] [CrossRef] [PubMed]
- Porres-Osante, N.; Sáenz, Y.; Somalo, S.; Torres, C. Characterization of beta-lactamases in faecal enterobacteriaceae recovered from healthy humans in Spain: Focusing on AmpC polymorphisms. Microb. Ecol. 2014, 70, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef]
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; McEwen, S.A.; Li, X.-Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The scourge of antibiotic resistance: The important role of the environment. Clin. Infect. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Walsh, F. Antibiotic resistance genes across a wide variety of metagenomes. FEMS Microbiol. Ecol. 2016, 92, fiv168. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mukhopadhyay, A.K.; Bhadra, R.K.; Ghosh, A.N.; Mitra, R.; Shimada, T.; Yamasaki, S.; Faruque, S.M.; Takeda, Y.; Colwell, R.R.; et al. Virulence Genes in Environmental Strains of Vibrio cholerae. Appl. Environ. Microbiol. 2000, 66, 4022–4028. [Google Scholar] [CrossRef]
- Altalhi, A.D.; Hassan, S.A. Bacterial quality of raw milk investigated by Escherichia coli and isolates analysis for specific virulence-gene markers. Food Control. 2009, 20, 913–917. [Google Scholar] [CrossRef]
- Paixão, A.C.; Ferreira, A.C.; Fontes, M.; Themudo, P.; Albuquerque, T.; Soares, M.C.; Fevereiro, M.; Martins, L.; de Sá, M.I.C. Detection of virulence-associated genes in pathogenic and commensal avian Escherichia coli isolates. Poult. Sci. 2016, 95, 1646–1652. [Google Scholar] [CrossRef]
- Salgueiro, V.; Manageiro, V.; Rosado, T.; Bandarra, N.M.; Botelho, M.J.; Dias, E.; Caniça, M. Snapshot of resistome, virulome and mobilome in aquaculture. Sci. Total. Environ. 2023, 905, 166351. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Nolan, L.K. Characterizing the APEC pathotype. Veter- Res. 2005, 36, 241–256. [Google Scholar] [CrossRef]
- Oh, J.; Kang, M.; Yoon, H.; Choi, H.; An, B.; Shin, E.; Kim, Y.; Kim, M.; Kwon, J.; Kwon, Y. The embryo lethality of Escherichia coli isolates and its relationship to the presence of virulence-associated genes. Poult. Sci. 2012, 91, 370–375. [Google Scholar] [CrossRef] [PubMed]
- De Carli, S.; Ikuta, N.; Lehmann, F.K.M.; da Silveira, V.P.; Predebon, G.d.M.; Fonseca, A.S.K.; Lunge, V.R. Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult. Sci. 2015, 94, 2635–2640. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M02-A12 Performance Standards for Antimicrobial Disk Susceptibility Test, 12th ed.; Approved Standards; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11.0. 2021. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf (accessed on 7 June 2022).
- Sogawa, K.; Watanabe, M.; Sato, K.; Segawa, S.; Ishii, C.; Miyabe, A.; Murata, S.; Saito, T.; Nomura, F. Use of the MALDI BioTyper system with MALDI–TOF mass spectrometry for rapid identification of microorganisms. Anal. Bioanal. Chem. 2011, 400, 1905–1911. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
| Isolate | Source Location | Size (bp) | GC Content (%) | Sequence Type | Average Coverage | % Good Targets cgMLST | Genome Accession Number |
|---|---|---|---|---|---|---|---|
| CP_Nsk1 | Nsukka | 5,252,065 | 51.8 | 881 | 41 | 99.2 | JALGBJ000000000 |
| CP_Nsk2 | Nsukka | 5,170,058 | 52.0 | 881 | 46 | 99.0 | JANBWY000000000 |
| CP_Nsk3 | Nsukka | 4,896,645 | 52.0 | 882 | 53 | 99.2 | JANBWX000000000 |
| CP_Nsk4 | Nsukka | 4,969,172 | 52.0 | 883 | 69 | 98.9 | JANBWW000000000 |
| CP_Nsk5 | Nsukka | 5,136,318 | 52.0 | 895 | 67 | 99.4 | JANBWN000000000 |
| CP_Nsk6 | Nsukka | 5,012,648 | 51.9 | 896 | 54 | 99.2 | JANBWM000000000 |
| CP_Nsk7 | Nsukka | 4,843,198 | 52.0 | 897 | 53 | 99.3 | JANBWL000000000 |
| CP_Igbk1 | Igboukwu | 4,648,030 | 52.0 | 884 | 82 | 99.6 | JANBWV000000000 |
| CP_Igbk2 | Igboukwu | 4,968,643 | 51.8 | 885 | 92 | 99.3 | JANBWU000000000 |
| CP_Igbk3 | Igboukwu | 4,766,390 | 52.0 | 886 | 68 | 98.9 | JANBWT000000000 |
| CP_Igbk4 | Igboukwu | 4,838,025 | 52.1 | 887 | 75 | 99.5 | JANDBI000000000 |
| CP_Igbk5 | Igboukwu | 4,943,623 | 52.1 | 888 | 67 | 98.9 | JANDBH000000000 |
| CP_Igbk6 | Igboukwu | 5,797,490 | 51.8 | 889 | 73 | 99.0 | JANBWS000000000 |
| CP_Igbk7 | Igboukwu | 5,295,614 | 51.7 | 890 | 47 | 98.1 | JANDBG000000000 |
| CP_Igbk8 | Igboukwu | 4,794,523 | 52.1 | 891 | 83 | 99.3 | JANBWR000000000 |
| CP_Igbk9 | Igboukwu | 5,430,528 | 51.5 | 892 | 61 | 98.6 | JANBWQ000000000 |
| CP_Igbk10 | Igboukwu | 5,192,886 | 51.9 | 881 | 82 | 98.8 | JANBWP000000000 |
| CP_Igbk11 | Igboukwu | 4,937,101 | 52.0 | 893 | 49 | 99.3 | JANBWO000000000 |
| CP_Igbk12 | Igboukwu | 4,960,921 | 51.8 | 894 | 77 | 99.2 | JANDBF000000000 |
| CP_Igbk13 | Igboukwu | 5,162,285 | 51.9 | 881 | 38 | 98.6 | JANBWK000000000 |
| Isolate | Resistance Phenotype | Resistance Genes | Virulence Genes | Plasmid |
|---|---|---|---|---|
| CP_Nsk1 | AMC-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB6, CMY-46. | traT, irp2, fyuA | - |
| CP_Nsk2 | CN-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB6, CMY-46. | traT, irp2, fyuA | - |
| CP_Nsk3 | AMC-CD-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB18, CMY-77, CMY-108. | - | - |
| CP_Nsk4 | AMP-AMC-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, acrB, QnrB57, CMY-124. | - | - |
| CP_Nsk5 | AK | PmrF, mdfA, acrA, rsmA, KpnE, KpnF, KdpE, mdtB, mdtC, baeR, msbA, CRP, marA, H-NS, CMY-46, ampH, emrR, emrB, QnrB17, mdtG, GlpT, UhpT, PBP3, EF-Tu, soxS, marR. | - | - |
| CP_Nsk6 | AMP-AMC-CPD-FOX | KdpE, PmrF, mdfA, ampH, acrB, acrA, CRP, QnrB18, H-NS, mdtC, baeR, marA, msbA, mdtG, KpnE, KpnF, CMY-34, rsmA, emRB, emrR, GlpT, PBP3, UhpT, EF-Tu, soxS, marR, qnrB23, qnrB29, qnrB48, blaCMY-106, blaCMY-108, blaCMY-37 | - | - |
| CP_Nsk7 | AMC-CN-CPD-FOX-CTX | CMY-63, KdpE, QnrB6, acrA, PmrF, KpnF, KpnE, emrB, emrR, rsmA, msbA, CRP, baeR, mdtC, mdtB, H-NS, mdtG, mdfA, marA, ampH, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, qnrB41, blaCMY-46 | - | - |
| CP_Igbk1 | AMP-AMC-CD-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB6, qnrB9, blaCMY-25, blaCMY-124, blaCMY-2 | - | - |
| CP_Igbk2 | AMP-AMC-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB17, CMY-129, | - | - |
| CP_Igbk3 | AMP-AMC-CD-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB6, CMY-35, qnrB9, blaCMY-2 | - | - |
| CP_Igbk4 | AMP-AMC-CD-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB18, CMY-124, qnrB48, qnrB23, qnrB29 | - | - |
| CP_Igbk5 | AMP-CPD-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB6, CMY-71 | - | - |
| CP_Igbk6 | AMP-AMC-CPD-FOX | msbA, kdpE, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB16, CMY-106 | - | - |
| CP_Igbk7 | FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB16, CMY-124 | irp2, fyuA | - |
| CP_Igbk8 | AMP-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB16, CMY-106 | - | - |
| CP_Igbk9 | - | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB17, CMY-77 | - | - |
| CP_Igbk10 | - | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB6, CMY-46 | traT, irp2, fyuA | Col440I, IncFIB(K) |
| CP_Igbk11 | AMC-FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB18, CMY-108, qnrB48, qnrB23, qnrB29 | - | - |
| CP_Igbk12 | FOX | msbA, kdpE, PmrF, baeR, mdtC, mdtB, mdfA, marA, rsmA, KpnE, KpnF, CRP, ampH, emrB, emrR, H-NS, mdtG, UhpT, PBP3, GlpT, EF-Tu, soxS, marR, acrA, QnrB17, CMY-46, qnrB77 | traT | IncFIB(pHCM2) |
| CP_Igbk13 | AMC-CTX | KdpE, acrA, H-NS, mdtB, mdtC, baeR, rsmA, mdfA, CRP, emrB, emrR, PmrF, msbA, marA, KpnE, KpnF, QnrB6, ampH, mdtG, PBP3, UhpT, GlpT, EF-Tu, soxS, marR, qnrB13, blaCMY-46 | irp2, fyuA, traT | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okafor, A.C.; Rosel, A.C.; Ogbo, F.C.; Adetunji, C.O.; Imarhiagbe, O.; Gamp, L.; Stöger, A.; Allerberger, F.; Ruppitsch, W. Antibiotic Resistance Hotspot: Comparative Genomics Reveals Multiple Strains of Multidrug-Resistant Citrobacter portucalensis in Edible Snails. Int. J. Mol. Sci. 2024, 25, 9889. https://doi.org/10.3390/ijms25189889
Okafor AC, Rosel AC, Ogbo FC, Adetunji CO, Imarhiagbe O, Gamp L, Stöger A, Allerberger F, Ruppitsch W. Antibiotic Resistance Hotspot: Comparative Genomics Reveals Multiple Strains of Multidrug-Resistant Citrobacter portucalensis in Edible Snails. International Journal of Molecular Sciences. 2024; 25(18):9889. https://doi.org/10.3390/ijms25189889
Chicago/Turabian StyleOkafor, Arthur C., Adriana Cabal Rosel, Frank C. Ogbo, Charles O. Adetunji, Odoligie Imarhiagbe, Lukas Gamp, Anna Stöger, Franz Allerberger, and Werner Ruppitsch. 2024. "Antibiotic Resistance Hotspot: Comparative Genomics Reveals Multiple Strains of Multidrug-Resistant Citrobacter portucalensis in Edible Snails" International Journal of Molecular Sciences 25, no. 18: 9889. https://doi.org/10.3390/ijms25189889
APA StyleOkafor, A. C., Rosel, A. C., Ogbo, F. C., Adetunji, C. O., Imarhiagbe, O., Gamp, L., Stöger, A., Allerberger, F., & Ruppitsch, W. (2024). Antibiotic Resistance Hotspot: Comparative Genomics Reveals Multiple Strains of Multidrug-Resistant Citrobacter portucalensis in Edible Snails. International Journal of Molecular Sciences, 25(18), 9889. https://doi.org/10.3390/ijms25189889







