Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment
Abstract
:1. Introduction
2. What Are Bacteriophages?
3. Phage-Based Therapy
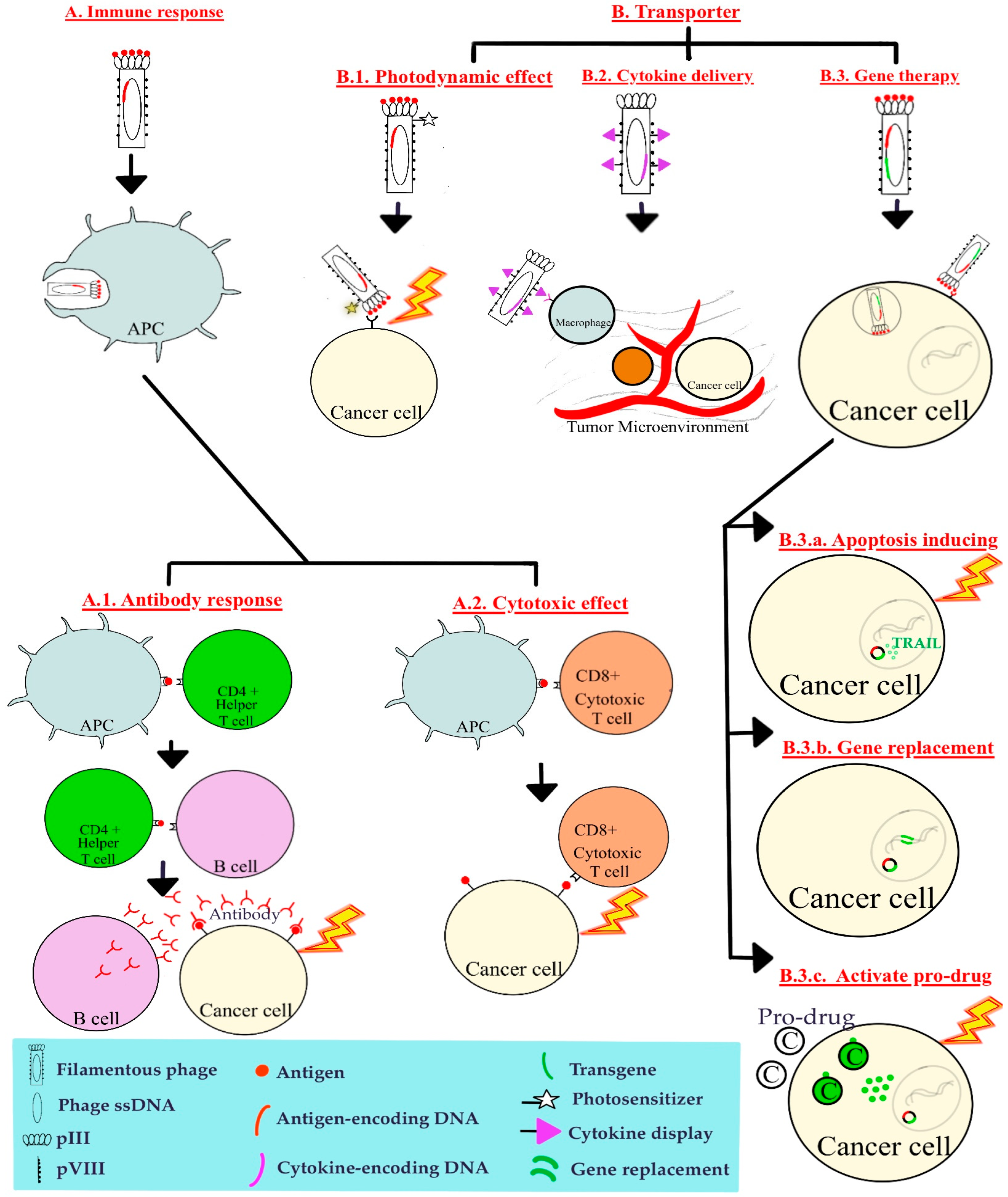
4. Main Mechanisms of Phage-Based Therapies
5. Current Phage-Based Therapies across Various Cancer Types
5.1. Breast Cancer
5.2. Colorectal Cancer
5.3. Lung Adenocarcinoma
5.4. Hepatocellular Carcinoma
5.5. B Cell Lymphoma
5.6. Multiple Myeloma
5.7. Cervical Cancer
5.8. Neuroendocrine Pancreatic Tumor
5.9. Melanoma
5.10. Chondrosarcoma
5.11. Glioblastoma
| Phage | Peptide Display | Mechanism | Result | Year | |
|---|---|---|---|---|---|
| Breast cancer (BALB/c mice) | T7 phage | Hsp 27 | Induction of antibodies | Significantly developed smaller average tumor weight (1.5 ± 0.2 g vs. 2.5 ± 0.5 g) in mice, which were subcutaneously injected with 4T1 breast carcinoma cell. | 2008 [49] |
| Lung carcinoma (mice) | T4 phage | mFlt4 | Induction of antibodies | Prolonged survival in mice, which were injected with Lewis lung carcinoma cells | 2009 [57] |
| Lung carcinoma (C57BL/6J mice) | T4 phage | mVEGFR2 | Induction of antibodies | Obvious inhibition of tumor growth and significant extension of survival length | 2011 [58] |
| Cancer associated with human HPV type 16 (C57BL/6 mice) | λ- phage | HPV E7 | Induction of cytotoxic immune response | Significant inhibition of tumor growth in mice, which were subcutaneously injected TC-1 cells | 2011 [73] |
| Breast cancer (BALB/c mice) | T7 phage | H-2k(d)-restricted CTL epitode (derived from rat HER2/neu) | Induction of cytotoxic immune response | Protected mice against HER2 positive tumor challenge in both prophylactic and therapeutic setting | 2012 [50] |
| B-cell lymphoma (BALB/c mice) | M13 phage (pVIII) | Chemically fuse single-chain variable fragment-BCL1 | Induction of antibodies | Induced B cell lymphoma Idiotype- specific IgG leads to significant survival benefit in the murine B cell lymphoma | 2013 [69] |
| Multiple myeloma (clinical phase I/II trial, 15 patients) | M13 phage | Chemically link patient-specific purified paraprotein | Induction of idiotype-specific antibodies | Decrease or stabilize paraprotein level in patients | 2014 [68] |
| Hepatocellular Carcinoma (BALB/c mice) | MS2 phage | GE11 peptide | Introducing IncRNA MEG3 transgene into EGFR positive HCC cell lines | Significant inhibition of tumor growth | 2016 [64] |
| Pancreatic neuroendocrime tumor (Men1 tumor suppressor gene KO mice) | Hybrid AAV/phage | Octreotide peptide (binding to SSTR2 on cancer cell) | Introducing TNF transgene directly into cancer cell | Induced apoptosis of cancer cell resulting in lowered tumor metabolism, reduced tumor size (73 ± 21%) and improved mice survival | 2016 [76] |
| Hepatocellular carcinoma (mice) | λ-phage (gp D) | Human Aspartate β-hydroxylase (ASPH) derived proteins | Induction of cytotoxic immune response | Significantly delayed HCC growth and progression in mice, which had subcutaneous implantation of a syngeneic BNL HCC cell lines | 2017 [62] |
| Breast cancer (BALB/c mice) | λF7 phage (gpD) | AE37 (Ii-Key/Her-2/neu 776–790) | Induction of cytotoxic immune response | Promising prophylactic and therapeutic effects against HER2 overexpressing cancer in BALB/c mice | 2018 [46] |
| Melanoma (mice) | T7 phage | 11-AA and 34-AA peptides | Induction of antibodies | 11-AA peptides showed better antibody stimulation than longer peptides (34-AA peptides) | 2018 [79] |
| Lung carcinoma (BALB/c or C57BL/6 mice) | T4 phage | VEGFR2 | VEGFR2 delivery (binding to VEGF), altering tumor microenvironment | Suppress tumor growth and decrease microvascular density in murine models of Lewis lung carcinoma | 2019 [59] |
| Glioblastoma (immunodeficient nude mice) | Hybrid AAV/phage | RGD4C ligand (binding to αvβ3 and αvβ5 on cancer cell) | Introducing Grp78-HSVtk transgene directly into cancer cell to activate ganciclovir (pro-drug) by phosphorylation | RGD4C/AAVP-Grp78-HSVtk plus ganciclovir inhibits tumor growth, and efficacy is boosted by Temozolomide | 2019 [87] |
| Lung Cancer (A549 human lung adenocarcinoma cells) | Hybrid AAV2/phage | RGD4C ligand (binding to αvβ3 and αvβ5 on cancer cell) | Tumor suppressor p53 gene replacement | Bacteriophage vector is efficiently and selectively delivered CRISPR-Cas9 transgene to A549 human lung adenocarcinoma cells | 2020 [56] |
| Melanoma (BALB/c mice) | T7 phage | pep42 | Introducing expression cassette of GM-CSF transgene into cancer cells | Inhibited tumor growth (B16F10 melanoma cells) by 72% compared to untreated control | 2020 [80] |
| Colorectal cancer (BALB/c mice) | M13 phage (pVIII) | GM-CSF (a potent activator for STAT5 signaling in murine macrophage) | Antitumor cytokine therapy, altering tumor microenvironment | Significantly reduced the tumor size (>50%) in mice, which had subcutaneously injected murine CRC cancer cell line CT26 | 2021 [38] |
| Hepatocellular Carcinoma (HCC cell line) | MS2 phage | GE-11 peptide | Introducing microRNA-21-sponge and pre-microRNA-122 transgenes into HCC cells | Decreased proliferation, migration and invasion of HCC cells | 2021 [65] |
| Breast cancer (△16HER2 transgenic mice) | M13 phage (pIII) | △16HER2 | Induction of anti-HER2 antibody | Significantly delayed tumor onset and reduced tumor growth rate in △16HER2 transgenic mice | 2022 [48] |
| Melanoma (mice) | Qβ phage | Sialyl Lewis antigen | Induction of antibodies | Significantly reduced tumor development in mice metastatic cancer model | 2023 [81] |
| Colorectal cancer (colon cancer cell line) | M13 phage (pIII) conjugated with photosensitizer Rose Bengal | Disulfide-constrained peptide nonamer (CPIEDRPMC) | Photodynamic anticancer effect | Colon cancer cell line (HT29) showed prominent decrease in cell viability | 2024 [52] |
| Hepatocellular carinoma (human HCC cell lines) | Hybrid AAV/phage | RGD4C ligand (binding to αvβ3 and αvβ5 on cancer cell) | Introducing TRAIL gene directly into cancer cell | Induced apoptosis in human HCC cell lines (Huh-7 and HepG2) | 2024 [63] |
| Malignant melanoma (female C57BL/6NCrl mice) | M13 phage (pIII or pVIII) | MAGE-A1 peptide | Induction of antibodies (anti-MAGE-A1 antibody) and cytotoxic immune response | Anti-MAGE-A1 antibody exhibited a binding capability to B16F10 tumor cells in vitro. Splenocytes demonstrated enhanced CTL cytotoxicity against B16F10 cells | 2024 [78] |
| Chondrosarcoma (BALB/c nu/nu mice) | Hybrid AAV2/phage | RGD4C ligand (binding to αvβ3 and αvβ5 on cancer cell) | Introducing human sTRAIL transgene directly into cancer cell | Decreased in tumor size mediated by tumor cell apoptosis (measured by tumor luminescence values as fold change) in BALB/c nu/nu mice implanted with human chondrosarcoma cells (SW1353) | 2024 [85] |
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services#:~:text=In%202022%2C%20there%20were%20an,women%20die%20from%20the%20disease (accessed on 7 July 2024).
- Centers for Disease Control and Prevention. Smoking and Cancer. Available online: https://www.cdc.gov/tobacco/campaign/tips/diseases/cancer.html#:~:text=Cancer%20refers%20to%20diseases%20in,able%20to%20invade%20other%20tissues (accessed on 7 July 2024).
- Diallo, K.; Dublanchet, A. A Century of Clinical Use of Phages: A Literature Review. Antibiotics 2023, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses. Virus Taxonomy. Available online: https://ictv.global/report/genome (accessed on 7 July 2024).
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W. The first phage electron micrographs. Bacteriophage 2011, 1, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. [Google Scholar] [CrossRef]
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P.E.; Cristofoli, F.; Antoni, L.D.; Beccari, T.; et al. Bacteriophages presence in nature and their role in the natural selection of bacterial populations. Acta Biomed. 2020, 91, e2020024. [Google Scholar]
- Fong, K.; Wong, C.W.Y.; Wang, S.; Delaquis, P. How Broad Is Enough: The Host Range of Bacteriophages and Its Impact on the Agri-Food Sector. Phage 2021, 2, 83–91. [Google Scholar] [CrossRef]
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.S.; Novik, G.I. Bacteriophages Receptors, Mechanisms of Phage Adsorption and Penetration into Host Cell. Pol. J. Microbiol. 2010, 59, 145–155. [Google Scholar] [CrossRef]
- Kasman, L.M.; Porter, L.D. Bacteriophages. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493185/ (accessed on 7 July 2024).
- McNair, K.; Bailey, B.A.; Edwards, R.A. PHACTS, a computational approach to classifying the lifestyle of phages. Bioinformatics 2012, 28, 614–618. [Google Scholar] [CrossRef]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis-lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef]
- Ponchon, L.; Mangenot, S.; Boulanger, P.; Letellier, L. Encapsidation and transfer of phage DNA into host cells: From in vivo to single particles studies. Biochim. Biophys. Acta 2005, 1724, 255–261. [Google Scholar] [CrossRef]
- Bloch, H. Experimental investigation on the relationships between bacteriophages and malignant tumors. Arch. Virol. 1940, 1, 481–496. [Google Scholar]
- Petrov, G.; Dymova, M.; Richter, V. Bacteriophage-Mediated Cancer Gene Therapy. Int. J. Mol. Sci. 2022, 23, 14245. [Google Scholar] [CrossRef] [PubMed]
- Palma, M. Aspects of Phage-Based Vaccines for Protein and Epitope Immunization. Vaccines 2023, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef]
- Islam, M.S.; Fan, J.; Pan, F. The power of phages: Revolutionizing cancer treatment. Front. Oncol. 2023, 13, 1290296. [Google Scholar] [CrossRef]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. Phage Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Wojciechowska, R.; Górski, A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Human Virome and Disease: High-Throughput Sequencing for Virus Discovery, Identification of Phage-Bacteria Dysbiosis and Development of Therapeutic Approaches with Emphasis on the Human Gut. Viruses 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Wong, S.; St Jean, J.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism to Cross Epithelial Cell Layers. mBio 2017, 8, e01874-17. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Haq, I.U.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: A review. Virol. J. 2012, 9, 9. [Google Scholar] [CrossRef]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vaccines Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef]
- Foglizzo, V.; Marchiò, S. Bacteriophages as Therapeutic and Diagnostic Vehicles in Cancer. Pharmaceuticals 2021, 14, 161. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Gupta, S.L.; Khan, N.; Basu, S.; Soni, V. B-Cell-Based Immunotherapy: A Promising New Alternative. Vaccines 2022, 10, 879. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mirshekari, H.; Moosavi, B.S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and Phage-inspired Nanocarriers for Targeted Delivery of Therapeutic Cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chang, Y.C.; Hu, C.W.; Kao, C.Y.; Yu, Y.A.; Lim, S.K.; Mou, K.Y. Development of a Novel Cytokine Vehicle Using Filamentous Phage Display for Colorectal Cancer Treatment. ACS Synth. Biol. 2021, 10, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Krebsbach, P.H. Gene Therapy: Design and Prospects for Craniofacial Regeneration. J. Dent. Res. 2009, 88, 585–596. [Google Scholar] [CrossRef]
- Mittal, M.; Kumari, A.; Paul, B.; Varshney, A.; Bhavya; Saini, A.; Verma, C.; Mani, I. Challenges and Opportunities of Gene Therapy in Cancer. OBM Genetics 2024, 8, 219. [Google Scholar] [CrossRef]
- Jia, L.T.; Chen, S.Y.; Yang, A.G. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat. Rev. 2012, 38, 868–876. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef]
- Rigg, A.; Sikora, K. Genetic prodrug activation therapy. Trends Mol. Med. 1997, 3, 359–366. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef] [PubMed]
- Barati, N.; Razazan, A.; Nicastro, J.; Slavcev, R.; Arab, A.; Mosaffa, F.; Nikpoor, A.R.; Badiee, A.; Jaafari, M.R.; Behravan, J. Immunogenicity and antitumor activity of the superlytic λF7 phage nanoparticles displaying a HER2/neu-derived peptide AE37 in a tumor model of BALB/c mice. Cancer Lett. 2018, 424, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Voutsas, I.F.; Gritzapis, A.D.; Mahaira, L.G.; Salagianni, M.; Hofe, E.V.; Kallinteris, N.L.; Baxevanis, C.N. Induction of potent CD4+ T cell-mediated antitumor responses by a helper HER-2/neu peptide linked to the Ii-Key moiety of the invariant chain. Int. J. Cancer 2007, 121, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lamolinara, A.; Conti, L.; Giangrossi, M.; Cui, L.; Morelli, M.B.; Amantini, C.; Falconi, M.; Bartolacci, C.; Andreani, C.; et al. HER2-Displaying M13 Bacteriophages induce Therapeutic Immunity against Breast Cancer. Cancers 2022, 14, 4054. [Google Scholar] [CrossRef] [PubMed]
- Shadidi, M.; Sørensen, D.; Dybwad, A.; Furset, G.; Sioud, M. Mucosal vaccination with phage-displayed tumour antigens identified through proteomics-based strategy inhibits the growth and metastasis of 4T1 breast adenocarcinoma. Int. J. Oncol. 2008, 32, 241–247. [Google Scholar] [CrossRef]
- Pouyanfard, S.; Bamdad, T.; Hashemi, H.; Bandehpour, M.; Kazemi, B. Induction of protective anti-CTL epitope responses against HER-2-positive breast cancer based on multivalent T7 phage nanoparticles. PLoS ONE 2012, 7, e49539. [Google Scholar] [CrossRef]
- Marcellinaro, R.; Spoletini, D.; Grieco, M.; Avella, P.; Cappuccio, M.; Troiano, R.; Lisi, G.; Garbarino, G.M.; Carlini, M. Colorectal Cancer: Current Updates and Future Perspectives. J. Clin. Med. 2023, 13, 40. [Google Scholar] [CrossRef]
- Turrini, E.; Ulfo, L.; Costantini, P.E.; Saporetti, R.; Giosia, M.D.; Nigro, M.; Petrosino, A.; Pappagallo, L.; Kaltenbrunner, A.; Cantelli, A.; et al. Molecular engineering of a spheroid-penetrating phage nanovector for photodynamic treatment of colon cancer cells. Cell Mol. Life Sci. 2024, 81, 144. [Google Scholar] [CrossRef]
- Seguin, L.; Durandy, M.; Feral, C.C. Lung Adenocarcinoma Tumor Origin: A Guide for Personalized Medicine. Cancers 2022, 14, 1759. [Google Scholar] [CrossRef]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Mogi, A.; Kuwano, H. TP53 Mutations in Nonsmall Cell Lung Cancer. J. Biomed. Biotechnol. 2011, 2011, 583929. [Google Scholar] [CrossRef] [PubMed]
- Yang Zhou, J.; Suwan, K.; Hajitou, A. Initial Steps for the Development of a Phage-Mediated Gene Replacement Therapy Using CRISPR-Cas9 Technology. J. Clin. Med. 2020, 9, 1498. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Ren, Z.J.; Zhao, M.Y.; Wang, X.B.; Zuo, S.G.; Yu, F. Antitumor activity of endogenous mFlt4 displayed on a T4 phage nanoparticle surface. Acta Pharmacol. Sin. 2009, 30, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Yu, F.; Zuo, S.G.; Zhao, M.Y.; Wang, X.B.; Wang, X.C.; Chen, Y.; Wu, Z.P.; Ren, Z.J. Inhibition of tumor angiogenesis in lung cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine 2011, 29, 5802–5811. [Google Scholar] [CrossRef]
- Zuo, S.G.; Dai, G.P.; Wang, L.P.; Wen, Y.Q.; Huang, Z.; Yang, W.Y.; Ma, W.L.; Ren, X.Q. Suppression of angiogenesis and tumor growth by recombinant T4 phages displaying extracellular domain of vascular endothelial growth factor receptor 2. Arch. Virol. 2019, 164, 69–82. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Liver Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/liver-cancer-statistics/ (accessed on 7 July 2024).
- Tejeda-Maldonado, J.; García-Juárez, I.; Aguirre-Valadez, J.; Gonzalez-Aguirre, A.; Vilatoba-Chapa, M.; Armengol-Alonso, A.; Escobar-Penagos, F.; Torre, A.; Sanchez-Avila, J.F.; Carrillo-Perez, D.L. Diagnosis and treatment of hepatocellular carcinoma: An update. World J. Hepatol. 2015, 7, 362–376. [Google Scholar] [CrossRef]
- Iwagami, Y.; Casulli, S.; Nagaoka, K.; Kim, M.; Carlson, R.I.; Ogawa, K.; Lebowitz, M.S.; Fuller, S.; Biswas, B.; Stewart, S.; et al. Lambda phage-based vaccine induces antitumor immunity in hepatocellular carcinoma. Heliyon 2017, 3, e00407. [Google Scholar] [CrossRef]
- Sittiju, P.; Wudtiwai, B.; Chongchai, A.; Hajitou, A.; Kongtawelert, P.; Pothacharoen, P.; Suwan, K. Bacteriophage-based particles carrying the TNF-related apoptosis-inducing ligand (TRAIL) gene for targeted delivery in hepatocellular carcinoma. Nanoscale 2024, 16, 6603–6617. [Google Scholar] [CrossRef]
- Chang, L.; Wang, G.; Jia, T.; Zhang, L.; Li, Y.; Han, Y.; Zhang, K.; Lin, G.; Zhang, R.; Li, J.; et al. Armored long non-coding RNA MEG3 targeting EGFR based on recombinant MS2 bacteriophage virus-like particles against hepatocellular carcinoma. Oncotarget. 2016, 7, 23988–24004. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Zhang, R.; Peng, R.; Li, J. Delivery of microRNA-21-sponge and pre-microRNA-122 by MS2 virus-like particles to therapeutically target hepatocellular carcinoma cells. Exp. Biol. Med. 2021, 246, 2463–2472. [Google Scholar] [CrossRef]
- PDQ Adult Treatment Editorial Board. B-Cell Non-Hodgkin Lymphoma Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US): Bethesda, MD, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK592890/ (accessed on 7 July 2024).
- Brown, S.L.; Miller, R.A.; Horning, S.J.; Czerwinski, D.; Hart, S.M.; Mcelderry, R.; Basham, T.; Warnke, R.A.; Merigan, T.C.; Levy, R. Treatment of B-cell Lymphomas with Anti-idiotype Antibodies Alone and in Combination with Alpha Interferon. Blood 1989, 73, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Roehnisch, T.; Then, C.; Nagel, W.; Blumenthal, C.; Braciak, T.; Donzeau, M.; Bohm, T.; Flaig, M.; Bourquin, C.; Oduncu, F.S. Phage idiotype vaccination: First phase I/II clinical trial in patients with multiple myeloma. J. Transl. Med. 2014, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Roehnisch, T.; Then, C.; Nagel, W.; Blumenthal, C.; Braciak, T.; Donzeau, M.; Bohm, T.; Bourquin, C.; Oduncu, F. Chemically linked phage idiotype vaccination in the murine B cell lymphoma 1 model. J. Transl. Med. 2013, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Institute for Quality and Efficiency in Health Care. Overview: Cervical cancer. In InformedHealth.org [Internet]; Institute for Quality and Efficiency in Health Care: Cologne, Germany, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279259/ (accessed on 7 July 2024).
- World Health Organization. Comprehensive Cervical Cancer Control: A Guide to Essential Practice, 2nd ed.; World Health Organization: Geneva, Switzerland, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK269619/ (accessed on 7 July 2024).
- Ghaemi, A.; Soleimanjahi, H.; Gill, P.; Hassan, Z.M.; Razeghi, S.; Fazeli, M.; Razavinikoo, S.M.H. Protection of Mice by a λ-Based Therapeutic Vaccine against Cancer Associated with Human Papillomavirus Type 16. Intervirology 2011, 54, 105–112. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Raphael, M.J.; Chan, D.L.; Law, C.; Singh, S. Principles of diagnosis and management of neuroendocrine tumours. CMAJ 2017, 189, E398–E404. [Google Scholar] [CrossRef]
- Smith, T.L.; Yuan, Z.; Cardó-Vila, M.; Claros, C.S.; Adem, A.; Cui, M.H.; Branch, C.A.; Gelovani, J.G.; Libutti, S.K.; Sidman, R.L.; et al. AAVP displaying octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine tumors of the pancreas. Proc. Natl. Acad. Sci. USA 2016, 113, 2466–2471. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Brišar, N.; Šuster, K.; Brezar, S.K.; Vidmar, R.; Fonović, M.; Cör, A. An Engineered M13 Filamentous Nanoparticle as an Antigen Carrier for a Malignant Melanoma Immunotherapeutic Strategy. Viruses 2024, 16, 232. [Google Scholar] [CrossRef]
- Shukla, G.S.; Sun, Y.J.; Pero, S.C.; Sholler, G.S.; Krag, D.N. Immunization with tumor neoantigens displayed on T7 phage nanoparticles elicits plasma antibody and vaccine-draining lymph node B cell responses. J. Immunol. Methods 2018, 460, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Myung, H. Engineered Bacteriophage T7 as a Potent Anticancer Agent in vivo. Front. Microbiol. 2020, 11, 491001. [Google Scholar] [CrossRef] [PubMed]
- Rashidijahanabad, Z.; Ramadan, S.; O’Brien, N.A.; Nakisa, A.; Lang, S.Y.; Crawford, H.; Gildersleeve, J.C.; Huang, X.F. Stereoselective Synthesis of Sialyl Lewisa Antigen and the Effective Anticancer Activity of Its Bacteriophage Qβ Conjugate as an Anticancer Vaccine. Angew. Chem. Int. Ed. Engl. 2023, 62, e202309744. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, K.P.; Devesa, S.S.; Bray, F.; Troisi, R.; Jonasdottir, T.J.; Bruland, O.S.; Grotmol, T. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005). Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Damron, T.A.; Ward, W.G.; Stewart, A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer Data Base Report. Clin. Orthop. Relat. Res. 2007, 459, 40–47. [Google Scholar] [CrossRef]
- Gazendam, A.; Popovic, S.; Parasu, N.; Ghert, M. Chondrosarcoma: A Clinical Review. J. Clin. Med. 2023, 12, 2506. [Google Scholar] [CrossRef]
- Chongchai, A.; Bentayebi, K.; Chu, G.; Yan, W.; Waramit, S.; Phitak, T.; Kongtawelert, P.; Pothacharoen, P.; Suwan, K.; Hajitou, A. Targeted treatment of chondrosarcoma with a bacteriophage-based particle delivering a secreted tumor necrosis factor-related apoptosis-inducing ligand. Mol. Ther. Oncol. 2024, 32, 200805. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M.; et al. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 2019, 11, e8492. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.D.; Cao, Y.; Wang, Z.Y.; Yang, T.; Wang, J.H. M13 phage: A versatile building block for a highly specific analysis platform. Anal. Bioanal. Chem. 2023, 415, 3927–3944. [Google Scholar] [CrossRef]
- Baas, J.; Senninger, N.; Elser, H. Das Retikuloendotheliale System. Eine Ubersicht über Funktion, Pathologie und neuere Messmethoden [The reticuloendothelial system. An overview of function, pathology and recent methods of measurement]. Z. Gastroenterol. 1994, 32, 117–123. [Google Scholar] [PubMed]
- Veeranarayanan, S.; Azam, A.H.; Kiga, K.; Watanabe, S.; Cui, L. Bacteriophages as Solid Tumor Theragnostic Agents. Int. J. Mol. Sci. 2021, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liang, X.J. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin. J. Cancer 2012, 31, 100–109. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, Y.; Shen, Y.; Cao, L.; Zhang, W.; Guan, X. Analysis of different HER-2 mutations in breast cancer progression and drug resistance. J. Cell Mol. Med. 2015, 19, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Corrigendum: Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2021, 12, 775758. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Cheng, P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188434. [Google Scholar] [CrossRef]
- Cieslewicz, M.; Tang, J.; Yu, J.L.; Cao, H.; Zavalijevski, M.; Motoyama, K.; Lieber, A.; Raines, E.W.; Pun, S.H. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Natl. Acad. Sci. USA 2013, 110, 15919–15924. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Expanded Access. Available online: https://www.fda.gov/news-events/public-health-focus/expanded-access (accessed on 7 July 2024).
- Pirnay, J.P.; Verbeken, G. Magistral Phage Preparations: Is This the Model for Everyone? Clin. Infect. Dis. 2023, 77, S360–S369. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
| Phage Type | Class | Family | Genus | Genome | Size (kb) | Proteins | Morphology | Life Cycle (in Bacteria) |
|---|---|---|---|---|---|---|---|---|
| M13 | Faserviricetes | Inoviridae | Inovirus | ssDNA | 6.4 | 11 | Filamentous | Lysogenic |
| λ | Caudoviricetes | Siphoviridae | Lambdavirus | dsDNA | 48.5 | 73 | Icosahedral head with tail | Lytic/ lysogenic |
| T4 | Caudoviricetes | Straboviridae | Tequatrovirus | dsDNA | 168 | 289 | Icosahedral head with tail | Lytic |
| T7 | Caudoviricetes | Autographiviridae | Teseptimavirus | dsDNA | 40 | 55 | Icosahedral head with tail | Lytic |
| MS2 | Leviviricetes | Fiersviridae | Emesvirus | ssRNA | 3.6 | 4 | Icosahedral | Lytic |
| Qβ | Leviviricetes | Fiersviridae | Qubevirus | ssRNA | 4.2 | 4 | Icosahedral | Lytic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, V.Y.; Yeh, T.-Y. Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 9938. https://doi.org/10.3390/ijms25189938
Ooi VY, Yeh T-Y. Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment. International Journal of Molecular Sciences. 2024; 25(18):9938. https://doi.org/10.3390/ijms25189938
Chicago/Turabian StyleOoi, Vivian Y., and Ting-Yu Yeh. 2024. "Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment" International Journal of Molecular Sciences 25, no. 18: 9938. https://doi.org/10.3390/ijms25189938







