Whole Mitochondrial Genome Sequencing Analysis of Canine Testicular Tumours
Abstract
:1. Introduction
2. Results
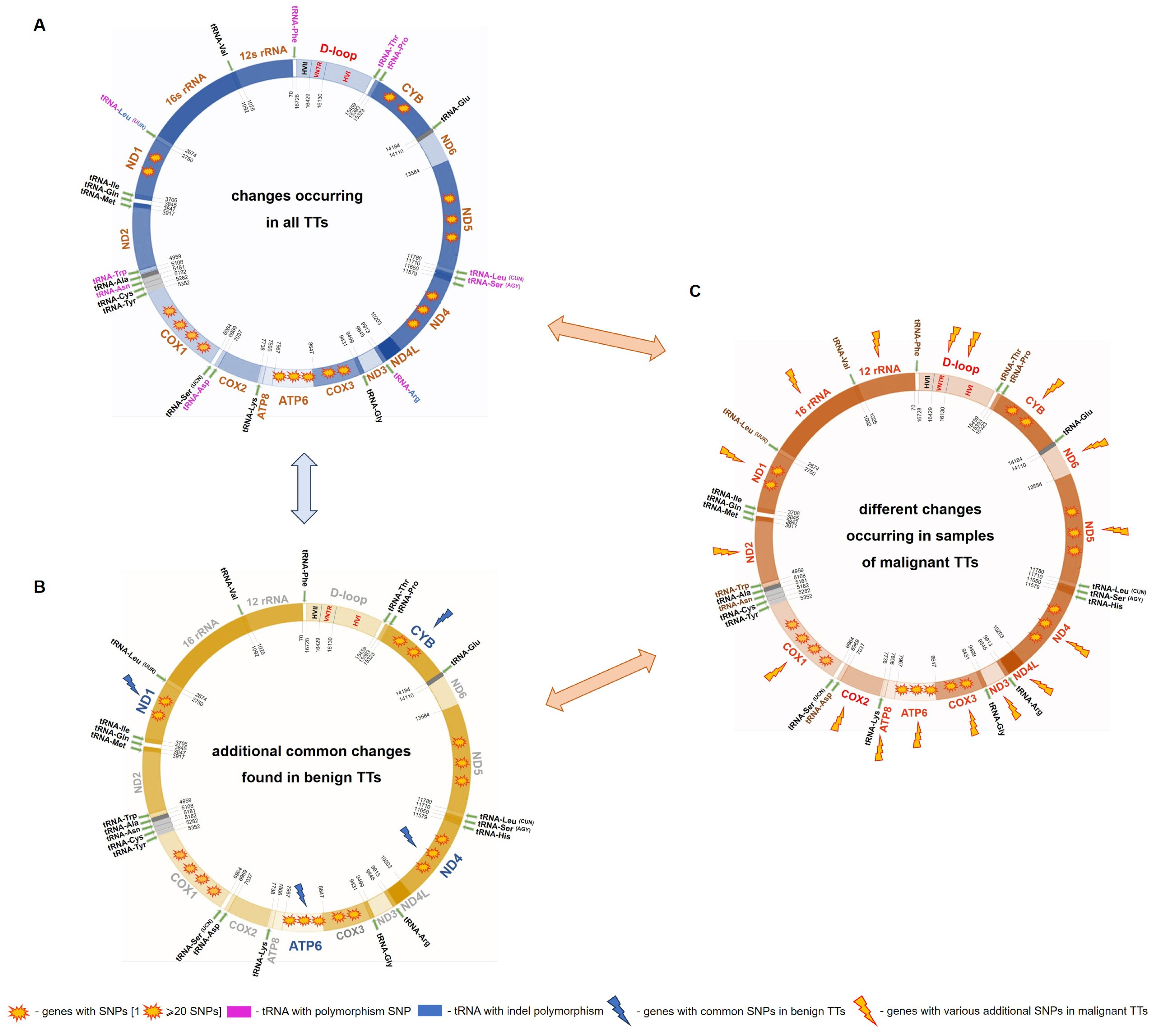
2.1. mtDNA Changes in TTs
2.2. mtDNA Alterations in Benign TTs
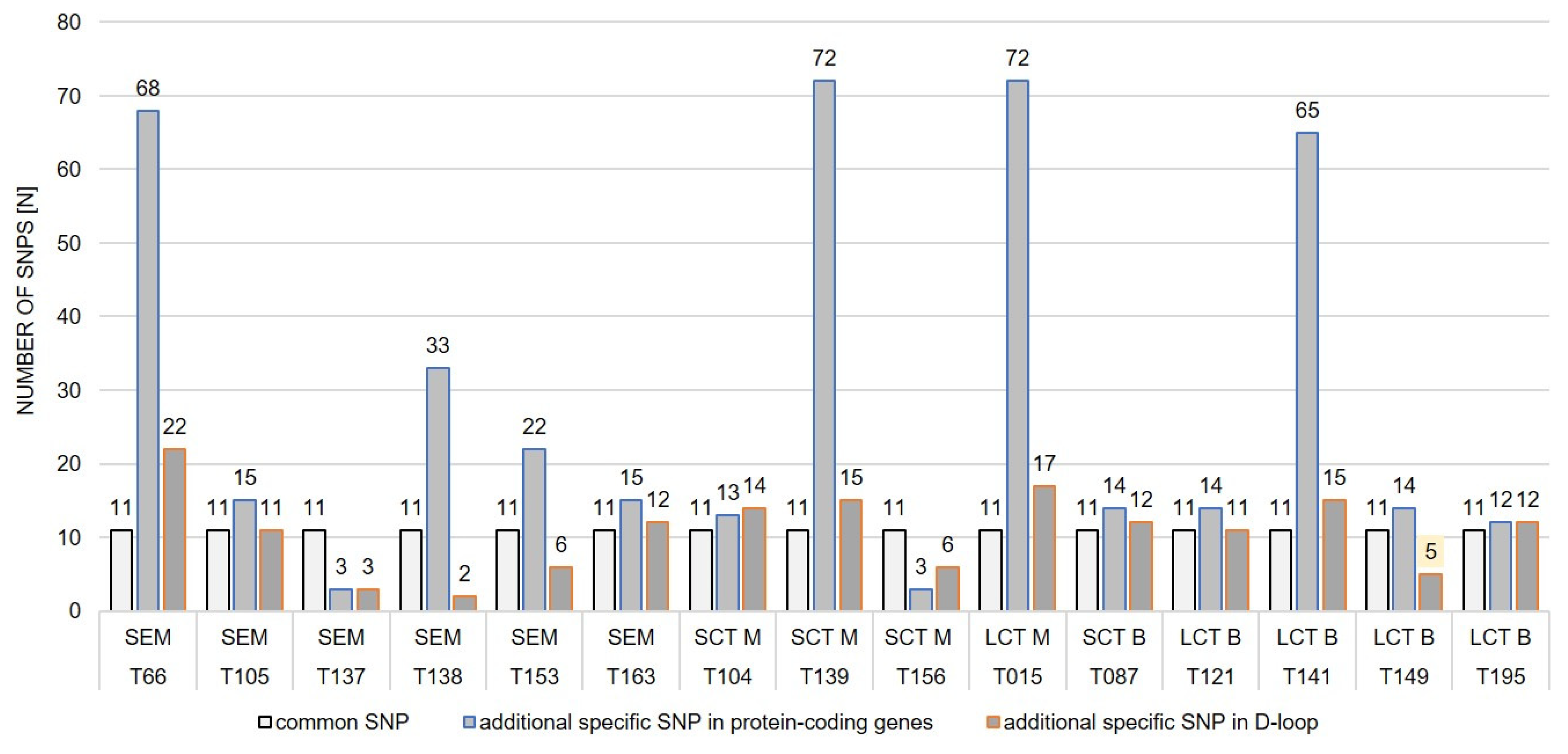
2.3. mtDNA Variants of Benign vs. Malignant TTs
2.4. mtDNA Changes and Characteristics of Dogs
3. Discussion
3.1. Instability of Mitochondrial DNA
3.2. Identified mtDNA Changes
3.3. Oxidative Phosphorylation Complex I
3.4. Comparison of mtDNA Changes of Benign vs. Malignant TTs
3.5. tRNA Variants
4. Materials and Methods
4.1. Samples
4.2. Patient Eligibility
4.3. Sample Information
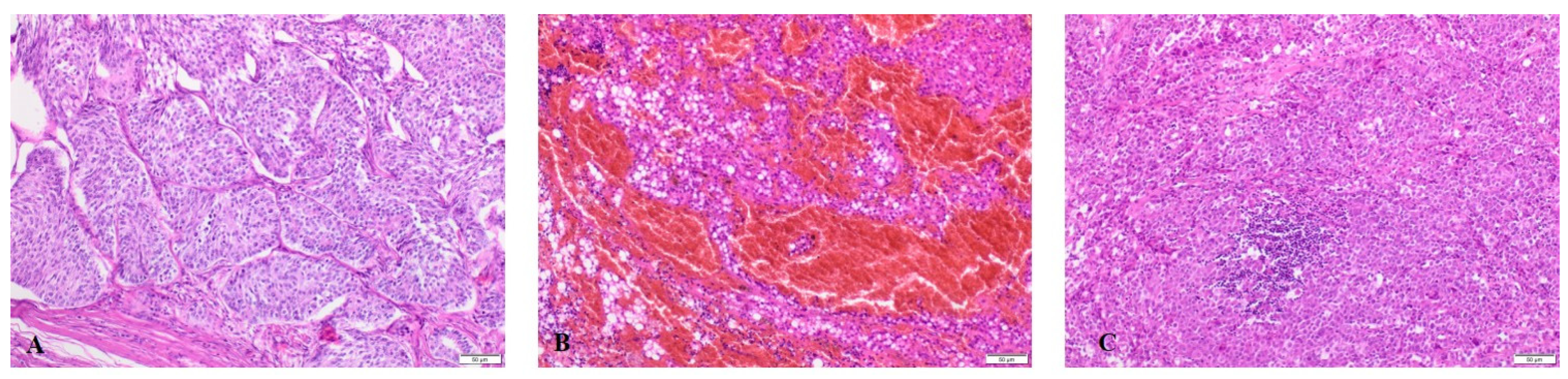
4.4. Histopathology
4.5. Molecular Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manuali, E.; Forte, C.; Porcellato, I.; Brachelente, C.; Sforna, M.; Pavone, S.; Ranciati, S.; Morgante, R.; Crescio, I.M.; Ru, G.; et al. A Five-Year Cohort Study on Testicular Tumors from a Population-Based Canine Cancer Registry in Central Italy (Umbria). Prev. Vet. Med. 2020, 185, 105201. [Google Scholar] [CrossRef] [PubMed]
- Nodtvedt, A.; Gamlem, H.; Gunnes, G.; Grotmol, T.; Indrebo, A.; Moe, L. Breed Differences in the Proportional Morbidity of Testicular Tumours and Distribution of Histopathologic Types in a Population-Based Canine Cancer Registry. Vet. Comp. Oncol. 2011, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.R.; Vita, S.; Marruchella, G.; Di Francesco, G. Canine Testicular Tumours: A Retrospective Investigation in Abruzzo and Molise, Italy. Vet. Ital. 2012, 48, 335–339. [Google Scholar]
- Ciaputa, R.; Brambilla, E.; Godizzi, F.; Dzimira, S.; Zebrowski, K.; Kandefer-Gola, M.; Nowak, M.; Grieco, V. First Report of Primary Testicular Leiomyosarcoma in Two Dogs. BMC Vet. Res. 2023, 19, 101. [Google Scholar] [CrossRef]
- Nascimento, H.H.L.; Santos, A.D.; Prante, A.L.; Lamego, E.C.; Tondo, L.A.S.; Flores, M.M.; Fighera, R.A.; Kommers, G.D. Testicular Tumors in 190 Dogs: Clinical, Macroscopic and Histopathological Aspects. Pesqui. Veterinária Bras. 2020, 40, 525–535. [Google Scholar] [CrossRef]
- Bush, J.M.; Gardiner, D.W.; Palmer, J.S.; Rajpert-De Meyts, E.; Veeramachaneni, D.N. Testicular Germ Cell Tumours in Dogs Are Predominantly of Spermatocytic Seminoma Type and Are Frequently Associated with Somatic Cell Tumours. Int. J. Androl. 2011, 34, 288–295. [Google Scholar] [CrossRef]
- Fan, T.M.; de Lorimier, L.P. Tumors of the Male Reproductive System. In Withrow & MacEwen’s Small Animal Clinical Oncology, 4th ed.; Withrow, S.J., Vail, D.M., Eds.; Elsevier Inc.: St. Louis, MO, USA, 2007; pp. 637–648. [Google Scholar]
- Grieco, V.; Riccardi, E.; Greppi, G.F.; Teruzzi, F.; Iermano, V.; Finazzi, M. Canine Testicular Tumours: A Study on 232 Dogs. J. Comp. Pathol. 2008, 138, 86–89. [Google Scholar] [CrossRef]
- Choi, U.S.; Kim, H.W.; Choi, J.H.; Kim, H.J.; Jang, J.; Lee, C.W. Sertoli Cell Tumor Accompanied by Pancytopenia in a Dog. J. Vet. Clin. 2008, 25, 523–525. [Google Scholar]
- Sapierzynski, R.; Wojtczak, M. Sertolioma U Dwóch Bliźniaczych Sznaucerów Olbrzymich. Życie Weter. 2013, 88, 492–495. [Google Scholar]
- Marfe, G.; De Martino, L.; Tafani, M.; Irno-Consalvo, M.; Pasolini, M.P.; Navas, L.; Papparella, S.; Gambacurta, A.; Paciello, O. A Multicancer-Like Syndrome in a Dog Characterized by P53 and Cell Cycle-Checkpoint Kinase 2 (Chk2) Mutations and Sirtuin Gene (Sirt1) Down-Regulation. Res. Vet. Sci. 2012, 93, 240–245. [Google Scholar] [CrossRef]
- Tkaczyk-Wlizlo, A.; Kowal, K.; Slaska, B. Mitochondrial DNA Alterations in the Domestic Dog (Canis Lupus Familiaris) and Their Association with Development of Diseases: A Review. Mitochondrion 2022, 63, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Tkaczyk-Wlizlo, A.; Pierzchala, M.; Gawor, J.; Slaska, B. Molecular Differences in Mitochondrial DNA Genomes of Dogs with Malignant Mammary Tumours. Vet. Comp. Oncol. 2022, 20, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, A.C.; Soares, P.; van Asch, B.; Amorim, A.; Cirnes, L.; Maximo, V.; Cassali, G.D. An Assessment of the Clonality of the Components of Canine Mixed Mammary Tumours by Mitochondrial DNA Analysis. Vet. J. 2009, 182, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Slaska, B.; Grzybowska-Szatkowska, L.; Bugno-Poniewierska, M.; Gurgul, A.; Smiech, A.; Rozanska, D.; Dudka, J. Relevance of Molecular Changes in the Nd4 Gene in German Shepherd Dog Tumours. Pol. J. Vet. Sci. 2016, 19, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Smiech, A.; Slaska, B.; Surdyka, M.; Grzybowska-Szatkowska, L.; Lopuszynski, W.; Rozanska, D. Identification of Additional Mitochondrial DNA Mutations in Canine Mast Cell Tumours. Acta Vet. Scand. 2016, 58, 28. [Google Scholar] [CrossRef] [PubMed]
- Strakova, A.; Ni Leathlobhair, M.; Wang, G.D.; Yin, T.T.; Airikkala-Otter, I.; Allen, J.L.; Allum, K.M.; Bansse-Issa, L.; Bisson, J.L.; Castillo Domracheva, A.; et al. Mitochondrial Genetic Diversity, Selection and Recombination in a Canine Transmissible Cancer. eLife 2016, 5, e14552. [Google Scholar] [CrossRef]
- Bautista-Gomez, L.; Martinez-Castaneda, S. Identification of Mitochondrial DNA Transfer in Canine Transmissible Venereal Tumours Obtained from Dogs in Mexico. Mitochondrial DNA Part A 2017, 28, 645–649. [Google Scholar] [CrossRef]
- Kowal, K.; Tkaczyk-Wlizlo, A.; Jusiak, M.; Grzybowska-Szatkowska, L.; Slaska, B. Canis Mitosnp Database: A Functional Tool Useful for Comparative Analyses of Human and Canine Mitochondrial Genomes. J. Appl. Genet. 2023, 64, 515–520. [Google Scholar] [CrossRef]
- Dzimira, S.; Kandefer-Gola, M.; Kubiak-Nowak, D. Multiple Primary Testicular Tumours in the Dog—A Case Report. Acta Vet. Brno. 2017, 86, 373–378. [Google Scholar] [CrossRef]
- Usman, M.B.M.; Bhuvaneshwari, V. Surgical Management of Testicular Tumour in a Dalmatian Dog. Indian Vet. J. 2018, 95, 73–74. [Google Scholar]
- Qasemi, M.; Sur, V.P.; Simonik, O.; Postlerova, P.; Skrobanek, P.; Hradec, T.; Boublikova, L.; Zamecnik, L.; Buchler, T.; Neuzil, J.; et al. Sperm Mitochondria Dysfunction in Response to Testicular Cancer. Eur. J. Clin. Investig. 2023, 54, e14146. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Albers, P.; Muller, S.C.; von Ruecker, A.; Bastian, P.J. Circulating Mitochondrial DNA in the Serum of Patients with Testicular Germ Cell Cancer as a Novel Noninvasive Diagnostic Biomarker. BJU Int. 2009, 104, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, K.; Kowal, K.; Tkaczyk-Wlizło, A.; Śmiech, A.; Ślaska, B. Mutations and Polymorphisms in Mitochondrial Genome of Dogs with Solid Mammary Carcinoma—A Preliminary Study. Med. Weter 2023, 79, 566–572. [Google Scholar] [CrossRef]
- Kopinski, P.K.; Singh, L.N.; Zhang, S.; Lott, M.T.; Wallace, D.C. Mitochondrial DNA Variation and Cancer. Nat. Rev. Cancer 2021, 21, 431–445. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Grzybowska-Szatkowska, L.; Ciesielka, M.; Rzymowska, J. The Role of Mitochondria in Carcinogenesis. Int. J. Mol. Sci. 2021, 22, 5100. [Google Scholar] [CrossRef]
- Dias, T.R.; Agarwal, A.; Pushparaj, P.N.; Ahmad, G.; Sharma, R. New Insights on the Mechanisms Affecting Fertility in Men with Non-Seminoma Testicular Cancer before Cancer Therapy. World J. Mens Health 2020, 38, 198–207. [Google Scholar] [CrossRef]
- Ji, J.; Xu, M.; Huang, Z.; Li, L.; Zheng, H.; Yang, S.; Li, S.; Jin, L.; Ling, X.; Xia, Y.; et al. Mitochondrial DNA Sequencing and Large-Scale Genotyping Identifies Mt-Nd4 Gene Mutation M.11696g>a Associated with Idiopathic Oligoasthenospermia. Oncotarget 2017, 8, 52975. [Google Scholar] [CrossRef]
- Amor, H.; Hammadeh, M.E. A Systematic Review of the Impact of Mitochondrial Variations on Male Infertility. Genes 2022, 13, 1182. [Google Scholar] [CrossRef]
- Hesser, A.; Darr, C.; Gonzales, K.; Power, H.; Scanlan, T.; Thompson, J.; Love, C.; Christensen, B.; Meyers, S. Semen Evaluation and Fertility Assessment in a Purebred Dog Breeding Facility. Theriogenology 2017, 87, 115–123. [Google Scholar] [CrossRef]
- Bin, Y.; Wang, X.; Zhao, L.; Wen, P.; Xia, J. An Analysis of Mutational Signatures of Synonymous Mutations across 15 Cancer Types. BMC Med. Genet. 2019, 20, 190. [Google Scholar] [CrossRef]
- Baranowska, I.; Jaderlund, K.H.; Nennesmo, I.; Holmqvist, E.; Heidrich, N.; Larsson, N.G.; Andersson, G.; Wagner, E.G.; Hedhammar, A.; Wibom, R.; et al. Sensory Ataxic Neuropathy in Golden Retriever Dogs Is Caused by a Deletion in the Mitochondrial Trnatyr Gene. PLoS Genet. 2009, 5, e1000499. [Google Scholar] [CrossRef] [PubMed]
- Baiker, K.; Hofmann, S.; Fischer, A.; Godde, T.; Medl, S.; Schmahl, W.; Bauer, M.F.; Matiasek, K. Leigh-Like Subacute Necrotising Encephalopathy in Yorkshire Terriers: Neuropathological Characterisation, Respiratory Chain Activities and Mitochondrial DNA. Acta Neuropathol. 2009, 118, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Tkaczyk-Wlizło, A.; Pierzchała, M.; Ślaska, B. Evaluation of the Trna-Leu (Uur) Gene Haplotype Profile Observed in Canine Mammary Gland Tumours Based on Comparative Analysis with the Mt-Tl1 Human Gene. Ann. Anim. Sci. 2022, 22, 915–922. [Google Scholar] [CrossRef]
- American Kennel Club. Available online: www.Akc.Org (accessed on 31 August 2024).
- Harvey, N.D. How Old Is My Dog? Identification of Rational Age Groupings in Pet Dogs Based upon Normative Age-Linked Processes. Front. Vet. Sci. 2021, 8, 643085. [Google Scholar] [CrossRef]
- Kennedy, P.C.; Cullen, J.M.; Edwards, J.F.; Goldschmidt, M.H.; Larsen, S.; Munson, L.; Nielson, S. Histological Classifications of Tumors of the Genital System of Domestic Animals. World Health Organ. Int. Histol. Classif. Tumors Domest. Anim. 1998, IV, 17–18. [Google Scholar]
- Imes, D.L.; Wictum, E.J.; Allard, M.W.; Sacks, B.N. Identification of Single Nucleotide Polymorphisms within the Mtdna Genome of the Domestic Dog to Discriminate Individuals with Common Hvi Haplotypes. Forensic Sci. Int. Genet. 2012, 6, 630–639. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, S.E.; Jeong, H.W.; Ha, J.H. The Complete Nucleotide Sequence of the Domestic Dog (Canis familiaris) Mitochondrial Genome. Mol. Phylogenetics Evol. 1998, 10, 210–220. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Team, U. Unipro Ugene: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Ślaska, B.; Surdyka, M.; Brodzki, A.; Nisztuk, S.; Gurgul, A.; Bugno-Poniewierska, M.; Śmiech, A.; Różańska, D.; Orzelski, M. Mitochondrial D-Loop Mutations Can Be Detected in Sporadic Malignant Tumours in Dogs. Bull. Vet. Inst. Pulawy 2014, 58, 631–637. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. Expasy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. Sift Missense Predictions for Genomes. Nat. Protoc. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deleage, G. Sopma: Significant Improvements in Protein Secondary Structure Prediction by Consensus Prediction from Multiple Alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deléage, G. Nps@: Network Protein Sequence Analysis. Trends Biochem. Sci. 2000, 3, 147–150. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E. Hgvs Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef]
| List of Common Polymorphisms Occurring in Each Dog with TT | |||||
| Gene/Region | Reference Sequence | Sequence Variant | Codon Change/Position in tRNA | Amino Acid Change/tRNA Region | SIFT 1 for Non-Synonymous Changes |
| tRNA Leu (UUR) | m.2678 | m.2678_2679insG | 8_9 | between the acceptor stem and DHU stem | - |
| m.2683G | m.2683G>A | 13 | DHU arm | ||
| COX1 | m.5367C | m.5367C>T | CTG → TTG | p.Leu7= | |
| m.5444T | m.5444T>C | GCT → GCC | p.Ala32= | ||
| m.6065A | m.6065A>G | GGA → GGG | p.Gly239= | ||
| ATP6 | m.8368C | m.8368C>T | CTC → CTT | p.Leu135= | |
| COX3 | m.8807G | m.8807G>A | TGC → TAC | p.Cys55Tyr | tolerant |
| ND4L | m.9911_9912 | m.9911_9912insGT | ATG → GTG | p.Met1Val | intolerant |
| ND5 | m.13299T | m.13299T>A | TCA → ACA | p.Ser508Thr | tolerant |
| D-loop | m.15639T | m.15639T>G | - | - | - |
| m.15814C | m.15814C>T | ||||
| List of Additional Polymorphisms Occurring Only in Dogs with Benign TT | |||||
| Gene/Region | Reference Sequence | Sequence Variant | Codon Change/Position in tRNA | Amino Acid Change/tRNA Region | SIFT for Non-Synonymous Changes |
| ND1 | m.2962C | m.2962C>T | ATC → ATT | p.Ile= | - |
| m.3196T | m.3196T>C | CTT → CTC | p.Leu= | ||
| ATP6 | m.8281T | m.8281T>C | ATT → ATC | p.Ile= | |
| ND4 | m.10992G | m.10992G>A | TTG → TTA | p.Leu= | |
| CYB | m.15214G | m.15214G>A | TTG → TTA | p.Leu= | |
| Number | Breed/ Crossbreed | Sample Number | Age of Dog | Size of the Dog According to AKC 1 | Age Group | Abnormalities of Testes | Tumour Type 2 | Malignant/Benign |
|---|---|---|---|---|---|---|---|---|
| 1 | Crossbreed | T163 | 8 | M | senior | unilateral | SEM | malignant |
| 2 | T138 | 10 | bilateral | |||||
| 3 | T087 | 11 | unilateral + cryptorchidism | SCT | benign | |||
| 4 | T015 | 12 | geriatric | unilateral | LCT | malignant | ||
| 5 | German Shepherd | T104 | 10 | L | senior | unilateral + cryptorchidism | SCT | |
| 6 | T121 | unilateral | LCT | benign | ||||
| 7 | T149 | 11 | ||||||
| 8 | T156 | 14 | geriatric | SCT | malignant | |||
| 9 | Giant Schnauzer | T139 | 14 | |||||
| 10 | Golden Retriever | T195 | 9 | senior | LCT | benign | ||
| 11 | Labrador Retriever | T105 | 8 | SEM | malignant | |||
| 12 | Miniature Schnauzer | T141 | 14 | S | geriatric | LCT | benign | |
| 13 | Poodle (Miniature) | T153 | 15 | SEM | malignant | |||
| 14 | Prague Ratter | T137 | 14 | unilateral + cryptorchidism | ||||
| 15 | Standard Schnauzer | T066 | 10 | M | senior |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkaczyk-Wlizło, A.; Kowal, K.; Śmiech, A.; Ślaska, B. Whole Mitochondrial Genome Sequencing Analysis of Canine Testicular Tumours. Int. J. Mol. Sci. 2024, 25, 9944. https://doi.org/10.3390/ijms25189944
Tkaczyk-Wlizło A, Kowal K, Śmiech A, Ślaska B. Whole Mitochondrial Genome Sequencing Analysis of Canine Testicular Tumours. International Journal of Molecular Sciences. 2024; 25(18):9944. https://doi.org/10.3390/ijms25189944
Chicago/Turabian StyleTkaczyk-Wlizło, Angelika, Krzysztof Kowal, Anna Śmiech, and Brygida Ślaska. 2024. "Whole Mitochondrial Genome Sequencing Analysis of Canine Testicular Tumours" International Journal of Molecular Sciences 25, no. 18: 9944. https://doi.org/10.3390/ijms25189944






