Novel Function of Osteocalcin in Chondrocyte Differentiation and Endochondral Ossification Revealed on a CRISPR/Cas9 bglap–bglap2 Deficiency Mouse Model
Abstract
1. Introduction
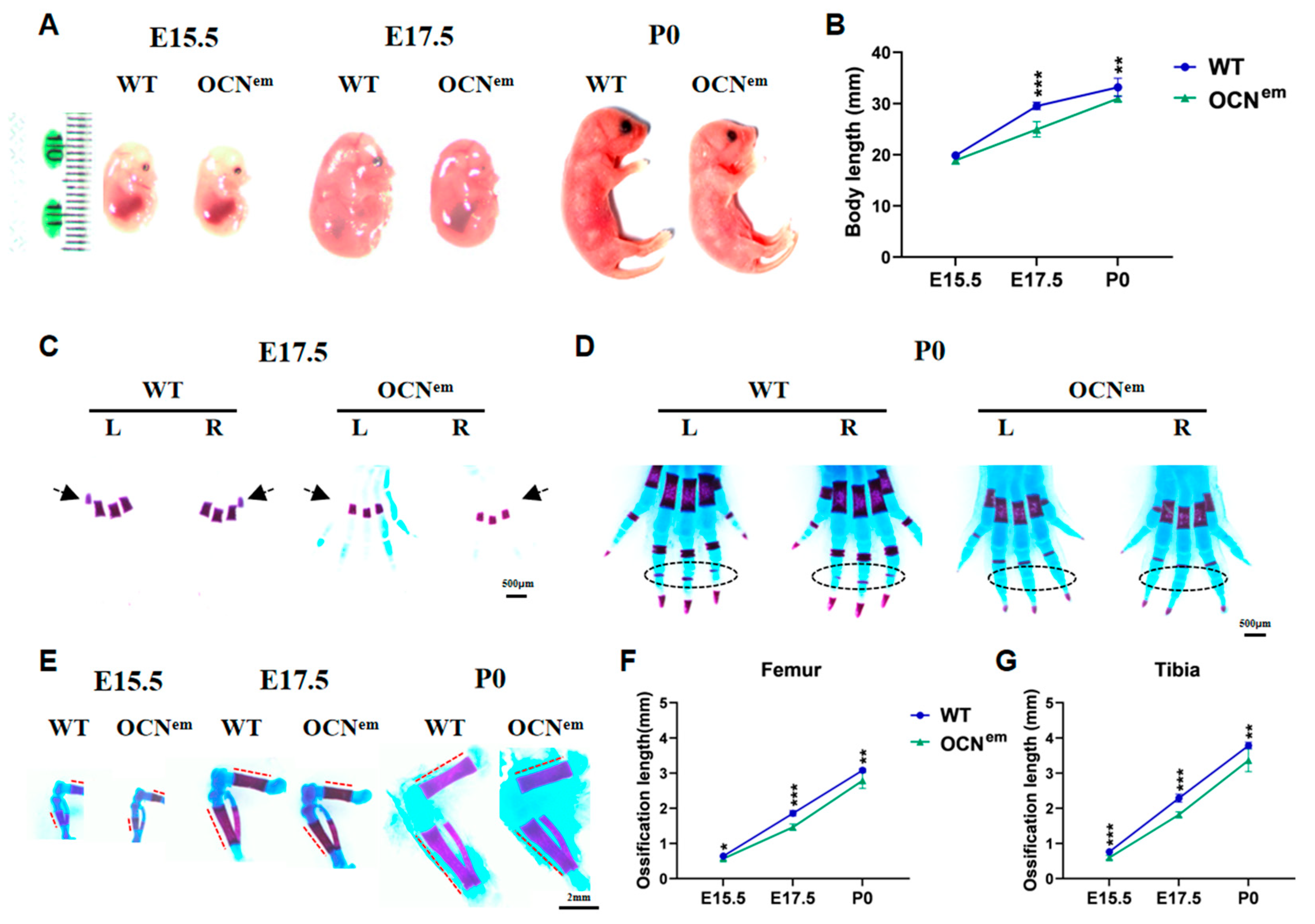
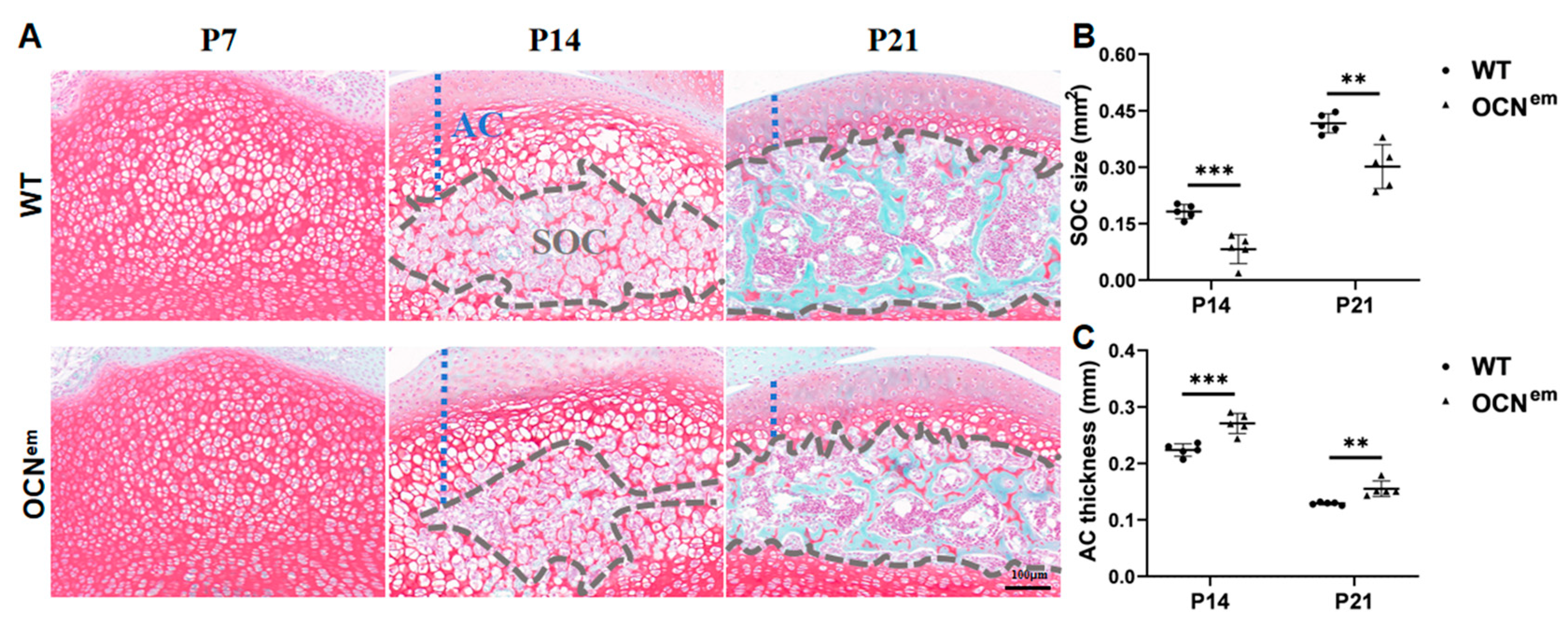
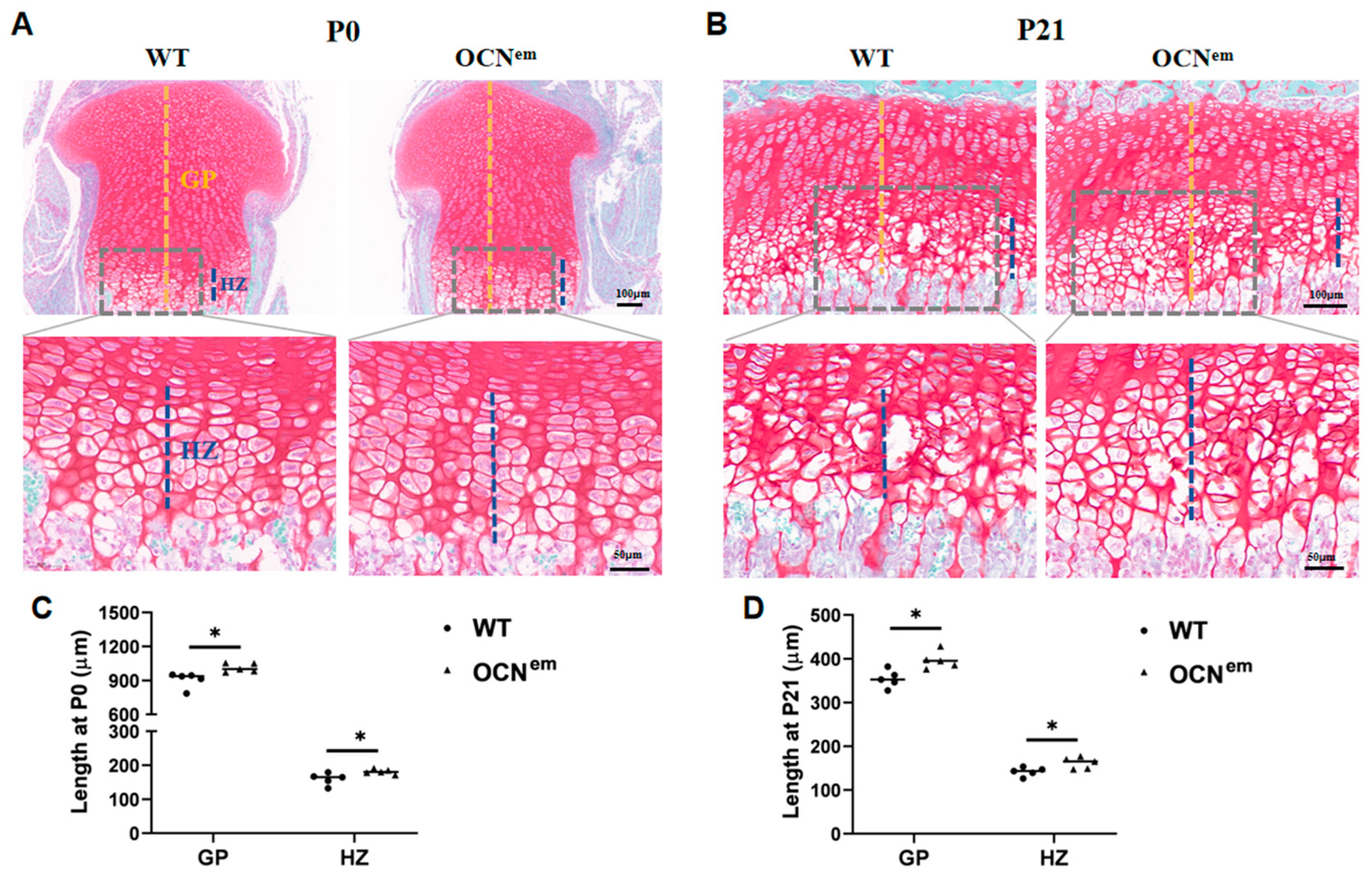
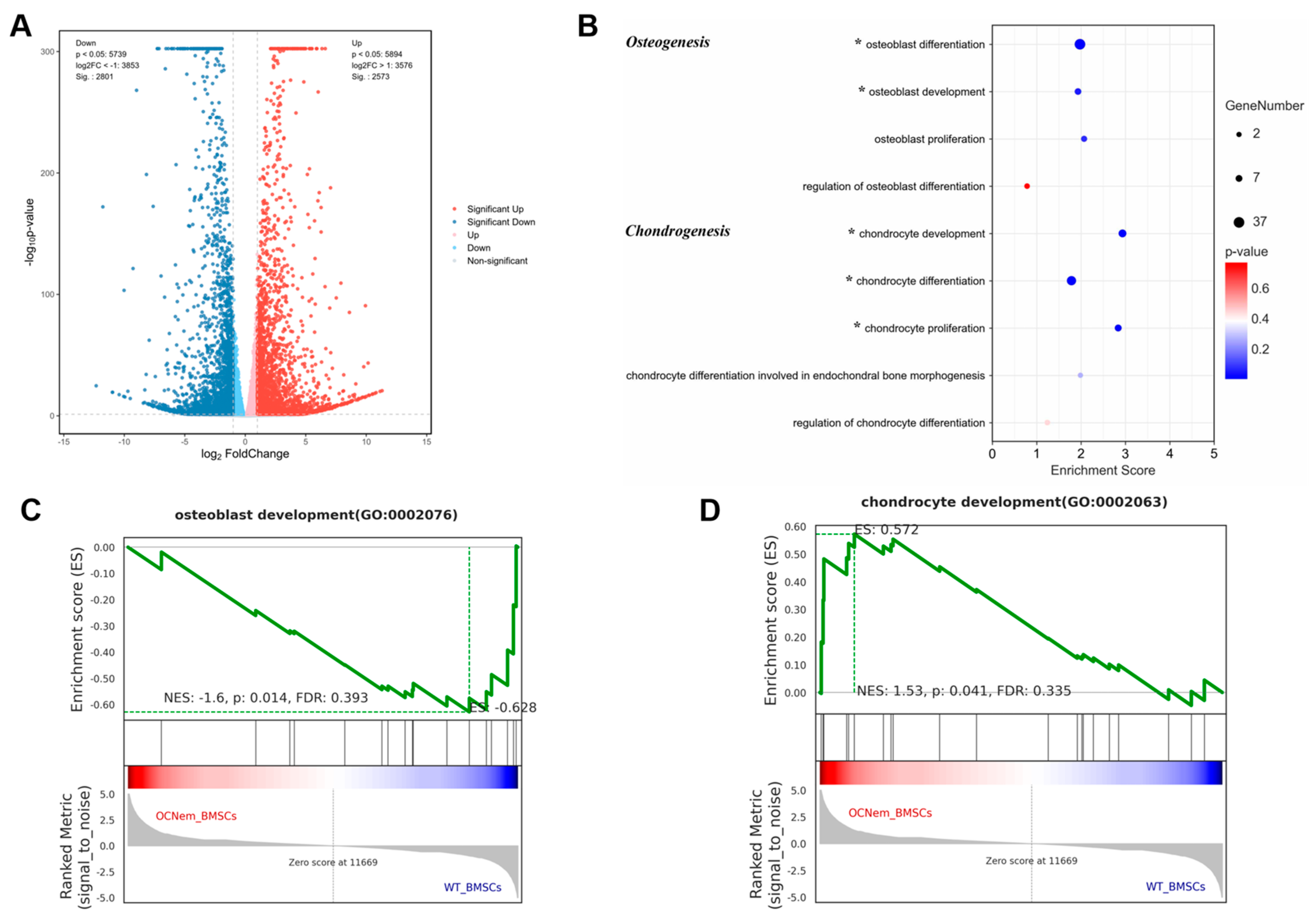
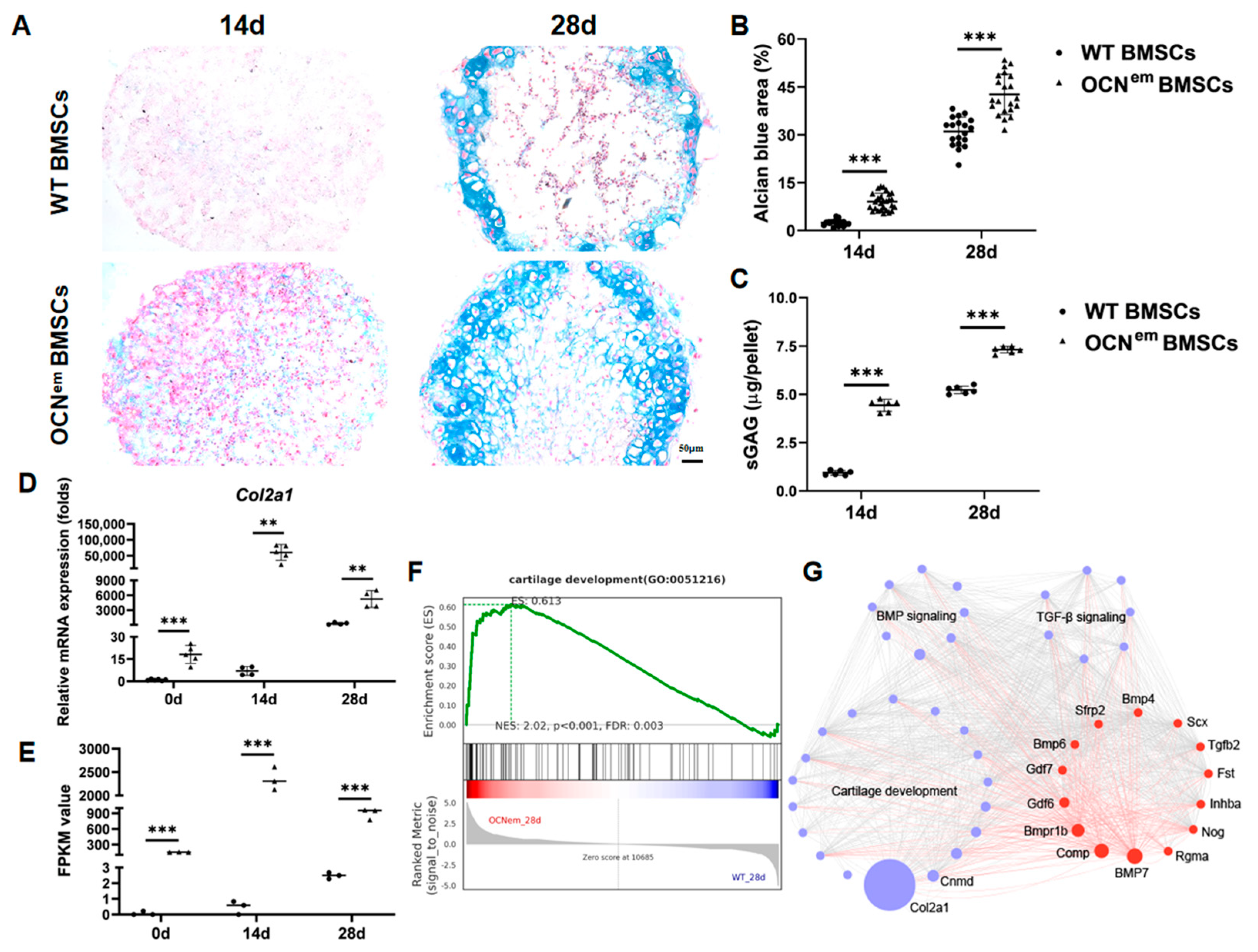
2. Results
3. Discussion
4. Materials and Methods
4.1. Generation of OCNem Mice and Housing
4.2. Whole-Mount Skeletal Staining
4.3. Histological and Immunofluorescence (IF) Staining
4.4. Isolation, Cultivation and Chondrogenic Differentiation of BMSCs
4.5. RNA-Sequencing and Bioinformatics Analyses
4.6. Alcian Blue/Nuclear Fast Red Staining of Chondrogenic Pellet
4.7. Sulfated Glycosaminoglycan (sGAG) Quantification
4.8. Real-Time Quantitative Reverse Transcription PCR (RT–qPCR)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wuelling, M.; Vortkamp, A. Chondrocyte proliferation and differentiation. Endocr. Dev. 2011, 21, 1–11. [Google Scholar] [PubMed]
- Mackie, E.J.; Ahmed, Y.A. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.; Beier, F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res. C Embryo Today 2014, 102, 74–82. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef]
- Grzelkowska-Kowalczyk, K. The importance of extracellular matrix in skeletal muscle development and function. In Composition and Function of the Extracellular Matrix in the Human Body; InTechOpen: London, UK, 2016; pp. 3–24. [Google Scholar]
- Bernabei, I.; So, A. Cartilage calcification in osteoarthritis: Mechanisms and clinical relevance. Nat. Rev. Rheumatol. 2023, 19, 10–27. [Google Scholar] [CrossRef]
- Grissom, S.K.; Semevolos, S.A. Role of cartilage and bone matrix regulation in early equine osteochondrosis. Bone Rep. 2023, 18, 101653. [Google Scholar] [CrossRef]
- Pullig, O.; Weseloh, G. Chondrocyte differentiation in human osteoarthritis: Expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif. Tissue Int. 2000, 67, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; El-Turk, C. Neonatal lethal osteochondrodysplasia with low serum levels of alkaline phosphatase and osteocalcin. J. Clin. Endocrinol. Metab. 2005, 90, 1233–1240. [Google Scholar] [CrossRef][Green Version]
- Romberg, R.W.; Werness, P.G. Inhibition of hydroxyapatite crystal growth by bone-specific and other calcium-binding proteins. Biochemistry 1986, 25, 1176–1180. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Moriishi, T.; Ozasa, R. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020, 16, e1008586. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Donoso, M.; Guevara, J.M. Morphological changes of physeal cartilage and secondary ossification centres in the developing femur of the house mouse (Mus musculus): A micro-CT based study. Anat. Histol. Embryol. 2019, 48, 117–124. [Google Scholar] [CrossRef]
- Setiawati, R.; Rahardjo, P. Bone development and growth. In Osteogenesis and Bone Regeneration; InTechOpen: London, UK, 2019; p. 10. [Google Scholar]
- Hu, L.; Yin, C. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Tatarczuch, L. The skeleton: A multi-functional complex organ: The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef]
- White, A.; Wallis, G. Endochondral ossification: A delicate balance between growth and mineralisation. Curr. Biol. 2001, 11, R589–R591. [Google Scholar] [CrossRef]
- Wilson, K.; Usami, Y. Analysis of Association between Morphometric Parameters of Growth Plate and Bone Growth of Tibia in Mice and Humans. Cartilage 2021, 13, 315S–325S. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Joyce, M.E.; Roberts, A.B. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J. Cell Biol. 1990, 110, 2195–2207. [Google Scholar] [CrossRef]
- Kawakami, Y.; Rodriguez-Leon, J. The role of TGFbetas and Sox9 during limb chondrogenesis. Curr. Opin. Cell Biol. 2006, 18, 723–729. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S. The roles and regulatory mechanisms of TGF-beta and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Chubinskaya, S.; Kuettner, K.E. Regulation of osteogenic proteins by chondrocytes. Int. J. Biochem. Cell Biol. 2003, 35, 1323–1340. [Google Scholar] [CrossRef]
- Flechtenmacher, J.; Huch, K. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996, 39, 1896–1904. [Google Scholar] [CrossRef]
- Badlani, N.; Oshima, Y. Use of bone morphogenic protein-7 as a treatment for osteoarthritis. Clin. Orthop. Relat. Res. 2009, 467, 3221–3229. [Google Scholar] [CrossRef]
- Hayashi, M.; Muneta, T. Intra-articular injections of bone morphogenetic protein-7 retard progression of existing cartilage degeneration. J. Orthop. Res. 2010, 28, 1502–1506. [Google Scholar] [CrossRef]
- Hurtig, M.; Chubinskaya, S. BMP-7 protects against progression of cartilage degeneration after impact injury. J. Orthop. Res. 2009, 27, 602–611. [Google Scholar] [CrossRef]
- Tang, C.Y.; Wu, M. Runx1 is a central regulator of osteogenesis for bone homeostasis by orchestrating BMP and WNT signaling pathways. PLoS Genet. 2021, 17, e1009233. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kung, L.H.; Rajpar, M.H. Increased classical endoplasmic reticulum stress is sufficient to reduce chondrocyte proliferation rate in the growth plate and decrease bone growth. PLoS ONE 2015, 10, e0117016. [Google Scholar] [CrossRef]
- Stickens, D.; Behonick, D.J. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883–5895. [Google Scholar] [CrossRef]
- Maridas, D.E.; Rendina-Ruedy, E. Isolation, Culture, and Differentiation of Bone Marrow Stromal Cells and Osteoclast Progenitors from Mice. J. Vis. Exp. 2018, 6, 56750. [Google Scholar]
- Xu, J.; Li, D. Comparison of skeletal and soft tissue pericytes identifies CXCR4(+) bone forming mural cells in human tissues. Bone Res. 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Park, J. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat. Commun. 2022, 13, 3960. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tsaryk, R. Poly(gamma-Glutamic Acid) as an Exogenous Promoter of Chondrogenic Differentiation of Human Mesenchymal Stem/Stromal Cells. Tissue Eng. Part. A 2015, 21, 1869–1885. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.-F.; Teng, B.; Li, J.-F.; Zhang, J.V.; Su, Z.; Ren, P.-G. Novel Function of Osteocalcin in Chondrocyte Differentiation and Endochondral Ossification Revealed on a CRISPR/Cas9 bglap–bglap2 Deficiency Mouse Model. Int. J. Mol. Sci. 2024, 25, 9945. https://doi.org/10.3390/ijms25189945
Yu X-F, Teng B, Li J-F, Zhang JV, Su Z, Ren P-G. Novel Function of Osteocalcin in Chondrocyte Differentiation and Endochondral Ossification Revealed on a CRISPR/Cas9 bglap–bglap2 Deficiency Mouse Model. International Journal of Molecular Sciences. 2024; 25(18):9945. https://doi.org/10.3390/ijms25189945
Chicago/Turabian StyleYu, Xiang-Fang, Bin Teng, Jun-Feng Li, Jian V. Zhang, Zhe Su, and Pei-Gen Ren. 2024. "Novel Function of Osteocalcin in Chondrocyte Differentiation and Endochondral Ossification Revealed on a CRISPR/Cas9 bglap–bglap2 Deficiency Mouse Model" International Journal of Molecular Sciences 25, no. 18: 9945. https://doi.org/10.3390/ijms25189945
APA StyleYu, X.-F., Teng, B., Li, J.-F., Zhang, J. V., Su, Z., & Ren, P.-G. (2024). Novel Function of Osteocalcin in Chondrocyte Differentiation and Endochondral Ossification Revealed on a CRISPR/Cas9 bglap–bglap2 Deficiency Mouse Model. International Journal of Molecular Sciences, 25(18), 9945. https://doi.org/10.3390/ijms25189945







