Cross-Species Studies Reveal That Dysregulated Mitochondrial Gene Expression and Electron Transport Complex I Activity Are Crucial for Sarcopenia
Abstract
:1. Introduction
2. Results
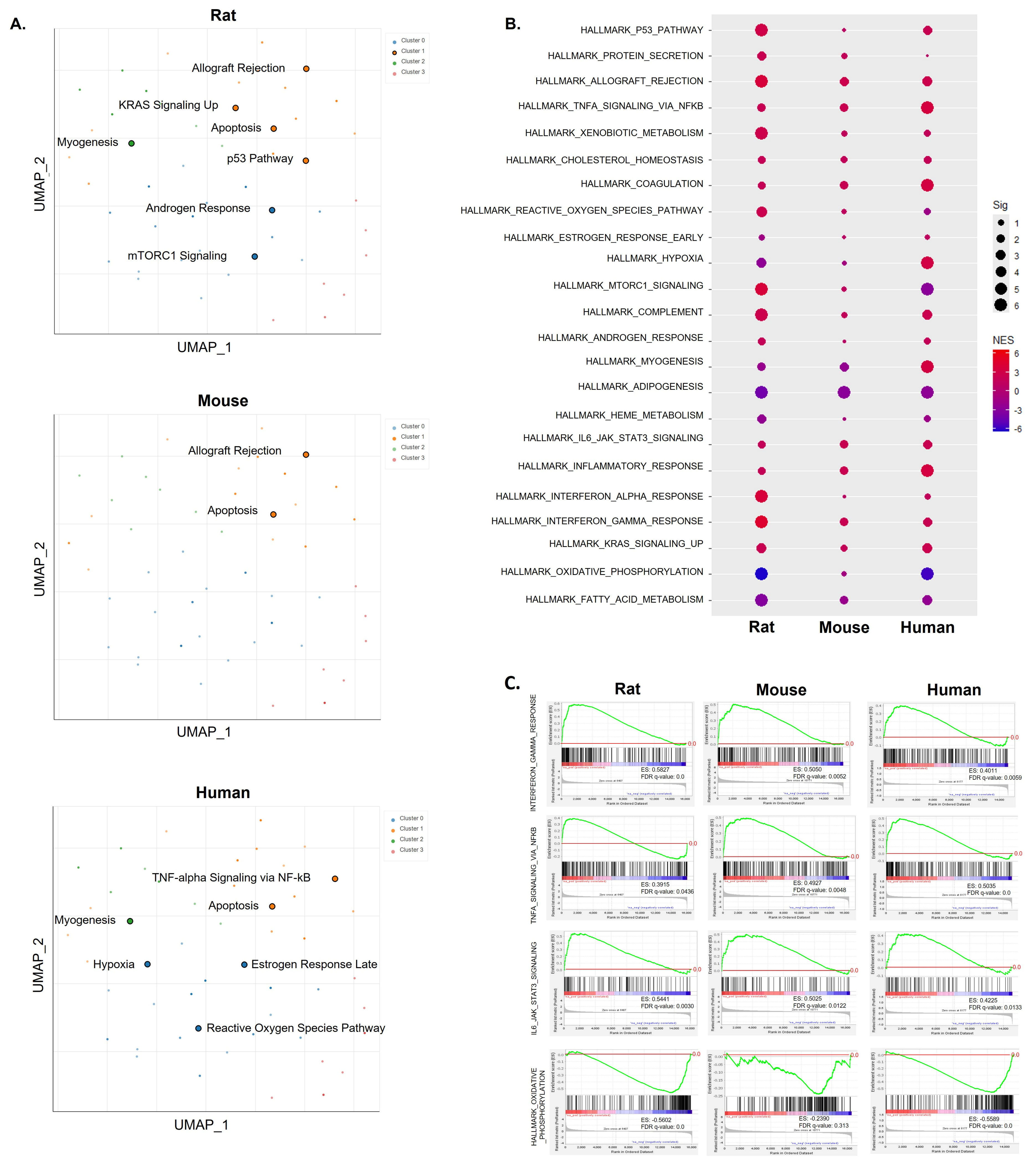
2.1. Common Pathways across Species Based on Hallmark Gene Set Analysis Using DEGs or Ranked Gene Lists
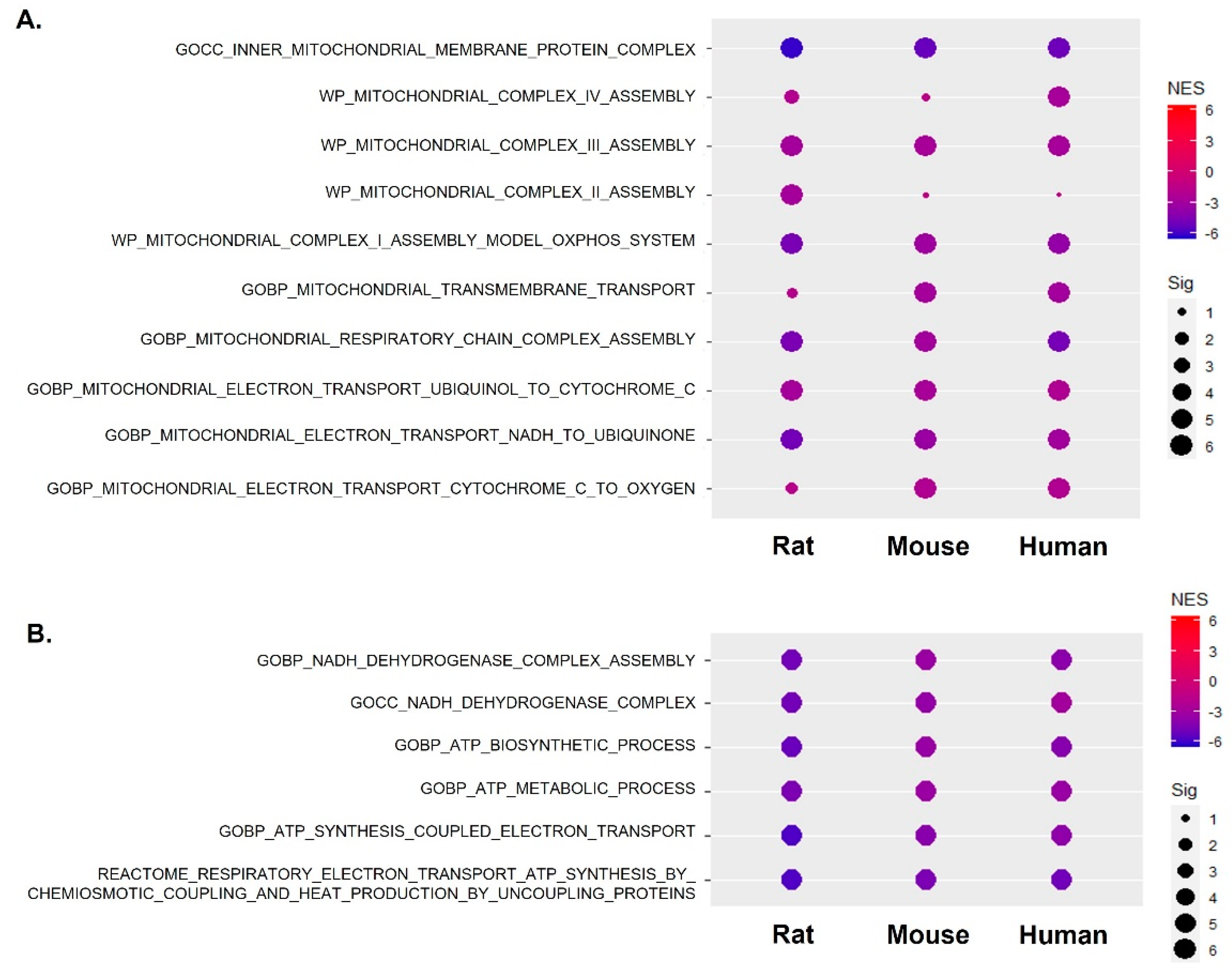
2.2. The Abnormal Metabolic Process Was Linked to Mitochondrial Respiratory Complexes across Species
2.3. Mitochondrial Complex I Activity along with Mitochondrial Gene Expression Was Significantly Suppressed across Species
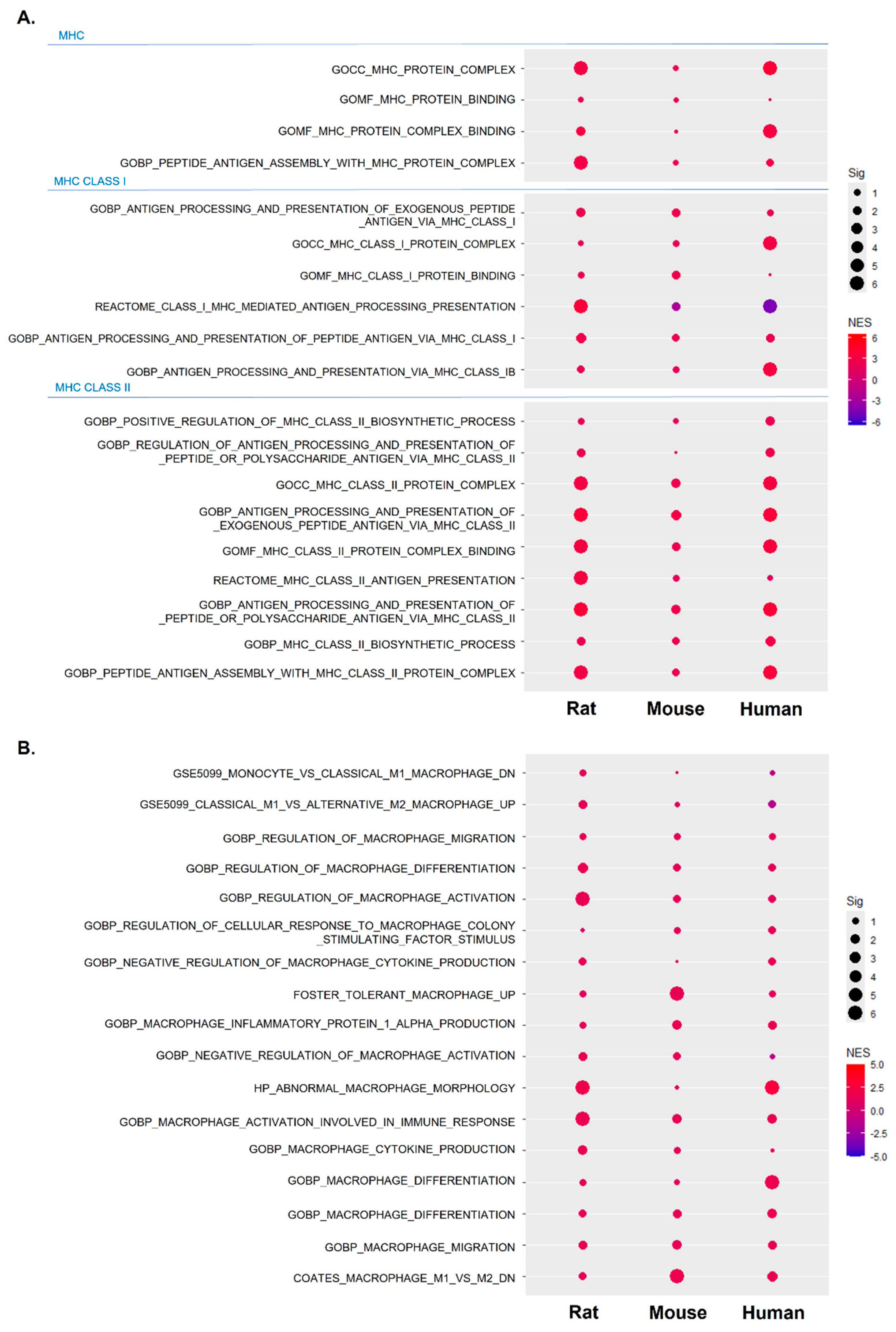
2.4. Inflammatory Responses of IFN-γ, TNF-α, and IL-6 Were Central Factors That Induced Abnormal Inflammatory Responses across Species
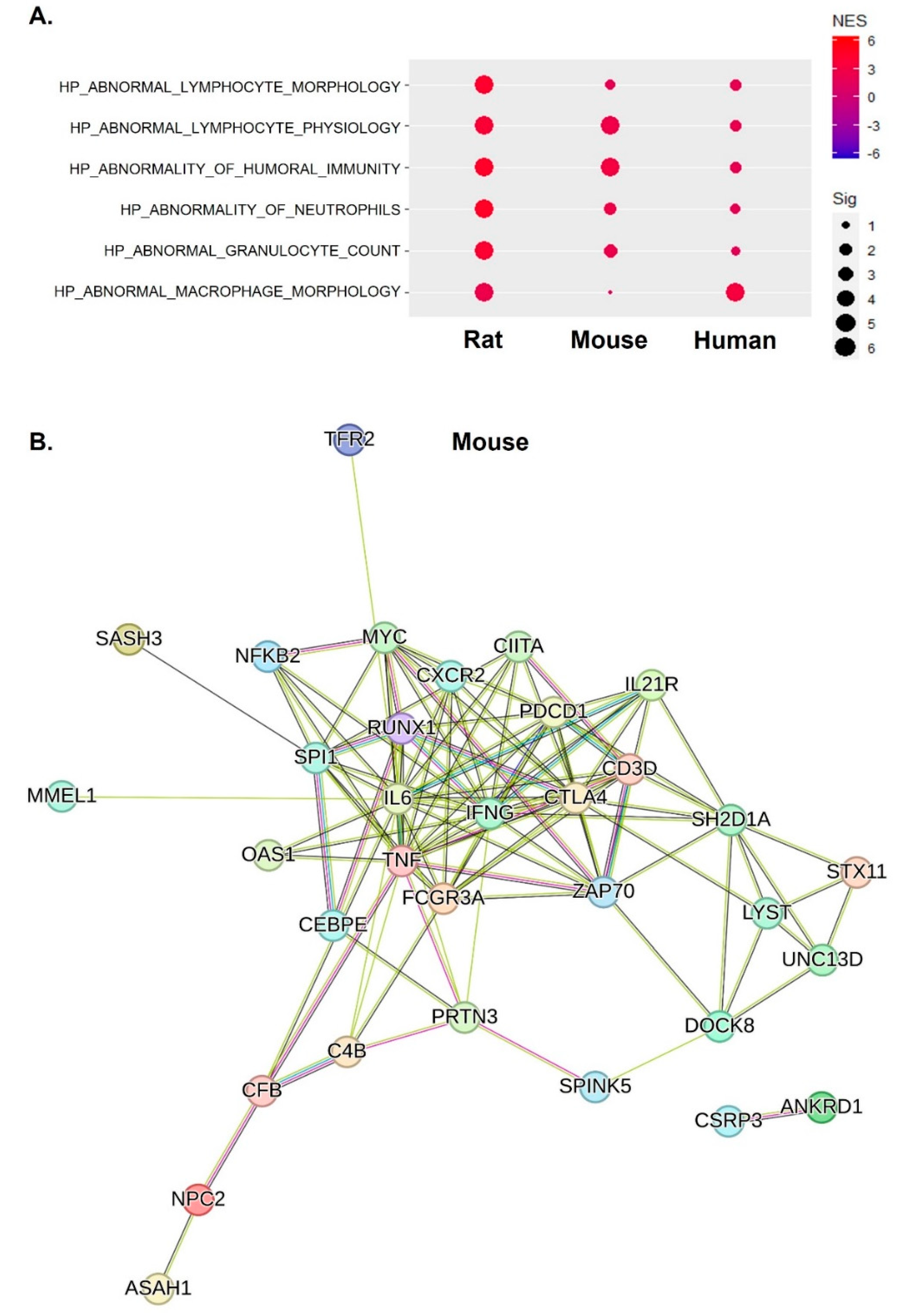
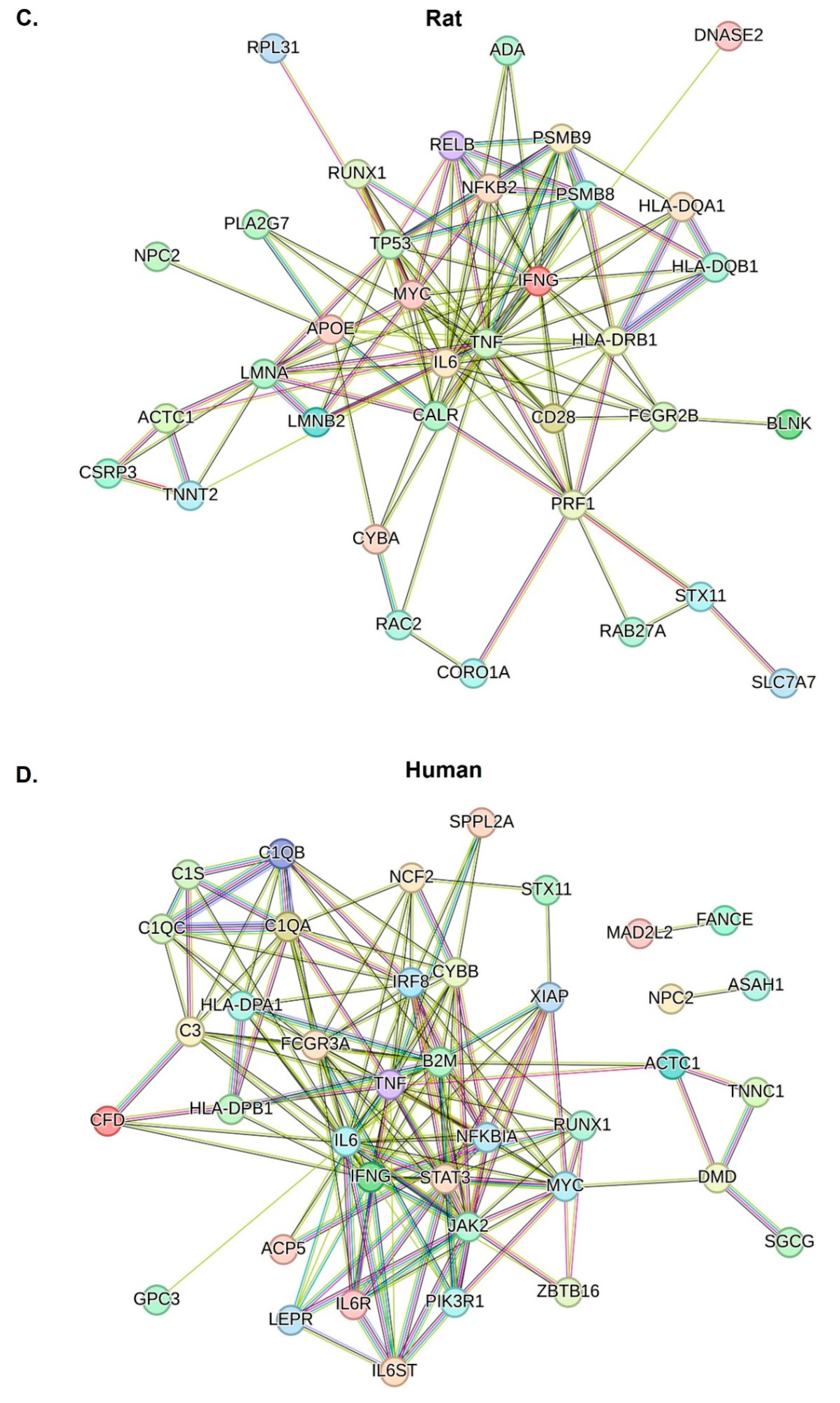
2.5. Increased and Dysregulated Macrophage Function Was Commonly Detectable across Species
3. Discussion and Conclusions
4. Methods
4.1. Gene Expression Data Acquisition
4.2. Metabolome Data Acquisition
4.3. Pathway Analysis
4.4. Data Visualization
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Rasmussen, B.B. Skeletal muscle protein balance and metabolism in the elderly. Curr. Aging Sci. 2011, 4, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Buglio, A.L.; Vendemiale, G. Mitochondrial Impairment in Sarcopenia. Biology 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Lenaz, G.; Bovina, C.; Castelluccio, C.; Fato, R.; Formiggini, G.; Genova, M.L.; Marchetti, M.; Pich, M.M.; Pallotti, F.; Castelli, G.P.; et al. Mitochondrial complex I defects in aging. Mol. Cell. Biochem. 1997, 174, 329–333. [Google Scholar] [CrossRef]
- Stefanatos, R.; Sanz, A. Mitochondrial complex I: A central regulator of the aging process. Cell Cycle 2011, 10, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- McElroy, G.S.; Chakrabarty, R.P.; D'Alessandro, K.B.; Hu, Y.-S.; Vasan, K.; Tan, J.; Stoolman, J.S.; Weinberg, S.E.; Steinert, E.M.; Reyfman, P.A. Reduced expression of mitochondrial complex I subunit Ndufs2 does not impact healthspan in mice. Sci. Rep. 2022, 12, 5196. [Google Scholar] [CrossRef] [PubMed]
- Signorile, A.; Rasmo, D.D. Mitochondrial Complex I, a Possible Sensible Site of cAMP Pathway in Aging. Antioxidants 2023, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.-Y.; Kim, D.K.; Mook-Jung, I. The role of mitochondrial DNA mutation on neurodegenerative diseases. Exp. Mol. Med. 2015, 47, e150. [Google Scholar] [CrossRef] [PubMed]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef]
- Bae, H.R.; Leung, P.S.C.; Tsuneyama, K.; Valencia, J.C.; Hodge, D.L.; Kim, S.; Back, T.; Karwan, M.; Merchant, A.S.; Baba, N.; et al. Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology 2016, 64, 1189–1201. [Google Scholar] [CrossRef]
- Bae, H.R.; Shin, S.-K.; Han, Y.; Yoo, J.-H.; Kim, S.; Young, H.A.; Kwon, E.-Y. D-Allulose Ameliorates Dysregulated Macrophage Function and Mitochondrial NADH Homeostasis, Mitigating Obesity-Induced Insulin Resistance. Nutrients 2023, 5, 4218. [Google Scholar] [CrossRef]
- Bae, H.R.; Shin, S.-K.; Yoo, J.-H.; Kim, S.; Young, H.A.; Kwon, E.-Y. Chronic inflammation in high-fat diet-fed mice: Unveiling the early pathogenic connection between liver and adipose tissue. J. Autoimmun. 2023, 139, 103091. [Google Scholar] [CrossRef]
- Memme, J.M.; Hood, D.A. Molecular Basis for the Therapeutic Effects of Exercise on Mitochondrial Defects. Front. Physiol. 2021, 11, 615038. [Google Scholar] [CrossRef]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Lee, C.-W.; Wu, H.-H.; Lin, W.-T.; Lee, O.K. Immunometabolism of macrophages regulates skeletal muscle regeneration. Front. Cell Dev. Biol. 2022, 10, 948819. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial respiratory complex I: Structure, function and implication in human diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef]
- Cui, C.-Y.; Driscoll, R.K.; Piao, Y.; Chia, C.W.; Gorospe, M.; Ferrucci, L. Skewed macrophage polarization in aging skeletal muscle. Aging Cell 2019, 18, e13032. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, N.; Qiao, B.; Zhang, F.; Wu, J.; Liu, C.; Li, Y.; Du, J. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1291–1305. [Google Scholar] [CrossRef]
- Liu, N.; Butcher, J.T.; Nakano, A.; Campo, A.D. Changes in macrophage immunometabolism as a marker of skeletal muscle dysfunction across the lifespan. Aging 2023, 15, 4035–4050. [Google Scholar] [CrossRef]
- Shin, H.E.; Won, C.W.; Kim, M. Metabolomic profiles to explore biomarkers of severe sarcopenia in older men: A pilot study. Exp. Gerontol. 2022, 167, 111924. [Google Scholar] [CrossRef]
- Uchitomi, R.; Hatazawa, Y.; Senoo, N.; Yoshioka, K.; Fujita, M.; Shimizu, T.; Miura, S.; Ono, Y.; Kamei, Y. Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci. Rep. 2019, 9, 10425. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Shin, S.-K.; Han, J.-W.; Kwon, E.-Y.; Bae, H.R. Cross-Species Studies Reveal That Dysregulated Mitochondrial Gene Expression and Electron Transport Complex I Activity Are Crucial for Sarcopenia. Int. J. Mol. Sci. 2024, 25, 10302. https://doi.org/10.3390/ijms251910302
Lee J-Y, Shin S-K, Han J-W, Kwon E-Y, Bae HR. Cross-Species Studies Reveal That Dysregulated Mitochondrial Gene Expression and Electron Transport Complex I Activity Are Crucial for Sarcopenia. International Journal of Molecular Sciences. 2024; 25(19):10302. https://doi.org/10.3390/ijms251910302
Chicago/Turabian StyleLee, Ji-Yoon, Su-Kyung Shin, Ji-Won Han, Eun-Young Kwon, and Heekyong R. Bae. 2024. "Cross-Species Studies Reveal That Dysregulated Mitochondrial Gene Expression and Electron Transport Complex I Activity Are Crucial for Sarcopenia" International Journal of Molecular Sciences 25, no. 19: 10302. https://doi.org/10.3390/ijms251910302





