The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Assessment of Risk of Bias in Included Studies
3. Results
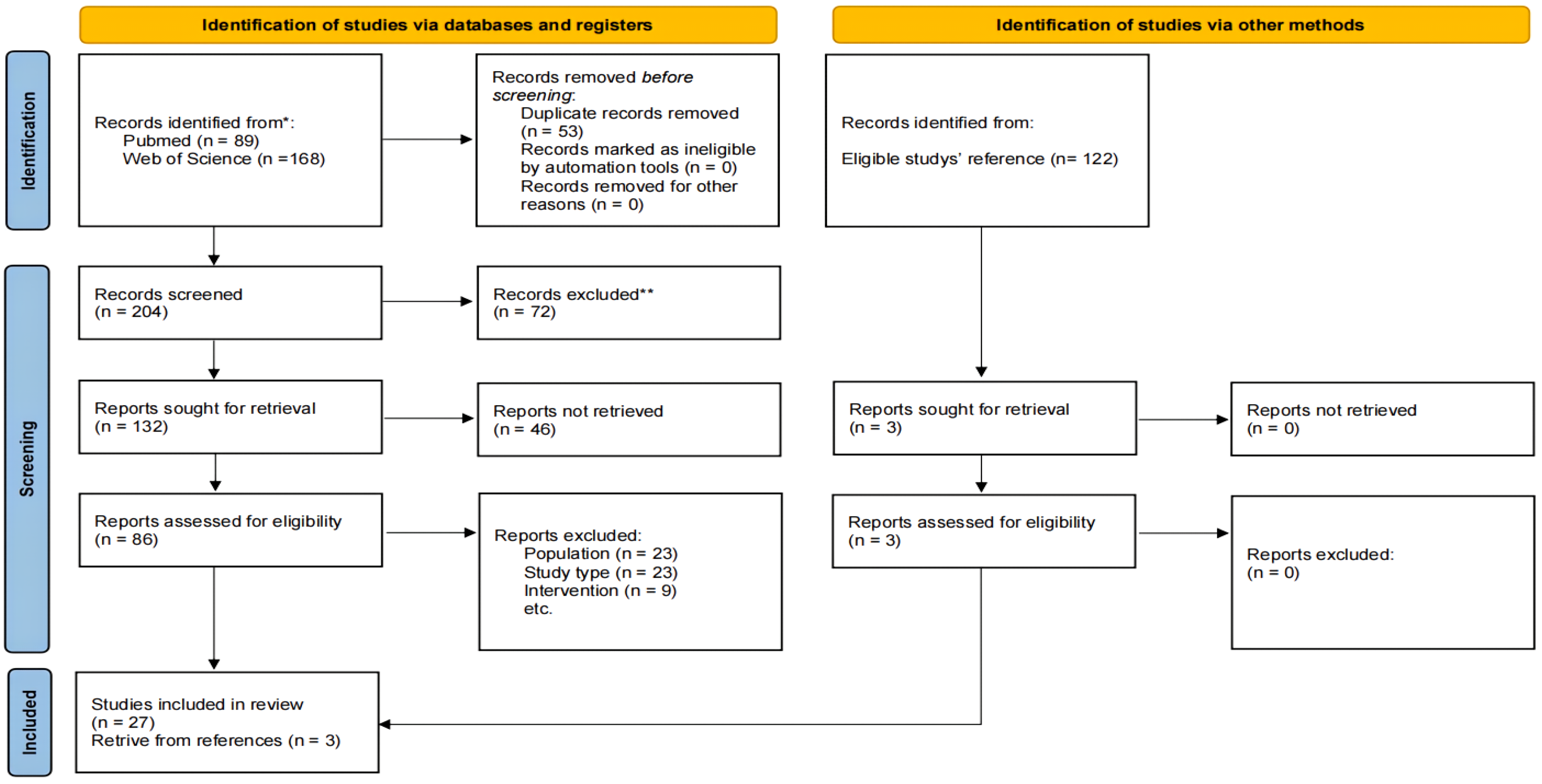
3.1. Search Results
3.2. Immune Checkpoint Inhibitors and Their Role in Melanoma Treatment
3.3. Cell Signaling Inhibitors
3.4. Indirect Kinase Inhibitors
3.5. Other Target Therapies
3.5.1. Engineering Target Drug Systems
3.5.2. MicroRNA Therapies
3.5.3. Old Drug, New Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gillard, M.; Cadieu, E.; De Brito, C.; Abadie, J.; Vergier, B.; Devauchelle, P.; Degorce, F.; Dréano, S.; Primot, A.; Dorso, L.; et al. Naturally occurring melanomas in dogs as models for non-UV pathways of human melanomas. Pigment. Cell Melanoma Res. 2014, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Prouteau, A.; André, C. Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes 2019, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Bastian, B.C.; Michael, H.T.; Webster, J.D.; Prasad, M.L.; Conway, C.M.; Prieto, V.M.; Gary, J.M.; Goldschmidt, M.H.; Esplin, D.G.; et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment. Cell Melanoma Res. 2014, 27, 37–47. [Google Scholar] [CrossRef] [PubMed]
- van der Weyden, L.; Brenn, T.; Patton, E.E.; Wood, G.A.; Adams, D.J. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J. Pathol. 2020, 252, e5505. [Google Scholar] [CrossRef]
- Bongiovanni, L.; Brachelente, C.; Dow, S.; Bergman, P.J. Editorial: Canine melanoma in comparative oncology: Translate research advances to develop new diagnostic and therapeutic options. Front. Vet. Sci. 2022, 9, 1127527. [Google Scholar] [CrossRef]
- Hernández, I.B.; Kromhout, J.Z.; Teske, E.; Hennink, W.E.; van Nimwegen, S.A.; Oliveira, S. Molecular targets for anticancer therapies in companion animals and humans: What can we learn from each other? Theranostics 2021, 11, 3882–3897. [Google Scholar] [CrossRef]
- Hardwick, L. A Comparative View on Molecular Alterations and Potential Therapeutic Strategies for Canine Oral Melanoma. Vet. Sci. 2021, 8, 286. [Google Scholar] [CrossRef]
- Regan, D.; Guth, A.; Coy, J.; Dow, S. Cancer immunotherapy in veterinary medicine: Current options and new developments. Vet. J. 2016, 207, 20–28. [Google Scholar] [CrossRef]
- Maeda, S. Second era of molecular-targeted cancer therapies in dogs. J. Vet. Med. Sci. 2023, 85, 790–798. [Google Scholar] [CrossRef]
- Modiano, J.F.; Ritt, M.G.; Wojcieszyn, J. The Molecular Basis of Canine Melanoma: Pathogenesis and Trends in Diagnosis and Therapy. J. Vet. Intern. Med. 1999, 13, 163–174. [Google Scholar] [CrossRef]
- Hernandez, B.; Adissu, H.A.; Wei, B.-R.; Michael, H.T.; Merlino, G.; Simpson, R.M. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, E394. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Collins, C.J.; Ye, F. Activation of the AKT and mammalian target of rapamycin pathways and the inhibitory effects of rapamycin on those pathways in canine malignant melanoma cell lines. Am. J. Vet. Res. 2009, 70, 263–269. [Google Scholar] [CrossRef]
- Alexander, A.; Huelsmeyer, M.; Mitzey, A.; Dubielzig, R.; Kurzman, I.; MacEwen, E.; Vail, D. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol. Immunother. 2006, 55, 433–442. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Bernabe, L.F.; Barnard, S.; Kisseberth, W.C.; Borgatti, A.; Henson, M.; Wilson, H.; Jensen, K.; Ito, D.; Modiano, J.F.; et al. Preclinical Evaluation of the Novel, Orally Bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in Spontaneous Canine Cancer: Results of a Phase I Study. PLoS ONE 2014, 9, e87585. [Google Scholar] [CrossRef]
- Kuroki, S.; Kobayashi, M.; Tani, H.; Miyamoto, R.; Kurita, S.; Tamura, K.; Ono, K.; Washizu, T.; Bonkobara, M. Selective growth inhibition by suppression of F1Fo ATPase in canine malignant melanoma cell lines. J. Vet. Pharmacol. Ther. 2017, 40, 101–104. [Google Scholar] [CrossRef]
- Inoue, K.; Ohashi, E.; Kadosawa, T.; Hong, S.-H.; Matsunaga, S.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Establishment and Characterization of Four Canine Melanoma Cell Lines. J. Vet. Med. Sci. 2004, 66, 1437–1440. [Google Scholar] [CrossRef]
- Chon, E.; Flanagan, B.; Rodrigues, L.C.d.S.; Piskun, C.; Stein, T.J. 6-Bromoindirubin-3′oxime (BIO) decreases proliferation and migration of canine melanoma cell lines. Vet. J. 2015, 205, 305–312. [Google Scholar] [CrossRef]
- Ohashi, E.; Hong, S.-H.; Takahashi, T.; Nakagawa, T.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Effect of retinoids on growth inhibition of two canine melanoma cell lines. J. Vet. Med. Sci. 2001, 63, 83–86. [Google Scholar] [CrossRef][Green Version]
- Yoshitake, R.; Saeki, K.; Watanabe, M.; Nakaoka, N.; Ong, S.; Hanafusa, M.; Choisunirachon, N.; Fujita, N.; Nishimura, R.; Nakagawa, T. Molecular investigation of the direct anti-tumour effects of nonsteroidal anti-inflammatory drugs in a panel of canine cancer cell lines. Vet. J. 2017, 221, 38–47. [Google Scholar] [CrossRef]
- Ishikawa, T.; Osaki, T.; Sugiura, A.; Tashiro, J.; Warita, T.; Hosaka, Y.Z.; Warita, K. Atorvastatin preferentially inhibits the growth of high ZEB-expressing canine cancer cells. Vet. Comp. Oncol. 2022, 20, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Michael, H.T.; Halsey, C.H.; Peer, C.J.; Adhikari, A.; Dwyer, J.E.; Hoover, S.B.; El Meskini, R.; Kozlov, S.; Ohler, Z.W.; et al. Synergistic targeted inhibition of MEK and dual PI3K/mTOR diminishes viability and inhibits tumor growth of canine melanoma underscoring its utility as a preclinical model for human mucosal melanoma. Pigment. Cell Melanoma Res. 2016, 29, 643–655. [Google Scholar] [CrossRef]
- Breit, M.N.; Kisseberth, W.C.; Bear, M.D.; Landesman, Y.; Kashyap, T.; McCauley, D.; Kauffman, M.G.; Shacham, S.; London, C.A. Biologic activity of the novel orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 against canine melanoma cell lines. BMC Vet. Res. 2014, 10, 160. [Google Scholar] [CrossRef]
- Gao, Y.; Packeiser, E.M.; Wendt, S.; Sekora, A.; Cavalleri, J.M.V.; Pratscher, B.; Alammar, M.; Hühns, M.; Brenig, B.; Junghanss, C.; et al. Cross-Species Comparison of the Pan-RAF Inhibitor LY3009120’s Anti-Tumor Effects in Equine, Canine, and Human Malignant Melanoma Cell Lines. Genes 2024, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Morris, J.S.; McDermott, M.R.; Lichty, B.D. Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol. Immunopathol. 2016, 169, 15–26. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Helfand, S.C.; Soergel, S.A.; Donner, R.L.; Gan, J.; Hank, J.A.; Lindstrom, M.J.; Sondel, P.M. Potential to involve multiple effector cells with human recombinant interleukin-2 and antiganglioside monoclonal antibodies in a canine malignant melanoma immunotherapy model. J. Immunother. Emphas. Tumor. Immunol. 1994, 16, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Topalian, S.L.; Schwartzentruber, D.J.; Weber, J.S.; Parkinson, D.R.; Seipp, C.A.; Einhorn, J.H.; White, D.E. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994, 271, 907–913. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, J.Y.; Lim, H.; Lee, S.H.; Moon, Y.J.; Pyo, H.J.; Ryu, S.E.; Shin, W.; Heo, Y.S. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 2017, 7, 5532. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Nemoto, Y.; Itamoto, K.; Tani, K.; Nakaichi, M.; Sakurai, M.; Sakai, Y.; Noguchi, S.; Kato, M.; Tsukui, T.; et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci. Rep. 2020, 10, 18311. [Google Scholar] [CrossRef] [PubMed]
- Kamoto, S.; Shinada, M.; Kato, D.; Yoshimoto, S.; Ikeda, N.; Tsuboi, M.; Yoshitake, R.; Eto, S.; Hashimoto, Y.; Takahashi, Y.; et al. Phase I/II Clinical Trial of the Anti-Podoplanin Monoclonal Antibody Therapy in Dogs with Malignant Melanoma. Cells 2020, 9, 2529. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Zheng, X.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Qian, Y.; Cui, P.; et al. Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 562315. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumor treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Jalali, S.A.; Mirzaei, H.R.; Grupp, S.A.; Suarez, E.R.; Rapôso, C.; Webster, T.J. CAR-T cells: Early successes in blood cancer and challenges in solid tumors. Acta Pharm. Sin. B 2021, 11, 1129–1147. [Google Scholar] [CrossRef]
- Forsberg, E.M.V.; Riise, R.; Saellström, S.; Karlsson, J.; Alsén, S.; Bucher, V.; Hemminki, A.E.; Bagge, R.O.; Ny, L.; Nilsson, L.M.; et al. Treatment with Anti-HER2 Chimeric Antigen Receptor Tumor-Infiltrating Lymphocytes (CAR-TILs) Is Safe and Associated with Antitumor Efficacy in Mice and Companion Dogs. Cancers 2023, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.S.; Denton, C.L.; Gustafson, D.L. Comparative analysis of MAPK and PI3K/AKT pathway activation and inhibition in human and canine melanoma: Pathway activation and inhibition in human and canine melanoma. Vet. Comp. Oncol. 2015, 13, 288–304. [Google Scholar] [CrossRef]
- Meier, F.; Busch, S.; Lasithiotakis, K.; Kulms, D.; Garbe, C.; Maczey, E.; Herlyn, M.; Schittek, B. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br. J. Dermatol. 2007, 156, 1204–1213. [Google Scholar] [CrossRef]
- Chen, X.; Liu, F.; Song, X.; Wang, Z.; Dong, Z.; Hu, Z.; Lan, R.; Guan, W.; Zhou, T.; Xu, X.; et al. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol. Carcinog. 2010, 49, 603–610. [Google Scholar] [CrossRef]
- Zeiser, R.; Andrlová, H.; Meiss, F. Trametinib (GSK1120212). Recent Results Cancer Res. 2018, 211, 91–100. [Google Scholar] [PubMed]
- Wei, B.R.; Hoover, S.B.; Peer, C.J.; Dwyer, J.E.; Adissu, H.A.; Shankarappa, P.; Yang, H.; Lee, M.; Peat, T.J.; Figg, W.D.; et al. Efficacy, Tolerability, and Pharmacokinetics of Combined Targeted MEK and Dual mTORC1/2 Inhibition in a Preclinical Model of Mucosal Melanoma. Mol. Cancer Ther. 2020, 19, 2308–2318. [Google Scholar] [CrossRef]
- Jang, S.; Strickland, B.; Finis, L.; Kooijman, J.J.; Melis, J.J.T.M.; Zaman, G.J.R.; Van Tornout, J. Comparative biochemical kinase activity analysis identifies rivoceranib as a highly selective VEGFR2 inhibitor. Cancer Chemother. Pharmacol. 2023, 91, 491–499. [Google Scholar] [CrossRef]
- Li, Q.; Kim, Y.-S.; An, J.-H.; Kwon, J.-A.; Han, S.-H.; Song, W.-J.; Youn, H.-Y. Anti-tumor effects of rivoceranib against canine melanoma and mammary gland tumour in vitro and in vivo mouse xenograft models. BMC Vet. Res. 2021, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Miyamoto, R.; Noguchi, S.; Kurita, S.; Nagashima, T.; Michishita, M.; Yayoshi, N.; Tamura, K.; Bonkobara, M. A canine case of malignant melanoma carrying a KIT c.1725_1733del mutation treated with toceranib: A case report and in vitro analysis. BMC Vet. Res. 2021, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Brocca, G.; Poncina, B.; Sammarco, A.; Cavicchioli, L.; Castagnaro, M. KIT Somatic Mutations and Immunohistochemical Expression in Canine Oral Melanoma. Animals 2020, 10, 2370. [Google Scholar] [CrossRef]
- Wood, E.A.; Lu, Z.; Jia, S.; Assumpção, A.L.F.V.; Van Hesteren, M.A.; Huelsmeyer, M.K.; Vail, D.M.; Pan, X. Pevonedistat targeted therapy inhibits canine melanoma cell growth through induction of DNA re-replication and senescence. Vet. Comp. Oncol. 2020, 18, 269–280. [Google Scholar] [CrossRef]
- Bernard, S.; Poon, A.C.; Tam, P.M.; Mutsaers, A.J. Investigation of the effects of mTOR inhibitors rapamycin and everolimus in combination with carboplatin on canine malignant melanoma cells. BMC Vet. Res. 2021, 17, 382. [Google Scholar] [CrossRef]
- Youssef, M.E.; Cavalu, S.; Hasan, A.M.; Yahya, G.; Abd-Eldayem, M.A.; Saber, S. Role of Ganetespib, an HSP90 Inhibitor, in Cancer Therapy: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2023, 24, 5014. [Google Scholar] [CrossRef]
- London, C.A.; Bear, M.D.; McCleese, J.; Foley, K.P.; Paalangara, R.; Inoue, T.; Ying, W.; Barsoum, J. Phase I evaluation of STA-1474, a prodrug of the novel HSP90 inhibitor ganetespib, in dogs with spontaneous cancer. PLoS ONE 2011, 6, e27018. [Google Scholar] [CrossRef]
- Tobin, S.J.; Chang, H.; Kent, M.S.; Davies, A.E. JARID1-targeted histone H3 demethylase inhibitors exhibit anti-proliferative activity and overcome cisplatin resistance in canine oral melanoma cell lines. Vet. Comp. Oncol. 2021, 19, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, T.; Yu, B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct. Target. Ther. 2023, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Hanazono, K.; Fu, D.-R.; Endo, Y.; Kadosawa, T.; Iwano, H.; Uchide, T. A new treatment for human malignant melanoma targeting L-type amino acid transporter 1 (LAT1): A pilot study in a canine model. Biochem. Biophys. Res. Commun. 2013, 439, 103–108. [Google Scholar] [CrossRef]
- Sato, A.; da Fonseca, I.I.M.; Nagamine, M.K.; de Toledo, G.F.; Olio, R.; Hernandez-Blazquez, F.J.; Yano, T.; Yeh, E.S.; Dagli, M.L.Z. Effects of Alpha-Connexin Carboxyl-Terminal Peptide (aCT1) and Bowman-Birk Protease Inhibitor (BBI) on Canine Oral Mucosal Melanoma (OMM) Cells. Front. Vet. Sci. 2021, 8, 670451. [Google Scholar] [CrossRef] [PubMed]
- Rozanov, D.V.; Golubkov, V.S.; Strongin, A.Y. Membrane type-1 matrix metalloproteinase (MT1-MMP) protects malignant cells from tumoricidal activity of re-engineered anthrax lethal toxin. Int. J. Biochem. Cell Biol. 2005, 37, 142–154. [Google Scholar] [CrossRef]
- Nishiya, A.T.; Massoco, C.O.; Felizzola, C.R.; Perlmann, E.; Batschinski, K.; Tedardi, M.V.; Garcia, J.S.; Mendonça, P.P.; Teixeira, T.F.; Dagli, M.L.Z. Comparative Aspects of Canine Melanoma. Vet. Sci. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Cejalvo, T.; Perisé-Barrios, A.J.; del Portillo, I.; Laborda, E.; Rodriguez-Milla, M.A.; Cubillo, I.; Vázquez, F.; Sardón, D.; Ramirez, M.; Alemany, R.; et al. Remission of Spontaneous Canine Tumors after Systemic Cellular Viroimmunotherapy. Cancer Res. 2018, 78, 4891–4901. [Google Scholar] [CrossRef]
- Laborda, E.; Puig-Saus, C.; Rodriguez-García, A.; Moreno, R.; Cascalló, M.; Pastor, J.; Alemany, R. A pRb-responsive, RGD-modified, and Hyaluronidase-armed Canine Oncolytic Adenovirus for Application in Veterinary Oncology. Mol. Ther. 2014, 22, 986–998. [Google Scholar] [CrossRef]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef]
- Ahn, J.O.; Lee, H.W.; Seo, K.W.; Kang, S.K.; Ra, J.C.; Youn, H.Y. Anti-tumor effect of adipose tissue derived-mesenchymal stem cells expressing interferon-β and treatment with cisplatin in a xenograft mouse model for canine melanoma. PLoS ONE 2013, 8, e74897. [Google Scholar] [CrossRef]
- Sell, M.C.; Ramlogan-Steel, C.A.; Steel, J.C.; Dhungel, B.P. MicroRNAs in cancer metastasis: Biological and therapeutic implications. Expert Rev. Mol. Med. 2023, 25, e14. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, R.; Mori, T.; Noguchi, S.; Akao, Y.; Maruo, K.; Kitade, Y. Synthetic microRNA-205 exhibited tumour suppression in spontaneous canine malignant melanoma by intratumoral injection. Vet. Comp. Oncol. 2019, 17, 407–412. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Inoue, J.; Iwasaki, R.; Terauchi, M.; Fujii, Y.; Ohta, M.; Hasegawa, T.; Mizuno, R.; Mori, T.; Inazawa, J. Therapeutic applications of local injection of hsa-miR-634 into canine spontaneous malignant melanoma tumors. Cancer Gene Ther. 2023, 30, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Noguchi, S.; Nishiyama, Y.; Matsuyama, S.; Mori, T.; Igase, M.; Mizuno, T.; Shimamura, S.; Shimada, T. MicroRNA-205 enhances the radiosensitivity of canine oral melanoma cells by inhibiting E2F1. Jpn. J. Vet. Res. 2019, 67, 151–161. [Google Scholar] [CrossRef]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef] [PubMed]
- Nishiya, A.T.; Nagamine, M.K.; da Fonseca, I.I.M.; Miraldo, A.C.; Scattone, N.V.; Guerra, J.L.; Xavier, J.G.; Santos, M.; Gomes, C.O.M.d.S.; Ward, J.M.; et al. Inhibitory Effects of a Reengineered Anthrax Toxin on Canine Oral Mucosal Melanomas. Toxins 2020, 12, 157. [Google Scholar] [CrossRef]
- Stevenson, V.B.; Perry, S.N.; Todd, M.; Huckle, W.R.; LeRoith, T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet. Pathol. 2021, 58, 692–698. [Google Scholar] [CrossRef]
- Chan, T.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Alsaihati, B.A.; Ho, K.-L.; Watson, J.; Feng, Y.; Wang, T.; Dobbin, K.K.; Zhao, S. Canine tumor mutational burden is correlated with TP53 mutation across tumor types and breeds. Nat. Commun. 2021, 12, 4670. [Google Scholar] [CrossRef]
- Utsugi, S.; Ogihara, K.; Naya, Y.; Sunden, Y.; Nakamoto, Y.; Okamoto, Y. Expression of L-type amino acid transporter 1 in canine and feline intracranial tumors. J. Vet. Med. Sci. 2022, 84, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Lisjak, A.; Lopes, B.C.; Pilla, R.; Nemec, A.; Suchodolski, J.S.; Tozon, N. A Comparison of the Oral Microbiota in Healthy Dogs and Dogs with Oral Tumors. Animals 2023, 13, 3594. [Google Scholar] [CrossRef] [PubMed]
- Kleber, K.T.; Iranpur, K.R.; Perry, L.M.; Cruz, S.M.; Razmara, A.M.; Culp, W.T.N.; Kent, M.S.; Eisen, J.A.; Rebhun, R.B.; Canter, R.J. Using the canine microbiome to bridge translation of cancer immunotherapy from pre-clinical murine models to human clinical trials. Front. Immunol. 2022, 13, 983344. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | Origin | Molecular Mutation Type | Ref. |
|---|---|---|---|
| 12 | Primary tumors of COM | Not mentioned | [13] |
| 23 | Primary tumors of COM | ||
| 50 | Primary tumors of COM | ||
| 17CM98 | Lymph node metastasis of COM | Not mentioned | [14] |
| 323610-3 | Not mentioned | BRAFWT | [15] |
| ChMC | COM | Not mentioned | [16] |
| CMeC-1 | Primary canine skin melanoma | Not mentioned | [17] |
| CMeC-2 | CMeC-1 Xenotransplantation lung metastasis | ||
| CML-1 | Primary COM | Not mentioned | [18] |
| CML-2 | Primary COM | ||
| CML-10C2 | Canine cutaneous melanoma | ||
| CML-6M | Lymph node metastasis of COM | ||
| CMM-1 | Primary oral melanoma from canine with lymph node metastasis | Not mentioned | [19] |
| CMM-2 | Primary COM with no metastasis | ||
| CMM7 | Not mentioned | Not mentioned | [20] |
| CMM8 | Not mentioned | ||
| CMM9 | Not mentioned | ||
| CMM10 | Not mentioned | ||
| CMM11 | Not mentioned | ||
| CMM12 | Not mentioned | ||
| HTR | Not mentioned | Not mentioned | [21] |
| ITP | Not mentioned | ||
| KMeC | Primary canine oral melanoma | Not mentioned | [17] |
| LMeC | Lymph node metastasis of COM | ||
| NIP | COM | Not mentioned | [16] |
| NML | Canine nail bed melanoma | ||
| OMJ | COM | Not mentioned | |
| PuMeC | Not mentioned | Not mentioned | [21] |
| UCDK9M1 | Skin metastasis of COM | NRASWT | [22] |
| UCDK9M2 | Lymph node metastasis COM | NRASWT | |
| UCDK9M3 | Primary COM | NRASWT | |
| UCDK9M4 | Primary COM | NRASWT | |
| UCDK9M5 | Lymph node metastasis of COM | NRAS Q61 mutation | |
| Mel 23 | primary COM | Not mentioned | [23] |
| Mel 36 | Metastatic lymph node | ||
| Mel 69 | primary COM | ||
| Mel 83 | primary COM | ||
| YCC | Not mentioned | Not mentioned | [21] |
| Jones | Primary tumor, COM | NRAS Q61 mutation | [22] |
| Parks | Primary tumor, COM | Not mentioned | |
| cRGO1 | Primary tumor, COM | BRAFWT, KRASWT, NRASG13R | [24] |
| cRGO1.2 | Lymph node metastasis of cRGO1 primary tumor, resistant to irradiation (0–6 Gy) | ||
| cRGO4 | Primary tumor, COM | BRAFWT, NRASWT, KRASWT | |
| cRGO6 | Cutaneous metastasis of COM | ||
| Mel1268 | Primary tumor, COM | ||
| Mel0910 | Primary cutaneous malignant melanoma | ||
| TLM1 | Primary tumor, COM | Not mentioned | [10] |
| Immunotherapeutic Strategy | Study Subject | Study Outcome | Ref. |
|---|---|---|---|
| Anti-ganglioside monoclonal antibodies: Murine Mab 14.G2a and its mouse-human chimera | In vitro experiment CML-2, CML-6M | CML-6M: mAbs 14.G2a (anti-GD2) and R24 (anti-GD3) mediate ADCC against the cell line. Combination with IL-2 enhanced the cytotoxicity. CML-2: no significant expression of GD2 or GD3, and hence, it was not responsive to the antibodies. | [27] |
| Immune Checkpoint Inhibition: Canine chimeric monoclonal antibody | A pilot clinical trial of seven dogs with OMM: stage II × 1; stage III × 2; stage IV × 4 | PR: 1/7 (stage II × 1) PD: 6/7 (stage III × 2; stage IV × 4) | [31] |
| Immune Checkpoint Inhibition: Anti-Podoplanin monoclonal antibody | Phase I/II clinical trial of three dogs with oral melanoma (stage I, stage III, and stage IV) with endogenously expressed canine podoplanin | SD: 1 case (stage I) PD: 2 cases (stage III, and stage IV) No severe adverse effects were observed. | [33] |
| Immune Checkpoint Inhibition: Anti-canine PD-1 monoclonal antibody | Pre-clinical study of 21 cases of OMM: 4 cases of Stage III and 17 cases of Stage IV | PR: 2 cases (Stage IV) SD: 5 cases (Stage IV) PD: 14 cases (Stage III × 4, Stage IV × 10) | [32] |
| CAR-TILs targeting HER2 | Pre-clinical trial of two dogs with HER2-positive aggressive melanoma. One dog with OMM (stage IV) and one dog with subungual melanoma (stage IV) | The dog with OMM experienced PD. The dog with subungual melanoma did not experience tumor recurrence within the follow-up period of the study and maintained a tumor-free status one-year post-treatment. | [37] |
| Inhibitor and Mechanism | Study Subject | Response Rate | Ref. |
|---|---|---|---|
| Rapamycin: Target cell signaling pathways mTOR | 12, 23, and 50 | 1. Decreases in phosphorylated mTOR expression and phosphorylated p70S6K expression. 2. Dose-dependent decrease in surviving tumor cell fraction.
| [13] |
| AZD6244: Selectively non-ATP competitive inhibitor of MEK1/2. Combine with rapamycin: Inhibitor of mTOR | CML-10C2, CML-6 M, 17CM98, Jones, Parks | 1. Canine melanoma cells exhibited sensitivity to AZD6244 and rapamycin.
2. The combination of AZD6244 and rapamycin demonstrated enhanced efficacy in reducing cell viability, evidenced by a greater reduction in IC50 values and more pronounced effects on inducing cell cycle arrest and apoptosis in melanoma cells. | [38] |
| GSK1120212: MEK inhibitor NVP-BEZ235: PI3K/mTOR inhibitor | UCDK9M1, UCDK9M2, UCDK9M3, UCDK9M4, UCDK9M5, Jones; Female nude mice with UCDK9M5 xenografts | 1. The drug combination synergistically reduced cell survival by activating caspase 3/7, altering cell cycle and Bcl-2 protein expression and inducing apoptosis. 2. In vivo, the drug combination targeted signaling pathways, enhancing the reduction of mediators p-ERK, p-AKT, p-S6, and 4E-BP1. | [22] |
| Trametibin: MEK inhibitor Sapanisertib: Inhibits both mTORC1 and mTORC2 complexes | UCDK9M1, CDK9M2, UCDK9M3, CDK9M5, Jones; Nude mice with UCDK9M5 and UCDK9M1 xenografts | 1. Trametinib IC50 ~ 10 nmol/L; 2. Sapanisertib IC50 10 ~ 100 nmol/L; 3. The combined in vitro therapy enhanced cytotoxicity (produced apoptosis and altered the cell cycle). 4. The combined in vivo therapy limited primary mucosal melanoma xenograft growth in mice and tumor dissemination in a metastasis model. | [42] |
| Rapamycin and everolimus, inhibitors of mTOR, synergize with platinum chemotherapy | CML-1, CML-6M, CML-10c2, 17CM98 | 1. Rapamycin or everolimus combined with carboplatin showed a synergistic reduction in cell viability. 2. Phosphorylated mTOR levels were reduced by rapamycin and everolimus in all cell lines. 3. Both mTOR inhibitors decreased the extracellular acidification rate, indicating reduced glycolytic activity in melanoma cells. | [48] |
| LY3009120: Binding to all RAF isoforms, inhibiting their activity and suppressing downstream signaling | cRGO1, cRGO1.2, Mel1268, Mel0910, cRGO4, cRGO6 | LY3009120 at the concentrations of 5 or 10 µM, cRGO1, cRGO1.2, cRGO4, cRGO6 Mel1268, and Mel0910 showed decreased cell proliferation by 90.58%, 80.09%, 94.82%, 93.52%, and 61.91%, respectively. | [24] |
| Toceranib: Tyrosine kinase inhibitor targeting KIT, PDGFR, VEGFR, and others | A dog with KIT mutation (c.1725_1733del) | The dog received toceranib orally every other day at a dosage of 2.6–2.9 mg/kg. Clinical signs such as halitosis, tumor hemorrhage, trismus, and facial edema improved, and the size of the metastatic lymph node reduced significantly by Day 15. The gingival tumor and associated masses in the masseter and pterygoid muscles decreased in size by Day 29 of treatment. However, toceranib treatment was terminated on Day 43 due to disease progression, and the dog died on Day 54. | [45] |
| Rivoceranib: VEGFR2 inhibitor | LMeC and LMeC cell-xenografted mice | 1. Rivoceranib induces G0/G1 cell cycle arrest through the downregulation of cyclin-D1, thereby inhibiting cell cycle progression in LMeC; inhibits the mobility of canine tumor cell lines in vitro in a dose-dependent manner; suppresses tumor growth in xenograft mouse models. 2. Rivoceranib probably inhibited cyclin-D1 and downregulated VEGFR2 phosphorylation to reduce canine cell line viability in vivo. Rivoceranib showed apoptotic and anti-angiogenic activity in vivo. | [44] |
| BIO: Serine/threonine kinase inhibitors targeting GSK-3β. | CML-10C2, UCDK9M2, UCDK9M3 | 1. BIO treatment at 5 μM for 72 h enhanced β-catenin-mediated transcriptional activity, indicating GSK-3β inhibition. 2. Reduced cell proliferation and migration were observed. | [18] |
| MLN4924: NAE inhibitor interferes with the function of specific proteins by blocking neddylation system | CML-1, CML-6M, 17CM98, CML-10C2 | 1. MLN4924 efficiently decreases the viability of CMM cell lines CML-1, CML-6 M, and CML-10C2 in vitro. 2. Canine melanoma cell line 17CM98 showed relative insensitivity to MLN4924 treatment. | [47] |
| Protease/Kinase Inhibitor | Study Subject | Response Rate | Ref. |
|---|---|---|---|
| STA-1474 | Phase I clinical trial (dog with OMM) | During the 4th STA-1474 treatment cycle, extravasation altered drug pharmacokinetics. A significant oral mass reduction was observed after 7 days, and a CT scan confirmed a partial response. | [50] |
| BCH and LPM | Cell lines (CMeC-1, CMeC-2, LMeC, PuMeC, KmeC) | 1. BCH and LPM inhibited cell growth of CMeC-1 in a dose-dependent manner. IC50 values for BCH or LPM were 43 ± 3.9 mM and 1.7 ± 0.19 μM, respectively. 2. LAT1 inhibitors enhance the inhibitory activities for cell growth produced by conventional anti-cancer drugs. | [53] |
| Verdinexor (KPT-335) | Cell lines (Mel 23, Mel 36, Mel 69, Mel 83) | 1. KPT-335 inhibited proliferation, blocked colony formation, and induced apoptosis at physiologically relevant drug concentrations. 2. Downregulation of the XPO1 protein while inducing an increase in XPO1 mRNA. 3. Upregulated expression of tumor suppressor proteins p53 and p21, promoting their nuclear localization. | [23] |
| Verdinexor (KPT-335) | Cell line (323610-3) | SINE compounds inhibited growth and induced apoptosis against melanoma cell lines, as evidenced by a 70 nM IC50 value for the 323610-3 melanoma cell line. | [15] |
| Oligomycin A (F1Fo ATPase inhibitor) | Cell lines (CMM-1, CMM-2, ChMC, NIP, NML, OMJ) | Oligomycin A showed potent cell growth inhibition activity against CMM-1, ChMC, and NIP, with IC50 values of 5.3 × 10−4–1.7 × 10−3 μM; NML, CMM-2, and OMJ were resistant to oligomycin A, with IC50 values of 5.0–10.5 μM. | [16] |
| HDIs | Cell lines (UCDK9M2, UCDK9M4) | 1. JARID1-targeted HDIs significantly reduced cell survival without affecting DNA damage repair kinetics and were effective against cisplatin-resistant cell lines. 2. HDIs delay the resolution of DNA damage markers, such as p53BP1 and phosphorylated γ-H2AX, suggesting a delay in DNA repair kinetics. | [51] |
| ACT1 and BBI | Cell line (TLM1) | 1. ACT1 alone did not significantly decrease cell viability. 2. BBI alone significantly decreased cell viability at a concentration of 400 µg/mL. 3. The combined treatment of aCT1 (200 µM) and BBI (400 µg/mL) significantly decreased cell viability more than either treatment alone. | [54] |
| Drug | Study Subject | Response Rate | Ref. |
|---|---|---|---|
| cAT-MSC-IFN-β | Cell line (LMeC) and BALB/c nude mouse xenografts | 1. In vitro efficiency: cAT-MSC-IFN-β can directly inhibit the growth of LMeC as compared to control (67.76% of control growth, p < 0.05). 2. LMeC cells co-cultured with cAT-MSC-IFN-β showed increases in the G0/G1 phase of the cell cycle compared to the controls (p < 0.01). G1 arrest occurred concurrently with a reduction in the percentage of S phase cells (p < 0.01 and p < 0.001). 3. In vivo efficiency: In BALB/c nude mouse xenografts with LMeC cells, cAT-MSC-IFN-β and low-dose cisplatin significantly reduced tumor volume compared to other groups. TUNEL assay confirmed apoptosis at the tumor site, showing the combination induced cell death. 4. Homing of cAT-MSC-IFN-β: Fluorescent microscopy analysis showed homing of cAT-MSC-IFN-β to the tumor site, implying that these modified stem cells were able to target the tumor microenvironment effectively. | [60] |
| Hyaluronidase-armed canine oncolytic adenovirus | Cell lines (17CM98, CML1) and CML1 xenograft model in nude mice | In vivo experiment: intratumoral treatment with ICOCAV17 reduced tumor volume five-fold compared to the PBS control and 2.7-fold compared to CAV2 at the end of the experiment, with prolonged survival. | [58] |
| Bacillus anthracis (anthrax) toxin | Five dogs with spontaneous OMM | 1. In vivo: no disease progression; 4 dogs showed tumor reduction varying from 12% to 63%. One dog showed a 20% increase in the tumor due to local edema. 2. Histopathology showed necrosis of tumor cells and blood vessel walls after treatment. 3. No significant systemic side effects were noted. | [67] |
| microRNA-205BP/S3 | Ten dogs with CMM: stage I × 2; stage II × 4; stage III × 3; stage IV × 1 | CR: 5/10 (I × 1, II × 3, III × 1) SD: 3/10 (I × 1, III × 2) PD: 2/10 (II × 1, IV × 1) No side effects were observed. Median overall survival: 340 days (n = 7). Median progression-free survival: 105 days (n = 6). | [62] |
| microRNA-634 | Cell lines (KMeC, CMM1, CMeC-1) | 1. miR-205 in canine melanoma cells heightened their radiation sensitivity, an effect linked to the suppressed activity of E2 transcription factor 1 (E2F1)-ATM signaling, which was confirmed by E2F1 knockdown and ATM knockout in the cell lines. 2. miR-205 overexpression led to a decrease in the expression of E2F1 and ATM, which are involved in the DNA damage response pathway. | [64] |
| microRNA-634 | Seven dogs with spontaneous CMM | 1. PR: 3 lesions; SD: 2 lesions; PD: 3 lesions. 2. The median treatment period was 53 d (range 23-652 d). miRNA was administered a median of seven times (range 4-76 times) at 2-10 nmol per dose. 3. Local administration to lung metastasis under ultrasound guidance induced tumor shrinkage. | [63] |
| Nonsteroidal Anti-Inflammatory Drugs | Cell lines (KMeC, LMeC, Mi, Pu, C1, C2, CMM7, CMM8, CMM9, MM10, CMM11, CMM12) | 1. NSAIDs exhibited anti-tumor effects only at high concentrations, suggesting their action might be mediated through COX/PG-independent pathways. 2. There was no strong correlation between the sensitivity to NSAIDs and the expression of COX-related molecules. 3. Transcriptome analysis of a melanoma cell line exposed to NSAIDs identified novel candidate genes potentially involved in the anti-tumor effects of NSAIDs, indicating that these effects might be mediated through mechanisms independent of COX inhibition. | [20] |
| Atorvastatin | Cell lines (YCC, ITP, HTR) | 1. Atorvastatin could potentially inhibit the growth of melanoma cells, especially those with high ZEB expression and mesenchymal-like properties. 2. The IC50 values for melanoma cell lines treated with atorvastatin ranged from 5.92 to 9.56 μM at 48 h, indicating a varying level of sensitivity among the melanoma cell lines tested. | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Gao, Y.; Deng, Y.; He, J.; Nolte, I.; Murua Escobar, H.; Yu, F. The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports. Int. J. Mol. Sci. 2024, 25, 10387. https://doi.org/10.3390/ijms251910387
He X, Gao Y, Deng Y, He J, Nolte I, Murua Escobar H, Yu F. The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports. International Journal of Molecular Sciences. 2024; 25(19):10387. https://doi.org/10.3390/ijms251910387
Chicago/Turabian StyleHe, Xiaohui, Yu Gao, Yuqing Deng, Junying He, Ingo Nolte, Hugo Murua Escobar, and Feng Yu. 2024. "The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports" International Journal of Molecular Sciences 25, no. 19: 10387. https://doi.org/10.3390/ijms251910387
APA StyleHe, X., Gao, Y., Deng, Y., He, J., Nolte, I., Murua Escobar, H., & Yu, F. (2024). The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports. International Journal of Molecular Sciences, 25(19), 10387. https://doi.org/10.3390/ijms251910387






