Hot Spots of Site-Specific Integration into the Sinorhizobium meliloti Chromosome
Abstract
1. Introduction
2. Results
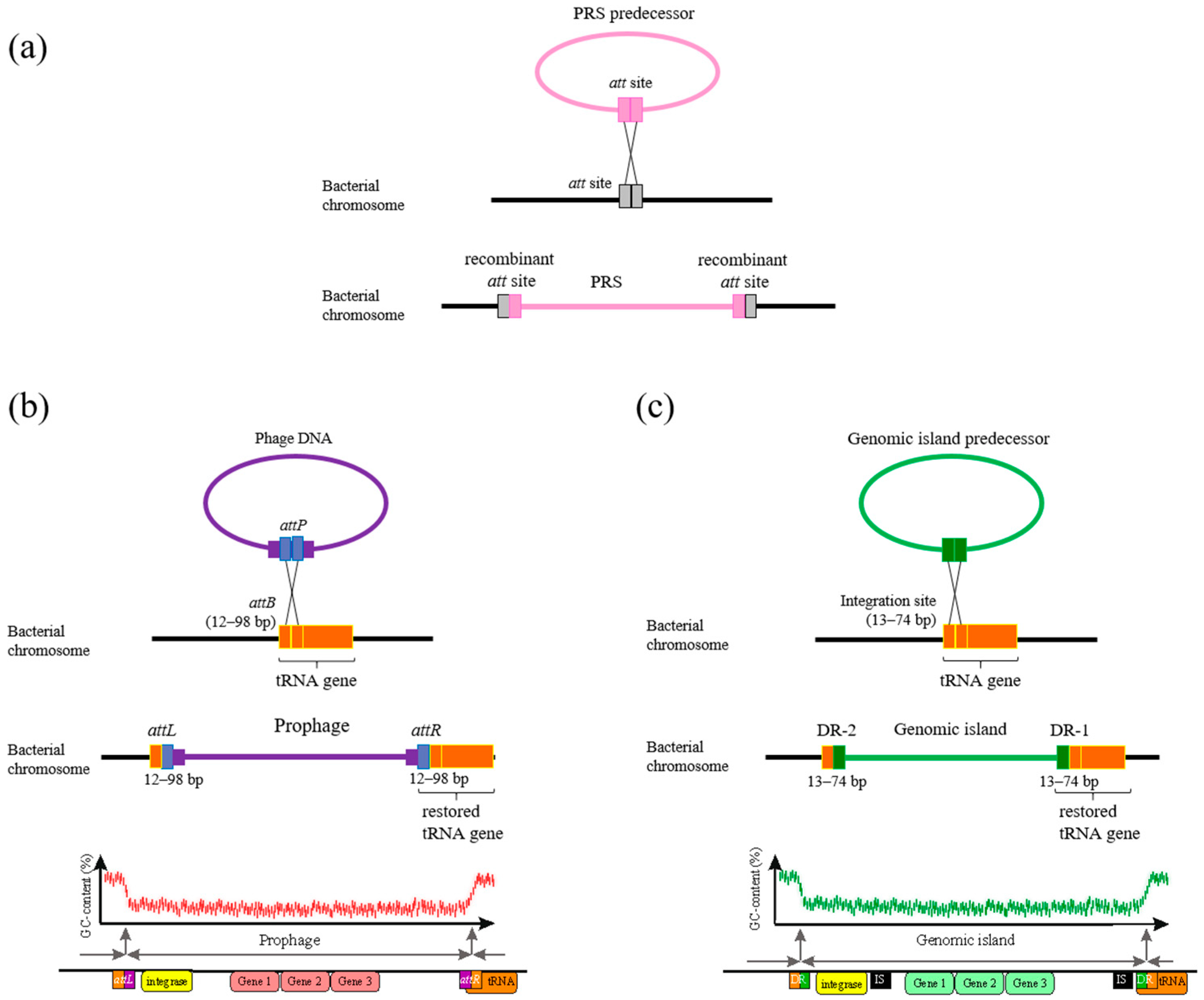
2.1. Phage-Related Sequences (PRSs)
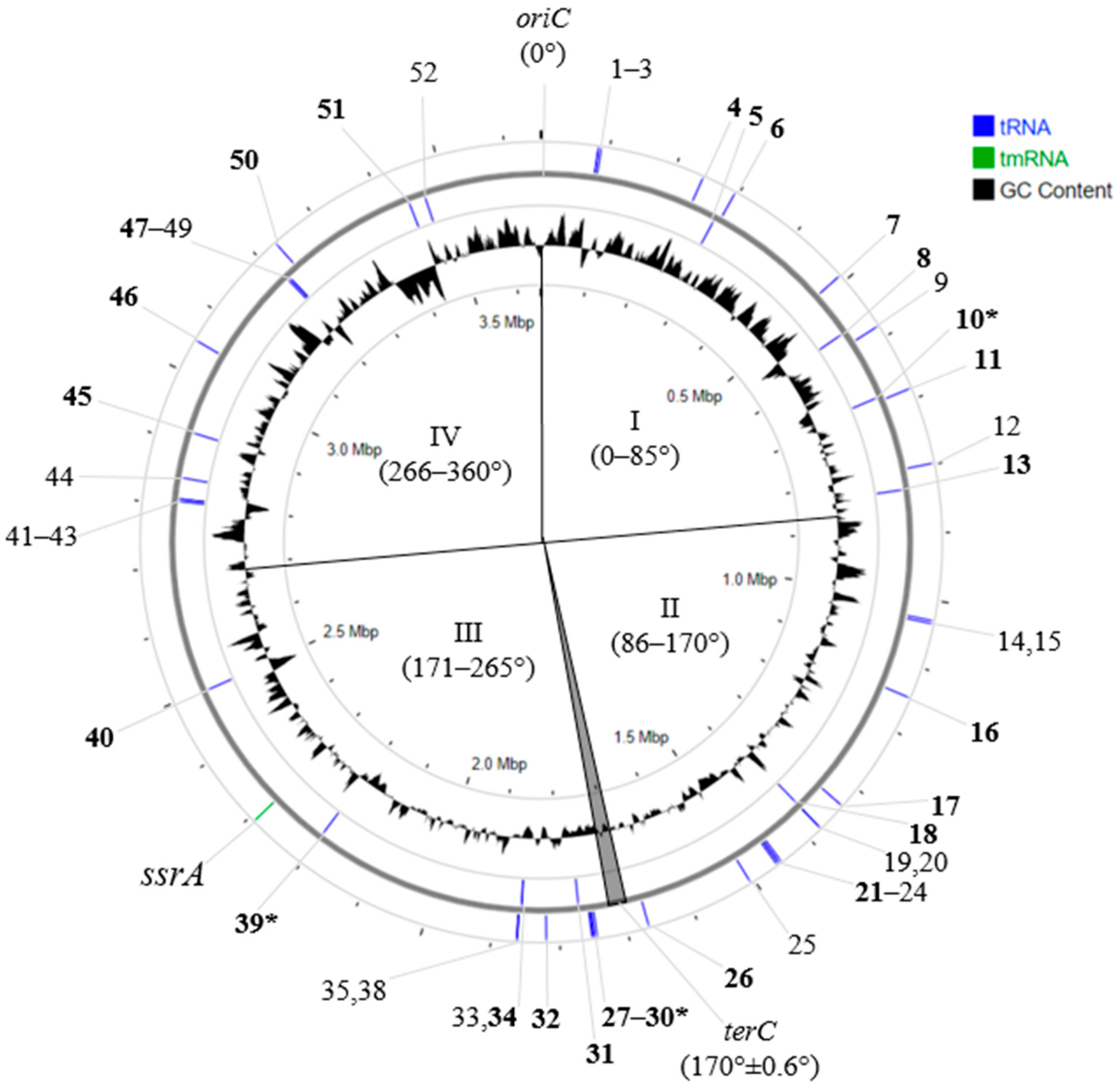
2.2. Site-Specific Integration of PRSs
2.3. tRNA Genes in PRSs (tRNAPRS)
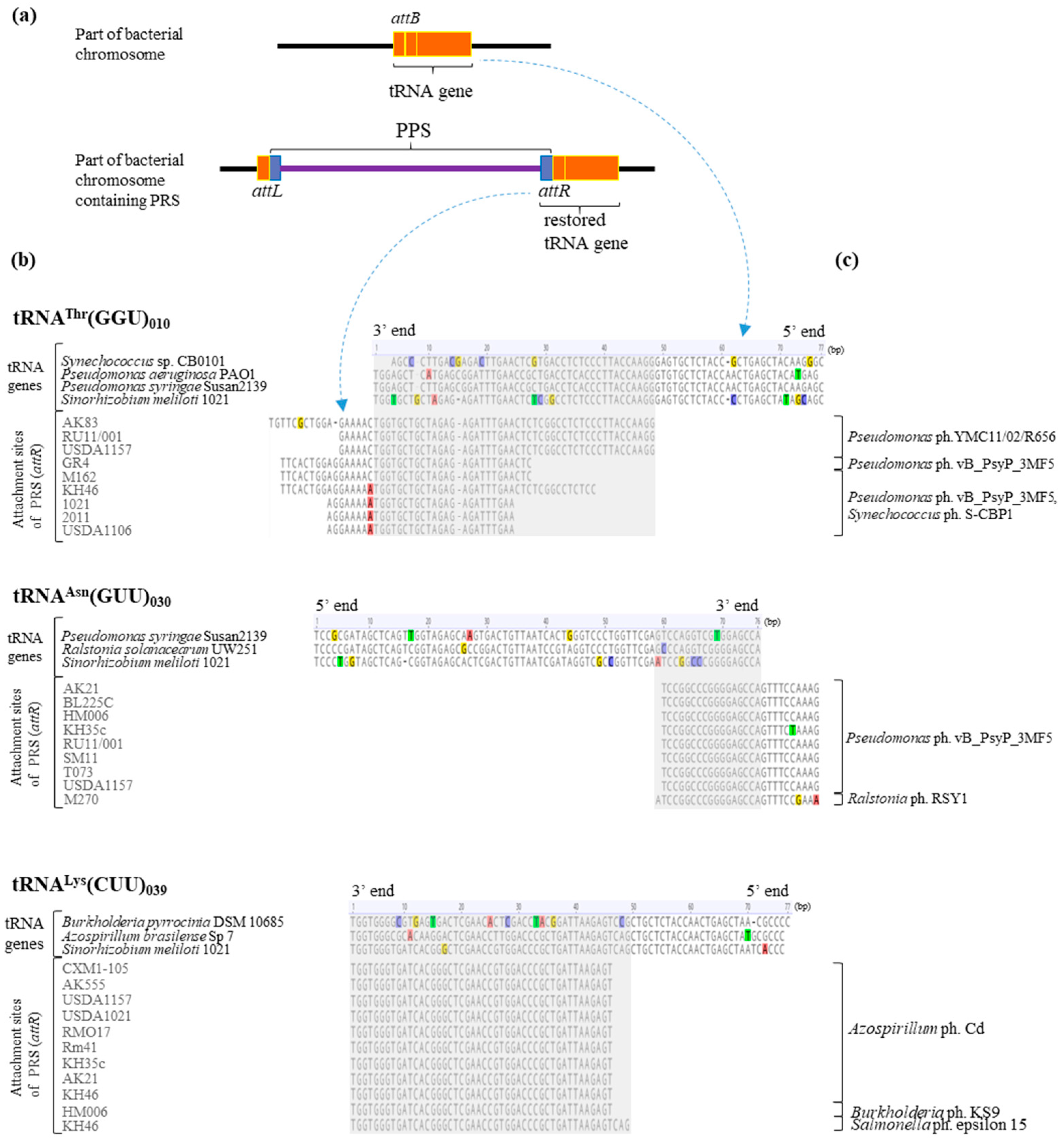
2.4. The Synteny of PRSs Integrated into Essential tRNA Genes
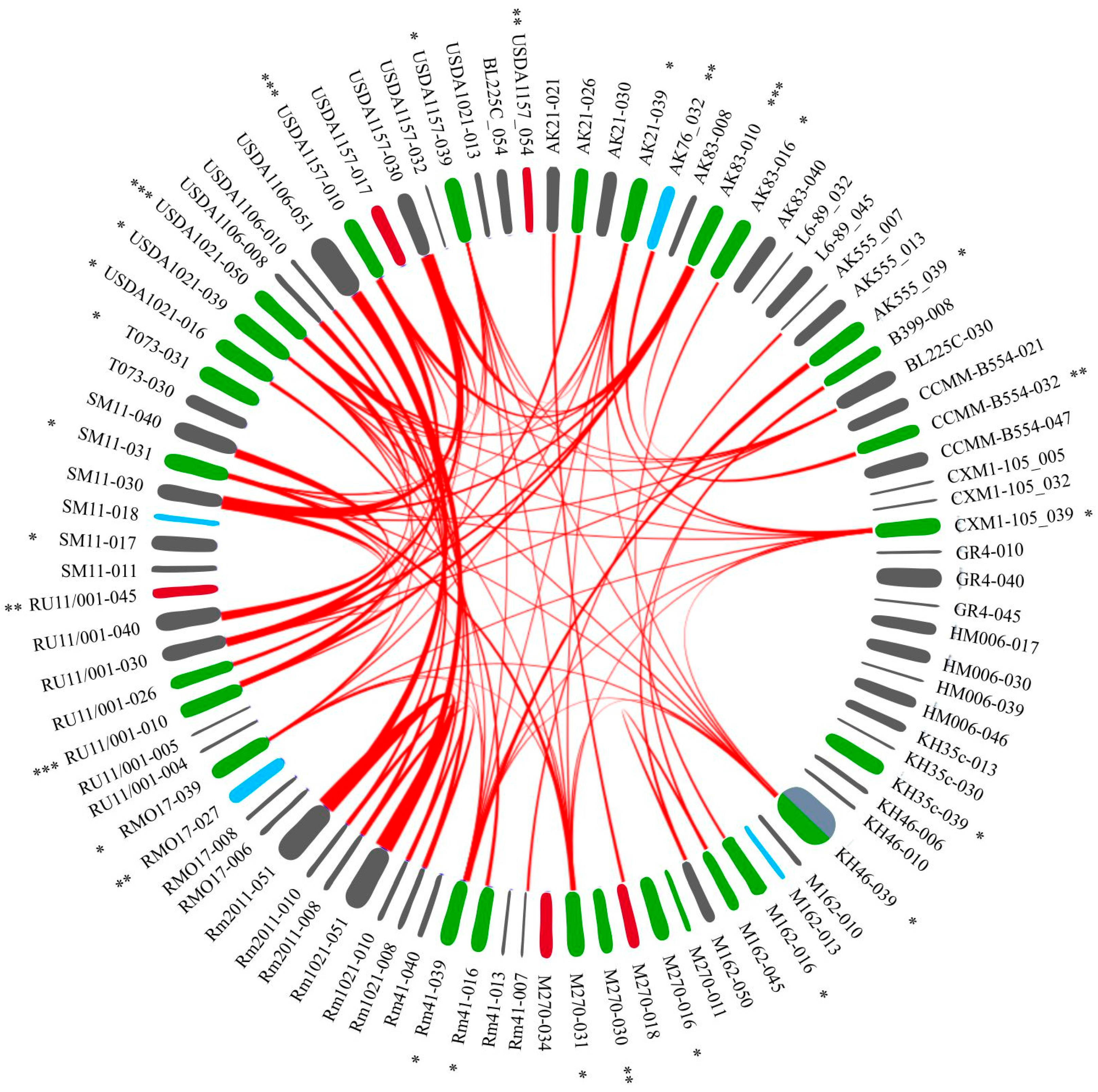
2.4.1. Analysis of 48 SBs between Ph/Ph Pairs
2.4.2. Analysis of 18 SBs between Ph/GI Pairs
2.4.3. Analysis of 17 SBs between GI/GI Pairs
2.4.4. Analysis of SBs between PRSs of Sinorhizobium spp.
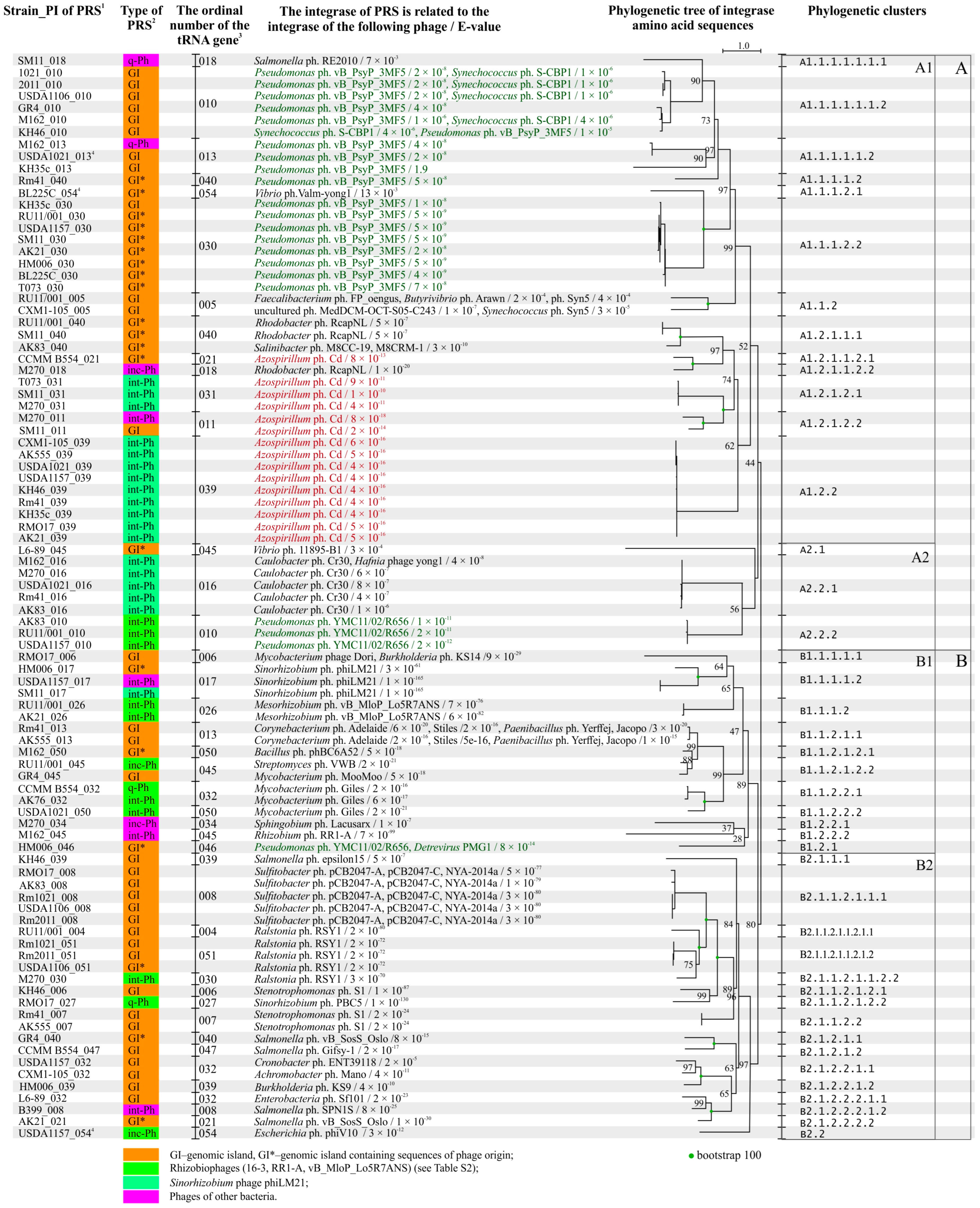
2.5. Analysis of Integrases Identified in PRSs
2.6. Analysis of PRS Attachment Sites (att)
3. Discussion
4. Materials and Methods
4.1. Bacterial Genomes Analyzed in This Study
4.2. List and Purpose of the Programs Used in This Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faruque, S.M.; Mekalanos, J.J. Phage-Bacterial Interactions in the Evolution of Toxigenic Vibrio cholerae. Virulence 2012, 3, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Soucy, S.M.; Huang, J.; Gogarten, J.P. Horizontal Gene Transfer: Building the Web of Life. Nat. Rev. Genet. 2015, 16, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Azarian, T.; Huang, I.-T.; Hanage, W.P. Structure and Dynamics of Bacterial Populations: Pangenome Ecology. In The Pangenome; Tettelin, H., Medini, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 115–128. ISBN 978-3-030-38280-3. [Google Scholar]
- Dobrindt, U.; Hochhut, B.; Hentschel, U.; Hacker, J. Genomic Islands in Pathogenic and Environmental Microorganisms. Nat. Rev. Microbiol. 2004, 2, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Ramisetty, B.C.M.; Sudhakari, P.A. Bacterial ‘Grounded’ Prophages: Hotspots for Genetic Renovation and Innovation. Front. Genet. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.; Hendrix, R.W. Bacteriophages and the Bacterial Genome. In The Bacterial Chromosome; Higgins, N.P., Ed.; ASM Press: Washington, DC, USA, 2014; pp. 39–52. ISBN 978-1-68367-204-3. [Google Scholar]
- Johnson, G.; Banerjee, S.; Putonti, C. Diversity of Pseudomonas aeruginosa Temperate Phages. mSphere 2022, 7, e01015-21. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Xu, X.; Rao, S.; Wen, H.; Han, Y.; Deng, A.; Zhang, Z.; Yang, Z.; Zhu, G. Whole-Genome Analysis Showed the Promotion of Genetic Diversity and Coevolution in Staphylococcus aureus Lytic Bacteriophages and Their Hosts Mediated by Prophages via Worldwide Recombination Events. Front. Microbiol. 2023, 14, 1088125. [Google Scholar] [CrossRef]
- Asadulghani, M.; Ogura, Y.; Ooka, T.; Itoh, T.; Sawaguchi, A.; Iguchi, A.; Nakayama, K.; Hayashi, T. The Defective Prophage Pool of Escherichia coli O157: Prophage–Prophage Interactions Potentiate Horizontal Transfer of Virulence Determinants. PLoS Pathog. 2009, 5, e1000408. [Google Scholar] [CrossRef]
- Castillo, A.; Tello, M.; Ringwald, K.; Acuña, L.G.; Quatrini, R.; Orellana, O. A DNA Segment Encoding the Anticodon Stem/Loop of tRNA Determines the Specific Recombination of Integrative-Conjugative Elements in Acidithiobacillus Species. RNA Biol. 2018, 15, 492–499. [Google Scholar] [CrossRef]
- Orellana, R.; Arancibia, A.; Badilla, L.; Acosta, J.; Arancibia, G.; Escar, R.; Ferrada, G.; Seeger, M. Ecophysiological Features Shape the Distribution of Prophages and CRISPR in Sulfate Reducing Prokaryotes. Microorganisms 2021, 9, 931. [Google Scholar] [CrossRef]
- Rocha, E.P.C.; Bikard, D. Microbial Defenses against Mobile Genetic Elements and Viruses: Who Defends Whom from What? PLoS Biol. 2022, 20, e3001514. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Lynch, K.H.; Dennis, J.J.; Wishart, D.S. PHAST: A Fast Phage Search Tool. Nucleic Acids Res. 2011, 39, W347–W352. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Roumiantseva, M.L.; Muntyan, V.S.; Cherkasova, M.E.; Saksaganskaya, A.S.; Andronov, E.E.; Simarov, B.V. Genomic Islands in Sinorhizobium meliloti Rm1021, Nitrogen-Fixing Symbiont of Alfalfa. Russ. J. Genet. 2018, 54, 759–769. [Google Scholar] [CrossRef]
- Finlay, B.B.; Falkow, S. Common Themes in Microbial Pathogenicity Revisited. Microbiol. Mol. Biol. Rev. 1997, 61, 136–169. [Google Scholar] [CrossRef]
- Escobar-Páramo, P.; Clermont, O.; Blanc-Potard, A.-B.; Bui, H.; Le Bouguénec, C.; Denamur, E. A Specific Genetic Background Is Required for Acquisition and Expression of Virulence Factors in Escherichia coli. Mol. Biol. Evol. 2004, 21, 1085–1094. [Google Scholar] [CrossRef]
- Middendorf, B.; Hochhut, B.; Leipold, K.; Dobrindt, U.; Blum-Oehler, G.; Hacker, J. Instability of Pathogenicity Islands in Uropathogenic Escherichia coli 536. J. Bacteriol. 2004, 186, 3086–3096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shestakov, S.V. How Does the Horizontal Gene Transfer in Bacteria Occur and than Is It Tied Up. Ecol. genet. 2007, 5, 12–24. [Google Scholar] [CrossRef][Green Version]
- Cherkasova, M.E.; Muntyan, V.S.; Saksaganskaia, A.S.; Simarov, B.V.; Roumiantseva, M.L. Sinorhizobium meliloti: Chromosomal Types and Genomic Islands. Ecol. Genet. 2019, 17, 23–38. [Google Scholar] [CrossRef]
- Roumiantseva, M.L.; Vladimirova, M.E.; Saksaganskaia, A.S.; Muntyan, V.S.; Kozlova, A.P.; Afonin, A.M.; Baturina, O.A.; Simarov, B.V. Ensifer meliloti L6-AK89, an Effective Inoculant of Medicago lupulina Varieties: Phenotypic and Deep-Genome Screening. Agronomy 2022, 12, 766. [Google Scholar] [CrossRef]
- Gal-Mor, O.; Finlay, B.B. Pathogenicity Islands: A Molecular Toolbox for Bacterial Virulence. Cell Microbiol. 2006, 8, 1707–1719. [Google Scholar] [CrossRef]
- Sekizuka, T.; Yamamoto, A.; Komiya, T.; Kenri, T.; Takeuchi, F.; Shibayama, K.; Takahashi, M.; Kuroda, M.; Iwaki, M. Corynebacterium ulcerans 0102 Carries the Gene Encoding Diphtheria Toxin on a Prophage Different from the C. diphtheriae NCTC 13129 Prophage. BMC Microbiol. 2012, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Ingmer, H.; Gerlach, D.; Wolz, C. Temperate Phages of Staphylococcus aureus. Microbiol. Spectrum 2019, 7, 7.5.1. [Google Scholar] [CrossRef]
- Kondo, K.; Kawano, M.; Sugai, M. Distribution of Antimicrobial Resistance and Virulence Genes within the Prophage-Associated Regions in Nosocomial Pathogens. mSphere 2021, 6, e00452-21. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.R.; Gao, L.A.; Shah, S.A.; López-Beltrán, A.; González-Delgado, A.; Martínez-Abarca, F.; Iranzo, J.; Redrejo-Rodríguez, M.; Zhang, F.; Toro, N. UG/Abi: A Highly Diverse Family of Prokaryotic Reverse Transcriptases Associated with Defense Functions. Nucleic Acids Res. 2022, 50, 6084–6101. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Melamed, S.; Leavitt, A.; Doron, S.; Bernheim, A.; Hör, J.; Garb, J.; Bechon, N.; Brandis, A.; Lopatina, A.; et al. An Expanded Arsenal of Immune Systems That Protect Bacteria from Phages. Cell Host Microbe 2022, 30, 1556–1569.e5. [Google Scholar] [CrossRef]
- Rousset, F.; Depardieu, F.; Miele, S.; Dowding, J.; Laval, A.-L.; Lieberman, E.; Garry, D.; Rocha, E.P.C.; Bernheim, A.; Bikard, D. Phages and Their Satellites Encode Hotspots of Antiviral Systems. Cell Host Microbe 2022, 30, 740–753.e5. [Google Scholar] [CrossRef]
- Kaneko, T. Complete Genomic Sequence of Nitrogen-Fixing Symbiotic Bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002, 9, 189–197. [Google Scholar] [CrossRef]
- Itakura, M.; Saeki, K.; Omori, H.; Yokoyama, T.; Kaneko, T.; Tabata, S.; Ohwada, T.; Tajima, S.; Uchiumi, T.; Honnma, K.; et al. Genomic Comparison of Bradyrhizobium japonicum Strains with Different Symbiotic Nitrogen-Fixing Capabilities and Other Bradyrhizobiaceae Members. ISME J. 2009, 3, 326–339. [Google Scholar] [CrossRef]
- Stiffler, A.K.; Hesketh-Best, P.J.; Varona, N.S.; Zagame, A.; Wallace, B.A.; Lapointe, B.E.; Silveira, C.B. Genomic and Induction Evidence for Bacteriophage Contributions to Sargassum-Bacteria Symbioses. Microbiome 2024, 12, 143. [Google Scholar] [CrossRef]
- Cheetham, B.F.; Katz, M.E. A Role for Bacteriophages in the Evolution and Transfer of Bacterial Virulence Determinants. Mol. Microbiol. 1995, 18, 201–208. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Aravind, L. Horizontal Gene Transfer in Prokaryotes: Quantification and Classification. Annu. Rev. Microbiol. 2001, 55, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Jain, R. Horizontal Gene Transfer Accelerates Genome Innovation and Evolution. Mol. Biol. Evol. 2003, 20, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Itoh, T.; Matsuda, H.; Gojobori, T. Biased Biological Functions of Horizontally Transferred Genes in Prokaryotic Genomes. Nat. Genet. 2004, 36, 760–766. [Google Scholar] [CrossRef]
- Pantůček, R.; Sedláček, I.; Indráková, A.; Vrbovská, V.; Mašlaňová, I.; Kovařovic, V.; Švec, P.; Králová, S.; Krištofová, L.; Kekláková, J.; et al. Staphylococcus edaphicus sp. Nov., Isolated in Antarctica, Harbors the mecC Gene and Genomic Islands with a Suspected Role in Adaptation to Extreme Environments. Appl. Environ. Microbiol. 2018, 84, e01746-17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiao, J.; Tian, C.-F. Adaptive Evolution of Rhizobial Symbiosis beyond Horizontal Gene Transfer: From Genome Innovation to Regulation Reconstruction. Genes 2023, 14, 274. [Google Scholar] [CrossRef]
- Nadeem, A.; Wahl, L.M. Prophage as a Genetic Reservoir: Promoting Diversity and Driving Innovation in the Host Community: BRIEF COMMUNICATION. Evolution 2017, 71, 2080–2089. [Google Scholar] [CrossRef]
- Braga, L.P.P.; Soucy, S.M.; Amgarten, D.E.; Da Silva, A.M.; Setubal, J.C. Bacterial Diversification in the Light of the Interactions with Phages: The Genetic Symbionts and Their Role in Ecological Speciation. Front. Ecol. Evol. 2018, 6, 6. [Google Scholar] [CrossRef]
- Martín-Galiano, A.J.; García, E. Streptococcus pneumoniae: A Plethora of Temperate Bacteriophages With a Role in Host Genome Rearrangement. Front. Cell. Infect. Microbiol. 2021, 11, 775402. [Google Scholar] [CrossRef]
- Mavrich, T.N.; Hatfull, G.F. Evolution of Superinfection Immunity in Cluster A Mycobacteriophages. mBio 2019, 10, e00971-19. [Google Scholar] [CrossRef]
- Gentile, G.M.; Wetzel, K.S.; Dedrick, R.M.; Montgomery, M.T.; Garlena, R.A.; Jacobs-Sera, D.; Hatfull, G.F. More Evidence of Collusion: A New Prophage-Mediated Viral Defense System Encoded by Mycobacteriophage Sbash. mBio 2019, 10, e00196-19. [Google Scholar] [CrossRef]
- Meaden, S.; Fineran, P.C. Bacterial Defense Islands Limit Viral Attack. Science 2021, 374, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.V.; Wenner, N.; Dulberger, C.L.; Rodwell, E.V.; Bowers-Barnard, A.; Quinones-Olvera, N.; Rigden, D.J.; Rubin, E.J.; Garner, E.C.; Baym, M.; et al. Prophages Encode Phage-Defense Systems with Cognate Self-Immunity. Cell Host Microbe 2021, 29, 1620–1633.e8. [Google Scholar] [CrossRef]
- Kozlova, A.P.; Muntyan, V.S.; Vladimirova, M.E.; Saksaganskaia, A.S.; Kabilov, M.R.; Gorbunova, M.K.; Gorshkov, A.N.; Grudinin, M.P.; Simarov, B.V.; Roumiantseva, M.L. Soil Giant Phage: Genome and Biological Characteristics of Sinorhizobium Jumbo Phage. IJMS 2024, 25, 7388. [Google Scholar] [CrossRef]
- Grainge, I.; Jayaram, M. The Integrase Family of Recombinases: Organization and Function of the Active Site. Mol. Microbiol. 1999, 33, 449–456. [Google Scholar] [CrossRef]
- Van Houdt, R.; Leplae, R.; Lima-Mendez, G.; Mergeay, M.; Toussaint, A. Towards a More Accurate Annotation of Tyrosine-Based Site-Specific Recombinases in Bacterial Genomes. Mobile DNA 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Van Der Meer, J.R.; Gaillard, M.; Harding, R.M.; Hood, D.W.; Crook, D.W. Genomic Islands: Tools of Bacterial Horizontal Gene Transfer and Evolution. FEMS Microbiol. Rev. 2009, 33, 376–393. [Google Scholar] [CrossRef]
- Guerrero-Bustamante, C.A.; Hatfull, G.F. Bacteriophage tRNA-Dependent Lysogeny: Requirement of Phage-Encoded tRNA Genes for Establishment of Lysogeny. mBio 2024, 15, e03260-23. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.P. Integration Sites for Genetic Elements in Prokaryotic tRNA and tmRNA Genes: Sublocation Preference of Integrase Subfamilies. Nucleic Acids Res. 2002, 30, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Grindley, N.D.F.; Whiteson, K.L.; Rice, P.A. Mechanisms of Site-Specific Recombination. Annu. Rev. Biochem. 2006, 75, 567–605. [Google Scholar] [CrossRef]
- Lysogeny: The λ Paradigm and the Role of Lysogenic Conversion in Bacterial Pathogenesis. In Molecular Genetics of Bacteria; ASM Press: Washington, DC, USA, 2007; Volume 8, pp. 343–376. ISBN 978-1-55581-399-4.
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded Prediction of Genomic Islands for Larger-Scale Datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- de Assis, J.C.S.; Gonçalves, O.S.; Fernandes, A.S.; de Queiroz, M.V.; Bazzolli, D.M.S.; Santana, M.F. Genomic Analysis Reveals the Role of Integrative and Conjugative Elements in Plant Pathogenic Bacteria. Mobile DNA 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Germon, P.; Roche, D.; Melo, S.; Mignon-Grasteau, S.; Dobrindt, U.; Hacker, J.; Schouler, C.; Moulin-Schouleur, M. tDNA Locus Polymorphism and Ecto-Chromosomal DNA Insertion Hot-Spots Are Related to the Phylogenetic Group of Escherichia coli Strains. Microbiology 2007, 153, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.H.; Touchon, M.; Cury, J.; Rocha, E.P.C. The Chromosomal Organization of Horizontal Gene Transfer in Bacteria. Nat. Commun. 2017, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Kopejtka, K.; Lin, Y.; Jakubovičová, M.; Koblížek, M.; Tomasch, J. Clustered Core- and Pan-Genome Content on Rhodobacteraceae Chromosomes. Genome Biol. Evol. 2019, 11, 2208–2217. [Google Scholar] [CrossRef]
- Leonard, A.C.; Grimwade, J.E. Building a Bacterial Orisome: Emergence of New Regulatory Features for Replication Origin Unwinding. Mol. Microbiol. 2005, 55, 978–985. [Google Scholar] [CrossRef]
- Kaguni, J.M. DnaA: Controlling the Initiation of Bacterial DNA Replication and More. Annu. Rev. Microbiol. 2006, 60, 351–371. [Google Scholar] [CrossRef]
- Mulcair, M.D.; Schaeffer, P.M.; Oakley, A.J.; Cross, H.F.; Neylon, C.; Hill, T.M.; Dixon, N.E. A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. Cell 2006, 125, 1309–1319. [Google Scholar] [CrossRef]
- Sibley, C.D.; MacLellan, S.R.; Finan, T. The Sinorhizobium Meliloti Chromosomal Origin of Replication. Microbiology 2006, 152, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Roumiantseva, M.L.; Muntyan, V.S.; Cherkasova, M.E.; Andronov, E.E.; Saksaganskaya, A.S.; Dzyubenko, E.A.; Dzyubenko, N.I.; Simarov, B.V. A Comparative Analysis of Genomic Characters of Reference Sinorhizobium meliloti Strains, the Alfalfa Symbionts (Review). S-kh. Biol. 2017, 52, 928–939. [Google Scholar] [CrossRef]
- Muntyan, V.S.; Cherkasova, M.E.; Andronov, E.E.; Simarov, B.V.; Roumiantseva, M.L. Occurrence of Islands in Genomes of Sinorhizobium Meliloti Native Isolates. Russ. J. Genet. 2016, 52, 1015–1022. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. In Gene Prediction; Kollmar, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1962, pp. 1–14. ISBN 978-1-4939-9172-3. [Google Scholar]
- Vladimirova, M.; Muntyan, V.; Kozlova, A.; Grudinin, M.; Roumiantseva, M. CRISPR/Cas in Genomes of Sinorhizobium meliloti. In Proceedings of the 20th International Multidisciplinary Scientific GeoConference Proceedings SGEM 2020; STEF92 Technology, Albena, Bulgaria, 18 August 2020; Volume 20, pp. 215–222. [Google Scholar]
- Vladimirova, M.; Afonin, A.; Muntyan, V.; Simarov, B.; Roumiantseva, M. Evaluation of Sinorhizobium meliloti Genomic Islands Inserted into the tRNA-Thr. In Proceedings of the 2020 Cognitive Sciences, Genomics and Bioinformatics (CSGB), IEEE, Novosibirsk, Russia, 6–10 July 2020; pp. 118–121. [Google Scholar]
- Kozlova, A.P.; Saksaganskaia, A.S.; Afonin, A.M.; Muntyan, V.S.; Vladimirova, M.E.; Dzyubenko, E.A.; Roumiantseva, M.L. A Temperate Sinorhizobium Phage, AP-16-3, Closely Related to Phage 16-3: Mosaic Genome and Prophage Analysis. Viruses 2023, 15, 1701. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, E.; Flores-Félix, J.D.; Kaur, S.; diCenzo, G.C.; Young, J.P.W.; Peix, A.; Velázquez, E. Reclassification of Type Strains of Rhizobium indigoferae and Sinorhizobium kummerowiae into the Species Rhizobium leguminosarum and Sinorhizobium meliloti, Respectively. Int. J. Syst. Evol. Microbiol. 2024, 74. [Google Scholar] [CrossRef]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; Van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of Morphology-Based Taxa and Change to Binomial Species Names: 2022 Taxonomy Update of the ICTV Bacterial Viruses Subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Nishimura, Y.; Shimizu, Y.; Nishiyama, H.; Yoshikawa, G.; Uehara, H.; Hingamp, P.; Goto, S.; Ogata, H. Linking Virus Genomes with Host Taxonomy. Viruses 2016, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Galardini, M.; Mengoni, A.; Brilli, M.; Pini, F.; Fioravanti, A.; Lucas, S.; Lapidus, A.; Cheng, J.-F.; Goodwin, L.; Pitluck, S.; et al. Exploring the Symbiotic Pangenome of the Nitrogen-Fixing Bacterium Sinorhizobium meliloti. BMC Genomics 2011, 12, 235. [Google Scholar] [CrossRef]
- Galardini, M.; Pini, F.; Bazzicalupo, M.; Biondi, E.G.; Mengoni, A. Replicon-Dependent Bacterial Genome Evolution: The Case of Sinorhizobium meliloti. Genome Biol. Evol. 2013, 5, 542–558. [Google Scholar] [CrossRef]
- diCenzo, G.C.; Finan, T.M. The Divided Bacterial Genome: Structure, Function, and Evolution. Microbiol. Mol. Biol. Rev. 2017, 81, e00019-17. [Google Scholar] [CrossRef]
- Bobay, L.-M. The Prokaryotic Species Concept and Challenges. In The Pangenome; Tettelin, H., Medini, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 21–49. ISBN 978-3-030-38280-3. [Google Scholar]
- Csiszovszki, Z.; Buzás, Z.; Semsey, S.; Ponyi, T.; Papp, P.P.; Orosz, L. immX Immunity Region of Rhizobium Phage 16-3 : Two Overlapping Cistrons of Repressor Function. J. Bacteriol. 2003, 185, 4382–4392. [Google Scholar] [CrossRef]
- Hudson, C.M.; Lau, B.Y.; Williams, K.P. Islander: A Database of Precisely Mapped Genomic Islands in tRNA and tmRNA Genes. Nucleic Acids Res. 2015, 43, D48–D53. [Google Scholar] [CrossRef]
- Vale, F.F.; HpGP Research Network; Roberts, R.J.; Kobayashi, I.; Camargo, M.C.; Rabkin, C.S. Gene Content, Phage Cycle Regulation Model and Prophage Inactivation Disclosed by Prophage Genomics in the Helicobacter pylori Genome Project. Gut Microbes 2024, 16, 2379440. [Google Scholar] [CrossRef]
- Rocha, E.P.C. Codon Usage Bias from tRNA’s Point of View: Redundancy, Specialization, and Efficient Decoding for Translation Optimization. Genome Res. 2004, 14, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Higgs, P.G. The Influence of Anticodon-Codon Interactions and Modified Bases on Codon Usage Bias in Bacteria. Mol. Biol. Evol. 2010, 27, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Ayan, G.B.; Park, H.J.; Gallie, J. The Birth of a Bacterial tRNA Gene by Large-Scale, Tandem Duplication Events. eLife 2020, 9, e57947. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.H.; Chihara, K.; Kondo, K.; Nakamura, T.; Ojima, S.; Tamura, A.; Yamashita, W.; Cui, L.; Takahashi, Y.; Watashi, K.; et al. Viruses Encode tRNA and Anti-Retron to Evade Bacterial Immunity 2023. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yang, J.Y.; Fang, W.; Miranda-Sanchez, F.; Brown, J.M.; Kauffman, K.M.; Acevero, C.M.; Bartel, D.P.; Polz, M.F.; Kelly, L. Degradation of Host Translational Machinery Drives tRNA Acquisition in Viruses. Cell Syst. 2021, 12, 771–779.e5. [Google Scholar] [CrossRef]
- Van Den Berg, D.F.; Van Der Steen, B.A.; Costa, A.R.; Brouns, S.J. Phage tRNAs Evade tRNA-Targeting Host Defenses through Anticodon Loop Mutations. eLife 2023, 12, e85183. [Google Scholar] [CrossRef]
- Wishart, D.S.; Han, S.; Saha, S.; Oler, E.; Peters, H.; Grant, J.R.; Stothard, P.; Gautam, V. PHASTEST: Faster than PHASTER, Better than PHAST. Nucleic Acids Res. 2023, 51, W443–W450. [Google Scholar] [CrossRef]
- Laslett, D. ARAGORN, a Program to Detect tRNA Genes and tmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-Line: Integrating Search and Context for Analysis of Transfer RNA Genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved Detection and Functional Classification of Transfer RNA Genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Kono, N.; Arakawa, K.; Sato, M.; Yoshikawa, H.; Tomita, M.; Itaya, M. Undesigned Selection for Replication Termination of Bacterial Chromosomes. J. Mol. Biol. 2014, 426, 2918–2927. [Google Scholar] [CrossRef]
- Lawrence, J.G.; Hendrickson, H. Lateral Gene Transfer: When Will Adolescence End? Mol. Microbiol. 2003, 50, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Salzberg, S.L. SkewIT: The Skew Index Test for Large-Scale GC Skew Analysis of Bacterial Genomes. PLoS Comput. Biol. 2020, 16, e1008439. [Google Scholar] [CrossRef] [PubMed]
- Stano, M.; Beke, G.; Klucar, L. viruSITE—Integrated Database for Viral Genomics. Database 2016, 2016, baw162. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]


| PRS * | Number of PRS Integrated into ** | Total Number of PRS *** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Essential tRNA Gene | Protein-Coding Sequence or Nonessential Region | |||||||||
| att+ | att− | att+ | att− | on Chrs | in Genomes | |||||
| Total | on Chrs | Total | on Chrs | Total | on Chrs | Total | on Chrs | |||
| GI | 54 | 51 | 0 | 0 | 0 | 0 | 0 | 0 | 51 | 54 |
| int-Ph | 30 | 30 | 0 | 0 | 11 | 2 | 12 | 3 | 35 | 53 |
| inc-Ph | 1 | 1 | 0 | 0 | 48 | 29 | 115 | 7 | 37 | 164 |
| q-Ph | 6 | 6 | 0 | 0 | 13 | 1 | 24 | 2 | 9 | 43 |
| In total: | 91 | 88 | 0 | 0 | 72 | 32 | 151 | 12 | 132 | 314 |
| Total number in genomes *** | 91 | 223 | ||||||||
| Total number on chrs *** | 88 | 44 | ||||||||
| PRSs Similarities to the following Phage 1 | PRSs 2: | in Total: | |||
|---|---|---|---|---|---|
| int-Ph | inc-Ph | q-Ph | |||
| Sinorhizobium phage | phiLM21 | 18 | 0 | 0 | 18 |
| Rhizobium phage | 16-3 | 2 | 1 | 2 | 5 |
| RR1-A | 4 | 0 | 0 | 4 | |
| Mesorhizobium phage | vB_MloP_Lo5R7ANS | 2 | 0 | 0 | 2 |
| Brucella phage | BiPBO1 | 1 | 0 | 0 | 1 |
| Sulfitobacter phage | pCB2047-A | 2 | 0 | 0 | 3 |
| NYA-2014a | 0 | 3 | 0 | 3 | |
| Enterobacteria phage | mEp235 | 0 | 0 | 2 | 2 |
| In total PRSs | 29 | 4 | 0 | 37 | |
| tRNA Isotype 1 | Ordinal Number of tRNA Gene in Chromosome | Anticodon | tRNA Isotype | Ordinal Number of tRNA Gene in Chromosome | Anticodon |
|---|---|---|---|---|---|
| tRNAAla | 028 | CGC | tRNAGln | 013 | CUG |
| 005 | GGC | 014 | UUG | ||
| 002, 042, 048 2 | UGC | tRNALys | 039 | CUU | |
| tRNAfMet | 003, 041, 047 2 | CAU | 032 | UUU | |
| tRNAMet | 045 | CAU | tRNAVal | 046 | GAC |
| tRNAIle | 040 3 | CAU | 018 | UAC | |
| 001, 043, 049 2 | GAU | tRNAGly | 009 | CCC | |
| tRNALeu | 044 | CAA | 052 | GCC | |
| 006 | CAG | 023 | UCC | ||
| 025 | GAG | tRNAArg | 007 | ACG | |
| 031 | UAA | 050 | CCU | ||
| 026 | UAG | 033 | UCU | ||
| tRNAGlu | 037 | CUC | tRNAPro | 015 | CGG |
| 035, 036, 038 | UUC | 017 | GGG | ||
| tRNASer | 051 | CGA | 034 | UGG | |
| 016 | GCU | tRNAAsn | 030 | GUU | |
| 004 | GGA | tRNACys | 029 | GCA | |
| 027 | UGA | tRNAHis | 012 | GUG | |
| tRNAThr | 008 | CGU | tRNAPhe | 011 | GAA |
| 010 | GGU | tRNATrp | 024 | CCA | |
| 021 | UGU | tRNATyr | 022 | GUA | |
| tRNAAsp | 019, 020 | GUC | Total: 20 | 52 | 42 |
| tRNA Gene(s) in PRS 1 | PRS 2 | Characteristics of PRSs | S. meliloti Strain | ||
|---|---|---|---|---|---|
| tRNA Gene 3 | Similarity to Phage 4 | Sequences Similar to 5 | |||
| Leu(UAG) | int-Ph | 026 | Mesorhizobium phage vB_MloP_Lo5R7ANS 6 | - | AK21 |
| Val(CAC) | int-Ph | 016 | Sinorhizobium phage phiLM21 | - | AK83 |
| Ser(GCU) | int-Ph | - | Ruegeria phage DSS3-P1, Loktanella phage pCB2051-A | - | |
| fMet(CAU) | int-Ph | 039 | Sinorhizobium phage phiLM21 | - | AK555 |
| fMet(CAU) | int-Ph | 039 | Sinorhizobium phage phiLM21 | - | CXM1-105 |
| Met(CAU) | int-Ph | 031 | Sinorhizobium phage phiLM21 | - | T073 |
| fMet(CAU) | int-Ph | 016 | Sinorhizobium phage phiLM21 | - | M162 |
| Met(CAU) | |||||
| fMet(CAU) | q-Ph | 017 | Sulfitobacter phage NYA-2014a | - | USDA1157 |
| Thr(GGU) | GI | 030 | - | Rhizobium phage vB_RleM_PPF1, q-Ph | |
| Met(CAU) | int-Ph | 039 | Sinorhizobium phage phiLM21 | - | |
| fMet(CAU) | q-Ph | 027 | Rhizobium phage 16-3 | - | RMO17 |
| Met(CAU) | int-Ph | 016 | Sinorhizobium phage phiLM21 | - | Rm41 |
| fMet(CAU) | int-Ph | 039 | Sinorhizobium phage phiLM21 | - | |
| Leu(UAG) | int-Ph | 026 | Mesorhizobium phage vB_MloP_Lo5R7ANS | - | RU11/001 |
| Thr(GGU) | GI | 030 | - | Rhizobium phage vB_RleM_PPF1, q-Ph | |
| fMet(CAU) | int-Ph | 045 | Rhizobium phage 16-3 | - | |
| Met(CAU) | int-Ph | 016 | Sinorhizobium phage phiLM21 | - | USDA1021 |
| Thr(GGU) | int-Ph | 050 | Rhizobium phage RR1-A | - | |
| Met(CAU) | int-Ph | 016 | Sinorhizobium phage phiLM21 | - | M270 |
| fMet(CAU) | inc-Ph | 018 | Sulfitobacter phage NYA-2014a | - | |
| Met(CAU) | int-Ph | 031 | Sinorhizobium phage phiLM21 | - | |
| fMet(CAU) | inc-Ph | - | Paracoccus phage Shpa | - | |
| Thr(GGU) | GI | 030 | - | Rhizobium phage vB_RleM_PPF1, q-Ph | SM11 |
| Met(CAU) | int-Ph | 031 | Sinorhizobium phage phiLM21 | - | |
| Strain | NCBI BioSample | Strain | NCBI BioSample | Strain | NCBI BioSample |
|---|---|---|---|---|---|
| Sinorhizobium meliloti | |||||
| AK21 I | SAMN08428886 | HM006 | SAMN07175160 | RMO17 | SAMN02952139 |
| AK83 I | SAMN00017059 | KH35c | SAMN07175161 | GR4 | SAMN02603224 |
| L6-AK89 I | SAMN22420025 II | KH46 | SAMN07175162 | CCMM B554 (FSM-MA) | SAMN06284128 |
| AK76 I | SAMN17104055 III | USDA1021 | SAMN07175167 | Rm41 | SAMEA2272434 |
| AK170 I | SAMN10256575 IV | USDA1157 | SAMN07175169 | T073 | SAMN07175166 |
| AK555 I | SAMN08826593 IV | USDA1106 | SAMN07175168 | M270 | SAMN07175164 |
| CXM1-105 I | SAMN08826592 IV | 1021 | SAMEA3283068 | BL225C | SAMN00017103 |
| B399 | SAMN06229775 | 2011 | SAMN02603522 | RU11/001 | SAMEA3146337 |
| B401 | SAMN06227501 | M162 | SAMN07175163 | SM11 | SAMN02603056 |
| Sinorhizobium medicae | |||||
| ml49 | SAMN38634768 | WSM1115 | SAMN23416899 | ml4 | SAMN38634762 |
| ml2 | SAMN38634765 | ml60 | SAMN38634769 | ml18 | SAMN38634763 |
| SU277 | SAMN30617559 | WSM419 | SAMN02598363 | ml29 | SAMN38634766 |
| ml42 | SAMN38634767 | ml20 | SAMN38634754 | T2 | SAMN18332160 |
| T10 | SAMN18332696 | ||||
| Sinorhizobium kummerowiae/S. meliloti V | Stappia indica | ||||
| CIP 108026 | SAMN38748741 | CCBAU 71714 | SAMN33342371 | PHM037 | SAMN13341662 |
| Pseudomonas syringae | Pseudomonas aeruginosa | Ralstonia solanacearum | |||
| Susan2139 | SAMN19068034 | PAO1 | SAMN02603714 | UW251 | SAMN22612269 |
| Azospirillum brasilience | Burkholderia pyrrocinia | Salmonella enterica | |||
| Sp7 | SAMN03159499 | DSM 10685 | SAMN03651233 | LT2 | SAMN02604315 |
| Jiella pelagia | Octadecabacter arcticus | Octadecabacter antarcticus | |||
| HL-NP1 | SAMN31329523 | 238 | SAMN02603620 | 307 | SAMN02603621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladimirova, M.E.; Roumiantseva, M.L.; Saksaganskaia, A.S.; Muntyan, V.S.; Gaponov, S.P.; Mengoni, A. Hot Spots of Site-Specific Integration into the Sinorhizobium meliloti Chromosome. Int. J. Mol. Sci. 2024, 25, 10421. https://doi.org/10.3390/ijms251910421
Vladimirova ME, Roumiantseva ML, Saksaganskaia AS, Muntyan VS, Gaponov SP, Mengoni A. Hot Spots of Site-Specific Integration into the Sinorhizobium meliloti Chromosome. International Journal of Molecular Sciences. 2024; 25(19):10421. https://doi.org/10.3390/ijms251910421
Chicago/Turabian StyleVladimirova, Maria E., Marina L. Roumiantseva, Alla S. Saksaganskaia, Victoria S. Muntyan, Sergey P. Gaponov, and Alessio Mengoni. 2024. "Hot Spots of Site-Specific Integration into the Sinorhizobium meliloti Chromosome" International Journal of Molecular Sciences 25, no. 19: 10421. https://doi.org/10.3390/ijms251910421
APA StyleVladimirova, M. E., Roumiantseva, M. L., Saksaganskaia, A. S., Muntyan, V. S., Gaponov, S. P., & Mengoni, A. (2024). Hot Spots of Site-Specific Integration into the Sinorhizobium meliloti Chromosome. International Journal of Molecular Sciences, 25(19), 10421. https://doi.org/10.3390/ijms251910421









