Abstract
C10orf90, a tumor suppressor, can inhibit the occurrence and development of tumors. Therefore, we investigated the gene function of C10orf90 in various tumors using multiple pan-cancer datasets. Pan-cancer analysis results reveal that the expression levels of C10orf90 vary across different tumors and hold significant value in the clinical diagnosis and prognosis of patients with various tumors. In some cancers, the expression level of C10orf90 is correlated with CNV, DNA methylation, immune subtypes, immune cell infiltration, and drug sensitivity in the tumors. In particular, in COAD, the C10orf90 gene is implicated in multiple processes associated with COAD. Cell experiments demonstrate that C10orf90 suppresses the proliferation and migration of colon cancer cells while promoting apoptosis. In summary, C10orf90 plays a role in the onset and progression of various cancers and could potentially serve as an effective diagnostic and prognostic marker for cancer patients. Notably, in COAD, C10orf90 inhibits the proliferation and migration of colon cancer cells, induces apoptosis, and is linked to the advancement of colon cancer.
1. Introduction
The mechanisms underlying the initiation of cancer remain poorly understood. Notably, the DNA damage response and defective repair processes may contribute to increased genomic instability, a critical factor in cancer development. With the vigorous development of genomics, researchers have discovered that specific DNA segments on chromosomes tend to exhibit a significant number of gaps and breaks in a highly non-random manner under replication stress. This phenomenon can lead to instability in chromosomal genes, designating these specific breakage regions as fragile sites [1].
Chromosome 10 open reading frame 90 (C10orf90), also known as D7Ertd443e or FATS, is a fragile site gene located on chromosome 10q26.2, within the fragile region of chromosome FRA10F [2]. The C10orf90 gene is rich in AT repeat sequences, with up to 56.8% AT base pairs. The cross-arrangement of double-stranded AT dinucleotide repeat sequences represents a fragile link, which is prone to gene breakage at this fragile site [3]. At the same time, multiple specialized DNA polymerases are required for the replicative synthesis of C10orf90 to overcome differences in replication pausing caused by repetitive sequence elements or specific DNA sequence elements [4,5]; this requirement greatly increases the instability of the C10orf90 gene. This explains the abnormal expression of C10orf90 in various tumors, which is closely related to the rapid proliferation of cancer.
Fragile sites are characterized as “fragile”, areas prone to genomic instability, and these sites are hot spots for chromosomal rearrangements in the early stages of cancer development. Frequent gene deletions, amplifications, and rearrangements in cancer cells aggravate the replication pressure of DNA and increase the instability of fragile sites, leading to DNA breaks and accelerating the development of early-stage cancer [6,7]. Research results suggest that the N-terminal region of the C10orf90 protein can bind to the p53 protein, mediate p53 oligomerization to suppress the degradation of the p53 protein, and thereby enhance the cellular DNA damage repair ability [8]. In addition, the C10orf90 protein can promote p21 acetylation, significantly decrease the activity of the proteasome subunit, attenuate the degradation rate of thep21 protein, and suppress cancer progression [9]. Some experimental evidence suggests that the C10orf90 gene can upregulate LC3I/II and ATG5 (markers of autophagy), increase the formation of mature autophagosomes and lysosomes, and promote cell apoptosis to inhibit NSCLC [10]. In addition, the deletion of C10orf90 can lead to mutations in NRAS (an oncogene) and BRAF (a proto-oncogene) [11], the latter two of which can confer greater therapeutic resistance and increased invasiveness to conjunctival melanoma [12,13]. Clinical data confirm a significant reduction in disease-free survival and a similar trend in 5-year overall survival for C10orf90-negative breast cancer patients compared to C10orf90-positive patients [14]. The C10orf90 gene has been identified as having a significant inhibitory impact on the advancement of various tumors, including non-small cell lung cancer, conjunctival melanoma, and breast cancer. This observation indicates that the biological function of the C10orf90 gene involves suppressing the proliferation of tumor cells. Previous studies have predominantly concentrated on exploring the genetic involvement of C10orf90 in individual cancer types, without conducting a comprehensive pan-cancer analysis of C10orf90, especially in COAD. Consequently, the objective of this study is to analyze the pan-cancer functionality of the C10orf90 gene in order to enhance the understanding of its biological role in different malignant tumors.
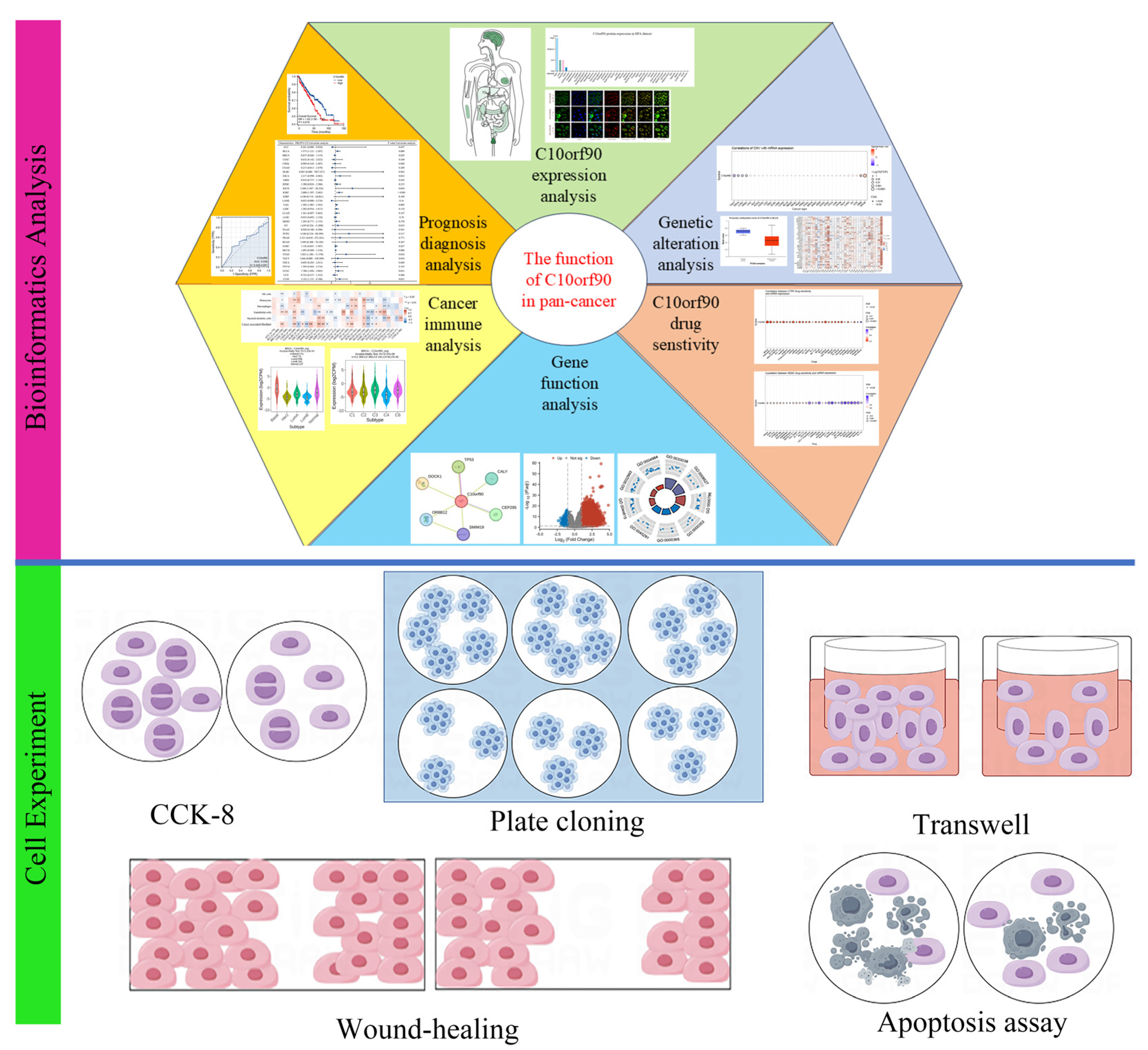
In this research (Figure 1), we utilized the GEPIA2, HPA, TCGA, and GTEx databases to examine the transcriptional and translational statuses of C10orf90. Using bioinformatic methods, we performed prognostic and diagnostic value analysis of the C10orf90 gene, analysis of gene copy number variation, and DNA methylation, among others. Furthermore, we performed various cell experiments, such as CCK-8, plate cloning, Transwell, wound-healing, and apoptosis assays, to clarify the possible function of the C10orf90 gene in inhibiting proliferation and migration in COAD cells. These experiments aimed to contribute additional data to elucidate the underlying biological mechanisms associated with the C10orf90 gene.
Figure 1.
Main workflow of this study.
2. Results
2.1. Expression and Subcellular Location of C10orf90
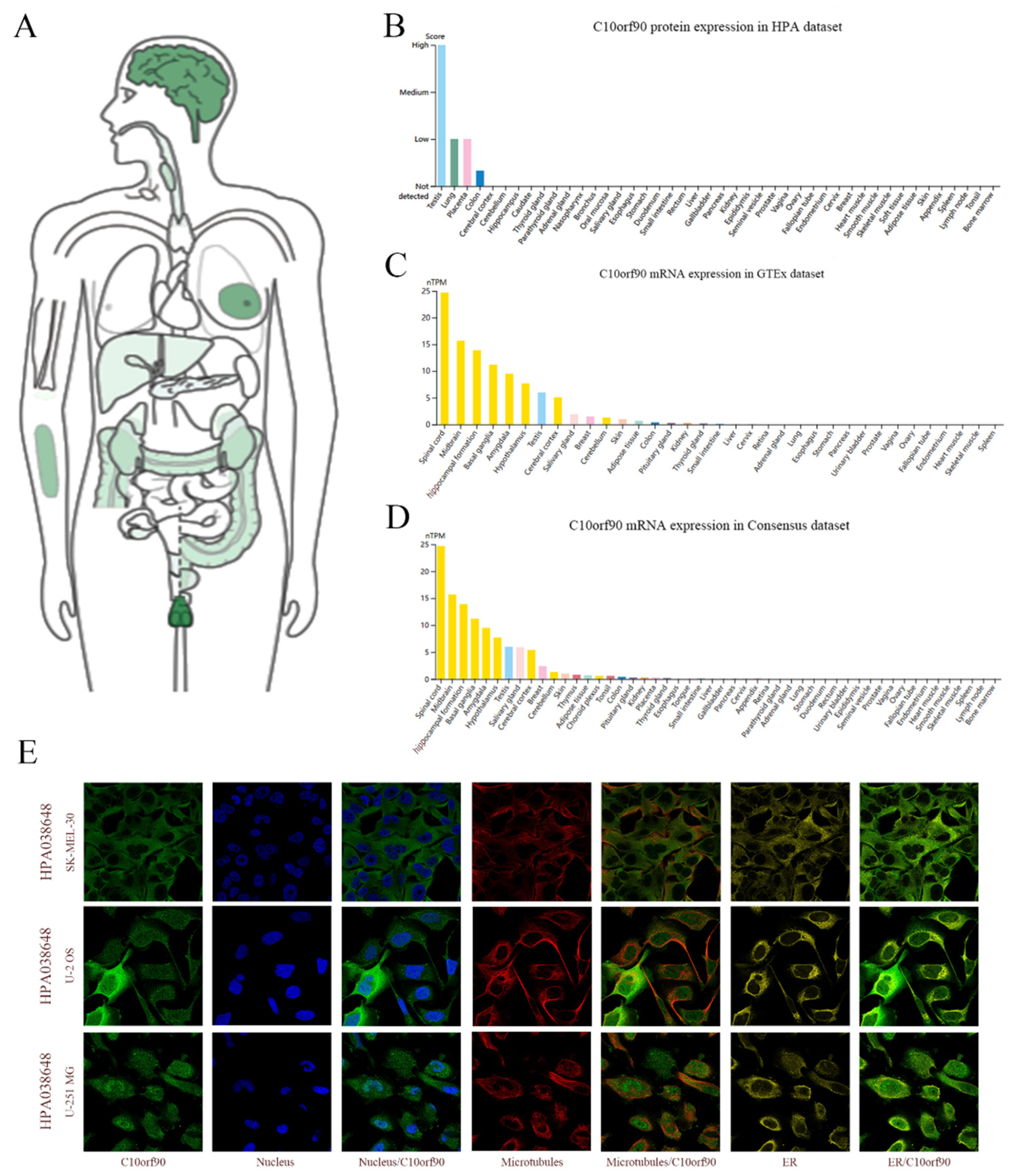
Utilizing the GEPIA2 and HPA databases, we performed a search for C10orf90 mRNA and protein expression. C10orf90 protein expression was detected in various organs and tissues, including the testis, brain, breast, forearm, colon, kidney, liver, and pancreas (Figure 2A). The findings from the HPA database indicate that the C10orf90 protein is predominantly expressed in the testis, lung, placenta, and colon (Figure 2B). The GTEx and Consensus dataset databases demonstrate that C10orf90 mRNA is mainly expressed in the spinal cord, brain, testis, salivary gland, breast, skin, adipose tissue, colon, and kidney (Figure 2C,D and Supplementary Table S1). Additionally, immunofluorescence localization of C10orf90 subcellular localization was obtained by staining the nucleus, microtubules, and endoplasmic reticulum in SK-MEL-30, U-2 OS, and U-251 MG cells. The results demonstrate that C10orf90 is primarily located in microtubules and cytoplasmic solutes (Figure 2E).
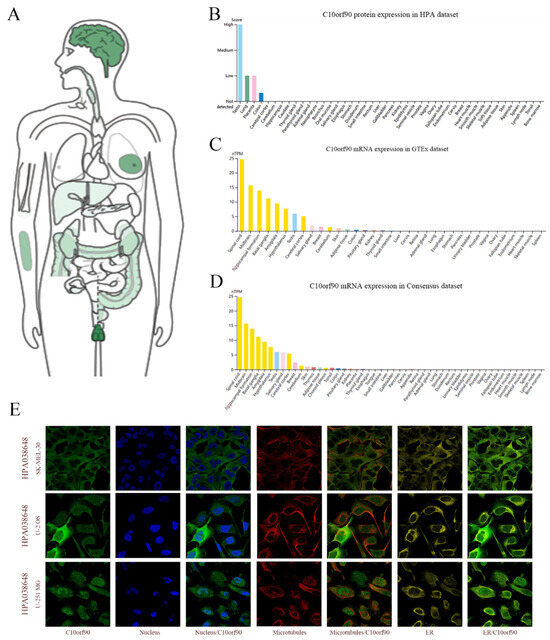
Figure 2.
Expression and subcellular location of C10orf90. (A) Expression of C10orf90 protein in normal human tissues in GEPIA2 database. (B) Expression of C10orf90 protein in HPA database (Different colors represent different tissues, and the same applies below). (C) Expression of C10orf90 mRNA in GTEx database. (D) Expression of C10orf90 mRNA in Consensus dataset database. (E) Immunofluorescence staining of the subcellular localization of C10orf90.
2.2. Expression of C10orf90 in Various Tumor Tissues
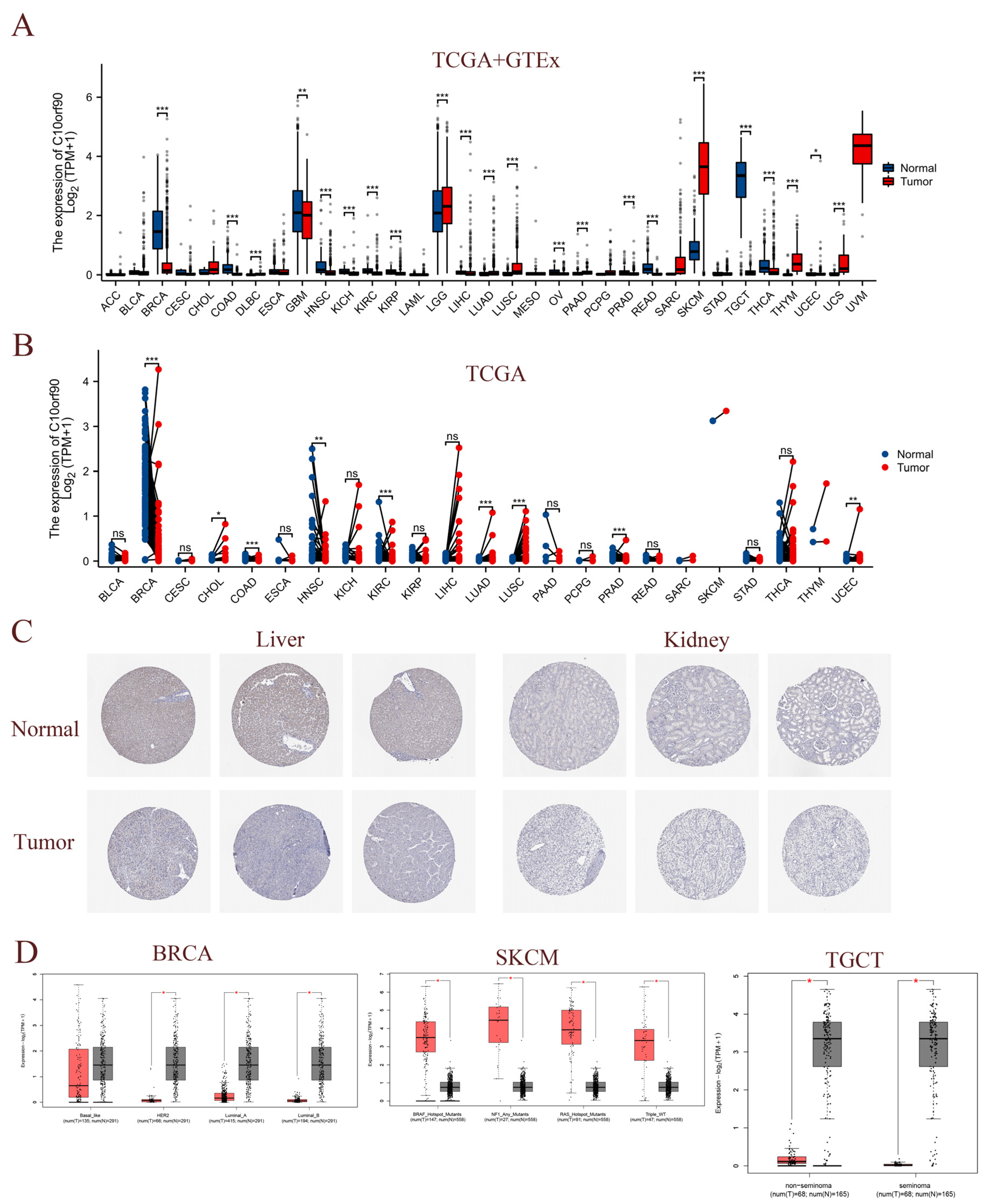
To assess the expression of C10orf90 in multiple cancerous tissues, we conducted a joint analysis of C10orf90 expression data from the TCGA and GTEx databases. This encompassed normal samples, tumor samples, and their corresponding adjacent tumor samples. A comparison of C10orf90 expression levels in normal tissues with those in DLBC, LGG, LUAD, LUSC, PAAD, SKCM, THYM, UCS, and UCEC revealed a significant upregulation in all of these tumor types (p < 0.05). Conversely, C10orf90 is downregulated in BRCA, COAD, GBM, HNSC, KICH, KIRC, KIRP, LIHC, OV, PRAD, READ, TGCT, and THCA (Figure 3A and Supplementary Table S2). The TCGA dataset analysis revealed varying levels of expression of C10orf90 in tumor tissues and paired normal tissues of the following cancers: BRCA, CHOL, COAD, HNSC, KIRC, LUAD, LUSC, PRAD, and UCEC (Figure 3B and Supplementary Table S3). In comparison to adjacent normal tissues, C10orf90 mRNA is significantly decreased in BRCA, COAD, HNSC, KIRC, and PRAD, while significantly increased in LUAD, LUSC, and UCEC (Supplementary Figure S1). Similarly, analysis of HPA data indicates that the levels of the C10orf90 protein expression are decreased in liver and kidney cancer tissues compared to normal tissues (Figure 3C). Furthermore, we examined the transcript levels of C10orf90 mRNA in different subtypes of various cancers, uncovering diverse expression patterns of C10orf90 mRNA in different subtypes of BRCA, SKCM, and TGCT (Figure 3D and Supplementary Figure S2).
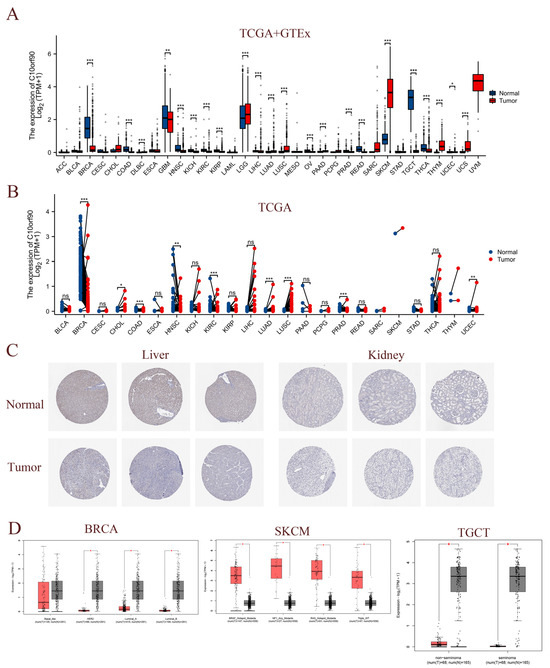
Figure 3.
Expression of C10orf90 in various tumor tissues. (A) Expression of C10orf90 mRNA in TCGA + GTEx database. (B) Expression of C10orf90 mRNA in TCGA database. (C) In HPA data, the expression of C10orf90 protein is lower in liver cancer and kidney cancer compared to normal tissues (antibody HPA075229). (D) GEPIA2 database analysis of C10orf90 expression in different subtypes of BRCA, SKCM, and TGCT. (* p < 0.05, ** p < 0.01, *** p < 0.001).
2.3. Prognostic Relevance of C10orf90 Expression in Various Tumors
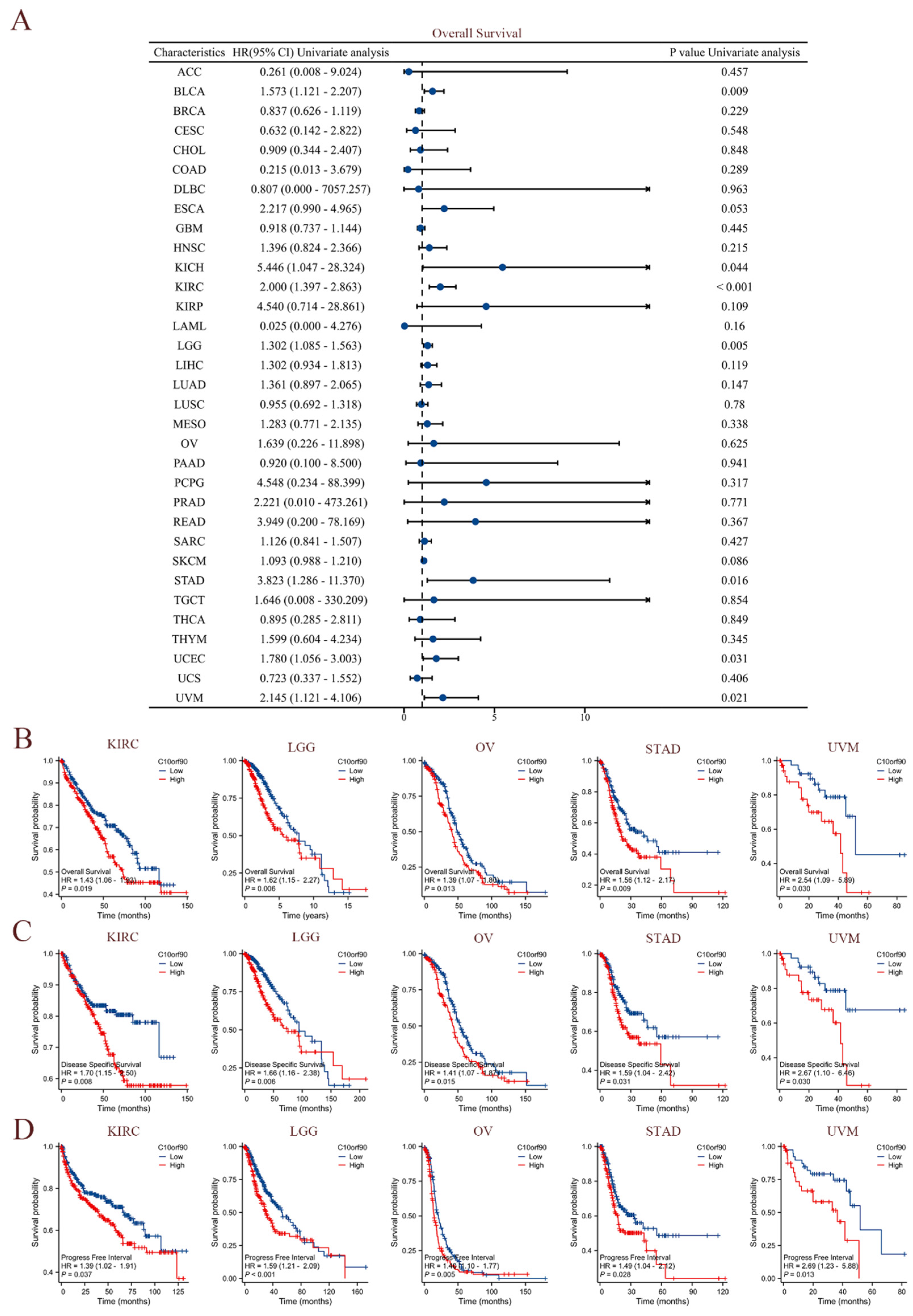
The clinical data sourced from TCGA were utilized to perform univariate Cox regression analysis, investigating the association between C10orf90 expression and overall survival (OS), disease-specific survival (DSS), and progression-free interval (PFI) across diverse cancer types. As demonstrated in Figure 4A, elevated levels of C10orf90 expression were significantly associated with worse overall survival rates among individuals diagnosed with BLCA, KICH, KIRC, LGG, STAD, UCEC, and UVM. Among these cancers, KIRC exhibited the most pronounced association with C10orf90. The forest plot and Cox assessment indicate that C10orf90 is an unfavorable factor for disease-specific survival in patients with BLCA, ESCA, KICH, KIRC, LGG, LIHC, MESO, and UVM (Supplementary Figure S3). In the analysis of cancer progression-free intervals, C10orf90 was identified as a potential risk factor associated with BLCA, KICH, KIRC, LGG, LIHC, PRAD, STAD, UCEC, and UVM. In addition, there is a significant correlation between high levels of C10orf90 expression and unfavorable prognostic outcomes in several types of cancer, including KIRC, LGG, OV, STAD, and UVM. Patients with high C10orf90 expression levels tended to have a worse prognosis compared to those with lower expression levels (Figure 4B–D). In conclusion, C10orf90 expression demonstrated prognostic relevance in different types of tumors.
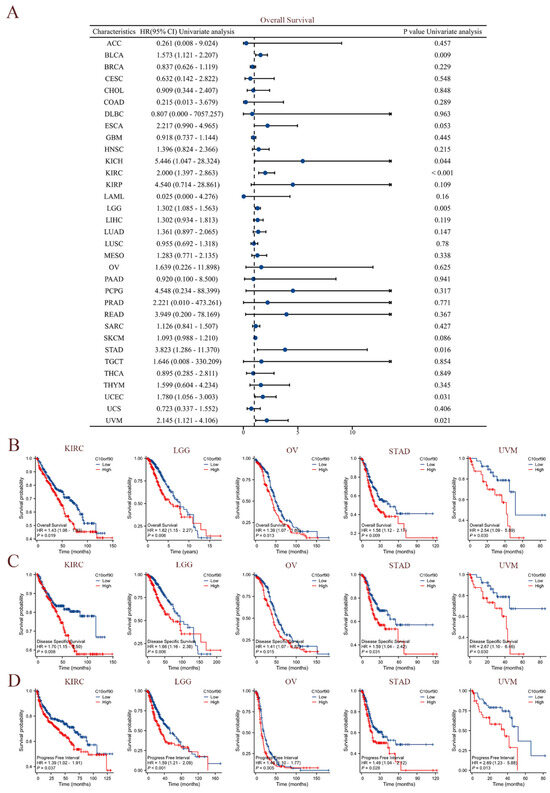
Figure 4.
Prognostic correlation of C10orf90 expression in multiple tumors. (A) The expression C10orf90 is associated with overall survival. (B) Overall survival K-M curves of C10orf90 expression in KIRC, LGG, OV, STAD, and UVM patients. (C) Disease-specific survival K-M curves of C10orf90 expression in KIRC, LGG, OV, STAD, and UVM patients. (D) Progression-free interval K-M curves of C10orf90 expression in KIRC, LGG, OV, STAD, and UVM patients.
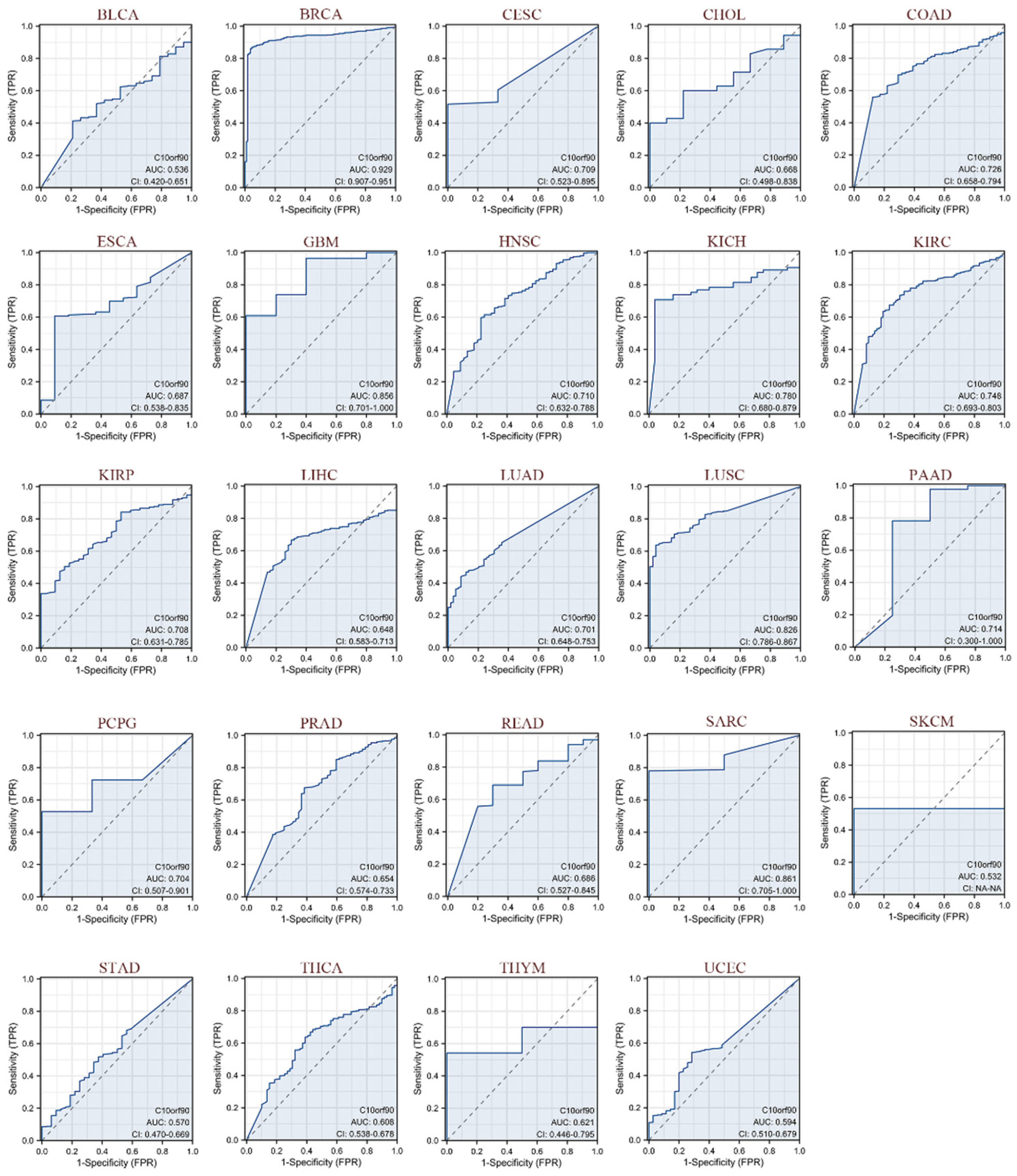
2.4. Diagnostic Value of C10orf90 in Various Cancers
As illustrated in Figure 5, we evaluated the diagnostic performance of C10orf90 in various cancers using receiver operating characteristic (ROC) curves. Our findings indicate that C10orf90 demonstrates favorable diagnostic value across multiple cancers, as evidenced by an area under the curve (AUC) exceeding 0.5 in 24 cancer types and even surpassing 0.7 in 13 types of cancers. C10orf90 exhibits certain accuracy and high diagnostic value in predicting BRCA (AUC = 0.929), CESC (AUC = 0.709), COAD (AUC = 0.726), GBM (AUC = 0.856), HNSC (AUC = 0.710), KICH (AUC = 0.780) and KIRP (AUC = 0.708). The remaining cancers for which the model exhibited accuracy were KIRC (AUC = 0.748), KIRP (AUC = 0.708), LUAD (AUC = 0.701), LUSC (AUC = 0.826), PAAD (AUC = 0.714), PCPG (AUC = 0.704) and SARC (AUC = 0.861).
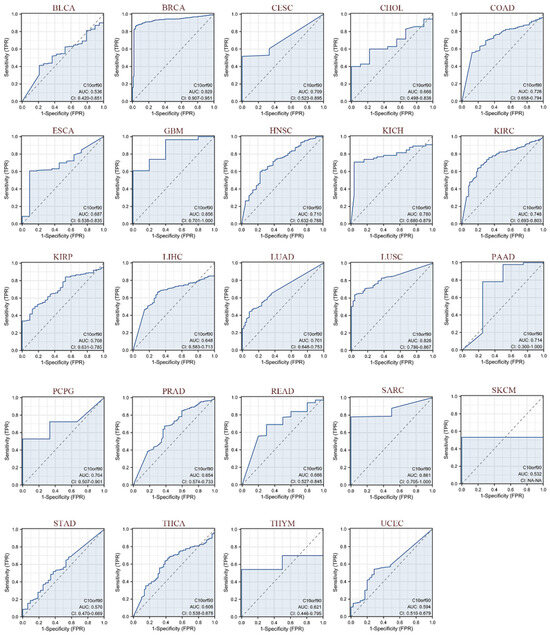
Figure 5.
ROC curves of C10orf90 in multiple cancers. Cancers with AUC > 0.5 include BLCA, BRCA, CESC, CHOL, COAD, ESCA, GBM, HNSC, KICH, KIRC, KIRP, LIHC, LUAD, LUSC, PAAD, PCPG, PRAD, READ, SARC, SKCM, STAD, THCA, THYM, and UCEC. (Blue curve is the ROC curve and the dotted line is the baseline).
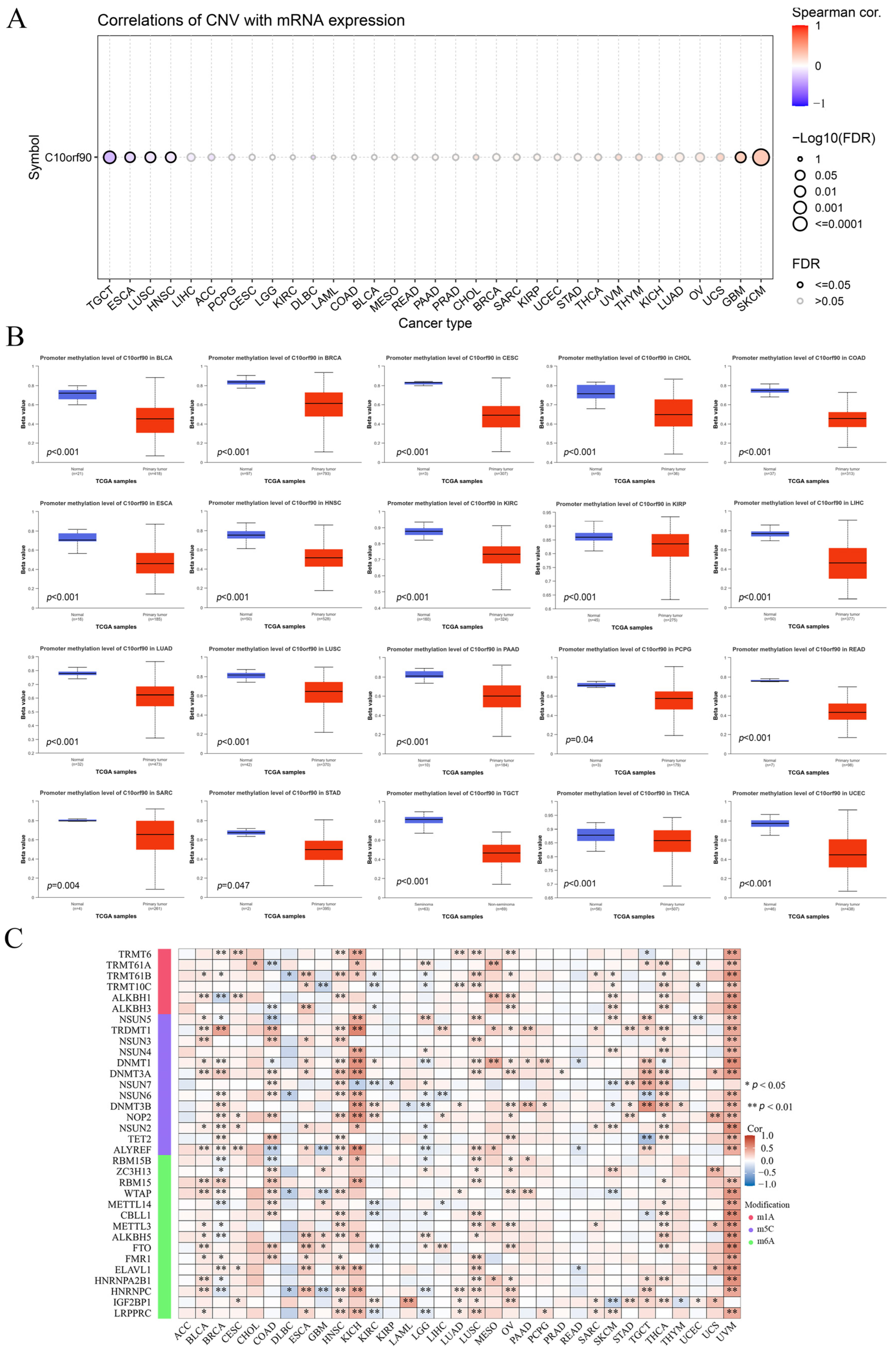
2.5. Association between C10orf90 Expression and CNV and Gene Methylation
We investigated the mechanisms behind gene copy number variation (CNV) and abnormal expression of C10orf90 mRNA in tumors. The results are presented in Figure 6A and Supplementary Figure S4. The expression of C10orf90 in patients with TGCT, ESCA, LUSC, and HNSC was negatively correlated with CNV. However, in patients with GBM and SKCM, the expression of C10orf90 mRNA was positively correlated with CNV expression, while the correlation was not significant in other tumor types. This suggests that CNV may not be the primary factor contributing to the abnormal expression of C10orf90. To address this issue, we assessed the promoter DNA methylation levels of C10orf90 in various tumors. This investigation focused on the epigenetic regulation of C10orf90 gene transcription (Figure 6B). The promoter of C10orf90 exhibits hypomethylation in various cancers, such as BLCA, BRCA, CESC, CHOL, COAD, ESCA, HNSC, KIRC, KIRP, LIHC, LUAD, LUSC, PAAD, PCPG, READ, SARC, STAD, TCGT, THCA, and UCEC. Similarly, the correlation between C10orf90 and genes related to N1-methyladenosine (m1A), 5-methylcytosine (m5C), and N6-methyladenosine(m6A) was assessed to explore the mechanisms underlying the inconsistent levels of C10orf90 promoter DNA methylation observed in multiple cancers. The results demonstrated that C10orf90 exhibited correlations with m1A, m5C, and m6A-related genes in most cancers, particularly in BLCA, HNSC, KICH, LUSC, OV, THCA, and UVM. In these cancers, C10orf90 was positively correlated with the majority of methylation-related genes (Figure 6C and Supplementary Table S4). The aforementioned results indicate that the C10orf90 gene may exert a functional influence by modulating CNV and gene methylation, thereby regulating gene expression levels in tumors.
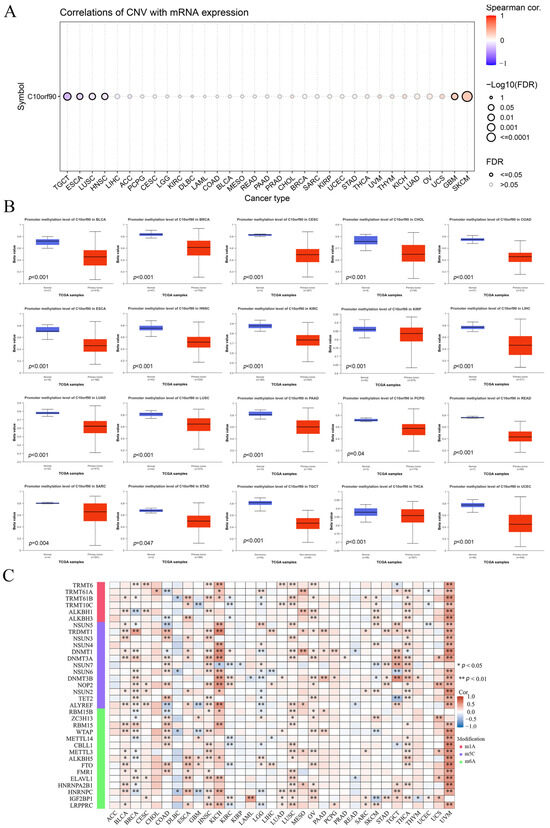
Figure 6.
Association between C10orf90 expression and CNV and gene methylation. (A) Correlation between CNV and C10orf90 expression in GSCA database. (B) Promoter methylation levels of C10orf90 in 20 cancers from UALCAN. (C) Correlation analysis of C10orf90 expression with mRNA modification methylation regulators.
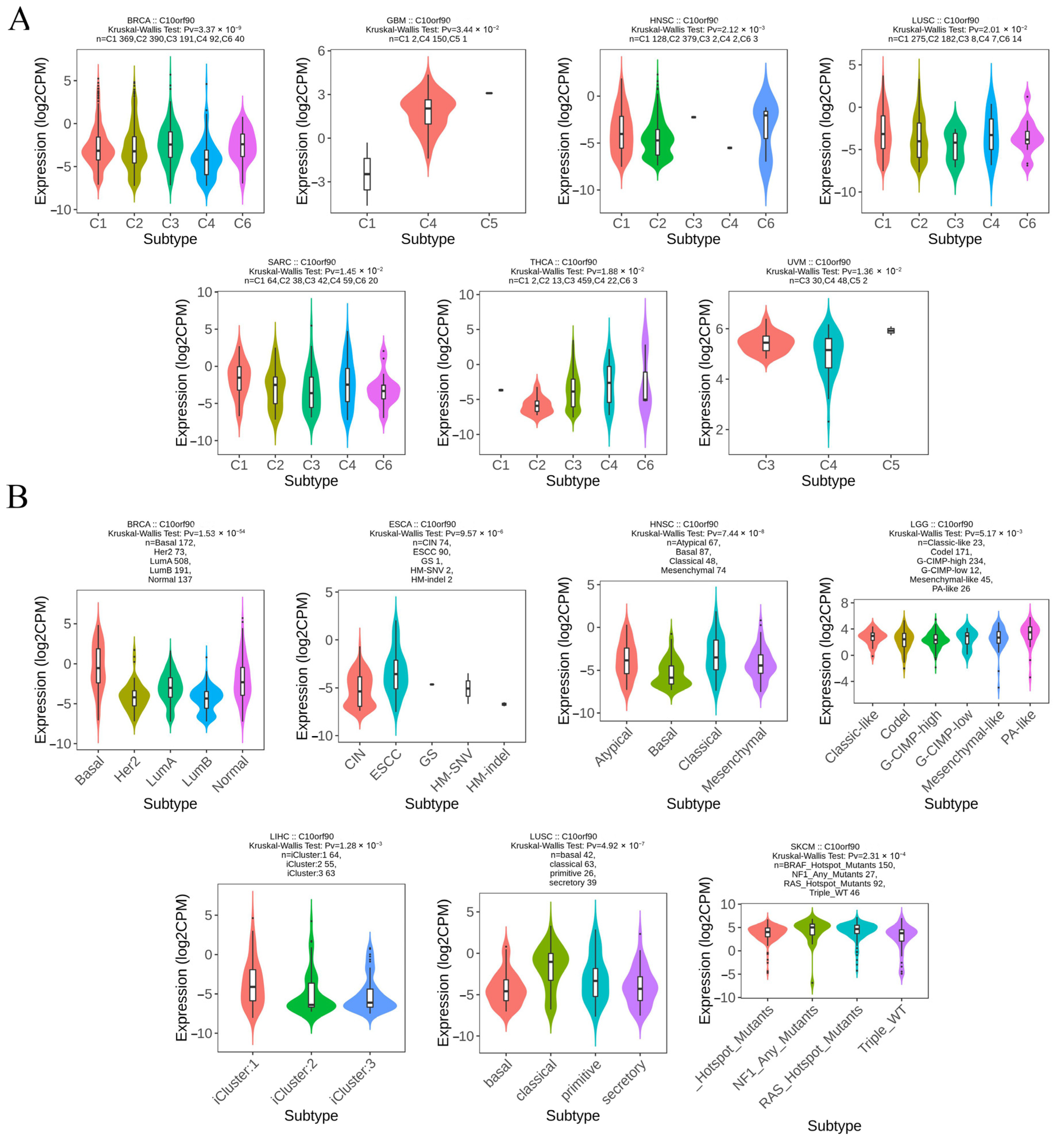
2.6. Expression of C10orf90 in Different Immune and Molecular Subtypes of Various Cancers
We analyzed the levels of C10orf90 expression across various cancer immune and molecular subtypes using the TISIDB database. The results, as illustrated in Figure 7A, demonstrate a notable disparity in the expression levels of C10orf90 across various immune subtypes of multiple cancers, including BRCA, GBM, HNSC, LUSC, SARC, THCA, and UVM. For the molecular subtypes (Figure 7B), differential expression of C10orf90 was observed among patients with BRCA, ESCA, HNSC, LGG, LIHC, LUSC, and SKCM. However, for other cancers, including immune subtypes such as BLCA, CHOL, ESCA, LGG, LIHC, SKCM, and USC, as well as molecular subtypes like GBM, the expression of C10orf90 is not statistically significant (Supplementary Figure S5).
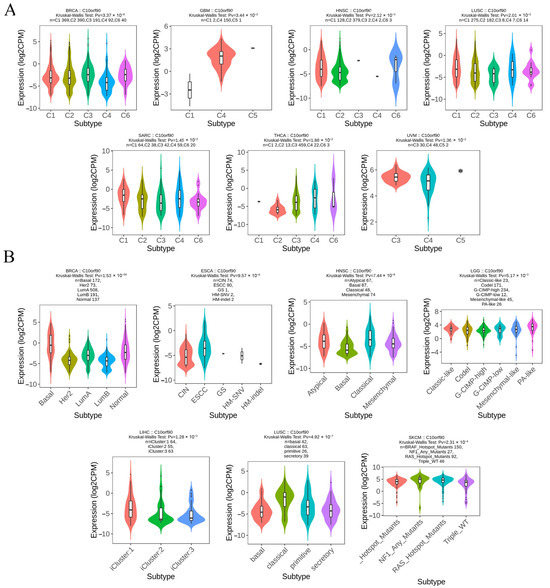
Figure 7.
Expression of C10orf90 in different immune and molecular subtypes of various cancers. (A) Correlation between C10orf90 and immune subtypes of cancers, including BRCA, GBM, HNSC, LUSC, SARC, THCA, and UVM. (B) Correlation between C10orf90 and cancer molecular subtypes, including BLCA, CHOL, ESCA, LGG, LIHC, SKCM, and USC.
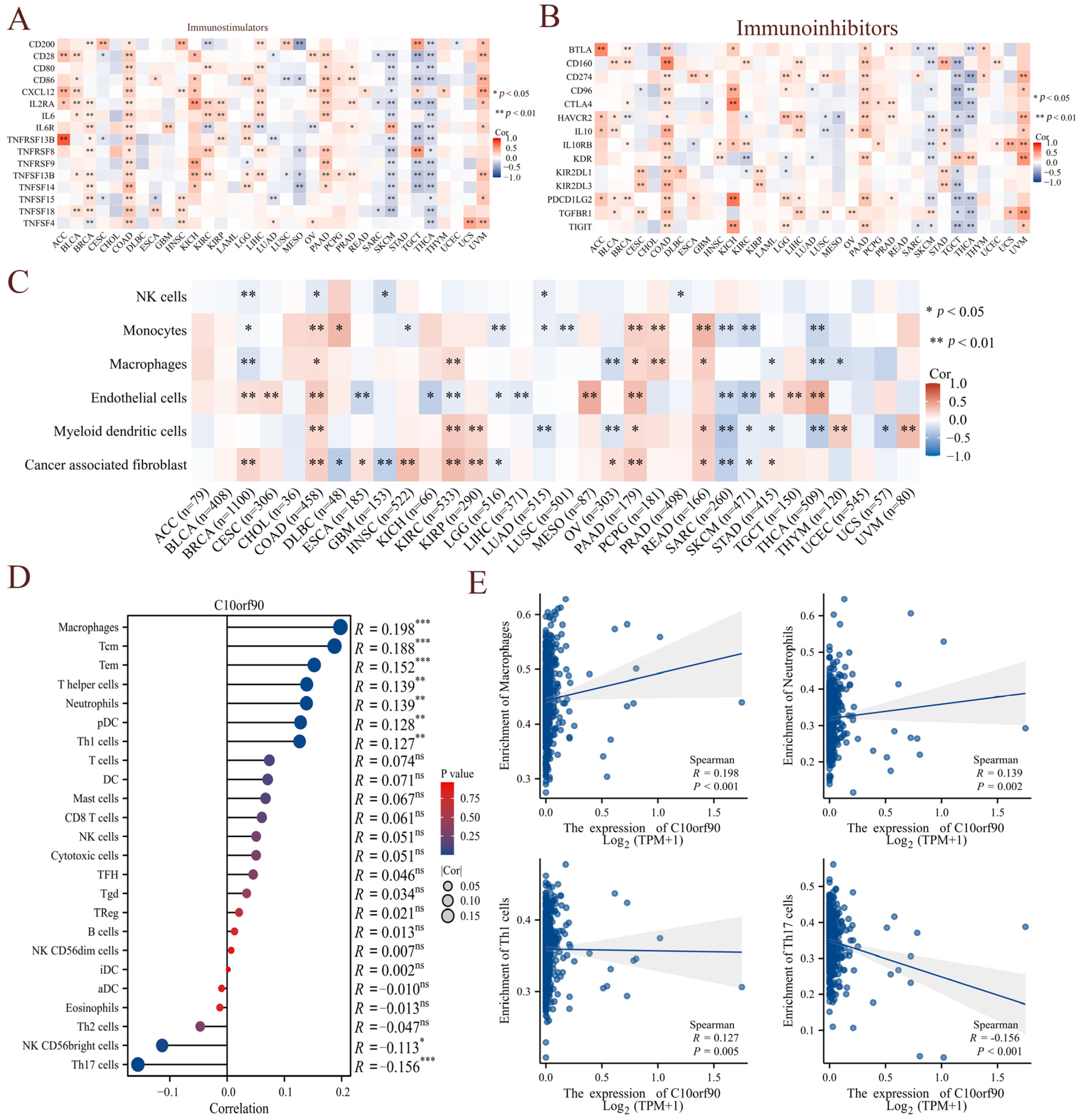
2.7. Correlation between C10orf90 Expression and Immune Infiltration
Given the presence of immune cell infiltration in the tumor microenvironment, which is closely linked to the efficacy of immunotherapy, we initially investigated the correlation between C10orf90 and genes identified as immune checkpoint genes. The results demonstrated that among the immunostimulators, C10orf90 exhibited a positive correlation with the immunostimulators in tumors such as COAD, PAAD, and UVM, and a negative correlation with SKCM, TGCT, and THCA cancers (Figure 8A and Supplementary Table S5). Conversely, C10orf90 demonstrated a similar pattern of correlation with immunoinhibitors in tumors such as COAD, PAAD, and UVM, with a negative correlation observed in SKCM, TGCT, and THCA cancers (Figure 8B and Supplementary Table S6). Subsequently, the correlation between C10orf90 expression in pan-cancer and the degree of tumor-infiltrating immune cells was validated using the TIMER database. The correlation coefficients of six tumor-infiltrating immune cells (NK cells, monocytes, macrophages, endothelial cells, myeloid dendritic cells, and cancer-associated fibroblasts) were collected and presented in a heatmap format (Figure 8C). The most pronounced correlation between C10orf90 expression levels and immune cell infiltration was observed in COAD, followed by BRCA and PAAD. Given the strongest association identified between C10orf90 and immune infiltration in COAD, our analysis focused on exploring the relationship between C10orf90 and 24 different subtypes of immune cells in COAD as a case study. The expression of the C10orf90 gene was found to be positively correlated with the presence of macrophages, Tcm, Tem, T helper cells, neutrophils, pDC, and Th1 cells. Conversely, it is negatively correlated with the presence of NK CD56 bright cells and Th17 cells (Figure 8D,E). These findings indicate that C10orf90 has the potential to influence the immune response by regulating genes related to immune modulation and the presence of immune infiltrating cells within tumors. This impact appears to be particularly significant in the context of COAD, as the extent of immune cell infiltration has been associated with the level of C10orf90 expression.
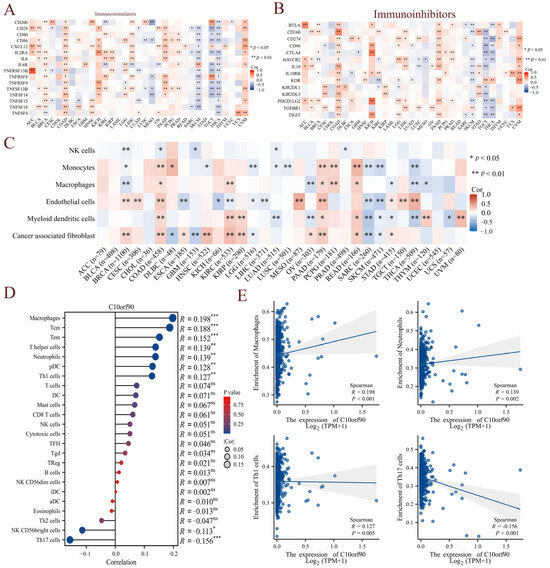
Figure 8.
Correlation between C10orf90 expression and immune infiltration. (A) Correlation between C10orf90 and immunostimulators. (B) Correlation between C10orf90 and immunoinhibitors. (C) Correlation between C10orf90 and immune cell infiltration. (D) Correlation between C10orf90 and immune cell subtypes in COAD. (E) Correlation between C10orf90 and macrophages, neutrophils, Th1 cells, and Th17 cells in COAD. (Blue points represent the expression levels of C10orf90 in different samples, and the shadow indicate the confidence intervals of the data) (* p < 0.05, ** p < 0.01, *** p < 0.001).
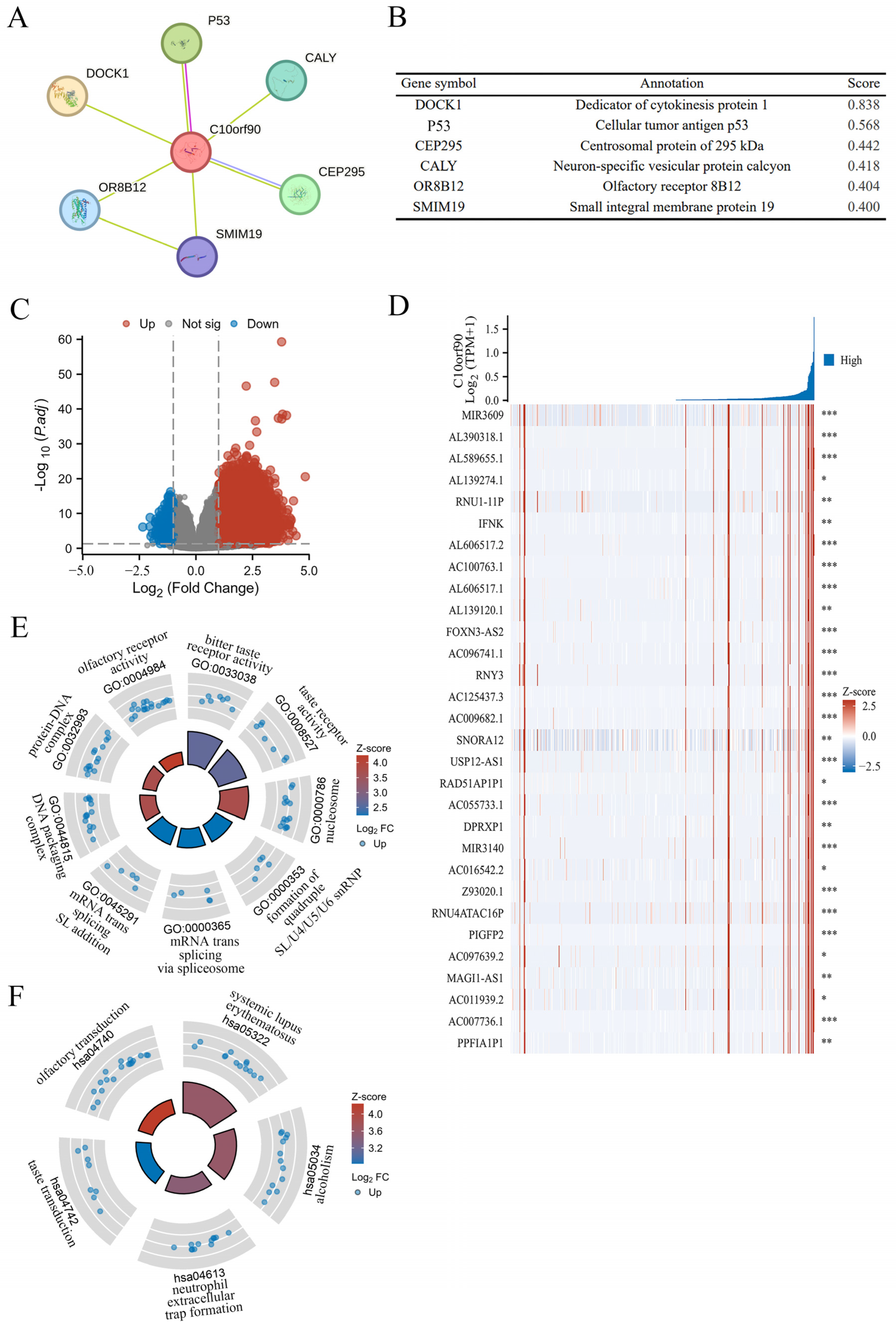
2.8. PPI Network and Gene Function Enrichment Analysis of C10orf90
Previous research findings indicate that C10orf90 expression is significantly downregulated in colon adenocarcinoma compared to normal and adjacent tissues, which has been demonstrated to have a high diagnostic value. In addition, the most significant correlation between the C10orf90 gene and immune infiltration in COAD was utilized as a case study to explore the biological functions of C10orf90 in COAD. This was achieved through protein–protein interaction (PPI) network analysis and gene function enrichment analysis of C10orf90. The PPI network analysis demonstrated that the C10orf90 protein may interact with associated proteins, including DOCK1, p53, CEP295, CALY, OR8B12, and SMIM19, with scores of 0.838, 0.568, 0.442, 0.418, 0.404 and 0.400, respectively (Figure 9A,B). The DOCK1 and p53 proteins exhibited a strong correlation with C10orf90 in colon cancer patients. DOCK1 has been demonstrated to facilitate the movement and infiltration of cancer cells, while p53 proteins are crucial in inhibiting cancer cell growth. Considering the biological functions of DOCK1 and p53 proteins, it is reasonable to suggest that C10orf90 could also have a significant impact on COAD tumorigenesis and progression.
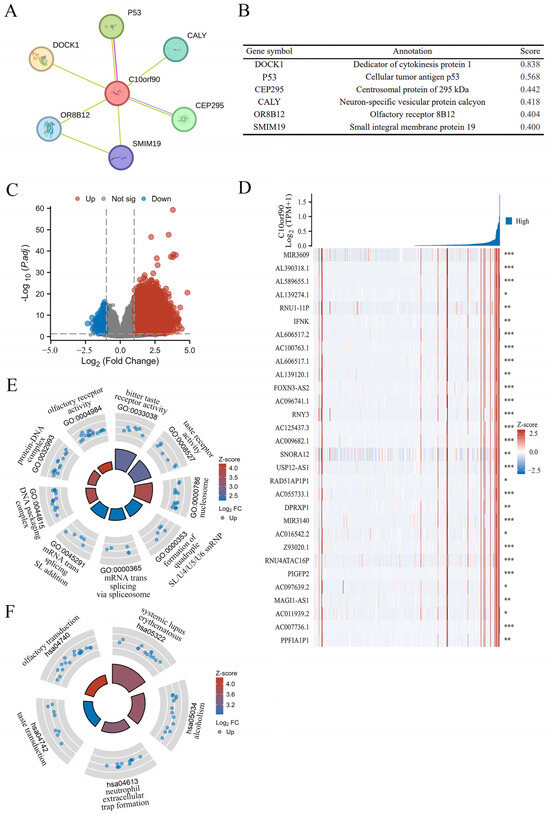
Figure 9.
PPI network and gene function enrichment analysis of C10orf90. (A) The C10orf90 protein interacts with a PPI network. (B) The PPI network co-expression score. (C) A volcano plot of significantly correlated genes with C10orf90 in COAD is presented. (D) Heatmap of the top 30 genes positively associated with the C10orf90 gene in COAD. (E) GO enrichment analysis. (F) KEGG enrichment analysis. (* p < 0.05, ** p < 0.01, *** p < 0.001).
Gene enrichment analysis revealed that there were 3256 differential genes associated with C10orf90 expression in COAD patients. Among these, 3218 were upregulated and 38 were down regulated (absLogFC > 1.5, p.adj < 0.01) (Figure 9C and Supplementary Table S7). Furthermore, the top 30 genes with the most significant positive correlation with the C10orf90 gene were displayed in the gene expression heat map (Figure 9D). Concurrently, the disproportionately low number of downregulated genes prompted the analysis of 3218 upregulated genes that are positively associated with C10orf90 gene expression for GO and KEGG pathway enrichment. At p.adj < 0.01, an analysis of GO enrichment revealed 11 biological processes (GO-BP), 13 cellular component (GO-CC), and 3 molecular function (GO-MF) pathways, in addition to 6 pathways identified by KEGG analysis (Supplementary Table S8). The bubble chart illustrated the top three enriched pathway information for BP, CC, and MF, as well as the top five pathways for KEGG (Figure 9E,F). The most significant findings of the GO analyses were the bitter taste receptor activity of MF (GO:0033038), the nucleosome of CC (GO:0000786), the quadruple SL/U4/U5/U6 snRNP of BP (GO:0000353), and the KEGG analysis was most notable for systemic lupus erythematosus (hsa05322). The gene enrichment analysis suggests that in COAD, the C10orf90 gene may influence tumor progression through the above-mentioned pathway and thus may be involved in the development of COAD.
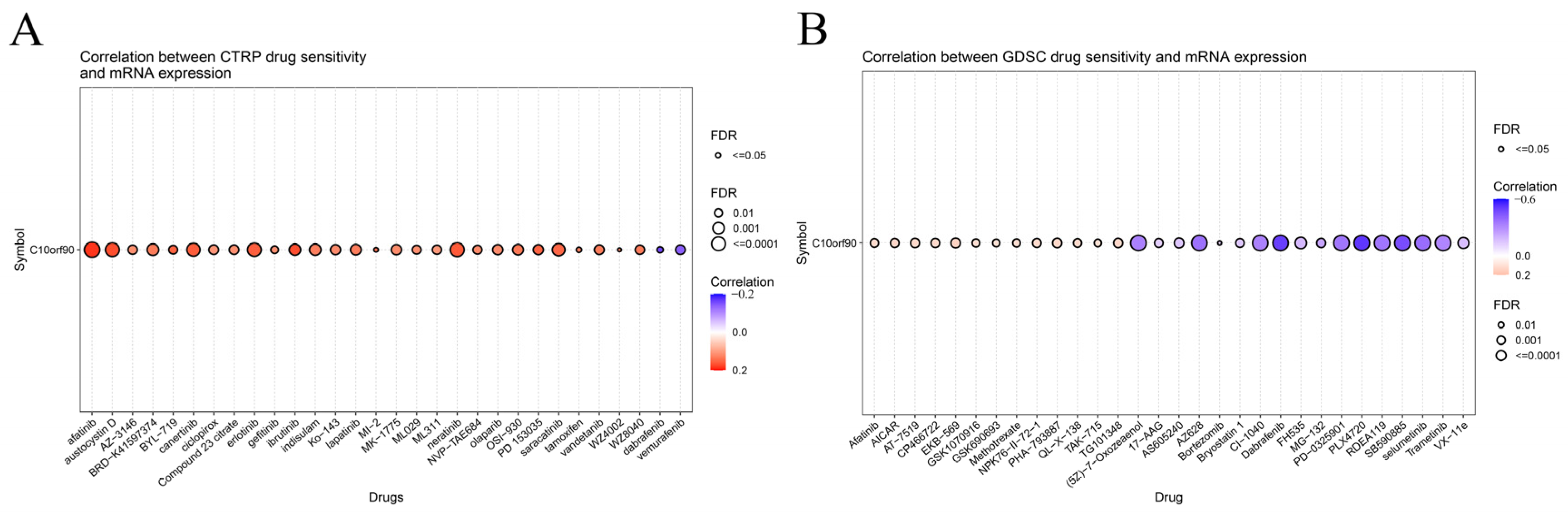
2.9. Sensitivity Analysis of C10orf90 Related Drugs
We investigated the drug sensitivity of C10orf90 expression in tumors. The CTPR database showed a correlation between C10orf90 expression and drug sensitivity. Several drugs positively correlate with C10orf90 expression, the top three being afatinib (an ErbB family blocker), ibrutinib (a Bruton’s tyrosine kinase inhibitor), and austocystin D (a DNA damage agent). In contrast, the 50% inhibitory concentration (IC50) values of the drugs dabrafenib (a BRAF inhibitor) and vemurafenib (a BRAF inhibitor) were negatively associated with C10orf90 expression. The inhibitory concentration (IC50) values were negatively correlated with C10orf90 expression (Figure 10A and Supplementary Table S9). Additionally, based on the GDSC drug sensitivity results, the top three drugs positively correlated with C10orf90 expression were EKB-569 (an EGFR-TK inhibitor), AICAR (an AMPK activator) and PHA-793887 (a CDK1 inhibitor). Conversely, the top three drugs negatively correlated with C10orf90 expression were PLX4720 (a ZAK inhibitor), dabrafenib, and SB590885 (a BRAF inhibitor) (Figure 10B and Supplementary Table S10).
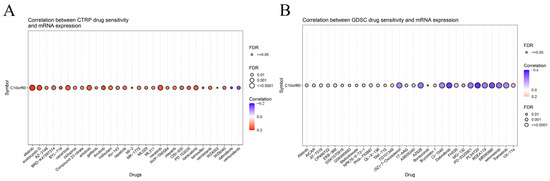
Figure 10.
Sensitivity analysis of C10orf90 related drugs. (A) Relationship between C10orf90 and CTRP drug sensitivity. (B) Relationship between C10orf90 and GDSC drug sensitivity.
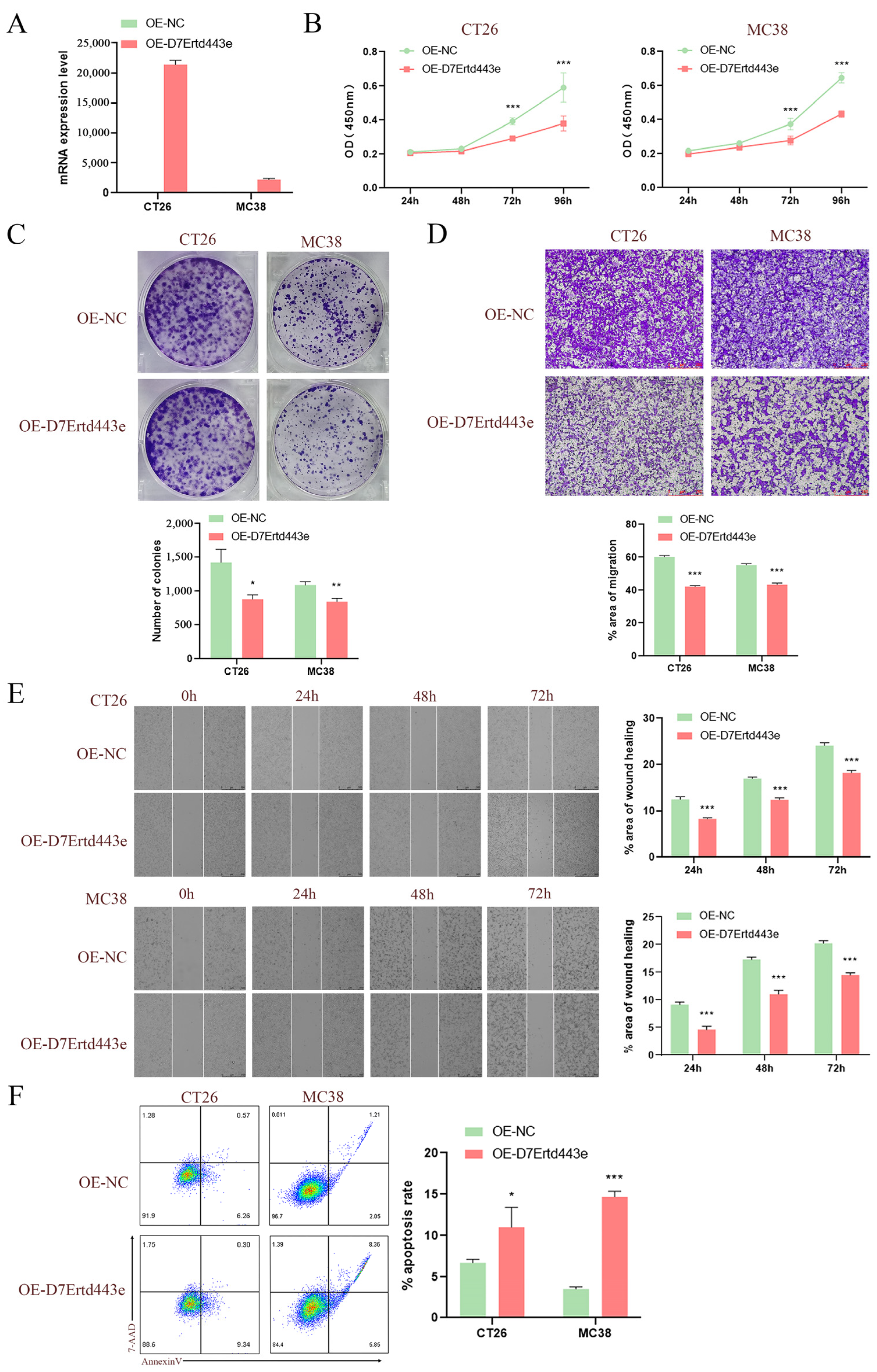
2.10. Overexpression of C10orf90 Inhibits Colon Cancer Cell Proliferation and Tumor Migration
Given that the C10orf90 gene is expressed at low levels in COAD, we utilized lentiviral constructs of two murine-derived colon cancer cell lines (CT26 and MC38) overexpressing the D7Ertd443e (C10orf90 homolog) gene to investigate the potential role of the D7Ertd443e gene in colon cancer. The results demonstrated the efficacy of the protocol for constructing CT26 and MC38 cells overexpressing the D7Ertd443e gene (Figure 11A). Subsequent to lentiviral infection, the results from CCK-8 and plate cloning assays demonstrated that the overexpression of D7Ertd443e inhibited COAD cell proliferation (Figure 11B,C). Furthermore, the Transwell and wound-healing assays revealed that overexpression of D7Ertd443e inhibited the migratory potential of the identified COAD cells (Figure 11D,E). It is noteworthy that overexpression of D7Ertd443e was found to induce apoptosis in COAD cells (Figure 11F). In conclusion, these findings indicate that D7Ertd443e (C10orf90 homolog) has the potential to significantly inhibit colon cancer cell proliferation and tumor migration while promoting apoptosis.
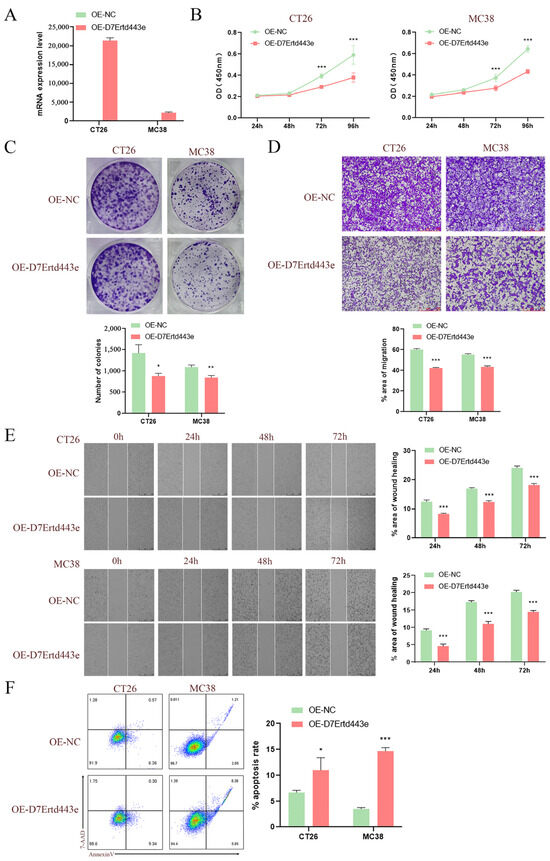
Figure 11.
Overexpression of C10orf90 inhibits colon cancer cell proliferation and tumor migration. (A) The mRNA expression level of D7Ertd443e in transfected cells. (B) CCK-8 assay of the impact of D7Ertd443e on tumor cell proliferation. (C) Plate cloning assay of the effect of D7Ertd443e on tumor cell proliferation. (D) Transwell assay of the impact of D7Ertd443e on tumor cell migration (scale: 250 μm). (E) Wound-healing assay of the effect of D7Ertd443e on tumor cell migration (scale: 500 μm). (F) Cell apoptosis assay of the effect of D7Ertd443e on tumor cell apoptosis. (Different colors represent cell density) (* p < 0.05, ** p < 0.01, *** p < 0.001).
3. Discussion
The C10orf90 gene is characterized by a distinctive fragile site structure, which is susceptible to disruption during the rapid proliferation of tumor cells. This can lead to genomic instability and impede the progression of cancer. Studies have demonstrated that the C10orf90 protein can inhibit the degradation rate of p53 and p21 proteins, thereby contributing to the occurrence and development of cancers, including non-small cell lung cancer, breast cancer, conjunctival melanoma, and pterygium and pinguecula. The actions of this protein have been shown to influence the survival outcomes and prognoses of individuals with these types of cancer. Therefore, we conducted a pan-cancer analysis of the C10orf90 gene using multiple databases and bioinformatics tools. Subsequently, we combined this analysis with cellular experiments to explore the potential role of C10orf90 in COAD.
The findings of the pan-cancer study suggest that the C10orf90 protein is present in a variety of bodily organs and tissues, including the testis, brain, breast, forearm, colon, kidney, liver, and pancreas. Subcellular localization suggests that C10orf90 functions in microtubules and cytoplasmic solutes. In comparison to normal tissues, C10orf90 is significantly upregulated in DLBC, LGG, LUAD, LUSC, PAAD, SKCM, THYM, UCS, and UCEC. Conversely, C10orf90 is downregulated in BRCA, COAD, GBM, HNSC, KICH, KIRC, KIRP, LIHC, OV, PRAD, READ, TGCT and THCA. In comparison to adjacent tissues, C10orf90 mRNA is significantly downregulated in BRCA, COAD, HNSC, KIRC, and PRAD, and significantly upregulated in LUAD, LUSC, and UCEC. Moreover, the protein expression level of C10orf90 is decreased in liver cancer and kidney cancer tissues, and the expression varies among different subtypes of BRCA, SKCM, and TGCT. These findings indicate that C10orf90 expression levels are heterogeneous across multiple tumors, suggesting the need for further investigation and analysis.
Our results indicate that C10orf90 has prognostic and diagnostic value in various cancers. Univariate Cox regression and Kaplan–Meier analysis demonstrated that C10orf90 expression was significantly associated with overall survival, disease-specific survival, and progression-free interval in KIRC, LGG, OV, STAD, and UVM. Patients with high C10orf90 expression exhibited a worse prognosis than those exhibiting low C10orf90 expression. However, it is important to note that the expression level of C10orf90 is generally downregulated in patients with tumors. While the low expression of the C10orf90 gene may not be a primary factor influencing survival rates, it still holds some prognostic significance. In terms of diagnostic value, the area under the ROC curve is above 0.5 in 24 types of cancer and exceeds 0.7 in 13 types of cancer, with BRCA having the highest AUC (0.929). It has been demonstrated that the expression of C10orf90 is downregulated in breast cancer samples and that cancer patients exhibit significant genetic variations in the C10orf90 gene, which are associated with susceptibility to breast cancer [15]. These findings indicate that C10orf90 has superior prognostic prediction and diagnostic value in these cancers, with potential applications in the clinical setting.
Tumor tissues exhibit genetic copy number variation and abnormal DNA methylation, which regulate cancer development [16,17]. Therefore, we analyzed the relationship between C10orf90 and CNV and DNA methylation using the GSCA and UALCAN databases. We observed a correlation between C10orf90 and CNV in 6 cancer types. Additionally, the promoter of C10orf90 exhibited hypomethylation in 20 cancer types. Furthermore, C10orf90 showed a correlation with m1A, m5C, and m6A-related genes in tumors. In BLCA, HNSC, KICH, LUSC, OV, THCA, and UVM, C10orf90 was positively correlated with the majority of methylation-related genes. DNA methylation is a common biochemical process that regulates gene expression and gene stability. However, in most tumor cells, aberrant methylation is prevalent in the promoters of some crucial tumor suppressor genes, leading to the dysregulation of DNA repair and chromosome stability genes. This, in turn, results in uncontrolled cancer development [18]. In conclusion, the results indicate that C10orf90 may exert gene functions in tumors by affecting gene copy number variation and DNA methylation to regulate gene expression levels.
The capacity of immune cells to eliminate tumor cells and impede cancer progression is a crucial aspect of cancer immunotherapy. Consequently, investigating the extent of immune cell infiltration within the tumor microenvironment is essential for advancing our understanding of this therapeutic approach [19]. Studies have demonstrated that C10orf90 can impede the TLR4-NF-κB activation pathway, inhibit the degradation of IκBα to suppress the NF-κB activation pathway and stimulate enhanced proliferation and activation of CD4+ T cells. The C10orf90 gene has been shown to promote the phenotypic shift from M2-like macrophages to anti-tumor M1-like macrophages. This ultimately inhibits melanoma and pancreatic tumorigenesis and development. This suggests that C10orf90 plays an important role in immune infiltration [20]. Consequently, we analyzed the expression levels of C10orf90 in immune subtypes and molecular subtypes of multiple cancers. The expression levels of C10orf90 are significantly different in the immune subtypes of tumors such as BRCA, GBM, HNSC, LUSC, SARC, THCA, and UVM. For molecular subtypes, C10orf90 exhibits different expression patterns in patients with BRCA, ESCA, HNSC, LGG, LIHC, LUSC, and SKCM. Immune checkpoint genes are mainly categorized into two groups: immune stimulation and immune inhibition. The data indicate that among immunostimulators, C10orf90 is positively correlated with immunostimulators in tumors such as COAD, PAAD, and UVM, while it is negatively correlated in cancers such as SKCM, TGCT, and THCA. In addition, C10orf90 is positively correlated with immunoinhibitors in tumors such as COAD, PAAD, and UVM, while it is negatively correlated in cancers such as SKCM, TGCT, and THCA. In the TIMER database, we identified a correlation between C10orf90 expression and six types of tumor-infiltrating immune cells, including NK cells, monocytes, macrophages, endothelial cells, myeloid dendritic cells, and cancer-associated fibroblasts. Interestingly, the C10orf90 gene shows the strongest association with immune infiltration in COAD. Further analysis revealed a strong positive correlation between C10orf90 gene expression and macrophages, Tcm, Tem, T helper cells, neutrophils, pDC, and Th1 cells in COAD. Conversely, a strong negative correlation was observed between NK CD56 bright cells and Th17 cells. According to reports, during the pathogenesis of COAD, M2-like macrophages secrete IL-1β, which induces Wnt signaling and supports tumor cell growth [21]. T helper cells are a crucial component of the adaptive immune system. Th1 cells can enhance the effector function of tumor-infiltrating lymphocytes and exert anti-tumor killing function, as evidenced by research in reference [22]. Th17 cells secrete IL-17A, which induces pyroptosis in colon cancer cells by stimulating the production of reactive oxygen species (ROS). Additionally, IL-17A recruits more CD8+ T cells to the tumor microenvironment, thereby demonstrating an anti-tumor immune response [23]. These findings suggest that C10orf90 might modulate tumor immunity through its influence on immune regulatory genes and immune infiltrating cells, especially in COAD. This highlights the importance of studying C10orf90 expression as part of tumor immunotherapy.
The PPI network was constructed using STRING, and the C10orf90 protein may be involved in interactions with related proteins, including DOCK1, p53, CEP295, CALY, OR8B12, and SMIM19. DOCK1 functions as a GTPase exchange factor that activates Rac proteins and stimulates actin polymerization at the membrane surface, thereby altering the cytoskeletal structure and cellular morphology to enhance the migration and invasive capabilities of tumor cells such as glioma, breast cancer, ovarian cancer, and other types of tumors [24]. The TP53 gene functions as a tumor suppressor by primarily promoting cellular apoptosis and facilitating the repair of DNA damage [25,26]. CEP295 is an essential protein for centriole formation and interacts directly with microtubules through its distinctive structural domains. It recruits upstream effectors in mitotic S and G2 phases, assembles centriole microtubules, and participates in post-translational modifications [27]. CALY is primarily involved in the clathrin-mediated endocytic machinery and regulates vesicular trafficking [28]. Although DOCK1, p53, CEP295, and CALY were identified as proteins interacting with C10orf90, further experimental validation of their interactions during carcinogenesis is necessary. In addition, we performed GO and KEGG pathway enrichment analyses. The differential genes linked to C10orf90 expression in COAD patients were subjected to analysis. The GO results showed that C10orf90 may regulate tumorigenesis and development through bitter taste receptor activity, taste receptor activity, nucleosome, DNA packaging complex, formation of quadruple SL/U4/U5/U6 snRNP and mRNA trans-splicing via spliceosome pathway effects. The KEGG analysis revealed that C10orf90 was associated with systemic lupus erythematosus, alcoholism, neutrophil extracellular trap formation, taste transduction, olfactory transduction, and neuroactive ligand-receptor interaction. These results indicate that in COAD, the C10orf90 gene plays important biological functions through multiple signaling pathways.
The relationship between C10orf90 and drug sensitivity was investigated using data from the CTPR and GDSC databases. The CTPR and GDSC databases identified 65 and 70 drugs, respectively, which may be associated with the C10orf90 gene. Some of these drugs include afatinib, ibrutinib, austocystin D, dabrafenib, vemurafenib, EKB-569, AICAR, PHA-793887, PLX4720, and SB590885. These drugs are used clinically to prevent cancer progression, with afatinib [29], ibrutinib [30], dabrafenib [31], vemurafenib [32], AICAR [33], and PLX4720 [34] being commonly used anti-cancer drugs for COAD patients. The C10orf90 gene may serve as a marker for predicting the therapeutic efficacy of afatinib, ibrutinib, dabrafenib, vemurafenib, AICAR, and PLX4720 in patients with COAD.
Finally, the bioinformatics results were used to confirm that overexpression of the C10orf90 gene could inhibit the proliferation and migration of colon cancer cells, induce apoptosis, and play a positive anticancer role at the cellular level. This was achieved through the utilization of the following assays: CCK-8, plate cloning, Transwell, wound healing, and apoptosis assays.
This study is subject to certain limitations. Despite utilizing multiple databases and bioinformatics tools to analyze the role of the C10orf90 gene in various cancers, there is still a lack of extensive clinical samples and experimental data for validation. In addition, we have preliminarily demonstrated that at the cellular level, high expression of C10orf90 could inhibit the proliferation and migration of colon cancer cells and induce apoptosis. Therefore, further validation in vivo through animal experiments is necessary, as well as excavating the deep molecular mechanisms to reveal the biological effects of the C10orf90 gene in COAD.
4. Materials and Methods
4.1. Investigation of the Expression Patterns and Subcellular Localization of C10orf90
We conducted a comprehensive search of the Gene Expression Profiling Interactive Analysis 2 (http://gepia2.cancer-pku.cn/#general/ accessed on 22 May 2024), the Human Protein Atlas (https://www.proteinatlas.org/ accessed on 22 May 2024), the Cancer Genome Atlas (https://cancergenome.nih.gov/ accessed on 22 May 2024), and the Genotype-Tissue Expression (https://gtexportal.org/ accessed on 22 May 2024) databases to retrieve protein and RNA expression profiles of C10orf90 from normal samples [35,36,37]. Then, we used log2 transformation to process the RNAseq data from TCGA and GTEx. We downloaded the subcellular localization of C10orf90 in human cancer cell lines (SK-MEL-30, U-2 OS, and U-251 MG) from the HPA database. In this context, green represents the C10orf90 protein, blue indicates the nucleus, red denotes microtubule proteins, and yellow signifies the endoplasmic reticulum. The 33 cancer types included adrenocortical carcinoma (ACC), bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), esophageal carcinoma (ESCA), glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), acute myeloid leukemia (LAML), Brain lower grade glioma (LGG), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), mesothelioma (MESO), ovarian serous cystadenocarcinoma (OV), pancreatic adenocarcinoma (PAAD), pheochromocytoma and paraganglioma (PCPG), prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ), sarcoma (SARC), skin cutaneous melanoma (SKCM), stomach adenocarcinoma (STAD), testicular germ cell tumors (TGCT), thyroid carcinoma (THCA), thymoma (THYM), uterine corpus endometrial carcinoma (UCEC), uterine carcinosarcoma (UCS), and uveal melanoma (UVM).
4.2. Analysis of Prognostic and Diagnostic Value of C10orf90
In the clinical relevance section of the Xiantao tool (https://www.xiantao.love/ accessed on 29 May 2024), patients underwent analysis utilizing Cox regression and the Kaplan–Meier method to assess prognostic factors such as overall survival, disease-specific survival, and progression-free interval. Statistical analysis was conducted using the Wilcoxon test, with p < 0.05 indicating a significant difference. The expression data of the C10orf90 gene were employed in the construction of the ROC curve, with the area under the curve serving as a metric to assess its accuracy.
4.3. Analysis of CNV and Gene Methylation Correlations
The “Mutation” component of the Gene Set Cancer Analysis database (http://bioinfo.life.hust.edu.cn/GSCA/ accessed on 29 May 2024) was employed to examine the association between C10orf90 and gene copy number variations across different types of tumors [38]. We utilized the University of Alabama at Birmingham Cancer Database (http://ualcan.path.uab.edu/ accessed on 29 May 2024) to investigate the DNA methylation status of the C10orf90 promoter [39]. The β value was employed to quantify the level of DNA methylation, with values indicating low methylation (β: 0.25–0.3) and high methylation (β: 0.5–0.7). The associations between C10orf90 and genes associated with m1A, m5C, and m6A modifications were assessed through a heatmap analysis.
4.4. Expression of C10orf90 across Various Immune and Molecular Subtypes of Cancer
We utilized the “Subtype” module within the TISIDB database (http://cis.hku.hk/TISIDB/ accessed on 2 June 2024) to investigate the expression patterns of C10orf90 across different immune and molecular subtypes present in various tumor types [40], such as C1 (wound healing), C2 (IFN-γ dominant), C3 (inflammatory), C4 (lymphocyte depleted), C5 (immune quiescent), and C6 (TGF-β dominant).
4.5. Relationship between C10orf90 Expression and Immune Infiltration
The XCELL algorithm, derived from the Tumor Immune Estimation Resource 2.0 database, was used to evaluate the impact of C10orf90 on immune cell infiltration within tumors [41]. At the same time, the Xiantao tool was used to investigate the correlation between C10orf90 in COAD patients and various immune cell subtypes, as well as its association with specific immune cell subpopulations.
4.6. Protein–Protein Interaction Network Analysis and Enrichment Analysis of Gene Functions
The Search Tool for the Retrieval of Interacting Genes/Proteins database (www.string-db.org/ accessed on 6 June 2024) was used for all protein–protein interaction data [42]. Based on the TCGA data, the genes COAD and C10orf90 were found to be co-expressed (with absLogFC > 1.5 and p < 0.01), followed by enrichment analyses using GO and KEGG databases.
4.7. Analysis of Drug Sensitivity in Association with C10orf90
Drug sensitivity of C10orf90 gene expression was analyzed using the Genomics of Drug Sensitivity in Cancer (https://www.cancerrxgene.org/ accessed on 6 June 2024) and the Cancer Therapeutics Response Portal (http://portals.broadinstitute.org/ctrp/ accessed on 6 June 2024) databases [43,44].
4.8. Cell Lines, Culture and Transfection
The mouse colon cancer cell lines CT26 and MC38 were obtained from the Cell Resource Center, IBMS, CAMS/PUMC (Beijing, China). The cell culture environment was set at 37 °C, 5% CO2, in appropriate humidity. Specific cell culture parameters included the use of 10% fetal bovine serum (FBS) in DMEM basal medium purchased from Pricella, Wuhan, China. Recombinant lentivirus (OE-D7Ertd443e) for D7Ertd443e overexpression and lentiviral vector (OE-NC) for negative control were purchased and infected into CT26 and MC38 cells. Moreover, 10 μg/mL of puromycin was incubated for 48 h to screen the infected cells.
4.9. QRT-PCR
RNA was extracted using the TRIzol method (TaKaRa, Kusatsu, Japan), reverse transcribed into cDNA, and used in a qRT-PCR kit (Vazyme, Nanjing, China) for detection. PCR detection included reactions at 95 °C (30 s), 95 °C (10 s), and 60 °C (15 s) for 40 cycles and 72 °C (30 s). The sequences for the mouse D7Ertd443e primers were (F) 5′-AGACCATTAAACACTTCCCTCCT-3′ and (R) 5′-AAGGGCAATAAAACCCAGGCA-3′.
4.10. CCK-8 Assay
CT26 and MC38 cells infected with OE-D7Ertd443e and OE-NC were inoculated with 2 × 103 cells in a 96-well plate, and 100 μL/well was cultured in the culture system for 24 h, 48 h, 72 h, and 96 h, respectively, and added to 10 μL of CCK-8 reagent (GLPBIO, Montclair, NY, USA); the OD values were measured after 1 h.
4.11. Plate Cloning Assay
CT26 and MC38 cells infected with OE-D7Ertd443e and OE-NC were inoculated with 2 × 103 cells in 6-well plates and cultured for 10 days. The cells were rinsed with PBS buffer, treated with methanol for 30 min for fixation, and subsequently stained with 0.1% crystal violet solution for 30 min. They were then washed with pure water, dried, and photographed.
4.12. Transwell Assay
CT26 and MC38 cell lines exposed to OE-D7Ertd443e and OE-NC were inoculated with 1 × 103 cells in the upper chamber of a Transwell kit (Corning, New York, NY, USA). The upper compartment was supplied with serum-free culture medium, while the lower compartment was supplied with DMEM culture medium supplemented with 20% FBS. The cells were rinsed with PBS buffer, treated with methanol for 30 min for fixation, and subsequently stained with 0.1% crystal violet solution for 30 min. They were then washed with pure water, dried, and photographed.
4.13. Wound-Healing Assay
CT26 and MC38 cells infected with OE-D7Ertd443e and OE-NC were inoculated with 1 × 106 cells in 6-well plates and cultured overnight. Parallel scratches were made on the cell layer with a 200 μL lance tip, washed three times with PBS buffer, and photographed at 0 h, 24 h, 48 h, and 72 h to observe changes in scratch width.
4.14. Apoptosis Assay
CT26 and MC38 cell lines infected with OE-D7Ertd443e and OE-NC were seeded in a 6-well plate at a density of 1 × 106 cells and then incubated for 24 h. The cells were then collected in flow-through tubes and washed with PBS buffer; they were stained with 5 μL of Annexin V-APC and 5 μL of 7-AAD (Beyotime, Shanghai, China) and detected by a machine.
4.15. Statistical Analysis
Biological information obtained in this study was analyzed in databases. All cell experiment data were statistically analyzed using GraphPad Prism 9.0. A t-test was employed for comparisons between two groups, while ANOVA was utilized for comparisons among multiple groups. Data are presented as the mean ± standard error of the mean (Mean ± SEM). p < 0.05 is considered statistically significant. (* p < 0.05, ** p < 0.01, *** p < 0.001).
5. Conclusions
C10orf90, a gene known for its tendency to be expressed at low levels in specific tissues, exhibits differential expression levels in different tumors. This gene has been demonstrated to possess certain diagnostic and prognostic values. Moreover, the expression of C10orf90 is correlated with several other factors, including CNV, DNA methylation, immune checkpoint genes, immune cell infiltration, and drug sensitivity in tumors. Specifically, in COAD, the C10orf90 gene is involved in several immune-related processes and can inhibit the proliferation and migration of colorectal cancer cells. Consequently, the C10orf90 gene shows promise as a diagnostic and prognostic biomarker in tumors, opening up new avenues for further research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms251910496/s1.
Author Contributions
Conception and design: C.R. Acquisition of data: C.R. and Y.Z. Drafting of the article: C.R. Study supervision: C.R., Y.Z., D.C., M.Z., P.Y., R.Z. and Y.L. Critical revision of the article: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [No. 82173087], and Guangdong Medical Research Foundation through [No. A2024236], and the Project in Key Areas of Guangdong Province’s General Colleges and Universities [No. 2024ZDZX2079].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the main text, figures, tables, and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Glover, T.W.; Berger, C.; Coyle, J.; Echo, B. DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum. Genet. 1984, 67, 136–142. [Google Scholar] [CrossRef]
- Ma, K.; Qiu, L.; Mrasek, K.; Zhang, J.; Liehr, T.; Quintana, L.G.; Li, Z. Common fragile sites: Genomic hotspots of DNA damage and carcinogenesis. Int. J. Mol. Sci. 2012, 13, 11974–11999. [Google Scholar] [CrossRef] [PubMed]
- Sarni, D.; Kerem, B. The complex nature of fragile site plasticity and its importance in cancer. Curr. Opin. Cell Biol. 2016, 40, 131–136. [Google Scholar] [CrossRef]
- Walsh, E.; Wang, X.; Lee, M.Y.; Eckert, K.A. Mechanism of Replicative DNA Polymerase Delta Pausing and a Potential Role for DNA Polymerase Kappa in Common Fragile Site Replication. J. Mol. Biol. 2013, 425, 232–243. [Google Scholar] [CrossRef]
- Li, S.; Wu, X. Common fragile sites: Protection and repair. Cell Biosci. 2020, 10, 29. [Google Scholar] [CrossRef]
- Palumbo, E.; Russo, A. Common fragile site instability in normal cells: Lessons and perspectives. Genes Chromosomes Cancer 2019, 58, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Irony-Tur Sinai, M.; Kerem, B. DNA replication stress drives fragile site instability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2018, 808, 56–61. [Google Scholar] [CrossRef]
- Yan, S.; Qiu, L.; Ma, K.; Zhang, X.; Zhao, Y.; Zhang, J.; Li, X.; Hao, X.; Li, Z. FATS is an E2-independent ubiquitin ligase that stabilizes p53 and promotes its activation in response to DNA damage. Oncogene 2014, 33, 5424–5433. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Mao, J.H.; Weise, A.; Mrasek, K.; Fan, X.; Zhang, X.; Liehr, T.; Lu, K.H.; Balmain, A.; et al. An HDAC1-binding domain within FATS bridges p21 turnover to radiation-induced tumorigenesis. Oncogene 2010, 29, 2659–2671. [Google Scholar] [CrossRef]
- Qiu, L.; Hu, L.; Wang, H.; Li, J.; Ruan, X.; Sun, B.; Zhi, J.; Zheng, X.; Gu, L.; Gao, M.; et al. FATS regulates polyamine biosynthesis by promoting ODC degradation in an ERβ-dependent manner in non-small-cell lung cancer. Cell Death Dis. 2020, 11, 839. [Google Scholar] [CrossRef]
- Kenawy, N.; Kalirai, H.; Sacco, J.J.; Lake, S.L.; Heegaard, S.; Larsen, A.C.; Finger, P.T.; Milman, T.; Chin, K.; Mosci, C.; et al. Conjunctival melanoma copy number alterations and correlation with mutation status, tumor features, and clinical outcome. Pigment Cell Melanoma Res. 2019, 32, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Randic, T.; Kozar, I.; Margue, C.; Utikal, J.; Kreis, S. NRAS mutant melanoma: Towards better therapies. Cancer Treat. Rev. 2021, 99, 102238. [Google Scholar] [CrossRef] [PubMed]
- Alqathama, A. BRAF in malignant melanoma progression and metastasis: Potentials and challenges. Am. J. Cancer Res. 2020, 10, 1103–1114. [Google Scholar] [PubMed]
- Zhang, J.; Wu, N.; Zhang, T.; Sun, T.; Su, Y.; Zhao, J.; Mu, K.; Jin, Z.; Gao, M.; Liu, J.; et al. The value of FATS expression in predicting sensitivity to radiotherapy in breast cancer. Oncotarget 2017, 8, 38491–38500. [Google Scholar] [CrossRef]
- Song, F.; Zhang, J.; Qiu, L.; Zhao, Y.; Xing, P.; Lu, J.; Chen, K.; Li, Z. A functional genetic variant in fragile-site gene FATS modulates the risk of breast cancer in triparous women. BMC Cancer 2015, 15, 559. [Google Scholar] [CrossRef]
- Liu, F.; Liao, Z.; Qin, L.; Zhang, Z.; Zhang, Q.; Han, S.; Zeng, W.; Zhang, H.; Liu, Y.; Song, J.; et al. Targeting VPS72 inhibits ACTL6A/MYC axis activity in HCC progression. Hepatology 2023, 78, 1384–1401. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Cao, Y.; Qin, J.; Song, X.; Zhang, Q.; Shi, Y.; Cao, L. DNA methylation, its mediators and genome integrity. Int. J. Biol. Sci. 2015, 11, 604–617. [Google Scholar] [CrossRef]
- Dercle, L.; Sun, S.; Seban, R.D.; Mekki, A.; Sun, R.; Tselikas, L.; Hans, S.; Bernard-Tessier, A.; Mihoubi, B.F.; Aide, N.; et al. Emerging and Evolving Concepts in Cancer Immunotherapy Imaging. Radiology 2023, 306, 32–46. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, K.; Zhang, J.; Zhu, J.; Xi, Q.; Wang, H.; Zhang, Z.; Cheng, Y.; Yang, G.; Liu, H.; et al. Loss of fragile site-associated tumor suppressor promotes antitumor immunity via macrophage polarization. Nat. Commun. 2021, 12, 4300. [Google Scholar] [CrossRef] [PubMed]
- Kaler, P.; Augenlicht, L.; Klampfer, L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: A crosstalk interrupted by vitamin D3. Oncogene 2009, 28, 3892–3902. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Voth Park, J.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.; Xu, Z.; Yu, S.; Huo, J.; Tuersun, A.; Zheng, M.; Zhao, J.; Zong, Y.; Lu, A. IL-17A-mediated mitochondrial dysfunction induces pyroptosis in colorectal cancer cells and promotes CD8 + T-cell tumour infiltration. J. Transl. Med. 2023, 21, 335. [Google Scholar] [CrossRef] [PubMed]
- Koubek, E.J.; Santy, L.C. Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement. Cells 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Strasser, A.; Kelly, G.L. Should mutant TP53 be targeted for cancer therapy? Cell Death Differ. 2022, 29, 911–920. [Google Scholar] [CrossRef]
- Boutelle, A.M.; Attardi, L.D. p53 and Tumor Suppression: It Takes a Network. Trends Cell Biol. 2021, 31, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Hsu, W.B.; Tsai, J.J.; Tang, C.J.; Tang, T.K. CEP295 interacts with microtubules and is required for centriole elongation. J. Cell Sci. 2016, 129, 2501–2513. [Google Scholar] [CrossRef]
- Xiao, J.; Dai, R.; Negyessy, L.; Bergson, C. Calcyon, a Novel Partner of Clathrin Light Chain, Stimulates Clathrin-mediated Endocytosis. J. Biol. Chem. 2006, 281, 15182–15193. [Google Scholar] [CrossRef]
- Hong, S.; Lin, H.; Wang, C.; Chang, C.; Lin, A.M.; Yang, J.C.; Lo, Y. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnol. 2019, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2023 update. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef]
- Hafliger, E.; Boccaccino, A.; Lapeyre-Prost, A.; Perret, A.; Gallois, C.; Antista, M.; Pilla, L.; Lecomte, T.; Scartozzi, M.; Soularue, E.; et al. Encorafenib plus cetuximab treatment in BRAF V600E-mutated metastatic colorectal cancer patients pre-treated with an anti-EGFR: An AGEO-GONO case series. Eur. J. Cancer (1990) 2022, 168, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, G.; Zheng, X.; Chang, W.; Fu, J.; Zhang, T.; Lin, Q.; Lv, Y.; Zhu, Z.; Tang, W.; et al. Treatment of metastatic colorectal cancer with BRAF V600E mutation: A multicenter real-world study in China. Eur. J. Surg. Oncol. 2023, 49, 106981. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Mao, M.; Tian, F.; Bornman, W.G.; Bollag, G.; Mills, G.B.; Powis, G.; Desai, J.; Gallick, G.E.; Davies, M.A.; Kopetz, S.; et al. Resistance to BRAF Inhibition in BRAF-Mutant Colon Cancer Can Be Overcome with PI3K Inhibition or Demethylating Agents. Clin. Cancer Res. 2013, 19, 657–667. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic. Acids. Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Human Genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Liu, C.; Hu, F.; Xia, M.; Han, L.; Zhang, Q.; Guo, A.; Wren, J. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor–immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic. Acids. Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic. Acids. Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.J.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.A.; Thompson, I.R.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic. Acids. Res. 2012, 41, D955–D961. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.G.; Seashore-Ludlow, B.; Cheah, J.H.; Adams, D.J.; Price, E.V.; Gill, S.; Javaid, S.; Coletti, M.E.; Jones, V.L.; Bodycombe, N.E.; et al. Correlating chemical sensitivity and basal gene expression reveals mechanism of action. Nat. Chem. Biol. 2016, 12, 109–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).